Abstract
Purpose
This study was conducted to evaluate potential pharmacokinetic interactions between docetaxel and atrasentan as part of a phase I/II clinical trial.
Methods
Patients with prostate cancer were treated with intravenous docetaxel (60-75 mg/m2) every three weeks and oral atrasentan (10 mg) daily starting on day 3 of cycle 1 and then given continuously. The pharmacokinetics of both drugs were evaluated individually (cycle 1, day 1 for docetaxel; day 21 for atrasentan) and in combination (cycle 2 day 1 for both drugs). Pharmacogenomics of alpha-1-acid glycoprotein (AAG) were also explored.
Results
Paired pharmacokinetic data sets for both drugs were evaluable in 21 patients. Atrasentan was rapidly absorbed and plasma concentrations varied over a 4 fold range at steady-state within a typical patient. The median apparent oral clearance of atrasentan was 17.4 L/h in cycle 1 and was not affected by docetaxel administration (p = 0.9). Median systemic clearance of docetaxel was 51.1 L/h on the first cycle and significantly slower (p = 0.01) compared to that obtained with co-administration of atrasentan, 61.6 L/h. Docetaxel systemic clearance in cycle 1 was 70.0 L/h in patients homozygous for a variant allele in AAG compared to 44.5 L/hr in those with at least one wild type allele (p = 0.03).
Conclusion
Genetic polymorphism in alpha-1-acid glycoprotein may explain some inter-patient variability in docetaxel pharmacokinetics. The systemic clearance of docetaxel is increased by approximately 21 percent when given concomitantly with atrasentan, however atransentan pharmacokinetics do not appear to be influenced by docetaxel administration.
Keywords: Atrasentan, Docetaxel, Pharmacokinetics, Pharmacogenomics, Prostate Cancer
Introduction
Docetaxel in combination with prednisone is a commonly prescribed treatment for men with castration-resistant, metastatic prostate cancer (CRPC) based on improvements in palliation, survival, and radiographic/prostate specific antigen response rates with this regimen as compared with older chemotherapeutic regimens [1,2]. To date, however, combining other agents with docetaxel has proven to be a formidable challenge in men with CRPC, with no agents showing an improvement over docetaxel/prednisone alone. In an attempt to improve upon the outcomes with docetaxel alone, we combined it with atrasentan, a highly potent and selective endothelin-A receptor antagonist being evaluated in phase III studies for the treatment of men with CRPC [3, 4]. The clinical report of the study was previously published [4], however the clinical pharmacology studies conducted in those patients were not available at the time of that publication and may provide insights into the clinical observations noted with this combination approach.
Atrasentan specifically antagonizes the endothelial-A (ETA) receptor. Blockade of this receptor inhibits cancer cell proliferation, augments chemotherapy-induced apoptosis by reversing ETA mediated cell survival and suppresses angiogenesis. [5]. Its effects on ETA and thus vascular tone suggest usefulness in reducing the metatstatic spread of prostate cancer cells, particularly to the bone as atansentan may also inhibit osteoblastic bone remodeling through this mechanism [6]. Docetaxel acts directly on the malignant cell, which will eventually lead to cell death through apoptosis, however ETA receptor binding can suppress taxane-induced apoptosis [7-8]. Thus it is reasonable to expect that the two drugs will act synergistically, one targeting the cancer cell, and the other decreasing the stimulus of the cancer cell to proliferate.
The rationale for evaluation of potential pharmacokinetic interactions between docetaxel and atrasentan was initially based on each agent’s common metabolism by the CYP450 3A enzyme family [9-10]. Atrasentan has been shown to cause an in vitro concentration dependent inhibition of CYP3A- and CYP2C9-dependent activities with IC50 values of 3.0 and 35 μM, respectively [10-11]. Furthermore, both atrasentan and docetaxel are highly bound to alpha-1-acid glycoprotein (AAG). The latter may be an important determinant of docetaxel clinical pharmacology since inter-patient variability in plasma AAG can be wide due to factors such as tobacco smoking and such variability can be correlated with both efficacy and toxicity [12-13]. Thus, we designed the phase II portion of this study with the objective of evaluating pharmacokinetic interaction between the two agents using individual patients as their own controls. We additionally explored the influence of genomic variants in a major plasma binding protein for both drugs, alpha 1-acid glycoprotein (AAG). This paper is the first report to describe the pharmacokinetics of both drugs when administered in combination.
Materials and Methods
Eligibility Criteria
Subjects participating in this study were required to have histologically confirmed metastatic prostate adenocarcinoma, with disease progression despite androgen deprivation therapy and a castrate level of testosterone (< 50 ng/dL). Patients had no malignancies within the last five years, with the exception of superficial non-melanoma skin cancer, and a life expectancy greater than six months. All participating men were castrated either surgically or pharmacologically at least three months prior to screening (testosterone value < 50 ng/dL). Pharmacologically castrated subjects were maintained on androgen suppression therapy for the duration of the study. A documented withdrawal period of 4-6 weeks was required for subjects who received anti-androgen therapy. The participants were required to have an adequate liver function (total bilirubin < 26.5 mol/L, and alanine transaminase and aspartate transaminase ≤ 1.5 times the upper limit of normal), and adequate renal function (creatinine clearance ≥ 40 mL/min). Additional details describing the eligibility are provided in the clinical manuscript [4]. This study was approved by the Duke University Institutional Review Board and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects signed an informed consent document prior to their inclusion in the study.
Study Design & Treatment Schedule
Docetaxel (60 or 75 mg/m2) was administered by intravenous infusion over one hour every 21 days without prednisone. One 10 mg soft-gelatin capsule of atrasentan was given by mouth daily starting on day 3 of cycle 1 and continuously thereafter.
Pharmacokinetic Studies
The pharmacokinetics of docetaxel were evaluated during and up to 72 hours after the first docetaxel infusion on day 1 of cycle 1 (alone) and day 22 (Day 1 of cycle 2, concurrent with atrasentan). Blood samples were collected from an IV access separate from that used for infusing drug at pre-dose, and at 30 min (during infusion), 58 min (end of infusion), 1.25, 1.5, 2, 4, 8, 24, 48, and 72 hours post-dose. All pharmacokinetic specimens were analyzed by the Pharmacology Core Lab of the WVU/Mary Babb Randolph Cancer Center.
Atrasentan pharmacokinetics were evaluated on an oral dose of 10 mg given to the same patients on day 21 of cycle 1 (alone; 20 days after first dose of docetaxel and 24 hr prior to the 2nd dose) and day 22 (concurrent with docetaxel). Blood samples for atrasentan analyses were collected at pre-dose, and at 15 min, 30 min, 1, 2, 4, 8, and 24 hours post-dose. All analytic and data analyses were conducted in the Pharmacology Core Lab whereas all genomic analyses occurred in the Molecular Medicine Core Lab both at the WVU/Mary Babb Randolph Cancer Center.
Pharmacokinetic Analyses
Docetaxel plasma concentrations were assayed using a validated LC-MS/MS method, modified from a previously published procedure [14]. The method employed liquid-liquid extraction of human plasma using 1:4 (v/v) mixture of acetonitrile-n-butyl chloride and paclitaxel as an internal standard. Samples were passed through Waters XTerra (2.1 × 100 mm) C18 3.5 μm analytical column and eluted with mobile mixture of methanol-water (70:30, v/v) containing 0.1% (v/v) formic acid at a flow rate of 0.3 ml/min. Detection was performed by tandem mass spectrometry in the positive-ion mode where the daughter ions of docetaxel (m/z 527.90) and the internal standard paclitaxel (m/z 286.20) were monitored (Micromass Quattro Micro, Waters, Milford, MA). The standard curve had a linear dynamic range of 0.5 to 100 nM. Inter-day and intra-day coefficients of variation for the assay were each < 5%.
Atrasentan plasma concentrations were determined using a validated HPLC-fluorescence assay method [15] with minor modifications. Atrasentan was extracted from plasma using 1:1 (v/v) mixture of hexane/tert-butylmethylether, however unlike the published method, the clean up step following reconstituting the extract was omitted. Separation of atrasentan from the internal standard ABT-790 was achieved using a C8 Waters Spherisorb (250 × 4.6 mm, 5.0 μm) analytical column and Adsorbsphere CN5 μm (7.5 × 4.6 mm) guard column. The LC mobile phase utilized was a mixture of 0.5 M K2HPO4 (pH 7.0)/acetonitrile/isopropanol/methanol 55:25:15:5, (v:v:v:v) and was delivered isocratically at a flow rate of 1.0 ml/min. Both compounds were detected using fluorescence using an excitation wavelength (λex) of 273 nm and emission (λem) of 320 nm. The standard curve linear dynamic range was 5.0 to 100 ng/mL. Inter-day and intra-day coefficients of variation for the assay were each < 15%.
Pharmacokinetic parameter estimates for each patient’s data sets were generated by the standard two-stage approach using non-compartmental techniques for atrasentan. A two-stage approach was also used for docetaxel, however compartmental modeling was conducted given our past experience with large data sets using this approach and the nature of the sampling schema. Selection of the most appropriate model for a data set was primarily based on the Akaike Information Criteria (AIC). All pharmacokinetic analyses were performed using WinNonlin Software (version 2.1, Pharsight Corporation, Mountain View, CA). The absolute dose (non-normalized) was utilized as the input variable for all analyses. Actual sample times obtained from initiation of the dose were calculated from the case report forms and used as primary input data.
AUC data provided in non-compartmental analyses were estimated using all samples obtained in the dosing interval without extrapolation. Compartmental analysis estimated AUC values from the modeled curve which was weighted by the inverse of the concentration squared. Initial parameter estimates and limits for all of the modeled analysis were program supplied.
Pharmacogenomic Analyses
Previously identified variants in ORM2 (one of the two genes which encode AAG) were analyzed from the patients’ peripheral white blood cell-derived DNA by the Molecular Medicine Core of the WVU/Mary Babb Randolph Cancer Center. Genomic DNA was extracted using a QIAGEN AllPrep DNA/RNA mini kit (Qiagen, Germantown, MD). Genotyping of ORM2 SNP rs2250242 was performed using a validated TaqMan® SNP Genotyping Assay (C_1191286_1), which consisted of a mix of unlabeled PCR primers and the TaqMan® minor groove binding group (MGB) probe (FAM™ and VIC® dye-labeled), on an ABI Prism 7000 Sequence Detection System (Applied Biosystems, newly named Life Technologies, Grand Island, NY.) In brief, 12.5 μL of TaqMan® Universal Master Mix (2X, No AmpErase® UNG), 11.25 ng of genomic DNA and 1.25 μL of TaqMan® SNP Genotyping Assay mix (20X) were added to each well of a 96-well plate to bring the final reaction volume to 25 μL/well. An additional two-duplicates, containing all PCR components except template DNA denoted no template controls (NTC), were used to ensure that the reagents were free of contamination. The PCR was performed with standard protocol (10 min at 95°C, 15 sec at 92°C and 1 min at 60°C for 40 cycles). Genotypes (allelic discrimination results) were determined in duplicate samples based on the two detectors (VIC & FAM) by ABI Prism 7000 v1.2.3 SDS Software (ABI, Foster City, CA). Any variant allele (non wild type) detected were subjected to at least three separate assay reactions and subsequent SNP analysis.
Statistical analyses were performed using the Wilcoxon Sign-Rank test with significance level of α = 0.05 used as the cutoff value.
Results
Blood samples for pharmacokinetic studies were obtained from twenty-eight of the thirty-two patients enrolled on the clinical study which included 24 Caucasian men and four African American men. The average age of the participants was 69.1 years (range 50 – 81 years). The average plasma AAG concentration was 789.3 mcg/mL, but varied over a 4 fold range (392-1545 mcg/mL). Only two of the 28 patients were actively smoking tobacco at the time of enrollment and the AAG concentrations in those individuals were in the upper quartile of patients in the study.
Atrasentan Pharmacokinetics
Matched sets of blood samples were available for analysis of atrasentan and estimation of pharmacokinetic parameters in 26 patients. Data from 4 of these patients were not used due to the unavailability of sufficient data points to estimate parameters in 3 of them and analytic interferences with atrasentan in the fourth patient’s baseline and subsequent plasma samples.
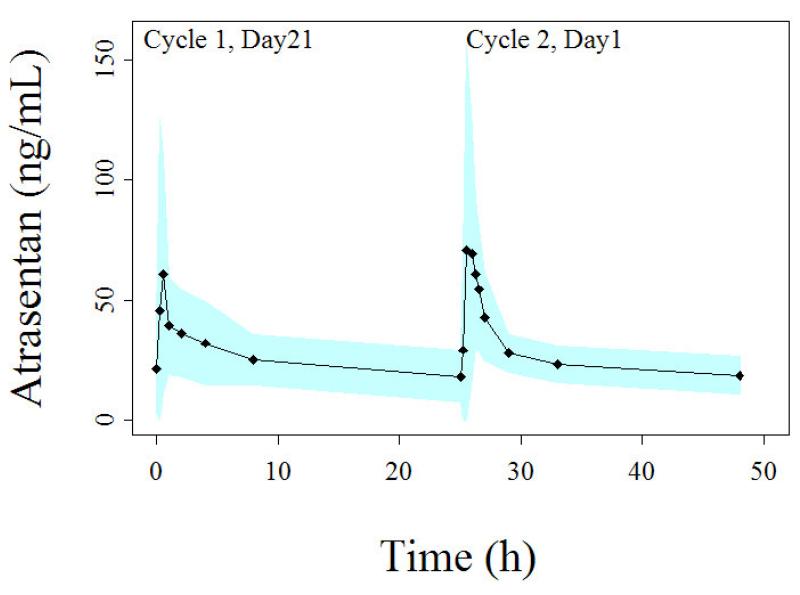
Atrasentan plasma concentrations increased rapidly following administration of the capsule (Fig. 1). Time of maximal concentration (Tmax) typically occurred approximately 30 minutes after drug administration.
Figure 1.
Plasma concentration-time profile of atrasentan following the administration of 10 mg oral daily doses. Profile on the left side represents atrasentan administered alone while the profile on the right side is concurrent with docetaxel administration on Cycle 2. Each point represents the average concentration of all patients and the shaded band represents the standard deviation.
Table 1 displays the statistical summaries of atrasentan pharmacokinetic parameters. Atrasentan median apparent oral clearance was 17.4 ± 9.1 L/h and 17.8 ± 6.2 L/h on cycle 1 and cycle 2, respectively (p = 0.9). The clearance varied over a 6 fold range on cycle 1 (CV 47%) and over 3 fold on cycle 2 (CV 29%). The apparent oral clearance of atrasentan was not significantly different when docetaxel was added on cycle 2 (p-value = 0.9). No association was found between atrasentan systemic clearance and patient body surface area (r2 = 0.09). The two patients who were active smokers upon entering this study had the lowest AUCs and fastest apparent oral atrasentan clearances (14.4 and 25.0 L/h m2).
Table 1.
Atrasentan pharmacokinetic parameters determined by non-compartmental analyses in 22 patients receiving 10 mg.
| Cmax (ng/mL) |
Cmin (ng/mL) |
Tmax (hr) |
AUC0-24 (ng/mL*h) |
CL/F (L/h) |
CL/F (L/h m2) |
Vz/F (L) |
||
|---|---|---|---|---|---|---|---|---|
| Cycle 1 Day 21 |
Mean | 85.6 | 15.8 | 0.8 | 603.8 | 19.4 | 9.5 | 510.6 |
| SD | 76.0 | 7.8 | 0.6 | 237.7 | 9.1 | 4.4 | 329.8 | |
| Median | 62.8 | 15.1 | 0.5 | 575.7 | 17.4 | 8.6 | 405.0 | |
| Range | 13.9-373.3 | 4.0-.39.0 | 0.3-2.1 | 199.9-1255.5 | 8.0-50.0 | 4.4-25.0 | 162.2-1341.8 | |
| Cycle 2 Day 1 |
Mean | 106.4 | 14.4 | 1.0 | 583.9 | 18.8 | 9.1 | 731.7 |
| SD | 92.4 | 5.1 | 0.6 | 174.2 | 6.2 | 2.6 | 1218.6 | |
| Median | 64.1 | 13.4 | 0.9 | 562.5 | 17.8 | 9.0 | 431.1 | |
| Range | 23.5-395.2 | 3.6-26.6 | 0.3-2.2 | 308.3-939.1 | 10.6-32.4 | 5.7-15.2 | 114.6-6020.4 |
Docetaxel Pharmacokinetics
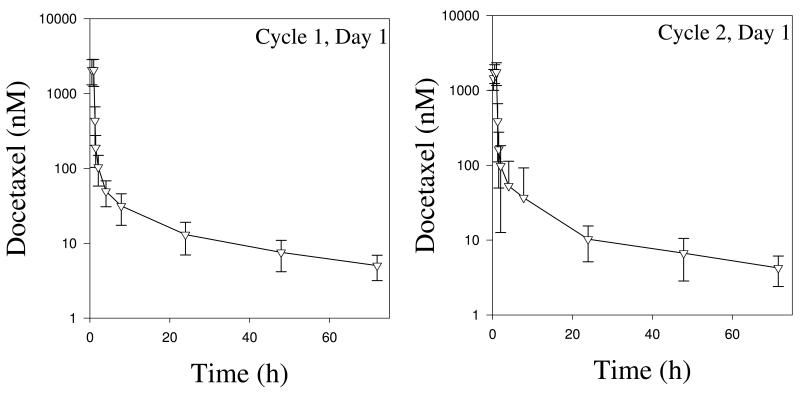
Matched sets of blood samples were available for analysis of docetaxel and subsequent estimation of its pharmacokinetic parameters in 21 patients. The semi log plots of plasma docetaxel concentration over time suggested triexponential decay following the infusion (Fig. 2). Plasma concentrations obtained during the infusion on the second cycle in 2 patients were extremely high due to probable contamination with the infusion fluid, thus were excluded from analysis. Plasma samples were not available from another two patients in cycle 2, while there were an insufficient number of samples to accurately estimate pharmacokinetic parameters in the third patient.
Figure 2.
Docetaxel plasma concentration-time profile following IV administration (60-75 mg/m2) of docetaxel alone (left panel) on day 1 of cycle 1 and co-administered with 10 mg atrasentan on day 1 of cycle 2 (right panel). Each point represents the average concentration of all patients and the error bars represent the standard deviation.
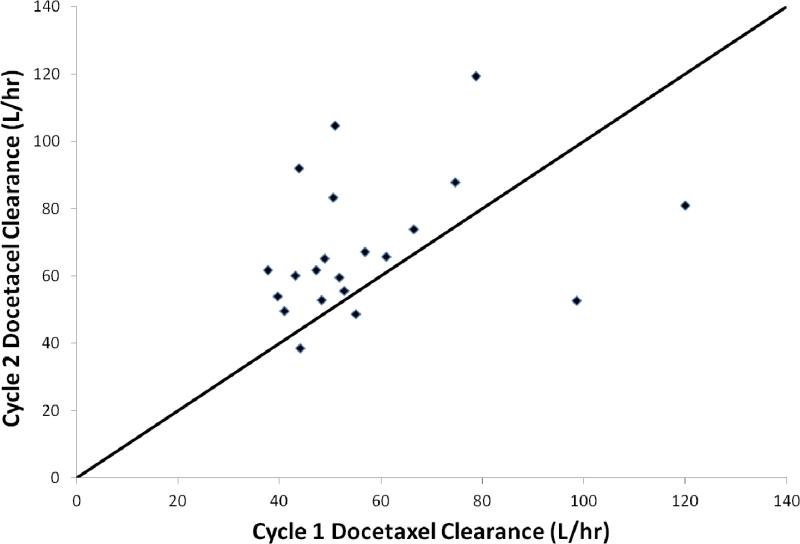
Compartmental analysis of the docetaxel pharmacokinetics was performed using both two and three compartment models. The three compartment model provided the best characterization of data in all but three patients. Overall statistical summaries for parameter estimation obtained from the assigned best fit model are shown in Table 2. Docetaxel systemic clearance varied over approximately a 3 fold range and was 20.5% faster when given concurrently with atrasentan (51.1 vs. 61.6 L/hr, cycles 1 and 2, respectively; p = 0.01; Fig. 3).
Table 2.
Docetaxel pharmacokinetic parameters determined in 21 patients by compartmental analyses.
| AUC/D (h/L) |
CL (L/hr) |
CL (L/m2) |
Vss (L) |
||
|---|---|---|---|---|---|
| Cycle 1 | Mean | 0.019 | 57.8 | 29.1 | 1160 |
| SD | 0.005 | 20.5 | 11.2 | 865 | |
| Median | 0.020 | 51.1 | 25.28 | 811 | |
| Range | 0.008-0.029 | 33.9-120 | 18.2-61.9 | 380.22-3605 | |
|
| |||||
| Cycle 2 | Mean | 0.016 | 67.3 | 33.8 | 1008 |
| SD | 0.005 | 21.0 | 11.6 | 745 | |
| Median | 0.015 | 61.6 | 30.8 | 741 | |
| Range | 0.008-0.027 | 36.4-119.2 | 17.5-64.1 | 152-2937 | |
Figure 3.
Percentage change in docetaxel systemic clearance from cycle 1 (no concurrent atrasentan) to cycle 2 (concurrent atrasentan) in 21 paired patients. Solid line represents a line of identity.
AAG Pharmacogenomic Analyses
Peripheral blood was available for extraction of leukocyte DNA in all 26 patients who underwent pharmacokinetic analyses. Only one of the six polymorphisms in ORM2 (rs250242; A_4069_G), one of the two AAG-encoding genes, displayed a minor allele frequency above 0.1 in this primarily Caucasian population of prostate cancer patients, and thus provided sufficient diversity to explore association with pharmacokinetic parameters. Pharmacokinetic data were used from the first cycle of therapy when drugs were given separately. Systemic docetaxel clearance was 70.0 L/hr in the twenty-four percent of patients homozygous (AA) for this minor/variant allele compared to 44.5 L/hr in those having at least one copy of the wild type allele (p = 0.03). Atrasentan apparent oral clearance was not significantly different when segregated in a similar manner (p = 0.16).
Discussion
We conducted clinical pharmacology studies to evaluate the disposition of atrasentan and docetaxel when used in combination for men with metastatic, castration-resistant prostate cancer. A change in docetaxel disposition during daily administration of atrasentan was observed, perhaps explaining the previously published clinical toxicology observations of this combination [4].
The pharmacokinetics of atrasentan are subject to alteration by biologically relevant changes in CYP3A4 activity. Xiong et al reported a 150% increase in atrasentan peak plasma concentration and 77% reduction in terminal half-life of the drug when it was administered with rifampin, a known inducing agent of CYP450 3A4 [16]. We did not observe any effect of concomitant docetaxel on the pharmacokinetics of atrasentan. Given our sample size we should have been able to observe approximately a 24 percent (or more) change in apparent oral clearance with 80 percent power. Area under the serum concentration-time curve values of atrasentan were similar whether the drug was administered alone or with docetaxel. This finding is consistent with previous knowledge that docetaxel does not induce or inhibit CYP3A4, the major CYP450 involved in the metabolism of atrasentan [17-18]. Furthermore, atransentan pharmacokinetic parameters observed on this study were similar to those reported using the drug as a single agent in both volunteers and patients with cancer [19-21].
Docetaxel is metabolized mainly by CYP450 3A4/5, and its half-life was significantly increased when concomitantly administered to rats with ketoconazole, a potent inhibitor of CYP450 3A4 [22]. Preclinical studies conducted in vitro showed that atrasentan can inhibit CYP450 3A4, however the maximal plasma concentrations observed in the patients on our study were approximately 10 fold lower than the in vitro IC50 value of 3 μM for inhibition. These data suggest that the systemic clearance of docetaxel is unlikely to be reduced by atrasentan based on metabolic inhibition. One previously published study evaluated the pharmacokinetic interaction of atrasentan on the CYP450 3A4 & 2C9 substrate, paclitaxel [23]. No significant difference in paclitaxel systemic clearance was observed, although the study was only designed to identify a comparatively large change (33%) in pharmacokinetic parameter estimates with 80 percent power [23]. We observed a statistically significant (21%) increase in docetaxel systemic clearance upon the co-administration of atrasentan.
An alternative preclinical model of drug disposition using a human colon adenocarcinoma cell line has shown that atrasentan significantly induces several drug transporters and CYP3A4/5 [24]. The concentrations required for such induction in that study (50 μM) were substantially higher than those found in the plasma of patients on this regimen (0.2μM), however the transport mechanisms for atrasentan suggest it has potential to accumulate in hepatocytes where P450s are found [25]. This would potentially lead to clinically significant metabolic induction and thus explain the changes in docetaxel clearance observed.
Another mechanism which could explain the change in docetaxel clearance in our study involves interaction at the plasma protein binding level. Docetaxel and atrasentan are both highly bound to plasma proteins (>99%), primarily human serum albumin and 1-acid glycoprotein [26]. Competition between the drugs for protein binding sites could result in higher unbound concentrations [27]. Given that the systemic clearance of docetaxel is well below that of liver blood flow, competitive displacement of the drug from protein binding sites could account for increased hepatic metabolism [28, 29]. Previous clinical studies have demonstrated systemic docetaxel clearance is sensitive to fluctuations in serum 1-acid glycoprotein concentrations encountered in patients, as described by reduced total plasma docetaxel clearance and lower toxicity in those with higher serum 1-acid glycoprotein [26, 30]. Thus, if atrasentan displaced docetaxel from binding (similar to a reduction in the protein concentration), an increase in toxicity, as was previously reported in these patients, and an increase in the total plasma clearance as noted here, would be anticipated [4, 31].
The functional significance of polymorphisms in genes which code for AAG i.e. ORM1 & ORM2 has been reported to a limited extent. Fitos et al described in vitro imatinib-AAG binding differences in human samples based on a variant F1-S associated with ORM1 [32]. Li et al found higher unbound quinidine systemic exposure in subjects with the same phenotype [33]. We chose to evaluate the pharmacologic relevance of the less studied polymorphisms in ORM2 since preliminary data suggested a limited number of evaluable polymorphisms but one in particular which had a reasonable expected allelic frequency for evaluation in the sample size anticipated for this study. Systemic clearance of docetaxel was approximately 1.6 fold higher in patients who were homozygous for the A_4069_G variant allele. We are unaware of any previously published studies which describe functionally significant associations with this variant, although a polymorphism located between ORM1 and ORM2 (rs1687390) has been correlated to the dosing requirements of warfarin (a known AAG binder) [34].
In summary, we observed a pharmacokinetic drug-drug interaction between atrasentan and docetaxel in the dose range used in clinical efficacy studies. While the precise mechanism behind this effect requires confirmatory studies, it could have important implication on use of use of any of the multiple drugs which are highly bound to AAG when these are combined with docetaxel. Our data also suggest future studies should confirm the functional significance of genetic polymorphism in the ORM2 gene on drug binding to AAG.
Acknowledgements
We wish to acknowledge the patients and Duke Cancer Center Clinical Trials staff for participation in this study.
This study was supported in part by an investigator initiated grant from Abbott Pharmaceuticals and NIH Grant 5P20RR016477 to the West Virginia IDeA Network for Biomedical Research Excellence. Dr. Petros also received support as the Mylan Chair of Pharmacology at West Virginia University.
Footnotes
Disclosures: None
References
- 1.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Opgenorth TJ, Adler AL, Calzadilla SV, Chiou WJ, Dayton BD, Dixon DB, et al. Pharmacological characterization of A-127722: an orally active and highly potent ETA-selective receptor antagonist. J Pharmacol Exp Ther. 1996;276:473–81. [PubMed] [Google Scholar]
- 4.Armstrong AJ, Creel P, Turnbull J, Moore C, Jaffe TA, Haley S, Petros W, Yenser S, Gockerman JP, Sleep D, Hurwitz H, George DJ. A phase I-II study of docetaxel and atrasentan in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2008 Oct 1;14(19):6270–6. doi: 10.1158/1078-0432.CCR-08-1085. [DOI] [PubMed] [Google Scholar]
- 5.Pinto A, Merino M, Zamora P, Redondo A, Castelo B, Espinosa E. Targeting the endothelin axis in prostate carcinoma. Tumour Biol. 2012;33:421–6. doi: 10.1007/s13277-011-0299-6. [DOI] [PubMed] [Google Scholar]
- 6.Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–66. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 7.Del Bufalo D, Di Castro V, Biroccio A, Varmi M, Salani D, Rosano L, et al. Endothelin-1 protects ovarian carcinoma cells against paclitaxel-induced apoptosis: requirement for Akt activation. Mol Pharmacol. 2002;61:524–532. doi: 10.1124/mol.61.3.524. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee S, Hussain M, Wang Z, Saliganan A, Che M, Bonfil D, et al. In vitro and in vivo molecular evidence for better therapeutic efficacy of ABT-627 and Taxotere combination in prostate cancer. Cancer Res. 2007;67:3818–3826. doi: 10.1158/0008-5472.CAN-06-3879. [DOI] [PubMed] [Google Scholar]
- 9.Fujita K. Cytochrome P450 and anticancer drugs. Curr Drug Metab. 2006;7:23–37. doi: 10.2174/138920006774832587. [DOI] [PubMed] [Google Scholar]
- 10.Jimeno A, Carducci M. Atrasentan: targeting the endothelin axis in prostate cancer. Expert Opin Investig Drugs. 2004;13:1631–40. doi: 10.1517/13543784.13.12.1631. [DOI] [PubMed] [Google Scholar]
- 11.Data on file, Abbott Pharmacetuitcals
- 12.Bruno R, Hille D, Riva A, Vivier N, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:187–96. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 13.Bruno R, Olivares R, Berille J, Chaikin P, Vivier N, Hammershaimb L, et al. α-1-Acid glycoprotein as an independent predictor for treatment effects and a prognostic factor of survival in patients with non-small cell lung cancer treated with docetaxel. Clin Cancer Res. 2003;9:1077–82. [PubMed] [Google Scholar]
- 14.Huang Q, Wang GJ, Sun JG, Hu XL, Lu YH, Zhang Q. Simultaneous determination of docetaxel and ketoconazole in rat plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:1009–18. doi: 10.1002/rcm.2903. [DOI] [PubMed] [Google Scholar]
- 15.Bryan PD, Sapochak LB, Tames MM, Padley RJ, El-Shourbagy TA. Determination of atrasentan by high performance liquid chromatography with fluorescence detection in human plasma. Biomed Chromatogr. 2001;15:525–33. doi: 10.1002/bmc.106. [DOI] [PubMed] [Google Scholar]
- 16.Xiong H, Carr RA, Locke CS, Katz DA, Achari R, Doan TT, et al. Dual effects of rifampin on the pharmacokinetics of atrasentan. J Clin Pharmacol. 2007;47:423–9. doi: 10.1177/0091270007299928. [DOI] [PubMed] [Google Scholar]
- 17.Puisset F, Chatelut E, Sparreboom A, Delord JP, Berchery D, Lochon I, et al. Dexamethasone as a probe for CYP3A4 metabolism: evidence of gender effect. Cancer Chemother Pharmacol. 2007;60:305–8. doi: 10.1007/s00280-006-0385-4. [DOI] [PubMed] [Google Scholar]
- 18.Nallani SC, Goodwin B, Buckley AR, Buckley DJ, Desai PB. Differences in the induction of cytochrome P450 3A4 by taxane anticancer drugs, docetaxel and paclitaxel, assessed employing primary human hepatocytes. Cancer Chemother Pharmacol. 2004;54:219–29. doi: 10.1007/s00280-004-0799-9. [DOI] [PubMed] [Google Scholar]
- 19.Verhaar MC, Grahn AY, Van Weerdt AW, Honing ML, Morrison PJ, Yang YP, Padley RJ, Rabelink TJ. Pharmacokinetics and pharmacodynamic effects of ABT-627, an oral ETA selective endothelin antagonist, in humans. Br J Clin Pharmacol. 2000;49:562–73. doi: 10.1046/j.1365-2125.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samara E, Dutta S, Cao G, Granneman GR, Dordal MS, Padley RJ. Single-dose pharmacokinetics of atrasentan, an endothelin-A receptor antagonist. J Clin Pharmacol. 2001;41:397–403. doi: 10.1177/00912700122010258. [DOI] [PubMed] [Google Scholar]
- 21.Ryan CW, Vogelzang NJ, Vokes EE, Kindler HL, Undevia SD, Humerickhouse R, et al. Dose-ranging study of the safety and pharmacokinetics of atrasentan in patients with refractory malignancies. Clin Cancer Res. 2004;10:4406–11. doi: 10.1158/1078-0432.CCR-04-0083. [DOI] [PubMed] [Google Scholar]
- 22.Back DJ, Tjia JF, Abel SM. Azoles, allylamines and drug metabolism. Br J Dermatol. 1992;39(126 Suppl):14–8. doi: 10.1111/j.1365-2133.1992.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 23.Chiappori AA, Haura E, Rodriguez FA, Boulware D, Kapoor R, Neuger AM, et al. Phase I/II study of atrasentan, an endothelin A receptor antagonist, in combination with paclitaxel and carboplatin as first-line therapy in advanced non-small cell lung cancer. Clin Cancer Res. 2008;14:1464–1469. doi: 10.1158/1078-0432.CCR-07-1508. [DOI] [PubMed] [Google Scholar]
- 24.Weiss J, Haefeli WE. Interaction potential of the endothelin-A receptor antagonist atrasentan with drug transporters and drug-metabolising enzymes assessed in vitro. Cancer Chemother Pharmacol. 2011;68:1093–8. doi: 10.1007/s00280-011-1715-8. [DOI] [PubMed] [Google Scholar]
- 25.Katz DA, Carr R, Grimm DR, Xiong H, Holley-Shanks R, et al. Organic anion transporting polypeptide 1B1 activity classified by SLCO1B1 genotype influences atrasentan pharmacokinetics. Clin Pharmacol Ther. 2006;79:186–96. doi: 10.1016/j.clpt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Urien S, Barre J, Morin C, Paccaly A, Montay G, Tillement JP. Docetaxel serum protein binding with high affinity to alpha 1-acid glycoprotein. Invest New Drugs. 1996;14:147–51. doi: 10.1007/BF00210785. [DOI] [PubMed] [Google Scholar]
- 27.Rolan PE. Plasma protein binding displacement interactions-why are they still regarded as clinically important? Br J Clin Pharmac. 1994;37:125–8. doi: 10.1111/j.1365-2125.1994.tb04251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKichan JJ. Influence of protein binding and use of unbound (free) drug concentrations. In: Burton ME, Shaw LM, Schentag JJ, Evans WE, editors. Applied pharmacokinetics & pharmacodynamics – principles of therapeutic drug monitoring. Lippincott Williams & Wilkins; Philadelphia: 2006. pp. 82–120. [Google Scholar]
- 29.Baker SD, Li J, ten Tije AJ, Figg WD, Graveland W, Verweij J, Sparreboom A. Relationship of systemic exposure to unbound docetaxel and neutropenia. Clin Pharmacol Ther. 2005;77:43–53. doi: 10.1016/j.clpt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Bruno R, Vivler N, Vergniol JC, De Phillips SL, Montay G, Sheiner LB. A population pharmacokinetic model for docetaxel (Taxotere): model building and validation. J Pharmacokinet Biopharm. 1996;24:153–72. doi: 10.1007/BF02353487. [DOI] [PubMed] [Google Scholar]
- 31.Minami H, Kawada K, Sasaki Y, Igarashi T, Saeki T, Tahara M, et al. Pharmacokinetics and pharmacodynamics of protein-unbound docetaxel in cancer patients. Cancer Sci. 2006;97:235–41. doi: 10.1111/j.1349-7006.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitos I, Visy J, Zsila F, Mády G, Simonyi M. Selective binding of imatinib to the genetic variants of human alpha 1-acid glycoprotein. Biochimica et Biophysica Acta. 2006;1760:1704–12. doi: 10.1016/j.bbagen.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Li J-H,, Xu J-Q,, Cao X-M,, Ni L,, Li Y,, Zhuang YY, Gong JB. Influence of the ORM1 phenotypes on serum unbound concentration and protein binding of quinidine. Clinica Chimica Acta. 2002;317:85–92. doi: 10.1016/s0009-8981(01)00763-x. [DOI] [PubMed] [Google Scholar]
- 34.Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, Wadelius C, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007;121:23–34. doi: 10.1007/s00439-006-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]