Abstract
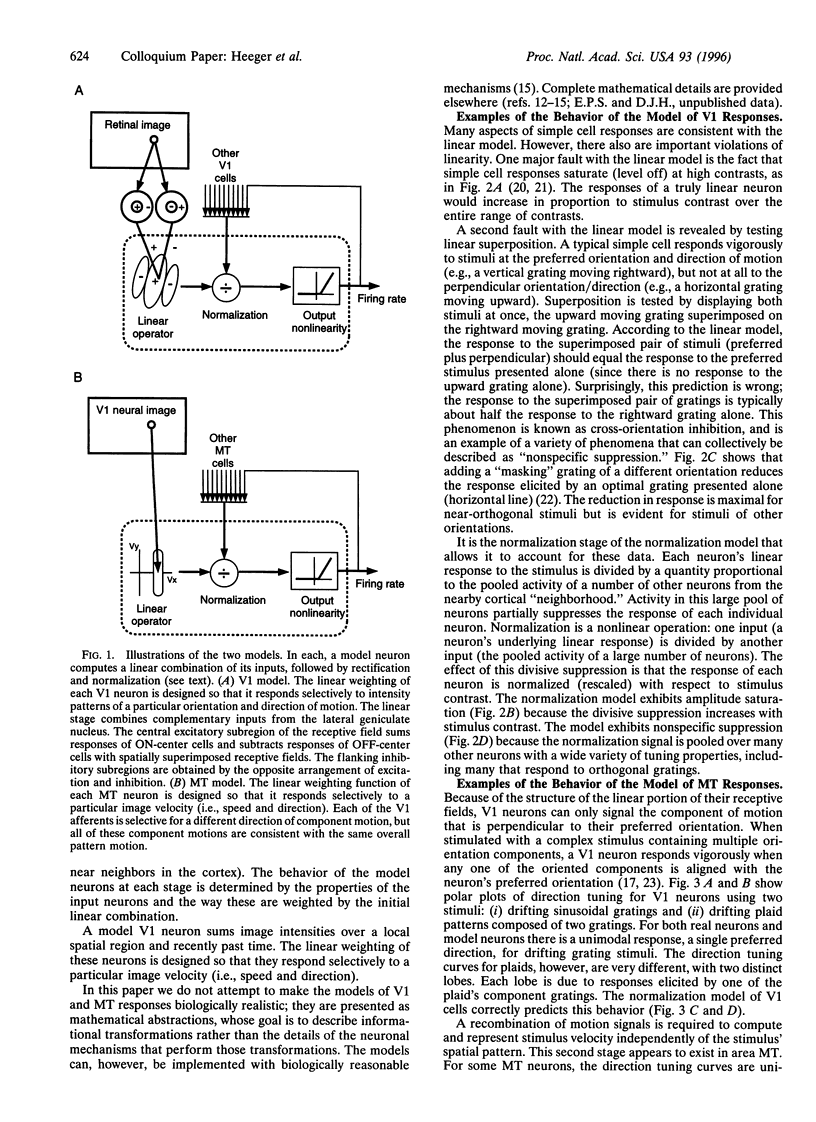
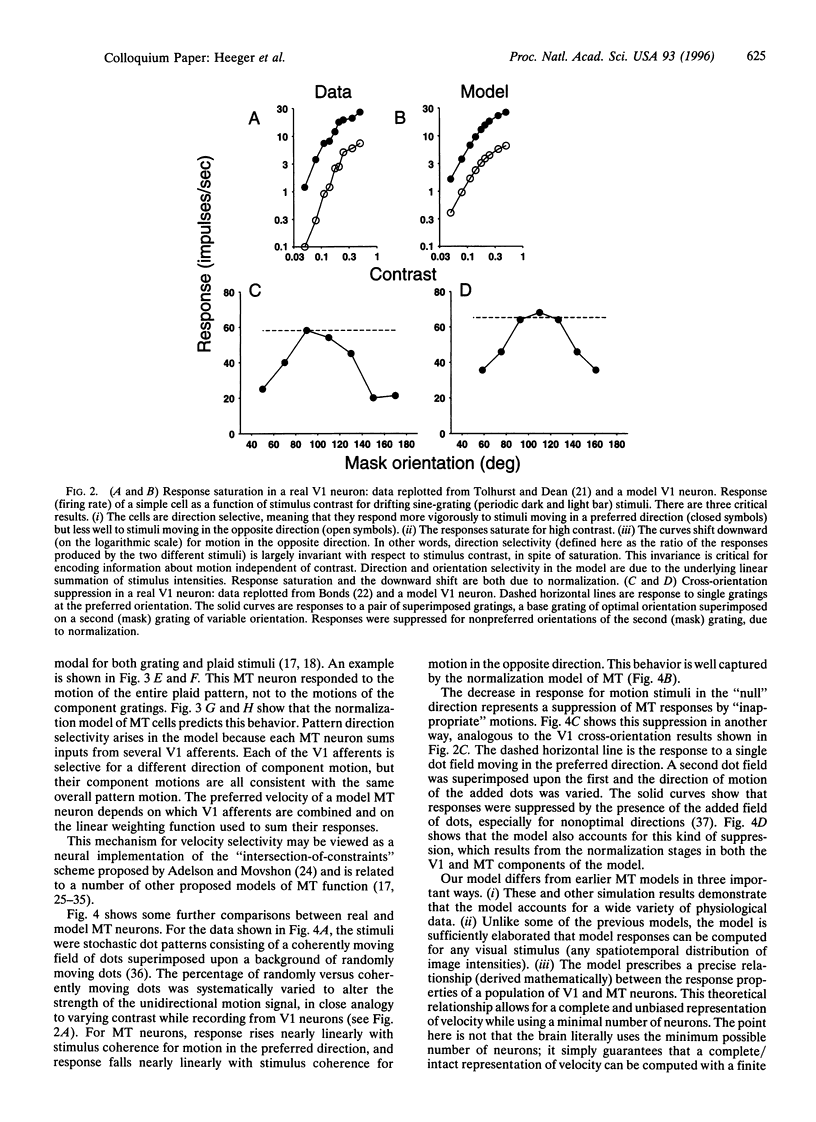
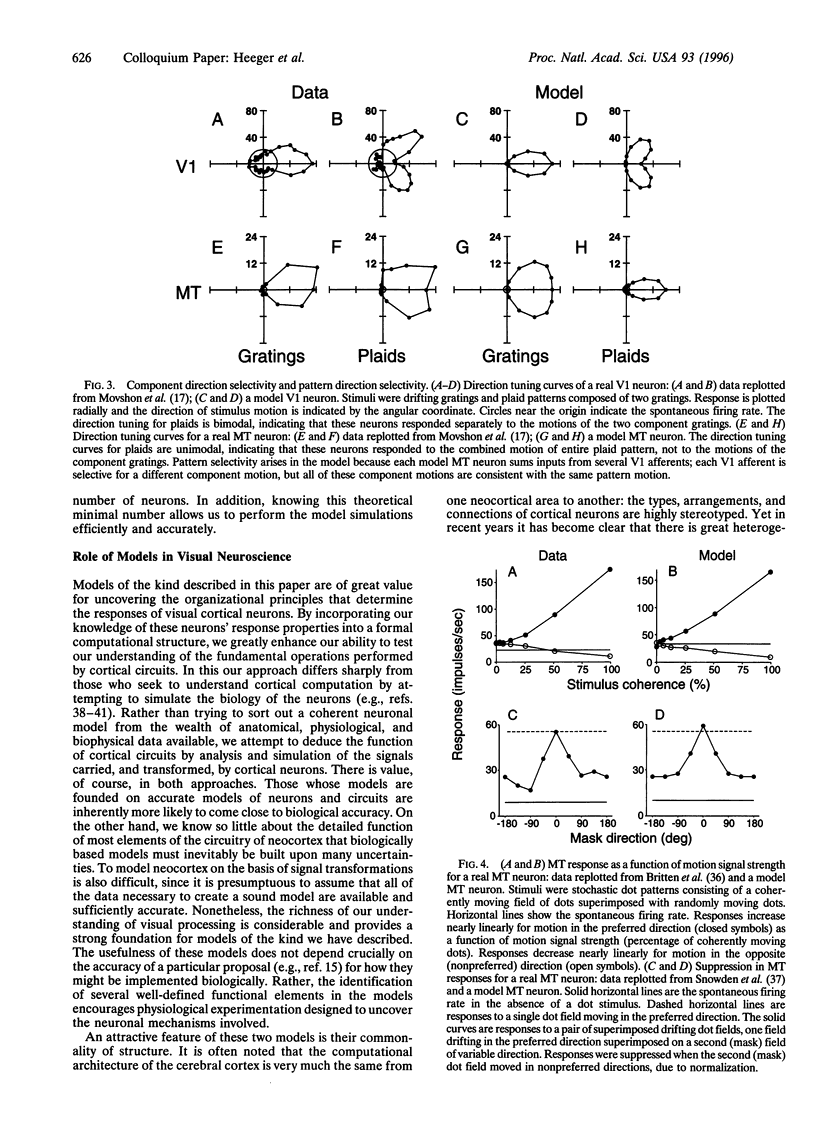
The visual responses of neurons in the cerebral cortex were first adequately characterized in the 1960s by D. H. Hubel and T. N. Wiesel [(1962) J. Physiol. (London) 160, 106-154; (1968) J. Physiol. (London) 195, 215-243] using qualitative analyses based on simple geometric visual targets. Over the past 30 years, it has become common to consider the properties of these neurons by attempting to make formal descriptions of these transformations they execute on the visual image. Most such models have their roots in linear-systems approaches pioneered in the retina by C. Enroth-Cugell and J. R. Robson [(1966) J. Physiol. (London) 187, 517-552], but it is clear that purely linear models of cortical neurons are inadequate. We present two related models: one designed to account for the responses of simple cells in primary visual cortex (V1) and one designed to account for the responses of pattern direction selective cells in MT (or V5), an extrastriate visual area thought to be involved in the analysis of visual motion. These models share a common structure that operates in the same way on different kinds of input, and instantiate the widely held view that computational strategies are similar throughout the cerebral cortex. Implementations of these models for Macintosh microcomputers are available and can be used to explore the models' properties.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelson E. H., Bergen J. R. Spatiotemporal energy models for the perception of motion. J Opt Soc Am A. 1985 Feb;2(2):284–299. doi: 10.1364/josaa.2.000284. [DOI] [PubMed] [Google Scholar]
- Adelson E. H., Movshon J. A. Phenomenal coherence of moving visual patterns. Nature. 1982 Dec 9;300(5892):523–525. doi: 10.1038/300523a0. [DOI] [PubMed] [Google Scholar]
- Albrecht D. G., Geisler W. S. Motion selectivity and the contrast-response function of simple cells in the visual cortex. Vis Neurosci. 1991 Dec;7(6):531–546. doi: 10.1017/s0952523800010336. [DOI] [PubMed] [Google Scholar]
- Albrecht D. G., Hamilton D. B. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982 Jul;48(1):217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- Albright T. D. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol. 1984 Dec;52(6):1106–1130. doi: 10.1152/jn.1984.52.6.1106. [DOI] [PubMed] [Google Scholar]
- Bernander O., Douglas R. J., Martin K. A., Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten K. H., Shadlen M. N., Newsome W. T., Movshon J. A. Responses of neurons in macaque MT to stochastic motion signals. Vis Neurosci. 1993 Nov-Dec;10(6):1157–1169. doi: 10.1017/s0952523800010269. [DOI] [PubMed] [Google Scholar]
- Carandini M., Heeger D. J. Summation and division by neurons in primate visual cortex. Science. 1994 May 27;264(5163):1333–1336. doi: 10.1126/science.8191289. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Albrecht D. G., Thorell L. G. Spatial frequency selectivity of cells in macaque visual cortex. Vision Res. 1982;22(5):545–559. doi: 10.1016/0042-6989(82)90113-4. [DOI] [PubMed] [Google Scholar]
- Douglas R. J., Martin K. A. A functional microcircuit for cat visual cortex. J Physiol. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman D. J., Van Essen D. C. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991 Jan-Feb;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Gizzi M. S., Katz E., Schumer R. A., Movshon J. A. Selectivity for orientation and direction of motion of single neurons in cat striate and extrastriate visual cortex. J Neurophysiol. 1990 Jun;63(6):1529–1543. doi: 10.1152/jn.1990.63.6.1529. [DOI] [PubMed] [Google Scholar]
- Grzywacz N. M., Yuille A. L. A model for the estimate of local image velocity by cells in the visual cortex. Proc R Soc Lond B Biol Sci. 1990 Mar 22;239(1295):129–161. doi: 10.1098/rspb.1990.0012. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger D. J. Model for the extraction of image flow. J Opt Soc Am A. 1987 Aug;4(8):1455–1471. doi: 10.1364/josaa.4.001455. [DOI] [PubMed] [Google Scholar]
- Heeger D. J. Modeling simple-cell direction selectivity with normalized, half-squared, linear operators. J Neurophysiol. 1993 Nov;70(5):1885–1898. doi: 10.1152/jn.1993.70.5.1885. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell J. H., Van Essen D. C. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol. 1983 May;49(5):1127–1147. doi: 10.1152/jn.1983.49.5.1127. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol. 1978 Oct;283:53–77. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlan S. J., Sejnowski T. J. A selection model for motion processing in area MT of primates. J Neurosci. 1995 Feb;15(2):1195–1214. doi: 10.1523/JNEUROSCI.15-02-01195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian N., Andersen R. A., Adelson E. H. Transparent motion perception as detection of unbalanced motion signals. III. Modeling. J Neurosci. 1994 Dec;14(12):7381–7392. doi: 10.1523/JNEUROSCI.14-12-07381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965 Dec;5(11):583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- Rodman H. R., Albright T. D. Single-unit analysis of pattern-motion selective properties in the middle temporal visual area (MT). Exp Brain Res. 1989;75(1):53–64. doi: 10.1007/BF00248530. [DOI] [PubMed] [Google Scholar]
- Snowden R. J., Treue S., Erickson R. G., Andersen R. A. The response of area MT and V1 neurons to transparent motion. J Neurosci. 1991 Sep;11(9):2768–2785. doi: 10.1523/JNEUROSCI.11-09-02768.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers D. C., Nelson S. B., Sur M. An emergent model of orientation selectivity in cat visual cortical simple cells. J Neurosci. 1995 Aug;15(8):5448–5465. doi: 10.1523/JNEUROSCI.15-08-05448.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst D. J., Dean A. F. Evaluation of a linear model of directional selectivity in simple cells of the cat's striate cortex. Vis Neurosci. 1991 May;6(5):421–428. doi: 10.1017/s0952523800001280. [DOI] [PubMed] [Google Scholar]
- Watson A. B., Ahumada A. J., Jr Model of human visual-motion sensing. J Opt Soc Am A. 1985 Feb;2(2):322–341. doi: 10.1364/josaa.2.000322. [DOI] [PubMed] [Google Scholar]
- Wilson H. R., Ferrera V. P., Yo C. A psychophysically motivated model for two-dimensional motion perception. Vis Neurosci. 1992 Jul;9(1):79–97. doi: 10.1017/s0952523800006386. [DOI] [PubMed] [Google Scholar]
- Wilson H. R., Kim J. A model for motion coherence and transparency. Vis Neurosci. 1994 Nov-Dec;11(6):1205–1220. doi: 10.1017/s0952523800007008. [DOI] [PubMed] [Google Scholar]
- van Santen J. P., Sperling G. Elaborated Reichardt detectors. J Opt Soc Am A. 1985 Feb;2(2):300–321. doi: 10.1364/josaa.2.000300. [DOI] [PubMed] [Google Scholar]



