Abstract
Vocalizations convey information about an individual’s motivational, internal, and social status. As circumstances change, individuals respond by adjusting vocal behavior accordingly. In European starlings, a male that acquires a nest site socially dominates other males and dramatically increases courtship song. Although circulating testosterone is associated with social status and vocal production it is possible that steroid receptors fine-tune status-appropriate changes in behavior. Here we explored a possible role for androgen receptors. Male starlings that acquired nest sites produced high rates of courtship song. For a subset of males this occurred even in the absence of elevated circulating testosterone. Immunolabeling for androgen receptors (ARir) was highest in the medial preoptic nucleus (POM) in males with both a nest site and elevated testosterone. For HVC, ARir was higher in dominant males with high testosterone (males that sang longer songs) than dominant males with low testosterone (males that sang shorter songs). ARir in the dorsal medial portion of the nucleus intercollicularis (DM) was elevated in males with high testosterone irrespective of dominance status. Song bout length related positively to ARir in POM, HVC and DM, and testosterone concentrations related positively to ARir in POM and DM. Results suggest the role of testosterone in vocal behavior differs across brain regions and support the hypothesis that testosterone in POM underlies motivation, testosterone in HVC relates to song quality, and testosterone in DM stimulates vocalizations. Our data also suggest that singing may influence AR independent of testosterone and that alternative androgen-independent pathways regulate status-appropriate singing behavior.
Keywords: androgen receptors, songbird, courtship, testosterone, social communication, motivation, dominance
Vocalizations allow an individual to convey information about current motivational state (e.g., fear, hunger or intention to breed), resource availability (e.g., food or territory) and social status (Bradbury and Vehrencamp, 1998). This information changes as circumstances change, and individuals respond by adjusting vocal behavior accordingly. For example, it is common for individuals that gain a territory or become socially dominant to increase the production of agonistic or courtship vocalizations (e.g., Boseret et al. (2006); Eens et al. (1993); Riters et al. (2004); Sartor and Ball (2005); Wiley et al. (1993)). While the steroid hormone testosterone (T) is known to facilitate seasonal, large scale changes in behavior (e.g., in seasonally breeding birds (Ball et al., 2004; Catchpole and Slater, 2008)), other factors such as steroid receptors may fine-tune changes in behavior to better reflect an individual’s current circumstances. Androgen receptors (ARs) within brain regions involved in sexual motivation and vocal production increase in association with seasonal increases in courtship singing and mating behavior (Gahr and Metzdorf, 1997; Pouso et al., 2010; Soma et al., 1999; Wacker et al., 2010). However, the role played by ARs in status-appropriate vocal production has not been well studied. The goal of the present study was to begin to fill this knowledge gap.
Male European starlings (Sturnus vulgaris) provide an excellent system in which to explore mechanisms underlying status-appropriate vocal behavior. In male starlings the acquisition of a nest site (or nest box in many studies) is an important first step in initiating breeding (Feare, 1984; Gwinner et al., 2002; Kessel, 1957). Prior to nest site acquisition males appear to ignore females and to tolerate other males (Feare, 1984; Kessel, 1957). However, once males acquire nest sites they respond to females with high rates of sexually-motivated song (Riters et al., 2000) and socially dominate other males (Sartor and Ball, 2005). Males that obtain nest boxes have higher concentrations of T than males without nest boxes (Riters et al., 2000), and castration eliminates both nest box occupancy and the production of sexually-motivated song (Pinxten et al., 2002). Male starlings with higher T are more likely to acquire a nest box and to show a secondary increase in T after acquiring a box (Gwinner and Gwinner, 1994; Gwinner et al., 2002). Furthermore, contact with a nest box alone is sufficient to cause a rise in T and luteinizing hormone (Gwinner et al., 2002).
A site in which T or its metabolites act(s) to modify sexually-motivated behavior is the medial preoptic nucleus, an AR-rich region that is central to male sexual behavior across vertebrates ((Ball and Balthazart, 2010; Balthazart and Ball, 2007; Crews, 2005; Hull and Dominguez, 2007); often referred to as POM in birds). Lesions to the POM in male starlings suppress sexually-motivated singing behavior but increase nonsexually-motivated song, highlighting a role for this region in adjusting song to match a specific social context (Alger et al., 2009; Alger and Riters, 2006; Riters and Ball, 1999). The volume of the POM is T sensitive (Balthazart et al., 2010), T implants in this area stimulate singing behavior in male canaries (Alward et al., 2013), and multiple studies link the POM to nest box ownership and sexually-motivated singing behavior in male starlings (Heimovics and Riters, 2005; Kelm et al., 2011; Kelm-Nelson et al., 2013; Riters et al., 2000).
In the POM, both T and its metabolite estradiol are implicated in the regulation of sexually-motivated behaviors (Balthazart and Surlemont, 1990; Christensen and Clemens, 1975; Clancy et al., 2000; Riters et al., 1998; Watson and Adkins-Regan, 1989), suggesting that androgens and/or estrogens may underlie changes in status-appropriate vocal behavior. We focus here on ARs because they are present at high densities in the POM (Balthazart et al., 1998; Smith et al., 1996), and past work in California mice shows that repeatedly winning territorial competitions (similar to a starling acquiring and defending a nest box) is tied to both a pulse of T and upregulation of AR in brain regions implicated in motivation (Fuxjager et al., 2010).
Circulating androgens increase AR expression in multiple brain regions (Kemppainen et al., 1992; Meek et al., 1997; Syms et al., 1985). This suggests that dynamic changes in testosterone (induced by the onset of the breeding season, agonistic or sexual interactions, or a change in dominance status) may modify activity in the POM (and a male’s associated motivational state) to promote status-appropriate vocal production. Pre-existing differences in AR may also underlie differences observed across individuals in singing behavior. Positive correlations have been found between AR mRNA and aggressive behavior in male and female dark-eyed juncos and between AR mRNA and T concentrations in males (Rosvall et al., 2012). However AR mRNA and T did not correlate in females suggesting that the two can be dissociated. Past studies also suggest that increases in AR following territorial competition may be brain region specific, may vary based on the behavioral context, or occur independent of circulating T (Fuxjager et al., 2010).
Our hypothesis was that the acquisition of dominance status and associated high T upregulates AR activity in the POM to promote sexually-motivated courtship singing (Figure 1A–C). Our data also allowed us to consider the additional (not mutually exclusive) possibilities that behavior influences AR activity independent of T and that alternative androgen-independent pathways may regulate status-appropriate singing behavior (Figure 1D and E). To begin to test these hypotheses, male starlings were given the opportunity to compete for nesting sites and behavior was observed in the presence of a female. Plasma T was measured before and after the study, and immunolabeled AR were quantified in POM in males with and without nest boxes. AR were also quantified in two additional AR-rich vocal control regions with which the POM interacts (Appeltants et al., 2000; Balthazart et al., 1992; Riters and Alger, 2004; Shaw and Kennedy, 2002; Smith et al., 1996), HVC (used as a proper name), and the dorsomedial portion of the nucleus intercollicularis (DM).
Figure 1.
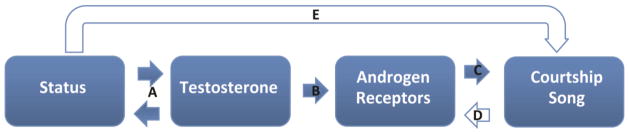
Schematic model of the hypotheses being tested. Our experiment was designed to provide insight into the hypothesis that A) the acquisition of dominance status and associated high T (variables that can influence each other reciprocally) B) upregulates AR in the POM and possibly HVC and DM C) to promote courtship singing. Our data also allowed us to consider the additional (not mutually exclusive) possibilities that D) behavior influences AR activity independent of T and that E) alternative androgen-independent pathways regulate status-appropriate singing behavior.
Methods
Animals
Male (n=20) and female starlings (n=4) were captured during winter 2009–2010 on a single farm in Madison, Wisconsin using baited fly-in traps. After capture, birds were housed indoors in stainless steel, single sex cages (91 cm x 47cm x 47 cm) in groups of 5 birds per cage in the University of Wisconsin’s Department of Zoology animal facilities. Food (Purina Mills Start and Grow Sunfresh Recipe, 61S3-IGH-G) and water were provided ad libitum. Each animal was assigned a numbered band for identification. All procedures and protocols adhered to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Housing conditions
After trapping, all birds were housed in captivity for at least one year. Prior to study initiation (beginning January, 2011), birds were placed on artificial photoperiods of 18h light (L):6h dark (D) for 6 weeks, followed by a photoperiod of 8L:16D for an additional 6 weeks. All birds in the study were exposed to these conditions in the same room at the same time. These photoperiod manipulations induce photosensitivity, a condition in which male starlings exposed to day lengths > 11h light per 24h period show increased gonad volume and plasma T concentrations typical of the spring breeding season (Dawson, 1983; Dawson et al., 2001). Immediately prior to releasing males into outdoor aviaries (described below), approximately 300 μl of blood was collected from each bird via alar venipuncture into heparin treated capillary-action centrifuge tubes and centrifuged at 3000 rpm for 20 min at 4°C. Approximately 50 μl blood plasma (supernatant) was then collected and stored at −80°C until assayed for T (12.5 μl is needed to run the assay described below). Groups of males were placed into one of four outdoor aviaries (five birds per aviary; aviary dimensions 2.13 m x 2.4 m x 1.98 m) with access to natural light, four nest boxes, perches, nesting material, birdbath, food and water ad libitum. Social groups were maintained from the beginning of light manipulations so that all individuals were familiar with their aviary-mates. Natural day length during this time (April 11–28, 2011) was approximately 13L:11D. Birds were allowed to habituate to aviaries for 13 days before the beginning of behavioral observations.
Behavioral observations
Behavioral observations were collected for 20 minutes on each of 4 days prior to brain collection for AR immunohistochemistry. Aviaries were observed in a rotating order. Four female conspecifics were used to stimulate male courtship song during observation periods on a daily rotating basis. On each observation day, a female conspecific was released into an aviary, and fresh nesting material (grass clippings and green leaves) was placed on the floor of the aviary. (A single female was used each day, rotating through all four aviaries.) After introduction of a female to an aviary, a single observer recorded behavior for 20 minutes. Bouts of gathering nesting material, feeding, drinking, and preening were recorded, with a bout defined as a behavior separated by more than two seconds from a previous occurrence. Additionally, the numbers of times males displaced others, entered a nest box, landed on a nest box perch, and looked into a box were recorded continuously. (Displacements were counted when a male approached to within approximately 5 cm of another and the approached male departed.) For singing behavior, the number of songs (song rate) and duration of each song (song bout length) in seconds were recorded. All birds were observed continuously for the full observation period (as in Heimovics and Riters (2005, 2008); Riters et al. (2005)). Starlings display a high level of site fidelity (remaining on and returning frequently to a single perch), and they are not constantly in motion or singing; thus continuous observations of multiple birds in a single flock (in some cases up to 12) are commonly performed in our lab (referenced above) and others (e.g., Eens et al. (1990)). After observations, the female conspecific was removed and returned to her home cage. Males were easily categorized as nest box owners or non-owners based on persistent proximity to the nest box.
Tissue preparation for immunohistochemistry
Following final observations, the female was removed from the aviary and males were sacrificed by rapid decapitation. A terminal trunk blood sample of approximately 1.5 ml was collected and approximately 150 μl of supernatant (plasma) collected and stored as described above for the pretest blood sample. The length and width of each testis was also measured at this time to the nearest tenth of a mm using calipers. Brains were immediately removed and submerged in a 5% acrolein (Sigma Aldrich Catalogue #110221) solution for 24 hours. Brains were then placed in 30% sucrose and stored at 4°C. Sucrose was replaced every 24 hours for three days. Brains were flash frozen in dry ice and stored at −80°C until slicing. They were then sectioned coronally at 40 μm on a cryostat at −17°C in triplicate serial sections and stored in antifreeze cryoprotectant (PBS, polyvinylpyrrolidone, sucrose and ethylene glycol mixture) at −20°C until immunohistochemistry. Storage in a cyroprotectant allows for the long-term storage of free-floating sections to reduce the loss of antigenicity (Hoffman and Le, 2004).
Testosterone assay and gonad area
Plasma T in the pre-experiment and terminal blood samples was measured with a commercial grade competitive immunoassay (EIA; Cayman Chemical, Ann Arbor, MI, USA, Catalog No. 582701) used in prior studies of songbirds including starlings (e.g. McGuire et al. (2013)). Samples were run in duplicate on two plates run simultaneously (using solutions mixed in the same batches), using the manufacturer’s protocol at a dilution of 1:8 for a total reaction volume of 50 μl (determined in pilot studies as the optimal concentration) in buffer solution and visualized at 405 nm with a BioTek 800 plate reader (#7331000, ELv800™, BioTek Instruments, Inc., Winooski, VT, USA). The 1:8 dilution was selected after pilot tests showed that this dilution made it so that samples were run on the ideal portion of the curve. Sensitivities of the commercial EIA according to the manufacturer’s specifications indicate a limit of detection: 80% B/B0: 6 pg/ml and sensitivity: 50% B/B0: 32 pg/ml. The assay is specific (cross reactivity to 5 α-DHT is 27.4%, and to 17β-estradiol <0.01%). The intra-assay Coefficient of Variations (CV) were 12.5% and 19.7%. The inter-assay CV was 13.7%. Gonad volume was determined by the elliptical volume equation (Volume= 4/3π (.5width2*.5length)) and averaged for both testes for each bird (Dawson, 2005; McGuire et al., 2013).
Antibody validation for immunohistochemistry
Western blot was used to test specificity of the antibody N-20 (sc-816, Santa Cruz Biotech) a polyclonal antibody raised against a peptide mapping at the N-terminus of AR of human origin that has been used in zebra finches, quail and chickens (Kharwar and Haldar, 2011; Shaw and Kennedy, 2002; Tang and Wade, 2010, 2011; Wu et al., 2010). Micropunched tissue from starling and mouse was homogenized with ice-cold lysis buffer (1.0 mL/100 mg of tissue) in pre-chilled centrifuge tubes. Lysis buffer consisted of 50 mM Tris–HCl, pH 7.4, 0.5% Na-deoxycholate, 1% NP-40, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, protease inhibitor cocktail (P8340, Sigma, St Louis, MO, USA) and phosphatase inhibitor cocktail (P0044, Sigma). Following tissue homogenization, samples were centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant was collected, and protein concentration was determined using BCA Protein Assay (Pierce Chemical Co., Rockford, IL, USA). Twenty micrograms of total protein from each bird and mouse were gel electrophoresed using a 4–20% precast Tris–HCl gel (Bio-Rad, Hercules, CA) and transferred to a PVDF membrane. Membranes were washed briefly in 0.1 M Tris buffered saline containing 0.05% Tween 20 (TBST) and blocked for 1 hr in 0.1 M TBST containing 5% nonfat dry milk with constant agitation at room temperature. The membrane was incubated with a primary antibody recognizing AR, N-20 (rabbit anti-AR, 1:200; sc-816, Santa Cruz Biotech), in TBST containing 5% nonfat dry milk overnight at 4°C with agitation, and washed 3 times for 5 min each in TBST. Following washes, the PVDF membrane was incubated with HRP-linked secondary antibody (goat anti-rabbit IgG, 1:10,000 Cell Signaling Technology, Beverly, MA, USA) for 1 hr at room temperature with agitation, and washed 3 times for 5 min each in TBST. Immunoreactive bands were detected using a chemiluminescence kit (ECL Plus, Amersham, Arlington Heights, IL, USA) and exposed to film (Hyperfilm-ECL, Amersham). Films for immunoblots were developed using an AGFA CP1000 X-ray film processor (AGFA, Nunawading, Australia). The results of the Western blot revealed a band at the expected molecular weight of ~110kDA (Figure 2). Additional lighter bands were observed at 75kD and 50kD. These bands may represent splice variants or a low level of nonspecific labeling.
Figure 2.
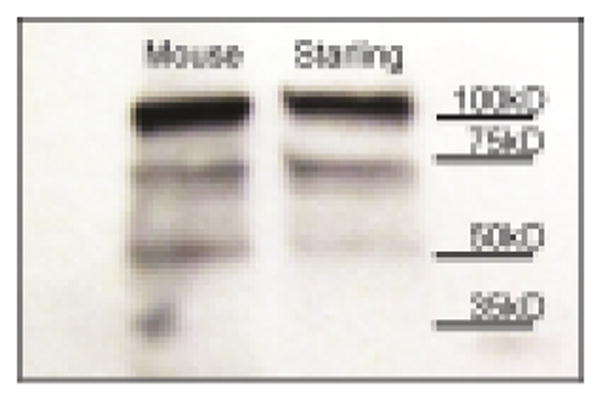
Western blot results for antibody N-20 (Santa Cruz Biotech). Mouse tissue was run alongside starling tissue as a positive control. Expected band for AR is at molecular weight 110kD.
As a side note, prior to piloting the N-20 antibody, we tested the specificity of PG-21 (06–680, Millipore), which has been widely used to examine AR in birds. Results of a Western Blot using the methods detailed above, including identical concentrations of primary and secondary antibodies, resulted in multiple bands well above and below the expected band at 110kD; therefore we did not use this antibody in the present study.
Immunohistochemistry
Immunohistochemistry was carried out using a protocol similar to Kelm et al. (2011). In short, tissue was rinsed in phosphate buffered saline (PBS), incubated for 15 min with 0.5% sodium borohydride, incubated for 10 min in 0.5% H2O2, blocked with 20% Normal Goat Serum and incubated with the primary anti-androgen receptor antibody prepared in rabbit (N-20, sc-816, Santa Cruz Biotech) at 1:500 for 24 hours. The following day tissue was incubated with the secondary antibody (Biotinylated goat anti-rabbit at 1:1000; Vector Laboratories, Burlingame, CA, USA). Secondary antibody was visualized using DAB as a chromogen. Sections were mounted on gelatin-coated slides, dehydrated and cover slipped. All tissue was run in a single batch. Some tissue was also processed without primary in order to examine nonspecific binding of the secondary antibody. Sections lacking primary showed no labeled neurons.
Quantification of immunolabeling
Using a Spot Camera (Diagnostic Instruments, Inc., Sterling Heights, MI, USA) connected to a Nikon microscope and a computer, images of brain sections were acquired. METAVUE (Fryer Company, Inc., Huntley, IL, USA) software was used to quantify androgen receptor labeling bilaterally on three serial sections. Sections were 40 μm thick and separated from one another by 80 μm. Thus sampling covered approximately 280 μm per hemisphere of each nucleus. This sampling method may produce an underestimate of total labeling density; however, because the same methods were used to analyze all birds, we are confident that relative density comparisons across groups provide accurate assessments of differences between birds in each condition. Measures were taken for each bird in the POM and two vocal control regions to which POM has been found to project directly (DM) or indirectly (HVC) (Appeltants et al., 2000; Riters and Alger, 2004). Labeling was not observed at high levels in several areas of potential interest (e.g., ventral tegmental area, periaqueductal gray, the robust nucleus of the arcopallium), thus measurements were not made in these areas. The experimenter used METAVUE software to set a threshold highlighting material on each section that a blind observer agreed represented labeling. A separate threshold was generated for each brain region of interest due to differences in background labeling across regions. The METAVUE autoscale function was used to calculate the correct exposure of each image as a percentage of the total range of light, which further reduced the variation in background among individuals. Pixel area total (number of pixels highlighting cells using the computer generated threshold) provides a measure of the approximate area covered by labeled receptors and has been previously used to measure steroid receptor immunolabeling (Senashova et al., 2012). Cell counts were not used for analysis as stained receptors overlapped (especially in HVC) and therefore would not be counted accurately. Measures in POM, HVC and DM were made within boxes or ovals (POM: 0.34mm x 0.35mm; HVC: 0.58mm x 0.43mm; DM (oval): 0.58mm x 0.37mm) centered within each region of interest (Figure 3), and measures were generated by the METAVUE software in each section in both hemispheres for each bird. In cases of tissue damage (n=2 for POM), labeling was quantified either on a fourth section or the individual was dropped from analysis for affected brain areas. The mean of all sections was used for statistical analysis.
Figure 3.
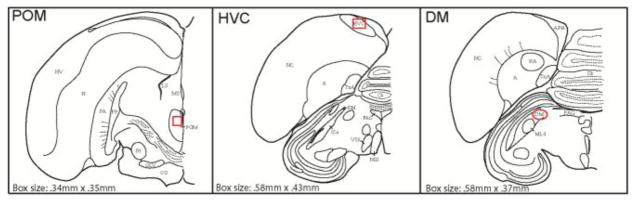
Approximate positions and sizes of boxes for label measurements in POM, HVC, and DM in illustrations of one hemisphere of coronal brain sections. Abbreviations: A, arcopallium; APH, area parahippocampalis, Cb: cerebellum, CO, optic chiasm; DM, dorsomedial portion of nucleus intercollicularis; GLV, Nucleus geniculatus lateralis, pars ventralis; HV, hyperstriatum ventral; ICo, nucleus intercollicularis, LS, lateral septum; MLd: nucleus mesencephalicus lateralis, pars dorsalis; MS, medial septum; N, nidopallium; NC, caudal nidopallium, NIII, third cranial nerve; PA, paleostriatum augmentatum; PAG, periaqueductal gray; pp, paleostriatum primitivum; POM, medial preoptic nucleus; RA, robust nucleus of the arcopallium; Rt, nucleus rotundus; TnA, nucleus taeniae of the amygdala; VTA, ventral tegmental area.
Statistical methods
Data were analyzed using the statistical software program Statistica (StatSoft 2001, Tulsa, OK). As detailed further in the Results section, factorial ANOVAs, Student’s t-tests, and stepwise multiple regression analyses were used to examine statistical relationships between nest box ownership, T concentrations, androgen receptor immunoreactivity (ARir), song (rate and bout length) and displacement behavior (the number of times a male was observed to displace another). For analyses of T concentrations we set undetectable T values at the limit of the assay (i.e., 6pg/ml). Homogeneity of variance and normality assumptions were tested using Levene’s Test, and outliers were identified using externally studentized residual plots and q-q plots. When data violated assumptions of parametric statistics, they were log transformed ((log(x+1)) as indicated in Results). When all birds were included (including birds with undetectable T), measures of post-experimental T were not normally distributed, and transformation of data did not correct this problem. We thus ran nonparametric Mann-Whitney U tests and Spearman rank correlations to analyze T data (detailed in Results). Effect sizes for pairwise comparisons were calculated using Cohen’s d (Ray and Shadish, 1996). For interpretation of d, the range is −3 to 3 representing standard deviations; d>.8 = strong effect; d>.5 = moderate effect; d>.2 = weak effect (Cohen, 1988). For factorial ANOVAs, significant main effects and interactions were analyzed using posthoc Fisher’s Least Significant Difference Tests. Effect size for significant results of factorial ANOVAs was calculated using eta2 (η2). For interpretation of η2, the range is 0–1 and represents the percentage of variance that can be accounted for by the effect tested; η2>0.14 = strong effect, η2>0.06 is a moderate effect, and η2>0.02 is a weak effect (Cohen, 1988).
Results
Testosterone concentrations and nest box ownership
All birds had undetectable concentrations of T (<6 pg/ml) at the beginning of the experiment; however, after 17 days of exposure to natural light conditions (approximately 13L:11D) in outdoor aviaries, mean T concentration was 507.37 pg/ml, standard error = 156.17 pg/ml. Males could easily be categorized as nest box owners (n = 9) or non-owners (n = 11) based on their propensity to remain near the entrance of the nest box. Although T concentrations tended to be higher in males with nest boxes (mean [SEM] =686.44 [225.55]; median =715.50) compared to those without nest boxes (mean [SEM] = 360.85 [214.13]; median =6) the difference was not statistically significant (Mann-Whitney U; U = 34.00, adj z = 1.23, p = 0.217). Upon closer inspection we found that 3 of the 9 nest box owners did not show detectable (>6 pg/ml as detectable by the assay) increases in T on the first compared to the last day of the experiment (in comparisons of the pre- and post-test measures). This was also the case for 6 of 11 non-owners. In contrast, the majority of owners (6 of the 9) had detectable increases in T, as did 5 of 11 non-owners (values shown in Table 1). For reference, T values reported in the males with detectable T in our study (mean T in box owners = 1027.67 pg/ml; mean T in non-owners = 789.06 pg/ml; Table 1) are similar to those obtained in other past studies in starlings living in stable flocks in captivity, which range from approximately a mean of 750 to 1600 pg/ml (Gwinner et al., 2002; Stevenson et al., 2009). A Student’s t-test was used to compare final T concentrations between the owners and non-owners exhibiting T increases. This concentration was not statistically different (t9=.53, p=0.61, Cohen’s d=0.47).
Table 1.
Groups of birds
Groups as determined by ownership status and T concentration
| Category | n | Starting [T] pg/ml [sem] |
Final [T] pg/ml [sem] |
Gonad Volume mm3 [sem] |
|---|---|---|---|---|
| Non-Owners without T increase | 6 | <6 pg/ml | <6 pg/ml | 452.37 [40.98] |
| Owners without T increase | 3 | <6 pg/ml | <6 pg/ml | 454.50 [57.96] |
| Non-Owners with T increase | 5 | <6 pg/ml | 789.06 [410.06] | 549.06 [44.89] |
| Owners with T increase | 6 | <6 pg/ml | 1027.67 [228.54] | 530.07 [40.98] |
Although T concentrations were below the level of assay detection in several males, gonads were fully recrudesced in all males (mean volume [SEM] for all birds = 500.17 mm3 [22.86] min = 285.66 mm3, max = 773.26 mm3). For reference, these values are almost identical to previous measures of gonad volume reported for breeding condition male starlings (e.g., mean = 502.0 mm3 in McGuire et al. (2013) and mean = approximately 500 mm3 [based on a figure] in Stevenson et al. (2009)). While the gonads of individuals that did not display an increase in T were slightly smaller (453.08 mm3 [23.79]) than those that did display a T increase (538.70 mm3 [33.23]), this difference was not significant (t18 = 2.01, p=0.06, Cohen’s d=0.90). It is also important to note that the smaller testes of birds with non-detectable T were still much larger than seasonally regressed testes (e.g. mean reported for regressed testes =76.0 mm3 (McGuire et al., 2013), mean = approximately 12.0 mm3 [from figure] in Stevenson et al. (2009), and mean =11.7 mm3 [0.85 SEM] in fall condition birds in our lab (unpublished data)). Given that T concentrations change dynamically in response to environmental and social factors (Goymann et al., 2007) and that T is known to alter AR activity (Burgess and Handa, 1993; Holmes and Wade, 2005; Meek et al., 1997; Menard and Harlan, 1993), we considered the presence or lack of measurable circulating T at the time of neural tissue collection an important, biologically relevant variable and included it in subsequent analyses.
Behavioral results and T status
Singing behavior
A factorial ANOVA was run with ownership status (owner versus non-owner) and T status (increased versus undetectable) entered as categorical variables and the number of complete songs entered as a dependent variable. Song data violated assumptions of normality and were log transformed (log(x+1)) (Lehner, 1996). Analysis revealed a significant main effect for ownership status (F1,16= 356.82, p=2.3x10−12, η2=0.45), with nest box owners singing at higher rates than non-owners (Figure 4A). Additionally, a significant main effect was found for T status (F1,16= 5.84, p=0.028, η2=0.007), with males that did show a rise in T singing at higher rates than males that did not show a rise in T (Figure 4A). Finally, the ownership x T status interaction was significant (F1,16= 11.80, p=0.003; η2=0.015). Results of significant Fisher’s LSD post hoc analyses are reported in Figure 4A. Briefly, the number of complete songs differed significantly between owners with and without T increases (p=0.001); between owners with a T increase and non-owners with and without a T increase (p=2.4x10−9 and p=4.6x10−10 respectively); and between owners without a T increase and non-owners with and without T increases (p=3.4x10−10 and p=1.0x10−10 respectively).
Figure 4.
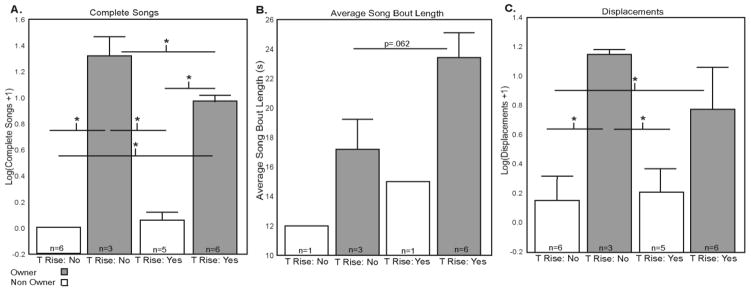
Differences in behavior in males with and without nest boxes (referred to as Owners and Non-owners, respectively), and with and without T increases. A) Log (Complete Songs+1), a measure of song rate. B) Average Bout Length, a measure of song quality only available for singing birds. For this analysis, only owners with and without T increases were compared (using Student’s T test) due to low sample sizes in other groups. C) Log (Displacements+1), a measure of agonistic/territorial behavior. Mean + SEM. * indicates p values of <.05 for post hoc comparisons run after significant main effects or interactions were identified. See text for main effects and exact p values.
Song Bout Length
Because song bout length can only be calculated for birds that sing, analysis of song bout length included only singing birds (n = 11). For this variable, the two non-owner groups only had an n of 1, so these groups were eliminated and a Student’s t-test was used to compare average bout length of the two groups of owners (the group with and without a rise in T). No significant differences in average song bout length were identified between the two groups (t7=−2.21, p=0.062, Cohen’s d=1.563), although males with a rise in T tended to sing longer bouts than those without a rise in T (Figure 4B).
Displacement Behavior
An ANOVA was run with mean log+1 transformed (to correct violations of assumptions of parametric statistics) displacements (the number of times a male was observed to displace another) entered as a dependent variable and with ownership status (owner versus non-owner) and T status (increased versus undetectable) entered as categorical variables. Results of the ANOVA revealed a significant main effect for ownership status (F1,16= 12.73, p=0.003; η2=0.48), with nest box owners displacing others at higher rates than non-owners (Figure 4C). No significant main effect was observed for T status (F1,16= 0.52, p=0.48), and no significant ownership x T status interaction was observed (F1,16= 0.96, p=0.34). Results of significant Fisher’s LSD post hoc analyses are reported in Figure 4C. Briefly, the number of displacements differed significantly between owners with a T increase and non-owners without an increase in T (p=0.036); and between owners without T increases and non-owners with and without T increases (p=0.015 and p=0.008 respectively).
Androgen receptor label, box ownership, and T status
POM
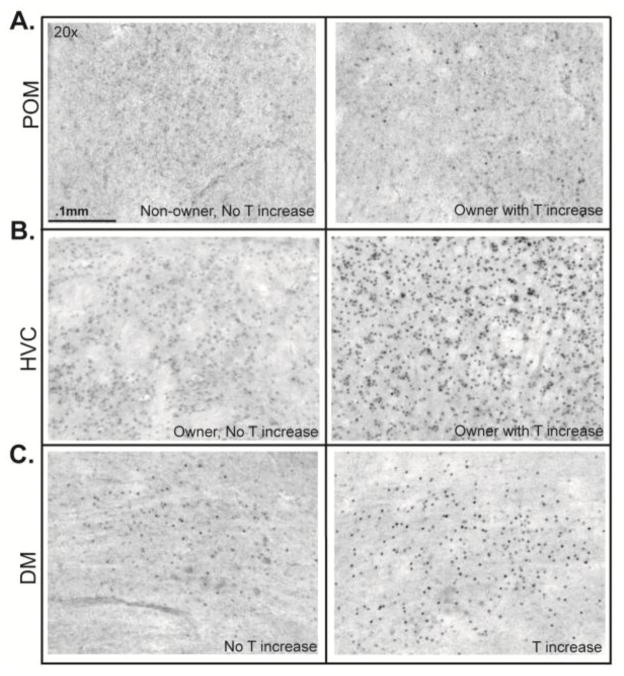
For all POM ARir analyses, two individuals were dropped because of tissue damage in this nucleus yielding n=18. A factorial ANOVA with ownership status (owner versus non-owner) and T status (increased versus undetectable) entered as categorical variables and ARir in POM entered as a dependent variable did not reveal main effects for ownership (F1,14= 2.10, p=0.169) or T status (F1,14= 4.51, p=0.052). However a significant ownership x T status interaction was observed (F1,14= 7.15, p=0.018, η2=0.09; Figure 5A; 6A). Results of significant Fisher’s LSD post hoc analyses are reported in Figure 5A. Briefly, ARir labeling differed significantly between owners with a T increase and non-owners without an increase in T (p=0.006), owners with and without an increase in T (p=0.009) and between owners and non-owners with an increase in T (p=0.005).
Figure 5.
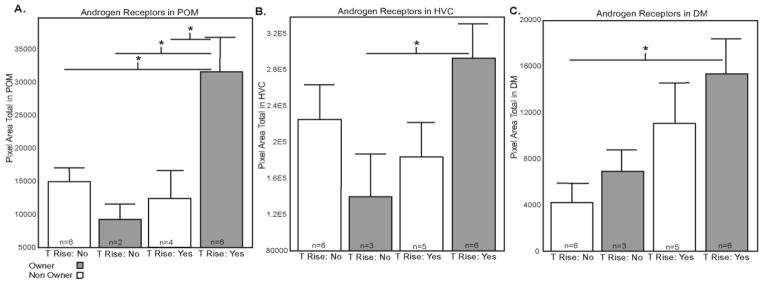
AR labeling in males with and without nest boxes (referred to as Owners and Non-owners, respectively), and with and without T increases. Mean Pixel Area Total +SEM. A) POM B) HVC C) DM. Mean + SEM. * indicates p values of <.05 for post hoc comparisons run after significant main effects or interactions were identified. See text for main effects and exact p values.
Figure 6.
Photomicrographs showing representative AR immunolabeling in POM, DM and HVC at 20X. A) POM staining in a non-owner with no T increase and an owner with a T increase. B) DM staining in a bird with no T increase and bird with a T increase. C) POM staining in an owner with no T increase and an owner with a T increase. Scale bar is indicated in the top left panel.
HVC
A factorial ANOVA with ownership status and T status entered as categorical variables and ARir in HVC entered as a dependent variable did not reveal significant main effects for ownership status (F1,16= 0.08, p=0.780) or T status (F1,16= 1.81, p=0.198). However a significant ownership x T status interaction was observed (F1,16= 5.47, p=0.033, η2=0.04; Figure 5B; 6B). Results of significant Fisher’s LSD posthoc analyses are reported in Figure 5B. Briefly, ARir labeling differed significantly between owners with a T increase and owners without an increase in T (p=0.028).
DM
A factorial ANOVA with ownership status and T status entered as categorical variables and ARir in DM entered as a dependent variable did not reveal significant main effects for ownership status (F1,16= 1.45, p=0.246); however a main effect was identified for T status (F1,16= 6.93, p=0.018, η2=0.10), with ARir higher in birds with a T increase than those with undetectable T Figure 5C; 6C). No significant ownership x T status interaction was observed (F1,16= 0.07, p=0.789). Results of significant Fisher’s LSD posthoc analyses are reported in Figure 5C. Briefly, ARir labeling differed significantly between owners with a T increase and non-owners without an increase in T (p=0.007).
Statistical contributions of nest box ownership, T, and androgen receptor label to behavior
Status-dependent behaviors
To provide insight into the hypothesis that individual differences in androgen receptor activity fine-tune status-appropriate singing behavior (Figure 1C), backward stepwise multiple regression analyses were used to determine the extent to which individual differences in ARir and T contribute statistically to social-status dependent behaviors. For each analysis, behavior was entered as a dependent variable, with song rate, song bout length, and displacement behavior included as dependent variables in separate analyses. Measures of T (post-experimental), gonad volume and ARir for a single brain region (ARir in POM, HVC, or DM) were entered as independent variables (these variables were not intercorrelated). Variables for inclusion in the model were selected based on F values. For backward regression, the F to remove was set to 3. Measures of ARir in POM, HVC, and DM were included as independent variables in separate analyses because they were intercorrelated, rendering inclusion in a single analysis inappropriate. Backwards stepwise multiple regression analyses indicated that ARir in POM, HVC, and DM contributed significantly to variance in song bout length (Figure 7, Table 2). Neither gonad volumes nor T concentrations reached threshold for inclusion in any of the models (Table 2). No models were returned in analyses of song rate or displacements.
Figure 7.
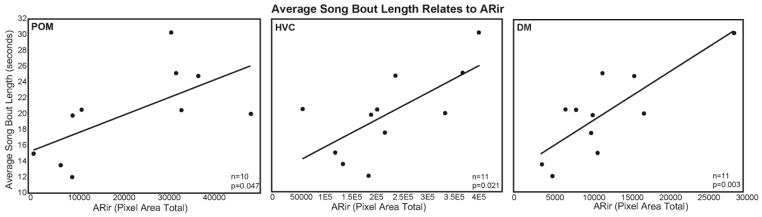
Scatterplots showing relationships between AR immunolabeling and average song bout length (seconds). A) POM B) HVC C) DM. Each point represents data from an individual. Presence of regression lines indicates significant correlations (p < 0.05).
Table 2.
Regression Results
Results of backwards stepwise multiple regression analyses show contributions of ARir to variance in song bout length
| Dependent Variable | Independent Variables | β | S.E. | t | p value |
|---|---|---|---|---|---|
| Average song bout length | |||||
| adj. R2= .34 p=.046 | ARir in POM | 0.0002 | 0.0001 | 2.35 | 0.046 |
| n=10 | T concentrations | excluded from model | n/a | n/a | n/a |
| Gonad volume | excluded from model | n/a | n/a | n/a | |
| adj. R2= .40 p=.021 | ARir in HVC | 0.00003 | 0.00001 | 2.79 | 0.021 |
| n=11 | T concentrations | excluded from model | n/a | n/a | n/a |
| Gonad volume | excluded from model | n/a | n/a | n/a | |
| adj. R2= .60 p=.003 | ARir in DM | 0.00060 | 0.0002 | 4.04 | 0.003 |
| n=11 | T concentrations | excluded from model | n/a | n/a | n/a |
| Gonad volume | excluded from model | n/a | n/a | n/a | |
Nonspecific behaviors
We ran an additional series of backward stepwise multiple regression analyses to provide insight into the extent to which statistical contributions of ARir to variance in behavior identified above were specific to status-dependent singing behavior. The analyses were identical to those described above except that feeding, drinking, and preening were entered as dependent measures (in place of song rate, song bout length, and displacement) in separate analyses. No significant models were returned for either preening or drinking. Neither ARir nor gonad volume contributed to variance in feeding; however, a trend was found for a positive correlation between feeding and T concentrations (adj. R2 = 0.15, p = 0.053).
Statistical contributions of T to androgen receptor label
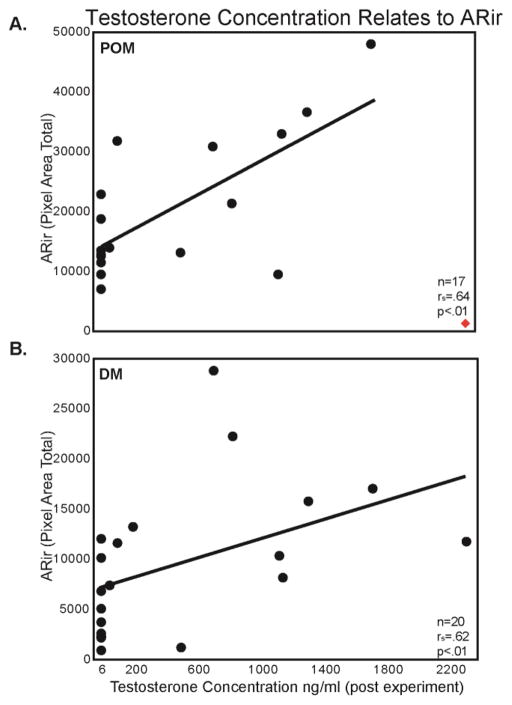
To provide insight into the hypothesis that elevations in T associated with social dominance increase ARir (Figure 1B), we ran 3 Spearman rank correlations (one for ARir in each brain region). T correlated positively with ARir in DM (rs = 0.62, p < 0.01; Figure 8) but not with ARir in POM (rs = 0.37, n.s) or HVC (rs = 0.17, n.s). For POM an influential outlier was identified (Figure 8A). As data were not normally distributed, parametric outlier identification approaches could not be used; however, this was the only point that when removed substantially altered results. Without this point T correlated positively with ARir in POM (rs = 0.64, p < 0.01; Figure 8A).
Figure 8.
Scatterplots showing relationships between AR immunolabeling and final T concentrations. A) POM B) DM. Each point represents data from an individual. Presence of line indicates a significant correlation (p < 0.05). Diamond in bottom right corner of POM figure, denotes an influential point that when included rendered the correlation nonsignificant. See text for additional details.
Discussion
Consistent with past studies (Riters et al., 2000; Sartor et al., 2005), only males with nest boxes produced high rates of song and dominated other males (as reflected in measures of displacement). Unlike past studies (Gwinner et al., 1987; Riters et al., 2000), plasma T was not significantly higher in males with compared to those without nest boxes. It is possible that T and nest box ownership are unrelated. This appears unlikely given multiple past studies demonstrating this link (reviewed in the introduction) and the trend in the present study for T to be higher in birds with nest boxes. Upon closer inspection, the lack of a significant difference was due to 3 birds that possessed nest boxes but had undetectable T (and relatively smaller gonads). These data may be interpreted to suggest that these birds were not in breeding condition, however this was not the case. Gonad volumes for all birds were enlarged and typical of breeding condition starlings (see Results). Secondly, starlings only occupy and defend nest boxes during the breeding season (Riters et al., 2000), which was observed even in 3 birds with undetectable T.
Mismatches between T and sexually-motivated behaviors are common. For example, in a variety of vertebrates, sexual behavior occurs even in the absence of elevated T ((Day et al., 2007; Foerster et al., 2002; Fusani et al., 2007; Moore, 1983; Moore and Kranz, 1983; Park et al., 2009; Park et al., 2004; Sinchak et al., 1996; Steiger et al., 2006; Wikelski et al., 2003); reviewed in Kempenaers et al. (2008)). Here, mismatches provide an opportunity to consider both T and dominance status independently. We found that dominant males (i.e. males with nest boxes) produced high rates of song and agonistic behavior irrespective of whether T concentrations were elevated or undetectable. This suggests that although T can certainly increase sexually-motivated behaviors, the possession of a nest site alone, even in the absence of elevated T, may also be capable of facilitating song and agonistic behaviors (Figure 1E).
In POM, ARir relates to dominance, testosterone and song
ARir in POM was elevated exclusively in males that both owned a nest box and showed a rise in T, offering some support to the hypothesis that elevated T in association with dominance status (Figure 1A) upregulates AR in this region (Figure 1B). The positive correlation between ARir in POM and T concentrations further supports this hypothesis. The analyses of AR and singing behavior suggest that both T-dependent and T-independent pathways may contribute to status-appropriate singing behavior. The finding that ARir in POM correlated positively with song bout length is consistent with the hypothesis that AR in POM stimulates sexually-motivated singing behavior (Figure 1C) or alternatively (and not mutually exclusively) that singing behavior upregulates AR in POM (Figure 1D). However, the presence of the 3 high singing birds with low T and low densities of AR in POM indicates that high T and high AR in POM are not necessary for an increase in the quantity of song produced by dominant birds. It may be that status-appropriate courtship song is not influenced by androgen activity in POM. This appears unlikely given a body of work demonstrating T in POM to facilitate a variety of sexually-motivated behaviors (Panzica et al., 1996; Riters et al., 1998). Furthermore, T implanted directly into the POM of castrated male canaries increased song rate (but not quality) (Alward et al., 2013). We therefore suggest that although singing behavior can be facilitated by T, additional androgen-independent mechanisms also exist (Figure 1E). This hypothesis is consistent with studies showing that although T strongly influences neural plasticity, additional T-independent factors, including singing behavior (Alvarez-Borda and Nottebohm, 2002; Sartor and Ball, 2005), social context (Boseret et al., 2006; Tramontin et al., 1999) and photoperiod (Bernard et al., 1997; Gulledge and Deviche, 1998) can all independently influence neural attributes.
One question is whether singing behavior differs qualitatively in dominant males with low compared to high T and AR in POM. Although we did not analyze this extensively, it is noteworthy that the 3 dominant males with low T tended to sing shorter songs than dominant males with high T. Male starlings produce long songs when singing to attract females (Riters et al., 2000), indicating that this attribute of song reflects sexual motivation. It is thus possible that the presence of high T and AR in the POM of dominant males functions to increase sexual motivation, which serves to promote the production of long song, perhaps by providing information about an individual’s motivational state to song control regions.
Finally, it has been suggested that the production of sexually-motivated behaviors in the absence of elevated AR activity can be maintained by activation of estrogen receptors (Canoine et al., 2007), or that continued sexual or agonistic behavior in the absence of elevated plasma T is dependent upon local steroid production within specific brain regions (Charlier et al., 2011; Park et al., 2004; Pradhan et al., 2010; Sinchak et al., 1996; Soma et al., 2008). An increase in neurosteroid activity may compensate for a lack of plasma T in birds with undetectable T. Future work is needed to examine this possibility and to examine potential roles for other non-hormonal neuromodulators.
In HVC, ARir relates to testosterone and song in dominant males
Males with nest boxes and elevated T had significantly higher ARir in HVC than males with nest boxes that did not show an increase in T. These data suggest that among males with nest boxes, a rise in T may be needed to increase AR density in HVC, providing support to the hypothesis that elevations in T associated with dominance status (Figure 1A) upregulate AR in HVC (Figure 1B). Androgens facilitate singing behavior and increase the volume of HVC (Sartor et al., 2005; Tramontin et al., 2003). Furthermore, the increased volume of HVC is enhanced by the presence of females (Boseret et al., 2006; Tramontin et al., 1999). Our finding that ARir only related to T status in males with nest boxes suggests that something about dominance status may modify this effect. Our findings are thus consistent with past data showing that HVC is sensitive to both androgens and external socially-relevant factors (in this case dominance status).
A positive correlation was also identified between mean song bout length and ARir in HVC; HVC regulates structural aspects of song production (Margoliash, 1997). Blocking AR in HVC in white-crowned sparrows disrupted song stereotypy but not rate, indicating that AR in HVC influence song structure or quality but not the motivation to sing (Meitzen et al., 2007). In starlings, females prefer males that sing long songs, which are more stereotyped than short songs (Eens et al., 1991; Gentner and Hulse, 2000). Thus ARir in HVC may alter the quality of song in males with nest boxes so that it is attractive to females (in agreement with Figure 1C). Consistent with this possibility, the 3 dominant males with low T and low ARir in HVC tended to sing shorter songs than dominant males with high T and high ARir in HVC. It is noteworthy that although birds with nest boxes sang at high rates and birds without nest boxes did not, ARir in HVC not differ significantly between these groups. We interpret this finding as consistent with data showing that T in HVC influences song quality but is not involved in the initiation of song (Brenowitz and Lent, 2002; Meitzen et al., 2007). Finally, the 3 high singing birds with nest boxes and low T and low AR in HVC indicate that androgen-independent pathways exist to induce status-appropriate singing behavior (Figure 1E) and that singing behavior may upregulate AR in HVC independent of T (Figure 1D). These possibilities are supported by past studies showing that although T induces remarkable plasticity in HVC, HVC plasticity can also be influenced by singing behavior and other factors independent of T (Hall and Macdougall-Shackleton, 2012; Sartor and Ball, 2005).
In DM, ARir relates to testosterone
ARir in DM was highest in males with elevated T, regardless of whether they owned a nest box or not, offering support for the hypothesis that T upregulates AR in this region (Figure 1B). This hypothesis was further supported by a positive correlation between T and ARir in DM. ARir in DM additionally correlated positively with average song bout length, supporting the hypothesis that AR in this region stimulate production of courtship song (Figure 1C), and alternatively that singing upregulates AR in this region (Figure 1D). The former idea is also supported by a past study showing that T implants in the intercollicular nucleus (of which DM is a part) stimulate courtship vocalizations, but not other courtship behaviors, in male ring doves (Cohen and Cheng, 1982). The finding that ARir in DM were elevated in birds with high T irrespective of dominance status differed from what was observed for POM and HVC. The meaning of this is not clear, but it may reflect differences in the sensitivity of DM to external factors compared to POM or HVC or differences in the function of AR in each of these regions.
Issues in the interpretation of steroid receptor immunolabeling data
ARs are traditionally thought to be located in the cytoplasm of a cell until they are driven into the nucleus by androgen binding. T increases ARir in several brain regions (Freeman et al., 1995; Lynch and Story, 2000; Meek et al., 1997; Smith et al., 1996; Soma et al., 1999). ARir in the present study may thus reflect either increased numbers of receptors or an increased level of bound AR present in the nucleus (which may be more easily visualized). Future studies are needed to distinguish between these possibilities. However, ARir was not uniformly higher in males with elevated T compared to males with undetectable T, and ARir measures did not correlate positively with T uniformly across brain regions, therefore, changes in nuclear translocation of AR may contribute to but are unlikely to explain all of the findings reported here. Finally, T increases the volume of the POM and song control nuclei (Charlier et al., 2008; Johnson and Bottjer, 1993). We did not collect tissue for Nissl staining and volume reconstruction and were unable to take this into account in our analysis. It is thus possible that T-dependent differences in the volume of nuclei influenced our measures of AR. However, one would predict that higher T would lead to larger nuclei and that expansion of a nucleus would cause labeling to “spread out” thereby reducing ARir measures so that males with the highest T would have the lowest measures of ARir. This was not the case, indicating that although this may have been a factor it was not solely contributing to the differences we observed in ARir.
Conclusions
The present findings are consistent with past studies indicating that the role of T in vocal behavior may differ across brain regions (Ball et al., 2004; Ball et al., 2002), with T in POM underlying the motivation to sing, T in HVC adjusting song quality, and T in DM stimulating vocal output. The data also suggest that social status may activate neural mechanisms both dependent and independent of T and AR to promote singing behavior and the possibility that singing behavior upregulates AR independent of T.
Highlights.
Dominant male starlings sang at high rates but did not have uniformly high T
Androgen receptor label (ARir) in vocal region DM related to T
ARir in POM and HVC were highest in males that were both dominant and had high T
ARir in POM and vocal control regions (DM and HVC) related to song bout length
Results suggest T-dependent and independent mechanisms regulate singing behavior
Acknowledgments
Funding provided by University of Wisconsin Zoology Department Meyer Fund to MAC. Support from the National Institute of Mental Health R01 MH080225 to LVR is also gratefully acknowledged. We also thank Dr. Ben Pawlisch, Dr. Cynthia Kelm-Nelson, and Dr. Jesse Ellis for laboratory assistance, Dr. M. Susan DeVries, and Devin Merullo for assistance on the manuscript, and Chris Elliott for starling care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alger SJ, Maasch SN, Riters LV. Lesions to the medial preoptic nucleus affect immediate early gene immunolabeling in brain regions involved in song control and social behavior in male European starlings. Eur J Neurosci. 2009;29:970–982. doi: 10.1111/j.1460-9568.2009.06637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger SJ, Riters LV. Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris) Behavioral Neuroscience. 2006;120:1326–1336. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Borda B, Nottebohm F. Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. J Neurosci. 2002;22:8684–8690. doi: 10.1523/JNEUROSCI.22-19-08684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Balthazart J, Ball GF. Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proc Natl Acad Sci U S A. 2013;110:19573–19578. doi: 10.1073/pnas.1311371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann N Y Acad Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Japanese quail as a model system for studying the neuroendocrine control of reproductive and social behaviors. ILAR J. 2010;51:310–325. doi: 10.1093/ilar.51.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23:137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Charlier TD, Barker JM, Yamamura T, Ball GF. Sex steroid-induced neuroplasticity and behavioral activation in birds. Eur J Neurosci. 2010;32:2116–2132. doi: 10.1111/j.1460-9568.2010.07518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Houbart M, Prins GS, Ball GF. Distribution of androgen receptor-immunoreactive cells in the quail forebrain and their relationship with aromatase immunoreactivity. J Neurobiol. 1998;35:323–340. [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Wilson EM, Ball GF. Immunocytochemical localization of androgen receptors in the male songbird and quail brain. J Comp Neurol. 1992;317:407–420. doi: 10.1002/cne.903170407. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Surlemont C. Andgrogen and Estrogen Action in the Preoptic Area and Activation of Copulatory Behavior in Quail. Physiol Behav. 1990;48:599–609. doi: 10.1016/0031-9384(90)90198-d. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Wilson FE, Ball GF. Testis-dependent and -independent effects of photoperiod on volumes of song control nuclei in American tree sparrows (Spizella arborea) Brain Res. 1997;760:163–169. doi: 10.1016/s0006-8993(97)00277-1. [DOI] [PubMed] [Google Scholar]
- Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria) J Neurobiol. 2006;66:1044–1060. doi: 10.1002/neu.20268. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of animal communication. Sinauer Associates, Inc; Sunderland, Massachusetts: 1998. [Google Scholar]
- Brenowitz EA, Lent K. Act locally and think globally: Intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proceedings of the National Academy of Sciences. 2002;99:12421–12426. doi: 10.1073/pnas.192308799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Hormonal regulation of androgen receptor mRNA in the brain and anterior pituitary gland of the male rat. Brain Res Mol Brain Res. 1993;19:31–38. doi: 10.1016/0169-328x(93)90145-f. [DOI] [PubMed] [Google Scholar]
- Canoine V, Fusani L, Schlinger B, Hau M. Low sex steroids, high steroid receptors: Increasing the sensitivity of the nonreproductive brain. Dev Neurobiol. 2007;67:57–67. doi: 10.1002/dneu.20296. [DOI] [PubMed] [Google Scholar]
- Catchpole C, Slater PJB. Bird song: biological themes and variations. 2. Cambridge University Press; Cambridge England; New York: 2008. [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Rapid action on neuroplasticity precedes behavioral activation by testosterone. Horm Behav. 2008;54:488–495. doi: 10.1016/j.yhbeh.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Newman AE, Heimovics SA, Po KW, Saldanha CJ, Soma KK. Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. J Neuroendocrinol. 2011;23:742–753. doi: 10.1111/j.1365-2826.2011.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen LW, Clemens LG. Blockade of testosterone-induced mounting behavior in the male rat with intracranial application of the aromatization inhibitor, androst-1,4,6,-triene-3,17-dione. Endocrinology. 1975;97:1545–1551. doi: 10.1210/endo-97-6-1545. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Zumpe D, Michael RP. Estrogen in the medial preoptic area of male rats facilitates copulatory behavior. Horm Behav. 2000;38:86–93. doi: 10.1006/hbeh.2000.1602. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Cohen J, Cheng MF. Effects of testosterone metabolites and estrogen in the midbrain control of courtship behavior in the male ring dove (Streptopelia risoria) Neuroendocrinology. 1982;34:64–74. doi: 10.1159/000123279. [DOI] [PubMed] [Google Scholar]
- Crews D. Evolution of neuroendocrine mechanisms that regulate sexual behavior. Trends Endocrinol Metab. 2005;16:354–361. doi: 10.1016/j.tem.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Dawson A. Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. General and comparative endocrinology. 1983;49:286–294. doi: 10.1016/0016-6480(83)90146-6. [DOI] [PubMed] [Google Scholar]
- Dawson A. The effect of temperature on photoperiodically regulated gonadal maturation, regression and moult in starlings potential consequences of climate change. Funct Ecol. 2005;19:995–1000. [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic Control of Seasonaility in Birds. J Biol Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Day LB, Fusani L, Hernandez E, Billo TJ, Sheldon KS, Wise PM, Schlinger BA. Testosterone and its effects on courtship in golden-collared manakins (Manacus vitellinus): Seasonal, sex, and age differences. Horm Behav. 2007;51:69–76. doi: 10.1016/j.yhbeh.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. On the function of singing and wing-waving in the European Starling Sturnus vulgaris. Bird Study. 1990;37:48–52. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Male Song as a Cue for Mate Choice in the European Starling. Behaviour. 1991;116:210–238. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Function of the song and song repertoire in the European starling (Sturnus vulgaris): an aviary experiment. Behaviour. 1993;125:51–66. [Google Scholar]
- Feare C. The starling. Oxford University Press; Oxford & New York: 1984. [Google Scholar]
- Foerster K, Poesel A, Kunc H, Kempenaers B. The natural plasma testosterone profile of male blue tits during the breeding season and its relation to song output. J Avian Biol. 2002;33:269–275. [Google Scholar]
- Freeman LM, Padgett BA, Prins GS, Breedlove SM. Distribution of androgen receptor immunoreactivity in the spinal cord of wild-type, androgen-insensitive and gonadectomized male rats. J Neurobiol. 1995;27:51–59. doi: 10.1002/neu.480270106. [DOI] [PubMed] [Google Scholar]
- Fusani L, Day LB, Canoine V, Reinemann D, Hernandez E, Schlinger BA. Androgen and the elaborate courtship behavior of a tropical lekking bird. Horm Behav. 2007;51:62–68. doi: 10.1016/j.yhbeh.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci USA. 2010;107:12393–12398. doi: 10.1073/pnas.1001394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M, Metzdorf R. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res Bull. 1997;44:509–517. doi: 10.1016/s0361-9230(97)00233-5. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH. Female European starling preference and choice for variation in conspecific male song. Anim Behav. 2000;59:443–458. doi: 10.1006/anbe.1999.1313. [DOI] [PubMed] [Google Scholar]
- Goymann W, Landys MM, Wingfield JC. Distinguishing seasonal androgen responses from male–male androgen responsiveness—Revisiting the Challenge Hypothesis. Horm Behav. 2007;51:463–476. doi: 10.1016/j.yhbeh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Gulledge CC, Deviche P. Photoperiod and testosterone independently affect vocal control region volumes in adolescent male songbirds. J Neurobiol. 1998;36:550–558. [PubMed] [Google Scholar]
- Gwinner H, Gwinner E. Effects of Testosterone on Nest-Box Occupation and Associated Behaviors by Male European Starlings (Sturnus vulgaris) Behaviour. 1994;129:141–148. [Google Scholar]
- Gwinner H, Gwinner E, Dittami J. Effects of Nestboxes on LH, Testosterone, Testicular Size, and the Reproductive Behavior of Male European Starlings in Spring. Behaviour. 1987;103:68–82. [Google Scholar]
- Gwinner H, Van’t Hof T, Zeman M. Hormonal and behavioral responses of starlings during a confrontation with males or females at nest boxes during the reproductive season. Horm Behav. 2002;42:21–31. doi: 10.1006/hbeh.2002.1795. [DOI] [PubMed] [Google Scholar]
- Hall ZJ, Macdougall-Shackleton SA. Influence of testosterone metabolites on song-control system neuroplasticity during photostimulation in adult European starlings (Sturnus vulgaris) PLoS One. 2012;7:e40060. doi: 10.1371/journal.pone.0040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol Behav. 2008;95:258–266. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Le WW. Just cool it! Cryoprotectant anti-freeze in immunocytochemistry and in situ hybridization. Peptides. 2004;25:425–431. doi: 10.1016/j.peptides.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wade J. Testosterone regulates androgen receptor immunoreactivity in the copulatory, but not courtship, neuromuscular system in adult male green anoles. J Neuroendocrinol. 2005;17:560–569. doi: 10.1111/j.1365-2826.2005.01339.x. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Bottjer SW. Hormone-induced changes in identified cell populations of the higher vocal center in male canaries. J Neurobiol. 1993;24:400–418. doi: 10.1002/neu.480240311. [DOI] [PubMed] [Google Scholar]
- Kelm CA, Forbes-Lorman RM, Auger CJ, Riters LV. Mu-opioid receptor densities are depleted in regions implicated in agonistic and sexual behavior in male European starlings (Sturnus vulgaris) defending nest sites and courting females. Behav Brain Res. 2011;219:15–22. doi: 10.1016/j.bbr.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Stevenson SA, Cordes MA, Riters LV. Modulation of male song by naloxone in the medial preoptic nucleus. Behavioral Neuroscience. 2013;127:451–457. doi: 10.1037/a0032329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempenaers B, Peters A, Foerster K. Sources of individual variation in plasma testosterone levels. Philos Trans R Soc Lond B Biol Sci. 2008;363:1711–1723. doi: 10.1098/rstb.2007.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen JA, Lane MV, Sar M, Wilson EM. Androgen receptor phosphorylation, turnover, nuclear transport, and transcriptional activation. Specificity for steroids and antihormones. Journal of Biological Chemistry. 1992;267:968–974. [PubMed] [Google Scholar]
- Kessel B. A Study of the Breeding Biology of the European Starling (Sturnus vulgaris) in NorthAmerica. Am Midl Nat. 1957;58:257–331. [Google Scholar]
- Kharwar RK, Haldar C. Reproductive phase dependent daily variation in melatonin receptors (Mel(1a) and Mel(1b)), androgen receptor (AR) and lung associated immunity of Perdicula asiatica. Comp Biochem Physiol A Mol Integr Physiol. 2011;159:119–124. doi: 10.1016/j.cbpa.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Lehner PN. Handbook of ethological methods. Cambridge University Press; Cambridge; New York: 1996. [Google Scholar]
- Lynch CS, Story AJ. Dihydrotestosterone and estrogen regulation of rat brain androgen-receptor immunoreactivity. Physiol Behav. 2000;69:445–453. doi: 10.1016/s0031-9384(99)00257-7. [DOI] [PubMed] [Google Scholar]
- Margoliash D. Functional organization of forebrain pathways for song production and perception. J Neurobiol. 1997;33:671–693. doi: 10.1002/(sici)1097-4695(19971105)33:5<671::aid-neu12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- McGuire NL, Koh A, Bentley GE. The direct response of the gonads to cues of stress in a temperate songbird species is season-dependent. PeerJ. 2013;1:e139. doi: 10.7717/peerj.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of Testosterone in Prepubertal and Postpubertal Male Hamsters: Dissociation of Effects on Reproductive Behavior and Brain Androgen Receptor Immunoreactivity. Horm Behav. 1997;31:75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 2007;27:12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard CS, Harlan RE. Up-regulation of androgen receptor immunoreactivity in the rat brain by androgenic-anabolic steroids. Brain Res. 1993;622:226–236. doi: 10.1016/0006-8993(93)90823-6. [DOI] [PubMed] [Google Scholar]
- Moore MC. Effect of female sexual displays on the endocrine physiology and behaviour of male white-crowned sparrows, Zonotrichia leucophrys. J Zool. 1983;199:137–148. [Google Scholar]
- Moore MC, Kranz R. Evidence for androgen independence of male mounting behavior in white-crowned sparrows (Zonotrichia leucophrys gambelii) Horm Behav. 1983;17:414–423. doi: 10.1016/0018-506x(83)90050-8. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: A key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Park JH, Bonthuis P, Ding A, Rais S, Rissman EF. Androgen- and estrogen-independent regulation of copulatory behavior following castration in male B6D2F1 mice. Horm Behav. 2009;56:254–263. doi: 10.1016/j.yhbeh.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Takasu N, Alvarez MI, Clark K, Aimaq R, Zucker I. Long-term persistence of male copulatory behavior in castrated and photo-inhibited Siberian hamsters. Horm Behav. 2004;45:214–221. doi: 10.1016/j.yhbeh.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Pinxten R, De Ridder E, Balthazart J, Eens M. Context-Dependent Effects of Castration and Testosterone Treatment on Song in Male European Starlings. Horm Behav. 2002;42:307–318. doi: 10.1006/hbeh.2002.1824. [DOI] [PubMed] [Google Scholar]
- Pouso P, Quintana L, Bolatto C, Silva AC. Brain androgen receptor expression correlates with seasonal changes in the behavior of a weakly electric fish, Brachyhypopomus gauderio. Horm Behav. 2010;58:729–736. doi: 10.1016/j.yhbeh.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Pradhan DS, Newman AE, Wacker DW, Wingfield JC, Schlinger BA, Soma KK. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm Behav. 2010;57:381–389. doi: 10.1016/j.yhbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JW, Shadish WR. How interchangeable are different estimators of effect size? J Consult Clin Psychol. 1996;64:1316–1325. doi: 10.1037//0022-006x.64.6.1316. [DOI] [PubMed] [Google Scholar]
- Riters LV, Absil P, Balthazart J. Effects of brain testosterone implants on appetitive and consummatory components of male sexual behavior in Japanese quail. Brain Res Bull. 1998;47:69–79. doi: 10.1016/s0361-9230(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Schroeder MB, Auger CJ, Eens M, Pinxten R, Ball GF. Evidence for opioid involvement in the regulation of song production in male European starlings (Sturnus vulgaris) Behavioral Neuroscience. 2005;119:245–255. doi: 10.1037/0735-7044.119.1.245. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB. Social status interacts with badge size and neuroendocrine physiology to influence sexual behavior in male house sparrows (Passer domesticus) Brain Behav Evol. 2004;63:141–150. doi: 10.1159/000076240. [DOI] [PubMed] [Google Scholar]
- Rosvall KA, Bergeon Burns CM, Barske J, Goodson JL, Schlinger BA, Sengelaub DR, Ketterson ED. Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proc Biol Sci. 2012;279:3547–3555. doi: 10.1098/rspb.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behavioral Neuroscience. 2005;119:233–244. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Balthazart J, Ball GF. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria) Horm Behav. 2005;47:467–476. doi: 10.1016/j.yhbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Senashova O, Reddy AP, Cameron JL, Bethea CL. The effect of citalopram on midbrain CRF receptors 1 and 2 in a primate model of stress-induced amenorrhea. Reprod Sci. 2012;19:623–632. doi: 10.1177/1933719111430992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw BK, Kennedy GG. Evidence for species differences in the pattern of androgen receptor distribution in relation to species differences in an androgen-dependent behavior. J Neurobiol. 2002;52:203–220. doi: 10.1002/neu.10079. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Roselli CE, Clemens LG. Levels of serum steroids, aromatase activity, and estrogen receptors in preoptic area, hypothalamus, and amygdala of B6D2F1 male house mice that differ in the display of copulatory behavior after castration. Behavioral Neuroscience. 1996;110:593–602. doi: 10.1037//0735-7044.110.3.593. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Prins GS. Use of PG-21 immunocytochemistry to detect androgen receptors in the songbird brain. J Histochem Cytochem. 1996;44:1075–1080. doi: 10.1177/44.9.8773574. [DOI] [PubMed] [Google Scholar]
- Soma KK, Hartman VN, Wingfield JC, Brenowitz EA. Seasonal changes in androgen receptor immunoreactivity in the song nucleus HVc of a wild bird. J Comp Neurol. 1999;409:224–236. [PubMed] [Google Scholar]
- Soma KK, Scotti MAL, Newman AEM, Charlier TD, Demas GE. Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol. 2008;29:476–489. doi: 10.1016/j.yfrne.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Steiger SS, Goymann W, Kempenaers B. Plasma steroid hormones in two Arctic-breeding shorebirds: Monogamy versus polygyny. Gen Comp Endocrinol. 2006;147:133–140. doi: 10.1016/j.ygcen.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Bernard DJ, Ball GF. Photoperiodic condition is associated with region-specific expression of GNRH1 mRNA in the preoptic area of the male starling (Sturnus vulgaris) Biol Reprod. 2009;81:674–680. doi: 10.1095/biolreprod.109.076794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syms AJ, Norris JS, Panko WB, Smith RG. Mechanism of androgen-receptor augmentation. Analysis of receptor synthesis and degradation by the density-shift technique. Journal of Biological Chemistry. 1985;260:455–461. [PubMed] [Google Scholar]
- Tang YP, Wade J. Sex- and age-related differences in ribosomal proteins L17 and L37, as well as androgen receptor protein, in the song control system of zebra finches. Neuroscience. 2010;171:1131–1140. doi: 10.1016/j.neuroscience.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Wade J. Developmental Changes in the Sexually Dimorphic Expression of Secretory Carrier Membrane Protein 1 and Its Co-Localisation with Androgen Receptor Protein in the Zebra Finch Song System. J Neuroendocrinol. 2011;23:584–590. doi: 10.1111/j.1365-2826.2011.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA. Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J Neurosci. 1999;19:476–483. doi: 10.1523/JNEUROSCI.19-01-00476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA. Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J Neurobiol. 2003;57:130–140. doi: 10.1002/neu.10263. [DOI] [PubMed] [Google Scholar]
- Wacker DW, Wingfield JC, Davis JE, Meddle SL. Seasonal Changes in Aromatase and Androgen Receptor, but not Estrogen Receptor mRNA Expression in the Brain of the Free-Living Male Song Sparrow, Melospiza melodia morphna. J Comp Neurol. 2010;518:3819–3835. doi: 10.1002/cne.22426. [DOI] [PubMed] [Google Scholar]
- Watson JT, Adkins-Regan E. Testosterone implanted in the preoptic area of male Japanese quail must be aromatized to activate copulation. Horm Behav. 1989;23:432–447. doi: 10.1016/0018-506x(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Hau M, Douglas Robinson W, Wingfield JC. Reproductive seasonality of seven neotropical passerine species. The Condor. 2003;105:683–695. [Google Scholar]
- Wiley RH, Piper WH, Archawaranon M, Wyrick Thompson E. Singing in relation to social dominance and testosterone in white-throated sparrows. Behaviour. 1993;127:175–190. [Google Scholar]
- Wu D, Tang YP, Wade J. Co-localization of sorting nexin 2 and androgen receptor in the song system of juvenile zebra finches. Brain Res. 2010;1343:104–111. doi: 10.1016/j.brainres.2010.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]