Abstract
Gait and balance deficits are a frequent complaint in MS but poorly captured by stopwatch-timed tests or rating scales. Body-worn accelerometers and gyroscopes are able to detect gait and balance abnormalities in people with MS who have normal walking speeds. Few longitudinal studies exist using this technology to study the evolution of mobility deficits. The purpose of this study was to determine if body-worn sensors detected any decline in gait and balance measures in people with MS over time. Twenty-seven people with MS (13 mildly disabled, Self-Rated Expanded Disability Status Scale 0 - 3.5; 14 moderately disabled, SR-EDSS 4.0 -5.5) who had normal walking speeds and 18 matched control subjects underwent gait and balance testing using body-worn sensors every 6 months for 18 months. While no parameter worsened over time, the moderately disabled MS cohort performed more poorly than the mildly disabled MS cohort who, in turn, were worse than control subjects for both objective and subjective walking and balance measures. Furthermore, the moderately disabled MS cohort demonstrated greater variation in between-visit performance than did the less disabled MS cohort or controls (Bonferroni-corrected p < 0.05). Variability may be a key indicator of worsening gait and balance disability in MS.
Keywords: multiple sclerosis, gait, postural balance, outcome assessment, rehabilitation, accelerometry
1. Introduction
Gait and balance impairment, the hallmark of MS, is a frequent and disabling complaint affecting about a quarter of people with MS at disease onset and nearly half by five years (1). Yet typical clinical measures capturing gait and balance dysfunction are insensitive to mild disease or subtle worsening. For instance, at least 20% slowing in the walking time is required for the Timed 25 Foot Walk (T25FW) to be considered clinically significant (2). The Expanded Disability Status Scale (EDSS), a physician-administered global rating scale of MS neurological disability, is notoriously slow to detect change and prone to intra- and inter-rater reliability problems (3). Because MS gait and balance problems occur early and commonly, measures of mobility dysfunction with greater sensitivity than the T25FW and EDSS may detect MS worsening sooner than the typical 2 year clinical trial needed to demonstrate clinical worsening (4). Improved establishment and monitoring of MS functional status is necessary to individualize MS treatment including optimizing disease-modifying therapy, identifying deficits with rehabilitation potential, and encouraging lifelong treatment adherence.
Until recently, specialized motion-analysis laboratories were required to capture sophisticated gait and balance data. Portable technologies now that collect equivalent data rapidly and with instant analysis are being widely explored for use in MS both in clinic and at home. These include accelerometers, pedometers, and pressure mats for gait analysis (5,6). Novel, synchronized, body-worn inertial sensors housing both accelerometers and gyroscopes derive data most closely matched to the 3-dimensional information obtained in a motion-analysis laboratory (7). We previously demonstrated that these body-worn sensors detected objective gait and balance deficits among people with MS who had normal walking speeds compared to matched control subjects when traditional T25FW could not suggesting improved sensitivity of these measures (8).
To our knowledge, no studies have examined the use of body-worn inertial sensors longitudinally in MS as a measure of functional decline. We followed our original cohort of MS subjects with normal walking speeds and matched control subjects every 6 months for 18 months and asked if the abnormal gait and balance parameters captured by body-worn sensors worsen over time in this cohort.
2. Patients
The Oregon Health & Science University Institutional Review Board approved the study in accordance with the Declaration of Helsinki. All subjects gave written informed consent prior to assessments.
Subject recruitment and sample size estimations are described in the baseline analysis paper (8). Briefly, people with MS of any type were included if their T25FW time was within two standard deviations (< 5 seconds, e.g. normal walking speed) of the age- and sex-matched control group, and had no other cause for gait or balance dysfunction. Visits were postponed by at least 60 days after an MS exacerbation.
3. Methods
3.1 Protocol
Two trials of the T25FW were recorded with a stopwatch and averaged according to instructions for the Multiple Sclerosis Functional Composite (9). Instrumented tasks were completed while subjects had portable body-worn sensors attached to their wrists, ankles, sternum and lumbar back according to previously described methods (8). For the gait task, subjects were instructed to stand up from a chair, walk 25 feet, turn, walk back to the chair and sit down, all “as quickly and safely as possible”. The balance task was completed by having subjects stand with arms crossed and feet placed by a template for 30 seconds in eyes opened (EO) and eyes closed (EC) conditions(10,11). Three trials of each instrumented task were completed, and the median and standard deviation over the 3 trials was calculated.
Self-reported gait and balance measures included the Multiple Sclerosis Walking Scale 12, V1 (MSWS12) and the Activities of Balance Confidence scale (ABC) (12,13). Subjects rated their MS disability using a self-rated EDSS (SR-EDSS) shown to correlate with the physician-rated version (14,15).
3.2 Equipment
A total of six body-worn sensors (Xsens, Enschede, The Netherlands www.xsens.com) each including a 3-dimentional gyroscope and tri-axial accelerometer sampling at 50 Hz were used, as previously described (8). The sensors were wired serially and connected to a portable data-receiver on a waist belt. The data-receiver then wirelessly streamed data to a laptop.
3.3 Data analysis
Gait and balance objective measures were automatically derived from acceleration and angular velocity signals using the APDM Mobility Lab software (APDM, Inc, Portland, OR, USA) and a user interface. Pre-processing of signals to extract gait and balance measures has been previously described (10,11). Briefly, the algorithm segments automatically the different parts of the gait task and provides separate analysis and measures for each part. Specifically, to analyze steady-state gait, after detecting sit-to stand and stand-to-sit transitions and turns, steps within turns and transitions were removed. Only the remaining steps, which were taken only during straight walking, were used for further analysis (11).
Here, we present those gait and balance measures that were significantly different between MS and control groups in our previously published paper (8). These included trunk yaw range of motion (Trunk ROM yaw) during gait, turning duration, sway acceleration amplitude (reported in this paper as the correlated measure of sway range in the mediolateral direction EC; Sway Range ML EC), and sway jerkiness EC ML (nJerk ML EC, Jerk is calculated from the first derivative of the acceleration) during quiet stance. In addition, we included other commonly reported mobility measures such as: gait velocity, sway area in the EO and EC conditions, and the percentage difference in area of sway between EO to EC condition (Sway Area Ratio, computed as the ratio between the median sway area in the EO and EC conditions normalized to EO).
3.4 Statistical analysis
Subjects from the baseline study (MS = 31, controls = 28) were included for analysis if they completed at least 2 of the 4 testing visits (MS = 27, controls = 18). The MS group was divided in two subgroups based on their initial SR-EDSS: MSmild (n = 13, SR-EDSS = 0 - 3.5), and MSmoderate (MSmod, n = 14, SR-EDSS = 4 - 5.5), similar to other studies (16).
Normality of the data was verified with the Shapiro-Wilk test before parametric analyses were performed. To assess the longitudinal changes in the self-rated and objective measures and the differences between the three groups (Controls, MSmild, MSmod), we performed a linear mixed model analysis considering group and time (sessions) as fixed factors. Significant main effects were subjected to post hoc Student's t-tests and Bonferroni corrected for multiple comparisons (specifically, group effect is corrected for 3 comparisons) (17).
As a secondary exploratory analysis, variability was assessed by the standard deviations within the three repetitions of the motor tasks (within-session) and between the different longitudinal visits (between-sessions). A 2X3 ANOVA, variability type (within-session, between-session) × group (Controls, MSmild, and MSmod) was used to investigate group differences among the within-session and between-session variability of the objective measures. Only the cases of significant group effect were subjected to post hoc Student's t-tests and Bonferroni corrected for multiple comparisons (group effect for 3 comparisons) to investigate if the between-session variability differed by group. All statistical analyses were made using NCSS Software, Kaysville, UT.
4. Results
The demographics of the 27 MS and 18 matched control subjects included in analysis are found in Table 1. MS subjects and controls were matched for age, sex, race and BMI. Mean MS disease duration was 10 years (median 5, range 0 - 46 years). All of the MS subjects had relapsing remitting disease, and about half (59%) were taking disease-modifying therapies. One MS subject took 4-aminopyridine, a symptomatic therapy that could affect walking speed, for one visit only but had no appreciable change in walking speed. The average SR-EDSS at baseline was 3.3 (0 - 5.5) with 13 in the mild disability group (MSmild, 2.2 average SR-EDSS) and 14 in the moderate disability group (MSmod, 4.3 average SR-EDSS). At the end of 18 months, the average change in SR-EDSS was - 0.24 (-2.5 to 2.0) for all MS, -0.21 (-2.0 to 1.5) for MSmild, and -0.27 (-2.5 to 2.0) for MSmod. Eighty-nine percent of the MS subjects completed at least 3 of 4 study visits while only 44% of the controls did likewise. Month 12 (third of four visits) had the least compliance with 36% of all subjects (30% MS and 44% Controls) missing this visit.
Table 1. Subject demographics.
| All MS | MSmild (baseline SR-EDSS 0 – 3.5) | MSmoderate (baseline SR-EDSS 4.0 – 5.5) | Controls | |
|---|---|---|---|---|
| N | 27, all RRMS | 13 | 14 | 18 |
| Average age, years (range) | 41 (24 – 67) | 40 (24-67) | 41 (26-59) | 34 (27 – 60) |
| Female, % | 67 | 69 | 64 | 78 |
| BMI, average | 26 | 24 | 28 | 23 |
| Caucasian race, % | 93 | 92 | 93 | 89 |
| Disease duration mean/median (range), years | 10 / 5 (0-46) | 10 / 7 (0 – 33) | 13 / 8 (1 – 46) | NA |
| On disease modifying therapy, % | 59 | 77 | 43 | NA |
| Patient-reported relapses, n | 8 | 1 | 7 (2 subjects with 2 relapses each) |
NA |
| Baseline SR-EDSS mean/median (range, 0-10) | 3.3 / 4.0 (0 - 5.5) | 2.2 / 2.0 (0 – 3.5) | 4.3 / 4.0 (4.0 – 5.5) | NA |
| 18 month SR-EDSS mean/median (range, 0-10) | 3.1 / 4.0 (0 – 6.0) | 2.0 / 2.0 (0 – 4.0) | 4.1 / 4.0 (2.5 – 6.0) | NA |
| 18 month change in SR-EDSS Mean (range) | -0.24 (-2.5 to 2.0) | -0.21 (-2.0 to +1.5) | -0.27 (-2.5 to +2.0) | NA |
BMI, body-mass index; RRMS, relapsing remitting multiple sclerosis; SR-EDSS, self-rated Expanded Disability Status Scale
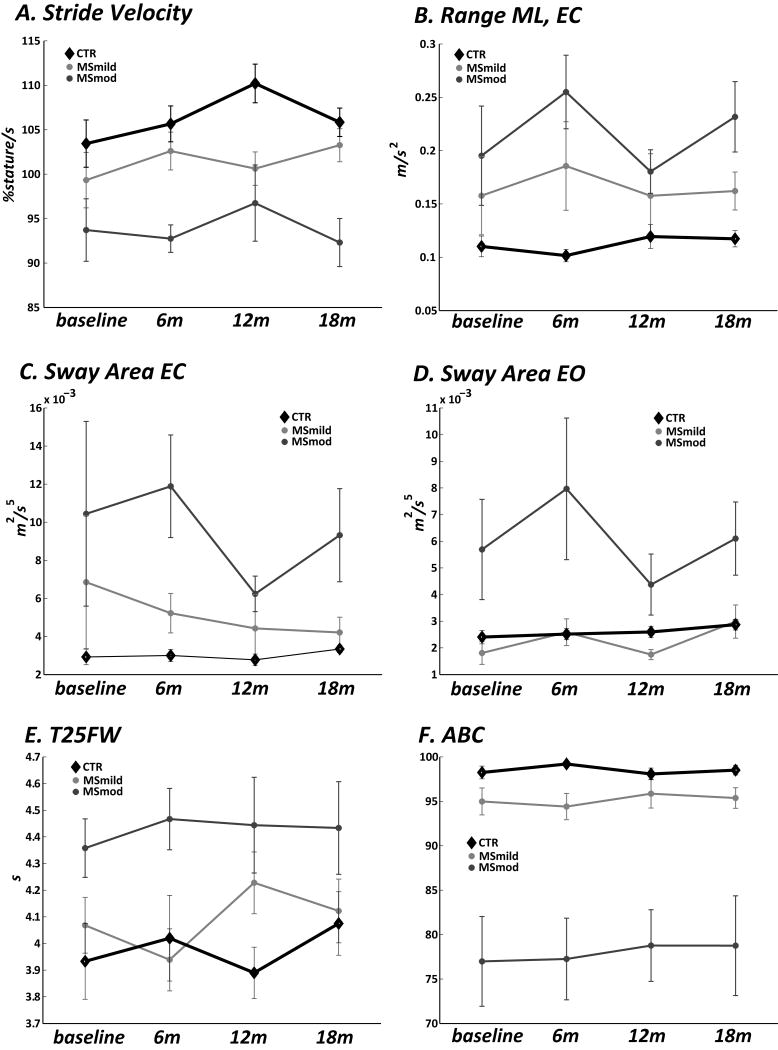
Objective gait and balance measures did not worsen over the 18 months testing period (Fig. 1A-F; see no significant Time or Interaction effect, Table 2) in any group. Similarly, the T25FW, disability (SR-EDSS), and self-rated gait (MSWS12) and balance (ABC) did not worsen over time in MS or controls (Table 2).
Figure 1.
Selected objective gait and balance measures captured by body-worn sensors every 6 months for 18 months include Stride Velocity (A.), sway range in the medio-lateral direction with eyes closed (Range ML EC, B.), Sway Area EC (C.), and Sway Area eyes open (EO, D.). Every 6 month collection of the Timed 25 Foot Walk (T25FW, E.) and the self rated balance measure Activities of Balance Confidence (ABC, F.) are also presented. No one measure worsened across time, however the moderately disabled MS cohort (MSmod) performed worse on all measures than the mildly disabled MS cohort (MSmild). The MSmild group was either worse or the same as the control group for the same measures. Bars represent standard error of the mean (SEM) among each group.
Table 2.
Results of objective measures (gait, turning, balance) captured by body-worn sensors at each visit along with the Timed 25 Foot Walk, self-rated balance (ABC), gait (MSWS-12), and disability (SR-EDSS) questionnaires for each visit. The linear mixed model results showed that there was no significant change in any parameter across time for any group (Time column) but that the measures differed by group (Control, MSmild, MSmod, Group column). The comparisons leading to significant separations between groups are noted (a, b, c). No single group changed across time differently than other groups (Interaction column).
| Baseline | Month 6 | Month 12 | Month 18 | Linear Mixed Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control n = 18 | Control n = 11 | Control n = 10 | Control n = 8 | ||||||||||
| MSmild n = 13 | MSmild n = 10 | MSmild n = 11 | MSmild n = 10 | ||||||||||
| MSmod n = 14 | MSmod n = 10 | MSmod n = 10 | MSmod n = 12 | ||||||||||
|
| |||||||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Time | Group | Interaction | |||
| Gait measures | |||||||||||||
|
| |||||||||||||
|
Stride Velocity (%height/s) |
Control | 103.4 | 2.6 | 105.6 | 3 | 110.2 | 3.2 | 105.8 | 4.1 | F-value | 0.7 | 13.8 | 0.4 |
|
|
|||||||||||||
| MSmild | 99.3 | 3.2 | 102.6 | 3.4 | 100.6 | 3.6 | 103.3 | 3.8 | p | 0.6 | <0.0001 a,b | 0.8 | |
|
|
|||||||||||||
| MSmod | 94.5 | 2.7 | 93.5 | 3.2 | 97.4 | 3.4 | 93 | 2.9 | |||||
|
| |||||||||||||
|
Trunk ROM Yaw (degrees) |
Control | 9.7 | 0.8 | 9.1 | 1 | 8.6 | 1.1 | 8.4 | 1.3 | F-value | 0.5 | 6 | 0.5 |
|
|
|||||||||||||
| MSmild | 11.1 | 1 | 11.2 | 1.1 | 12.2 | 1.2 | 12.1 | 1.2 | p | 0.7 | 0.003 c | 0.8 | |
|
|
|||||||||||||
| MSmod | 11.3 | 0.9 | 9.3 | 1 | 9.4 | 1.1 | 9.5 | 0.9 | |||||
|
| |||||||||||||
| Turning measures | |||||||||||||
|
| |||||||||||||
|
Turning duration (s) |
Control | 1.65 | 0.08 | 1.52 | 0.09 | 1.49 | 0.09 | 1.78 | 0.12 | F-value | 1.2 | 2.6 | 1 |
|
|
|||||||||||||
| MSmild | 1.84 | 0.09 | 1.65 | 0.1 | 1.63 | 0.1 | 1.64 | 0.11 | p | 0.3 | 0.07 | 0.4 | |
|
|
|||||||||||||
| MSmod | 1.73 | 0.08 | 1.85 | 0.09 | 1.71 | 0.1 | 1.77 | 0.09 | |||||
|
| |||||||||||||
| Balance measures | |||||||||||||
|
| |||||||||||||
|
Sway Area EO (m2/s5) |
Control | 0.0024 | 0.001 | 0.0025 | 0.0012 | 0.0026 | 0.0013 | 0.0029 | 0.0016 | F-value | 0.7 | 9.5 | 0.4 |
|
|
|||||||||||||
| MSmild | 0.0018 | 0.0013 | 0.0026 | 0.0014 | 0.0017 | 0.0015 | 0.003 | 0.0015 | p | 0.6 | 0.0001 a,b | 0.9 | |
|
|
|||||||||||||
| MSmod | 0.0057 | 0.0011 | 0.0078 | 0.0013 | 0.0042 | 0.0014 | 0.0058 | 0.0012 | |||||
|
| |||||||||||||
|
Sway Area EC (m2/s5) |
Control | 0.0029 | 0.0019 | 0.003 | 0.0024 | 0.0028 | 0.0024 | 0.0033 | 0.003 | F-value | 0.5 | 6.1 | 0.2 |
|
|
|||||||||||||
| MSmild | 0.0068 | 0.0025 | 0.0052 | 0.0027 | 0.0044 | 0.0027 | 0.0042 | 0.0027 | p | 0.7 | 0.003 a | 0.9 | |
|
|
|||||||||||||
| MSmod | 0.0101 | 0.0022 | 0.0112 | 0.0027 | 0.0061 | 0.0027 | 0.0088 | 0.0023 | |||||
|
| |||||||||||||
|
nJerk ML EC (unitless) |
Control | 2.89 | 0.17 | 2.91 | 0.21 | 2.91 | 0.21 | 2.82 | 0.26 | F-value | 0.8 | 9.1 | 0.1 |
|
|
|||||||||||||
| MSmild | 2.23 | 0.22 | 2.23 | 0.23 | 2.38 | 0.23 | 2.04 | 0.22 | p | 0.5 | 0.0002 a,c | 0.9 | |
|
|
|||||||||||||
| MSmod | 2.51 | 0.19 | 2.41 | 0.23 | 2.7 | 0.23 | 2.25 | 0.2 | |||||
|
| |||||||||||||
|
range ML EC (m/s2) |
Control | 0.11 | 0.02 | 0.1 | 0.03 | 0.12 | 0.03 | 0.12 | 0.04 | F-value | 0.4 | 9 | 0.3 |
|
|
|||||||||||||
| MSmild | 0.16 | 0.03 | 0.18 | 0.03 | 0.16 | 0.04 | 0.16 | 0.04 | p | 0.7 | 0.0002 | 0.9 | |
|
|
|||||||||||||
| MSmod | 0.19 | 0.03 | 0.24 | 0.04 | 0.18 | 0.03 | 0.22 | 0.03 | |||||
|
| |||||||||||||
|
Sway Area Ratio (unitless) |
Control | 6.5 | 9.6 | 8.6 | 11.7 | -1.55 | 11.8 | 11.9 | 14 | F-value | 0.7 | 5.6 | 0.5 |
|
|
|||||||||||||
| MSmild | 39.8 | 11.7 | 42.07 | 12.3 | 14.13 | 13.1 | 15.9 | 13.1 | p | 0.5 | 0.004 a,c | 0.8 | |
|
|
|||||||||||||
| MSmod | 30.8 | 10.3 | 33.8 | 12.4 | 31.6 | 12.4 | 35.1 | 11.7 | |||||
|
| |||||||||||||
| Self-rated measures and T25FW | |||||||||||||
|
| |||||||||||||
|
T25FW (s) |
Control | 3.9 | 0.1 | 4 | 0.2 | 3.9 | 0.2 | 4 | 0.2 | F-value | 0.2 | 7.4 | 0.4 |
|
|
|||||||||||||
| MSmild | 4.1 | 0.2 | 3.9 | 0.2 | 4.2 | 0.2 | 4.1 | 0.2 | p | 0.9 | 0.001 a,b | 0.9 | |
|
|
|||||||||||||
| MSmod | 4.3 | 0.1 | 4.4 | 0.2 | 4.3 | 0.2 | 4.4 | 0.1 | |||||
|
| |||||||||||||
|
ABC (unitless) |
Control | 98.2 | 2.8 | 99.2 | 4 | 98.1 | 3.8 | 98.5 | 4.2 | F-value | 0.02 | 43 | 0.06 |
|
|
|||||||||||||
| MSmild | 94.5 | 3.8 | 94.4 | 4 | 95.6 | 4 | 95.3 | 4 | p | 1 | <0.0001 a,b | 1 | |
|
|
|||||||||||||
| MSmod | 76.7 | 3.2 | 76.9 | 3.7 | 74.3 | 4 | 76.1 | 3.3 | |||||
|
| |||||||||||||
|
MS12W-1 2 (unitless) |
Control | 0 | 3.4 | 0.2 | 4.6 | 0.2 | 4.6 | 0.6 | 5.1 | F-value | 0.1 | 85 | 0.4 |
|
|
|||||||||||||
| MSmild | 2.3 | 4.6 | 4.6 | 4.8 | 4.1 | 4.8 | 3 | 4.8 | p | 0.9 | <0.0001 a,b | 0.9 | |
|
|
|||||||||||||
| MSmod | 42.5 | 3.9 | 33.7 | 4.5 | 37.5 | 4.8 | 35 | 4 | |||||
|
| |||||||||||||
| SR-EDSS | Msmild | 1.9 | 0.3 | 2.5 | 0.3 | 2.7 | 0.3 | 1.8 | 0.3 | F-value | 1.4 | 79 | 0.4 |
|
|
|||||||||||||
| MSmod | 4.3 | 0.2 | 4.2 | 0.3 | 4.6 | 0.3 | 4 | 0.2 | p | 0.2 | <0.0001 | 0.7 | |
= group differences between controls and MS moderate p < 0.05, Bonferroni adjusted for 3 comparisons.
= group differences between MS mild and MS moderate p < 0.05, Bonferroni adjusted for 3 comparisons.
= group differences between controls and MS mild p < 0.05, Bonferroni adjusted for 3 comparisons.
ABC, Activities of Balance Confidence scale; EC, eyes closed; EO, eyes open; m, meter; ML, medio-lateral; MSmod, MS moderately disabled; MSWS12, Multiple Sclerosis Walking Scale-12; nJERK, normalized Jerk; s, second; SR-EDSS, self-rated Expanded Disability Status Scale; T25FW, Timed 25 Foot Walk.
However, there was a group effect such that all but one objective measure separated the 3 groups (see Group effect column, Table 2). In general, MS mod performed the worse than MSmild, who in turn were worse than the Control group. Specifically, Stride Velocity was significantly slower and nJerk ML EC significantly reduced in MSmod compared to controls (Bonferroni adjusted p for 3 comparison <0.05, Fig.1, Table 2). Sway Area EO, Sway Area EC, range ML EC, and Sway Area Ratio were significantly greater in MSmod compared to controls (Bonferroni adjusted p for 3 comparisons <0.05, Fig. 1, Table 2). Similarly, Stride Velocity was significantly slower in MSmod compared to MSmild and Sway Area EO was greater in MSmod compared to MSmild (Bonferroni adjusted p for 3 comparison <0.05. Fig. 1, Table 2). In addition, Trunk ROM Yaw, Sway range ML EC, and Sway Area Ratio were significantly higher in MS mild compared to controls (Bonferroni adjusted p for 3 comparison <0.05, Fig. 1, Table 2). The T25FW showed significant differences between MSmod and MS mild, and MSmod and Controls, but not MSmild and Controls (Bonferroni adjusted p for 3 comparison <0.05, Fig. 1, Table 2).
Self-rated measures followed the pattern of the worst ratings in the MSmod compared to MSmild, and MSmild compared to Controls. Both the ABC and MSWS-12 were significantly worse (lower score for ABC, higher score for MSWS12) in MSmod compared to Controls and in MSmod compared to MSmild (Bonferroni adjusted p for 3 comparison <0.05, Fig. 1, Table 2).
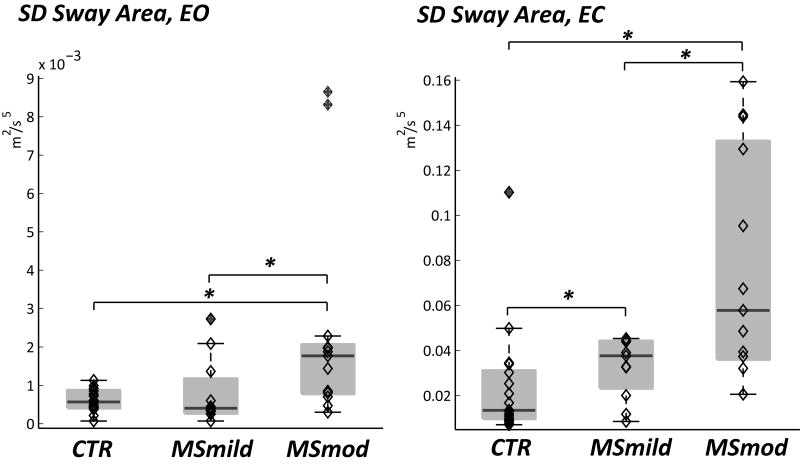
Within-session and between-session variability was evaluated as an exploratory analysis, expecting the same or greater between-session than within-session variability overall and more variability among the more disabled MS cohort. There was greater variability in the between-sessions SD than within-session SD for nJerk ML EC and Sway Area EC, however more within-session variability for Trunk ROM yaw and Sway Range ML EC (Table 3, Variability Type column). The Student's t-test demonstrated that the between-session variability for Sway Area EO and Sway Area EC were significantly greater in MSmod compared to controls and in MSmod compared to MS mild (Fig. 2, Table 3: Group column). Sway Area EC SD was also significantly greater in MSmild compared to Controls (Fig. 2, Table 3: Group column).
Table 3.
There was greater between-session variability (variability measured as standard deviation, SD, across three trials) than within-session variability for nJerk ML EC and Sway Area EC, however more within-session variability for Trunk ROM yaw and Sway Range ML EC (Variability Type column). Student's t-test demonstrated significantly greater between-session variability for Sway Area EO and Sway Area EC in MSmod compared to controls and in MSmod compared to MS mild (Group column). Sway Area EC between-session variability was also significantly greater in MSmild compared to Controls (Group column).
| Objective measures | CTR | MS mild | MS mod | t-test | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Variability type | Groups | Interaction | ||||
| Stride Velocity, SD | within-session between-sessions | 4.49 | 0.33 | 5.21 | 0.47 | 4.72 | 0.42 | F-value | 0.2 | 0.9 | 0.1 | |
| 3.92 | 0.68 | 4.84 | 0.52 | 4.85 | 0.97 | p-value | 0.6 | 0.4 | 0.8 | |||
|
| ||||||||||||
| Trunk ROM yaw, SD | within-session between-sessions | 2.67 | 0.30 | 3.11 | 0.24 | 2.80 | 0.24 | F-value | 46.0 | 0.6 | 0.8 | |
| 1.29 | 0.16 | 1.39 | 0.24 | 1.74 | 0.22 | p-value | <0.0001 | 0.5 | 0.4 | |||
|
| ||||||||||||
| Turning Duration, SD | within-session between-sessions | 0.104 | 0.016 | 0.177 | 0.038 | 0.235 | 0.054 | F-value | 0.2 | 3.2 | 1.7 | |
| 0.151 | 0.022 | 0.234 | 0.042 | 0.174 | 0.019 | p-value | 0.6 | 0.1 | 0.2 | |||
|
| ||||||||||||
| Sway Area EO, SD | within-session between-sessions | 0.00065 | 0.00015 | 0.00047 | 0.00016 | 0.00242 | 0.00112 | F-value | 0.1 | 5.3 | 1.4 | |
| 0.00060 | 0.00008 | 0.00081 | 0.00024 | 0.00293 | 0.00077 | *+ | p-value | 0.7 | 0.01 | 0.2 | ||
|
| ||||||||||||
| nJerk ML EC, SD | within-session between-sessions | 0.397 | 0.027 | 0.386 | 0.042 | 0.422 | 0.069 | F-value | 5.3 | 0.5 | 0.2 | |
| 0.490 | 0.063 | 0.467 | 0.083 | 0.566 | 0.056 | p-value | 0.02 | 0.6 | 0.8 | |||
|
| ||||||||||||
| Sway Range ML EC, SD | within-session between-sessions | 0.02132 | 0.00522 | 0.07927 | 0.04774 | 0.06246 | 0.01527 | F-value | 11.3 | 1.5 | 1.2 | |
| 0.00097 | 0.00016 | 0.00288 | 0.00132 | 0.00502 | 0.00178 | p-value | 0.002 | 0.2 | 0.3 | |||
|
| ||||||||||||
| Sway Area EC, SD | within-session between-sessions | 0.00081 | 0.00015 | 0.00553 | 0.00461 | 0.00268 | 0.00106 | F-value | 65.0 | 4.1 | 6.4 | |
| 0.02431 | 0.00308 | 0.04456 | 0.01243 | 0.07155 | 0.01429 | *+^ | p-value | <0.0001 | 0.02 | 0.004 | ||
MSmod vs CTR, between-session variability, p < 0.05
MSMod vs MSmild, between-session variability,y p < 0.05
MSmild vs CTR, between-session variability, p < 0.05
Figure 2.
Between-sessions variability quantified as standard deviation (SD) of Sway Area eyes open (EO) and eyes closed (EC). Boxplot and individual subjects are shown for controls, MSmild and MSmod. Variability was greater in the moderate MS group compared to the mildly disabled MS group and controls. * indicates a p<0.05.
5. Discussion
The objective gait and balance measures captured by body-worn sensors that were significantly different between MS subjects with normal walking speeds and matched controls at baseline did not worsen over 18 months, even when the MS cohort was separated into mild and moderate disability groups. Self-rated disability (SR-EDSS), gait (MSWS12) and balance (ABC) scales also did not worsen over the testing period. However, in general the moderate disability group both performed during testing and self-rated their abilities as worse than the mildly disabled MS group who in turn performed more poorly than the control group. Our exploratory analyses regarding variability in performance might suggest an increase in between-visit variability in the more disabled cohort; however some measures unexpectedly demonstrated greater within-session than between-session variability.
The lack of worsening of objective gait and balance parameters over the 18 months can be interpreted in several ways. There may, indeed, have been no worsening as supported by the static T25FW and self-rated measures. Ytterberg et al. followed 200 people with MS every six months for two years and was also unable to show a worsening trajectory in the T25FW, upper arm function, cognition, fatigue, depression, or activity level (18). Another possibility is that functional measures such as gait are subject to compensatory strategies that mask underlying deterioration in the short-term. Therefore, unlike MRI plaques which accumulate or enlarge thereby rendering them amenable to quantification, any gait and balance worsening during an 18 month period among this high-functioning cohort may have been subject to compensatory mechanisms. In fact, MS subjects rated their disability as improved (lower SR-EDSS score) at the end of 18 months than at the beginning which could indicate compensation for prior deficits (Table 1). The infrequent testing protocol many have been insufficient to demonstrate true fluctuations in abilities over time. Technical error is less likely a factor as similar sensors were able to capture significant gait and balance decline using the same testing protocol in Parkinson's disease over 18 months (19,20). A more frequent testing strategy such as in-home monitoring for longer or more frequent periods is now feasible with current wearable sensor technology.
We stratified MS subjects into a mild (EDSS 0 – 3.5) and moderate (EDSS 4.0 – 5.5) disability groups expecting a greater likelihood of detecting gait and balance decline in the more disabled group. The rationale for this expectation derives from natural history studies that show a steady rate of gait decline once a moderate level of disability is reached. In a British Columbian registry, Tremlett et al. demonstrated a six to seven year period of progression from EDSS 3.0 (unlimited walking range) to 6.0 (unilateral aid required to walk) regardless of time taken to reach an EDSS of 3.0 in nearly all people with MS (21). Confavreux and Vukusic recorded a roughly five year period for French patients to progress from an equivalent EDSS score of 4.0 to 6.0 regardless of MS subtype (16). The linear mixed model analysis did show a significantly worse performance in gait and balance measures and self-rated scales based on disability subtype, but no worsening over 18 months was detected among any subgroup.
We expected that in between session variability would be similar to or greater than within-session variability in performance as captured by the standard deviation of three trials, similar to the method used by Sosnoff et al. who found that variability as measured by the standard deviation of daily activity counts captured by accelerometers worn for 7 days correlated with MS disability, self-reported walking dysfunction, and ambulatory status (5). Variability in MS gait has been found in footfall patterns (22), joint angle positions (23), and lumbar accelerometry patterns (24), and is thought to reflect the overall health of the movement system. As such, increasing variability may be a better indicator of MS mobility decline than absolute values. For the most part, between-session variability was similar to or greater than within-session variability of the measures, however measures that had greater within-session than between-session variability (Trunk ROM yaw and Sway Range ML EC) suggests a low reliability for those particular measures in the present population. For those measures with greater between-session variability (Sway Area EO SD and Sway Area EC SD), the moderately disabled group was worse than the mild MS group, matching clinical experience of more disabled people with MS reporting “good” and “bad” days causing major impacts on day to day functioning.
There are several limitations of this study. 1. The sample size was based on a similar study in Parkinson's disease (11) which, due to differences in disease pathology and patient characteristics, may have underpowered this study. 2. Not all MS subjects and controls were tested at all four sessions. While potentially weakening the strength of the data, the results more closely approximate “real-world” use of body-worn sensors as an outpatient monitoring tool. 3. This study used an early generation of sensors that required serial wiring and was prone to data transmission problems. Current sensor technology is far more reliable, has immediate feedback of successful capture, and no longer requires serial wiring; the independent sensors are the size of a sport-watch and wirelessly transmit the data to a receiving device for instant computer analysis. 4. Recruitment of MS patients based on a normal walking speed may have introduced a recruitment bias. Separate cohorts of subjects with a range of baseline abilities will help determine the reproducibility, test-retest reliability, and specificity of the study findings to MS.
In conclusion, the gait and balance deficits in people with MS who have normal walking speeds captured by body-worn sensors do not worsen over 18 months in an every 6 month testing protocol. Performance on mobility testing and self-rated measures was worse in the moderately disabled group, better in the mildly disabled group, and best among controls. Variability in between-session performance may be greater in the more disabled MS cohort, suggesting that performance variability may be a better indicator of MS disability status and disease progression. Further testing is required to replicate these results, provide test-retest validation, refine a user-friendly testing protocol, and determine the specificity of the gait and balance abnormalities to MS.
Research Highlights.
We measured gait and balance using body-worn sensors in MS patients with normal walking speeds
Gait and balance did not worsen when tested every 6 months for total of 18 months
Performance on objective and subjective mobility measures segregated by global disability level.
Between-visit variability was highest in the most disabled MS cohort
Variability may be an indicator of MS disability and functional decline.
Acknowledgments
This work was supported by a Pilot Grant from the National MS Society [grant number GNEUR0615A]; and the Oregon Clinical and Translational Research Institute (OCTRI) [grant number UL1 RR024140] from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zwibel HL. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv Ther. 2009 Dec;26(12):1043–57. doi: 10.1007/s12325-009-0082-x. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman M, Moyer D, Norton J. The significant change for the Timed 25-foot Walk in the multiple sclerosis functional composite. Mult Scler. 2000 Aug;6(4):286–90. doi: 10.1177/135245850000600411. [DOI] [PubMed] [Google Scholar]
- 3.Goodkin DE, Cookfair D, Wende K, Bourdette D, Pullicino P, Scherokman B, et al. Inter- and intrarater scoring agreement using grades 1[spacing dot above]0 to 3[spacing dot above]5 of the Kurtzke Expanded Disability Status Scale (EDSS) Neurology. 1992 Apr;42(4):859–63. doi: 10.1212/wnl.42.4.859. [DOI] [PubMed] [Google Scholar]
- 4.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis. New England Journal of Medicine. 2012 Sep 20;367(12):1098–107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 5.Sosnoff JJ, Goldman MD, Motl RW. Real-life walking impairment in multiple sclerosis: preliminary comparison of four methods for processing accelerometry data. Mult Scler. 2010 Jul;16(7):868–77. doi: 10.1177/1352458510373111. [DOI] [PubMed] [Google Scholar]
- 6.Motl RW, Weimo Zhu, Park Y, McAuley E, Scott JA, Snook EM. Reliability of Scores From Physical Activity Monitors in Adults With Multiple Sclerosis. Adapted Physical Activity Quarterly. 2007 Jul;24(3):245–53. doi: 10.1123/apaq.24.3.245. [DOI] [PubMed] [Google Scholar]
- 7.Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB. The instrumented Timed Up and Go test: Potential outcome measure for disease modifying therapies in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010 Feb;81(2):171–6. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spain RI, St George RJ, Salarian A, Mancini M, Wagner JM, Horak FB, et al. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture. 2012 Apr;35(4):573–8. doi: 10.1016/j.gaitpost.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite measure (MSFC): an integrated approach to MS clinical outcome assessment. Multiple Sclerosis (13524585) 1999 Aug;5(4):244–50. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 10.Mancini M, Salarian A, Carlson-Kuhta P, Zampieri C, King L, Chiari L, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:59. doi: 10.1186/1743-0003-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salarian A, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Aminian K. iTUG, a Sensitive and Reliable Measure of Mobility. IEEE Trans Neural Syst Rehabil Eng. 2010 Jun;18(3):303–10. doi: 10.1109/TNSRE.2010.2047606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disability & Rehabilitation. 2006 Jun 30;28(12):789–95. doi: 10.1080/09638280500404289. [DOI] [PubMed] [Google Scholar]
- 13.Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability The 12-Item MS Walking Scale (MSWS-12) Neurology. 2003 Jan 14;60(1):31–6. doi: 10.1212/wnl.60.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Bowen J, Gibbons L, Gianas A, Kraft GH. Self-administered Expanded Disability Status Scale with functional system scores correlates well with a physician-administered test. Multiple Sclerosis (13524585) 2001 Jun;7(3):201–6. doi: 10.1177/135245850100700311. [DOI] [PubMed] [Google Scholar]
- 15.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983 Nov;33(11):1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 16.Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain. 2006 Mar 1;129(3):606–16. doi: 10.1093/brain/awl007. [DOI] [PubMed] [Google Scholar]
- 17.West B, Welch KB, Galecki AT. Linear mixed models : a practical guide using statistical software. Boca Raton, FL: Chapman & Hall/CRC; 2007. [Google Scholar]
- 18.Ytterberg C, Johansson S, Andersson M, Widén Holmqvist L, von Koch L. Variations in functioning and disability in multiple sclerosis. A two-year prospective study. J Neurol. 2008 Jul;255(7):967–73. doi: 10.1007/s00415-008-0767-0. [DOI] [PubMed] [Google Scholar]
- 19.Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB. Postural sway as a marker of progression in Parkinson's disease: a pilot longitudinal study. Gait Posture. 2012 Jul;36(3):471–6. doi: 10.1016/j.gaitpost.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salarian A, Zampieri C, Horak FB, Carlson-Kuhta P, Nutt JG, Aminian K. Analyzing 180 degree turns using an inertial system reveals early signs of progression of parkinson's disease. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2009. EMBC 2009. 2009:224–227. doi: 10.1109/IEMBS.2009.5333970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology. 2006 Jan 24;66(2):172–7. doi: 10.1212/01.wnl.0000194259.90286.fe. [DOI] [PubMed] [Google Scholar]
- 22.Sosnoff JJ, Sandroff BM, Motl RW. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait & Posture. 2012 May;36(1):154–6. doi: 10.1016/j.gaitpost.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Crenshaw SJ, Royer TD, Richards JG, Hudson DJ. Gait variability in people with multiple sclerosis. Mult Scler. 2006 Oct;12(5):613–9. doi: 10.1177/1352458505070609. [DOI] [PubMed] [Google Scholar]
- 24.Huisinga JM, Mancini M, St George RJ, Horak FB. Accelerometry Reveals Differences in Gait Variability Between Patients with Multiple Sclerosis and Healthy Controls. Ann Biomed Eng. 2012 Nov 18; doi: 10.1007/s10439-012-0697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]