Abstract
Methylmercury (MeHg) and prenatal stress (PS) are risk factors for neurotoxicity that may co-occur in human populations. Because they also share biological substrates and can produce common behavioral deficits, this study examined their joint effects on behavioral and neurochemical effects in male and female rats. Dams had access to 0, 0.5 or 2.5ppm MeHg chloride drinking water from two weeks prior to breeding through weaning. Half of the dams in each of these treatment groups also underwent PS on gestational days 16–17. This yielded 6 groups/gender: 0-NS, 0-PS, 0.5-NS, 0.5-PS, 2.5-NS, and 2.5-PS. Behavioral testing began in young adulthood and included Fixed Interval (FI) schedule-controlled behavior, novel object recognition (NOR) and locomotor activity, behaviors previously demonstrated to be sensitive to MeHg and/or mediated by brain mesocorticolimbic dopamine glutamate systems targeted by both MeHg and PS. Behavioral deficits were more pronounced in females and included impaired NOR recognition memory only under conditions of combined MeHg and PS, while non-monotonic reductions in FI response rates occurred, with greatest effects at the 0.5ppm concentration; the less reduced 2.5ppm FI response rates were further reduced under conditions of PS (2.5-PS). Correspondingly, many neurochemical changes produced by MeHg were only seen under conditions of PS, particularly in striatum in males and in hippocampus and nucleus accumbens in females, regions of significance to the mediation of FI and NOR performance. Collectively these findings demonstrate sex-dependent and non-monotonic effects of developmental MeHg exposure that can be unmasked or enhanced by PS, particularly for behavioral outcomes in females but for both sexes in neurochemical changes, that were observed at MeHg exposure concentrations that did not influence either reproductive outcomes or maternal behavior. Thus, assessment of risks associated with MeHg may be underestimated in the absence of other extant risk factors with which it may share common substrates and effects.
Keywords: methylmercury, prenatal stress fixed interval schedule, novel object recognition, corticosterone, catecholamines, indoleamines
1. Introduction
Methylmercury (MeHg) is a documented neurotoxicant, both in humans and in experimental animal models (Castoldi et al., 2008; Karagas et al., 2012). In some, but not all (Davidson et al., 2011) cohorts of children, prenatal MeHg in children has been associated with neurocognitive deficits, including increased diagnosis of attention deficit hyperactivity disorder and attention-related behaviors (Boucher et al., 2012), impaired visual recognition memory (Oken et al., 2005; Sagiv et al., 2012), and cognition and IQ (intelligence quotient) reductions (Jedrychowski et al., 2007; Lederman et al., 2008; Oken et al., 2008). Experimental animal studies likewise show deficits resulting from prenatal MeHg exposures in corresponding behavioral domains including deficits in learning, discrimination/transition reversal and working memory, increased perseverative behavior and increased behaviors interpreted as anxiety (Ceccatelli et al., 2013; Liang et al., 2009; Montgomery et al., 2008; Newland et al., 2013; Newland et al., 2004; Onishchenko et al., 2007; Peters et al., 2010; Yoshida et al., 2011).
In the human environment, exposures to MeHg inevitably occur in conjunction with other risk factors that can also adversely impact children’s neurodevelopmental trajectory. One such factor can be prenatal stress (PS). As mediated by the HPA (hypothalamic-pituitary-adrenal) axis, stress causes activation of the periventricular nucleus of the hypothalamus to release corticotrophin-releasing hormone and vasopressin. These in turn stimulate adrenocorticotrophin synthesis from the anterior pituitary corticotroph cells leading to the release of cortisol (corticosterone in rodents) from the adrenal cortex. The stress response also includes a glucocorticoid negative feedback system that involves both glucocorticoid and mineralocorticoid receptors, particularly in hippocampal systems as well as significant interactions with brain mesocorticolimbic systems (Herman et al., 2012).
PS has repeatedly been shown to produce deficits in cognitive and other behavioral functions in children (Davis and Sandman, 2010; Laplante et al., 2004; Lupien et al., 2009) and in animal models (Weinstock, 2011) that are similar to those associated with developmental MeHg exposure. These corresponding behavioral deficits may reflect the fact that MeHg and PS also share several biological substrates important to these behavioral functions. First, MeHg itself can interact with the HPA axis. Developmental exposure of rodents to 8 mg/kg MeHg on gestational day 15 produced protracted HPA axis hyperactivation, as indicated by 4-fold increases in corticosterone at PND90 (Carratu et al., 2008b). In addition, 2-fold increases in corticosterone and adrencorticotrophin hormone were found after exposures of adult male rats to only 5 ppb MeHg in drinking water over an 8 week period (Ortega et al., 1997). Increases in cortisol following MeHg have also been reported in ecotoxicology studies (Gharaei et al., 2011; Tan et al., 2009; Wada et al., 2009).
MeHg and PS also both impact brain mesocorticolimbic dopamine/glutamate pathways that mediate complex cognitive functions. Nucleus accumbens and prefrontal cortex mesocorticolimbic DA pathways, regions that receive hippocampal inputs, interact with the HPA axis, and that are also critical to complex cognition (Belujon and Grace, 2008), are susceptible to PS. In prefrontal cortex, for example, PS alters the ability of offspring to mount a homeostatic glutamatergic response to stress (Fumagalli et al., 2009), the response to anxiogenic stimuli (Mairesse et al., 2007), spine density and dendritic complexity (Mairesse et al., 2007; Murmu et al., 2006), and regulation of brain-derived neurotrophic factor (Fumagalli et al., 2004). In nucleus accumbens, PS reduces both cell number and volume (McClure et al., 2004), and enhances d-amphetamine sensitization (Henry et al., 1995). PS produces broad-based dopamine (DA) and glutamate changes in prefrontal cortex, nucleus accumbens and hippocampus (Berger et al., 2002). Similarly, developmental MeHg exposures influence dopamine and glutamate function in these pathways (Carratu et al., 2006; Castoldi et al., 2006; Coccini et al., 2011; Farina et al., 2011). Such effects have included reports of alterations in dopamine turnover in hippocampus and striatum (Bourdineaud et al., 2012; Lin et al., 2011), alterations in cortical D1 and D2 dopamine receptors (Coccini et al., 2011), and alterations in striatal dopamine release (Faro et al., 2007). The impact of MeHg on glutamatergic function includes inhibition of its uptake into astrocytes and over-activation of NMDA receptors (Aschner et al., 2007; Fitsanakis and Aschner, 2005; Xu et al., 2012).
Based on the widespread nature of stress, including prenatal stress, and thus its potential to co-occur in populations also exposed to MeHg, coupled with their shared biological substrates and common adverse outcomes, this study examined the hypothesis that combined exposures of rats to MeHg and PS could lead to enhanced effects manifest in common downstream behavioral and neurochemical markers, particularly those related to mesocorticolimbic dopamine/glutamate systems and function. Because effects of both MeHg and PS have been reported to differ by sex (Betts et al., 2012; Cory-Slechta et al., 2013; Llop et al., 2013; Rossi et al., 1997), effects were examined separately in males and females. Additionally, both a relatively low and higher dose of MeHg were explored in context with PS to provide some dose-effect information.
2. Methods and Materials
2.1 Breeding and Pup Care
Adult female Long-Evans rats (Charles River, Germantown, NY) were provided with drinking water containing 0, 0.5 or 2.5 ppm MeHg beginning 2.5 weeks prior to breeding with male Long-Evans rats from the same breeder. MeHg exposure of dams continued until offspring weaning. Breeding was accomplished by pairing 2 females with one male for a 4 day period. The presence of vaginal plugs or sperm in vaginal smears collected in the early morning was considered indicative of pregnancy and deemed gestational day 1. Females were subsequently individually housed for the duration of gestation and lactation. Half of the dams in each of the three MeHg treatment group were also subjected to immobilization restraint stress (prenatal stress, PS) on gestational days 16 and 17, while the other half of each group remained in their home cages and were not exposed to stress (NS). This resulted in a total of 6 treatment groups with numbers of dams as indicated: Control (0-NS; n=12), Stress (0-PS; n=11), low MeHg (0.5-NS; n=12), low MeHg and stress (0.5-PS; n=11), high MeHg (2.5-NS; n=10) and high MeHg and stress (2.5-PS; n=10). Dams were housed 2 per cage until breeding and individually thereafter in a vivarium room maintained at 22±2 °C with a 12-h light-dark cycle (lights on at 0700h). Rodent diet (Harlan Teklad Indianapolis, IN, maximum acceptable mercury level of 0.2 ppm) was provided ad libitum. All experiments were carried out according to NIH Guidelines and were approved by the University Committee of Animal Resources of University of Rochester School of Medicine and Dentistry.
Maternal behavior was recorded in a subset of dams from postnatal days 1–5 every 5 min for 55 min at both 10 a.m. and 4 p.m. Following birth, litter size, weight and sex ratios were determined. Litters were culled to no more than 8 at postnatal days 6–7, maintaining equal numbers of males and females if possible. Trunk blood and whole brain were collected from extra pups from each treatment group at this time for measurement of blood and brain Hg levels. At postnatal day 21, pups were weaned and pair housed by sex and treatment group for the duration of the study. High performance liquid chromatographic determinations of brain monoamines and serum corticosterone were measured in a subset of pups at 60 days of age. Behavioral testing in additional pups was initiated at 90 days of age, and included locomotor behavior, novel object recognition and fixed interval (FI) schedule-controlled behavior. An additional set of animals was retained without behavioral testing for the duration of behavioral testing. Brains were harvested from both behaviorally- and non-behaviorally tested offspring at the completion of behavioral testing. For all outcome measures, a single pup/sex/treatment group/dam was used to preclude litter specific effects.
2.2 MeHg Exposure
MeHg drinking solutions were made by dissolving methylmercury chloride in distilled deionized water. Precision of exposures was determined by TestAmerica Laboratories, inc. (North Canton, OH) in accordance with EPA Method 1630.
2.3 Prenatal Stress
On gestational days 16 and 17, dams assigned to PS groups were weighed and subjected to a widely employed restraint stress procedure consisting of three 45 min restraint sessions (precisely, 1000, 1300 and 1600h) in plastic cylindrical devices (Ward and Weisz, 1984). NS dams were weighed and subsequently left undisturbed in their home cages. This protocol, as used in our previous study of Pb and stress, elevated corticosterone levels and altered catecholamine levels in frontal cortex and nucleus accumbens of dams (Cory-Slechta et al., 2004). The choice of days 16 and 17 was timed to correspond to the development of key brain regions associated with the endpoints under study (Diaz et al., 1997; Yi et al., 1994).
2.4 Maternal Behavior Assessment
Maternal behavior was scored in the home cage from PND1–5 in a subset of dams from each treatment group (n=4/treatment group). Behavior was scored once every 5 min over a 55 min period both at 1000 and at 1600 hr on each of these days. Measures recorded included arched back nursing, blanket nursing, licking and grooming, licking pups and arched back nursing, passive nursing, no contact, and no contact mother resting, as previously described (Franks et al., 2009).
2.5 Behavioral Testing in Offspring
With the initiation of behavioral testing of offspring at 3 mos of age, rats were first calorically restricted sufficiently to produce a 2–3 gm weight loss per day until male pups reached approximately 300 g and females 250 g. At this point, caloric intake was regulated for the duration of the experiment to maintain the above-stated body weights as required for motivation for behavioral testing. Because animals were pair-housed, individual feeding was accomplished by separating the residents through the introduction of a cage divider at the time of feeding which remained in place for approximately 90–120 min.
2.5.1 Fixed Interval (FI) Schedule-Controlled Behavior
Prior studies have established the sensitivity of the FI schedule to MeHg (Reed and Newland, 2007b) and to mesocorticolimbic dopamine/glutamate mediation (Cory-Slechta et al., 2002; Cory-Slechta et al., 1998; Cory-Slechta et al., 1997), consequently it was used here to examine the impact of combined MeHg and PS. Behavioral testing was carried out on an FI 1 min schedule of food reinforcement in 30 min sessions 5 days a week (M-F) between 0900 and 1500h in operant chambers (30.5 cm × 24.5 cm × 21 cm; Med Associates Inc., St. Albans, Vermont) housed in sound-attenuated enclosures ventilated by a fan. Chambers were equipped with a grid floor, speaker, house light and three response levers configured horizontally on the front panel; only the left lever was active in these experiments. Behavioral contingencies and data were controlled by SoftCtrl™ Cumulative Record interface and Med-PC Version IV Research Control and Data Acquisition software.
Lever press response training was first carried out in automated overnight sessions as previously described (Cory-Slechta et al., 1985), after which the FI 1 min schedule was implemented. The FI 1 min schedule provides a reinforcer (45 mg food pellet; Bioserv, Frenchtown, NJ) for the first occurrence of a designated response (left lever press) after a 1 min interval had elapsed and initiated the next 1 min fixed interval. Responses during the 1 min interval itself had no programmed consequences. Sessions lasted 30 min.
After the completion of 36 sessions, a total of 3 consecutive sessions under extinction were carried out as a stressor challenge. During extinction, all conditions were identical to the FI schedule described above, but no food reward pellet was dispensed at the occurrence of the appropriate response. Following extinction, 1 session of FI 1min was carried out to restore responding, after which an FI 3min schedule with food reward was imposed for 4 sessions to determine whether acquisition of a new interval length would be affected by MeHg ± PS. Sample sizes were 10–11/group for males and 8–11/group for females.
Standard outcomes for FI performance (Kelleher and Morse, 1969) were used to measure various indices of response rate and timing of the interval, including overall response rate (total number of responses divided by total session time), post-reinforcement pause (time to the first response in the interval; a time which normally correlates with interval length) and run rate (number of responses per minutes during the interval, calculated without the postreinforcement puase to provide a measure of local response rate), and mean time between responses (interresponse time). Collectively, these measures assess efficiency of the acquisition of prototypical FI performance and provide information related to behavioral mechanisms in the case of treatment effects, e.g., rate of response vs. timing deficiencies.
2.5.2 Novel Object Recognition (NOR)
To further assess the learning/memory impacts of MeHg ± PS effects, NOR was measured as it also involves mediation by mesocorticolimbic systems (Antunes and Biala, 2012). NOR was carried out in an enclosed arena (60.2 cm × 60.2 cm × 44.0 cm) with blue walls in which two objects (drawer knobs) were located. Rats were placed individually in the arena in which the two objects were located for 5 min (session 1). Rats were subsequently returned to their home cage. Twenty four hours later, rats were placed back into the arena (session 2) in which one of the objects had now been replaced with a novel object and time spent with the novel object measured over 1 min intervals for an additional 5 min. Indices of recognition memory were calculated for average duration of time in contact with novel object (average duration of time with novel object/(average duration of time with novel object + average duration of time with familiar object)) as well as maximal duration of time spent with novel object (maximum duration (across all recorded episodes) of time spent with novel object/maximum duration of time spent with novel object + maximum duration of time spent with familiar object)). Sample sizes ranged from n=6–11/group.
2.5.3 Locomotor Activity
Developmental exposures to MeHg as well as to PS have previously been reported to alter motor activity (Emack and Matthews, 2011; Franco et al., 2006; Goulet et al., 2003; Kim et al., 2000; Montgomery et al., 2008; Patin et al., 2004), and such alterations could influence FI and/or NOR performance. Therefore, locomotor activity was measured in an automated open field arena (43.2 cm × 43.2 cm × 30.5 cm; Med Associates Inc., St. Albans, Vermont) housed in sound-attenuated enclosures ventilated by a fan for a 60 min period. Measures of locomotor activity were collected at each 5 min interval. Chambers were cleaned with Roccal between each behavioral test. Sample sizes ranged from n=4–11/group.
Behavioral testing was carried out in the same subset of animals across paradigms, in the order locomotor activity, FI schedule-controlled behavior and NOR.
2.6 Determination of Brain Monoamines
As noted above, both MeHg and PS alter brain mesocorticolimbic function. Levels of (DA (dopamine), DOPAC (dihydroxyphenylacetic acid), HVA (homovanillic acid), NE (norepinephrine), 5-HT (serotonin) and 5-HIAA (5 hydroxyindoleacetic acid) from frontal cortex, nucleus accumbens, striatum, hippocampus, hypothalamus, midbrain and olfactory bulb were analyzed using high performance liquid chromatography with electrochemical detection as described in detail previously (Cory-Slechta et al., 2009). Concentrations of neurotransmitters were expressed in terms of ng/mg protein. Protein levels were determined using commercially available BCA assay kit according to the manufacture’s instructions (Thermo Fisher Scientific Inc., Rockford, IL). DA turnover was calculated as the [DOPAC]/[DA] ratio.
2.7 Corticosterone Determinations
Approximately 200 µl of blood was collected into pre-chilled tubes that were spun at 3500 rpm for 10 min. Serum was separated and stored at −20°C until the time of the assay. Serum corticosterone was measured using the commercially available ImmuChem™ Double Antibody Corticosterone 125I kit according to the manufacturer’s instructions (MP Biomedicals, Orangeburg, NY). All standards and samples were run in duplicate, counted using a Cobra II Auto Gamma counter and expressed in ng/ml. The minimum detectable level in this assay was 7.7ng/ml.
2.8 Blood and Brain Mercury Determinations
Total mercury (Hg) levels in brain and blood were determined by cold vapor atomic absorption spectrophotometry using a flameless atomic absorption monitor (model 1235; Laboratory Data Control, Riviera Beach, FL) as previously described (Magos, 1971; Magos and Clarkson, 1972). Total Hg in brain was determined after digestion with 40% sodium hydroxide, and in blood after sample dilution with saline. For standards preparation, Mercury Reference Standard Solution (SM 114–100; Fisher Scientific, Fairmont, NJ) was used. Detection limits (LODs) and quantification limits were calculated from blank measurements following the recommendations of the International Union of Pure and Applied Chemistry (Clougherty et al., 2010). The LODs (3 × SD for blanks) were 25.1 ng/g for blood Hg and 8.0 ng/g for brain Hg, whereas limits of quantification were 83.6 ng/g and 26.6 ng/g, respectively. The method imprecision, calculated as the coefficient of variation for duplicate preparations measurements, was 4%. The analytical accuracy of Hg determination was evaluated using reference material (certified human blood samples from the Centre de Toxicologie du Quebec, International Comparison Program, Quebec, Canada). Results were 136 ng/g for lot PC-B-M-1208, compared with 138 ng/g (range, 104 –172 ng/g) and 35.6 ng/g for lot PC-B-M1214, compared with 35.9 ng/g (range 26.7– 45.1 ng/g), the results from the Centre de Toxicologie du Quebec. Participation in external quality control programs also rendered highly satisfactory data.
2.9 Statistical Analyses
Blood and brain Hg levels from postnatal days 6–7 and corticosterone from 2 mos of age were analyzed using three factor analysis of variance (ANOVA) with MeHg, PS and sex as between group factors, with subsequent post-hoc tests as appropriate dependent upon main effect or interaction outcomes. Maternal behavior was also analyzed by repeated measures ANOVA with MeHg and PS as between group factors and days as a within group factor. Subsequent one factor ANOVAs were carried out as appropriate dependent upon main effect or interaction outcomes. Offspring brain monoamines measured at 2 mos of age were analyzed separately for each sex with two factor ANOVAs, with MeHg and PS as between group factors and followed by post-hoc tests as appropriate dependent upon main effect or interaction outcomes. All behavioral measures in offspring, analyses were also analyzed separately for each sex. FI schedule controlled behavior was analyzed by repeated measures ANOVAs with MeHg and PS as between groups factors, and session as a within group factor separately for each sex. Locomotor activity was also analyzed by repeated measures ANOVAs with MeHg and PS as between groups factors, and 5 min interval as a within group factor separately for each sex. Subsequent one or two factor ANOVAs were carried out as appropriate dependent upon main effect or interaction outcomes. NOR session 2 was analyzed separately by sex using two factor ANOVAs with MeHg and PS as between group factors, with subsequent ANOVAs carried out as appropriate dependent upon main effect or interaction outcomes. P values ≤ 0.05 were considered statistically significant.
3. Results
3.1 Mercury Blood and Brain Concentrations
Both blood and brain total Hg concentrations obtained at postnatal days 6–7 increased significantly with increasing MeHg concentration in drinking water (Figure 1; F(2,75)=43.48, p<0.001 and F(2,75)=256.57, p<0.001 for blood and brain, respectively). Increases in brain Hg, but not in blood, were not proportional to increasing MeHg dose: ratios of total brain Hg levels at 2.5ppm relative to those at 0.5ppm averaged between 6.04–7.26 as compared to the 5-fold difference in nominal exposure concentrations (p<0.05), finding consistent with those reported by Newland et al. (Newland et al., 2008). No interactions with either sex or stress were found, so blood and brain Hg levels increased equivalently in males and females and in NS and PS treated rats.
Figure 1.
Group mean ± SE values of blood (left) and brain (right) Hg values (ng/g) measured at postnatal days 6–7 plotted for male and female groups in relation to MeHg dose (ppm). Both blood and brain Hg values increased with increasing dose of MeHg in drinking water; no effects of sex or stress or corresponding interactions were noted. n=6–8/treatment group.
3.2 Litter Size, Weight and Sex Ratios
Statistical analyses found no significant effects of MeHg ± PS on litter size, litter weight, sex ratio of litters or weight/pup (Table 1), although a trend towards an MeHg effect on litter weight was found (F(2,50)=2.84, p=0.068), likely due to the suggestively lower mean weights of the 0.5 MeHg ppm groups. However, when weight/pup ratios were calculated to normalize for litter number, no significant differences were evident.
Table 1.
Group Mean ± S.E. Litter Size, Weight and Sex Ratio
| Litter Size1 | Litter Weight2 | Weight/Pup3 | Sex Ratio4 | |
|---|---|---|---|---|
| 0-NS5 | 14.33 ± 0.69 | 88.89 ± 4.26 | 6.21 ± 0.11 | 0.600 ± 0.023 |
| 0-PS | 14.09 ± 1.04 | 88.00 ± 5.31 | 6.09 ± 0.13 | 0.539 ± 0.047 |
| 0.5-NS | 11.55 ± 1.34 | 71.10 ± 7.98 | 6.06 ± 0.16 | 0.531 ± 0.057 |
| 0.5-PS | 13.18 ± 1.26 | 77.95 ± 7.22 | 6.17 ± 0.27 | 0.485 ± 0.037 |
| 2.5-NS | 13.50 ± 1.18 | 89.38 ± 3.49 | 6.18 ± 0.12 | 0.489 ± 0.021 |
| 2.5-PS | 12.70 ± 1.41 | 85.15 ± 7.99 | 6.97 ± 0.54 | 0.524 ± 0.039 |
= total number of offspring measured at PND0
= group mean value at PND1
= Total litter weight/litter size
= numbers of males/total males and females
= 0-NS, control; 0-PS, stress; 0.5-NS, 0.5 MeHg, no stress; 0.5-PS, 0.5 MeHg and PS; 2.5-NS, 2.5 MeHg, no stress; 2.5-PS, 2.5 MeHg and prenatal stress
3.3 Maternal Behavior
Maternal behavior was recorded to determine whether any treatment-related differences might influence offspring outcomes. Changes in maternal behavior were extremely limited and not systematic (Tables 2 and 3). While significant MeHg-related changes were found for a.m. measures of no contact dam resting (Table 2), subsequent post-hoc tests failed to find any significant group differences at any given day. Similarly, significant stress-related changes were found for p.m. no contact dam resting (Table 3), but again subsequent post-hoc tests failed to find any significant group differences at any given day.
Table 2.
Group Mean ± S.E. A.M. Counts of Maternal Behavior
| No contact; dam resting |
No contact; dam active |
Pup Licking/ Grooming |
Pup licking & arched back nursing |
Arched back nursing |
Blanket nursing |
Passive nursing |
|
|---|---|---|---|---|---|---|---|
| Day 1 0NS1 | 3.8±2.4 | 26.3±7.7 | 1.3±1.3 | 6.3±2.4 | 21.3±9 | 0±0 | 1.3±1.3 |
| 0-PS | 3.8±3.8 | 8.8±5.5 | 0±0 | 6.3±3.8 | 37.5±9.2 | 1.3±1.3 | 2.5±2.5 |
| 0.5-NS | 11.3±2.4 | 16.3±10 | 1.3±1.3 | 1.3±1.3 | 23.8±8.5 | 2.5±1.4 | 3.8±2.4 |
| 0.5-PS | 5±5 | 18.8±9 | 1.3±1.3 | 3.8±2.4 | 28.8±10 | 0±0 | 2.5±1.4 |
| 2.5-NS | 2.5±2.5 | 0±0 | 0±0 | 8.8±5.5 | 38.8±6.3 | 3.8±2.4 | 6.3±2.5 |
| 2.5-PS | 1.3±1.3 | 5±3.6 | 0±0 | 8.8±1.3 | 38.8±5.5 | 1.3±1.3 | 5±3.5 |
| Day 2 0-NS | 17.5±10.1 | 5±3.5 | 0±0 | 5±2 | 28.8±10 | 0±0 | 3.8±3.8 |
| 0-PS | 21.3±9.4 | 11.3±4.3 | 1.3±1.3 | 5±2 | 20±11.7 | 0±0 | 1.3±1.3 |
| 0.5-NS | 23.8±10.1 | 13.8±8.8 | 0±0 | 5±2 | 17.5±6.6 | 0±0 | 0±0 |
| 0.5-PS | 10±6.8 | 3.8±2.4 | 1.3±1.3 | 10±2 | 32.5±7.8 | 2.5±2.5 | 0±0 |
| 2.5-NS | 5±5 | 5±2.9 | 2.5±2.5 | 3.8±2.4 | 32.5±7.2 | 1.3±1.3 | 10±6.1 |
| 2.5-PS | 2.5±1.4 | 8.8±3.1 | 0±0 | 6.3±3.8 | 25±9.4 | 0±0 | 12.5±11 |
| Day 3 0-NS | 33.8±9.7 | 6.3±3.8 | - | 1.3±1.3 | 18.8±9.7 | 0±0 | 0±0 |
| 0-PS | 6.3±6.3 | 7.5±3.2 | - | 8.8±.3.1 | 25±5.4 | 0±0 | 12.5±7.8 |
| 0.5-NS | 23.8±6.3 | 10±10 | - | 0±0 | 25±9.8 | 1.3±1.3 | 0±0 |
| 0.5-PS | 18.8±3.8 | 2.5±1.4 | - | 6.3±3.1 | 16.3±9.4 | 0±0 | 16.3±5.5 |
| 2.5-NS | 28.8±11.3 | 6.3±3.8 | - | 5±5 | 15±7.4 | 0±0 | 5±5 |
| 2.5-PS | 13.8±10.7 | 13.8±4.7 | - | 3.8±2.4 | 17.5±10 | 0±0 | 11.3±8.3 |
| Day 4 0-NS | 18.8±6.6 | 7.5±2.5 | 0±0 | 5±2 | 25±4.1 | 0±0 | 3.8±3.8 |
| 0-PS | 15±8.9 | 13.8±7.5 | 1.3±1.3 | 6.3±1.3 | 18.8±5.5 | 2.5±2.5 | 2.5±2.5 |
| 0.5-NS | 22.5±6.6 | 6.3±2.4 | 0±0 | 3.8±3.8 | 26.3±3.8 | 1.3±1.3 | 0±0 |
| 0.5-PS | 11.3±4.3 | 13.8±7.7 | 0±0 | 2.5±1.4 | 22.5±10 | 0±0 | 10±10 |
| 2.5-NS | 0±0 | 5±3.5 | 1.3±1.3 | 6.3±3.1 | 27.5±7.8 | 0±0 | 20±11.5 |
| 2.5-PS | 8.8±7.2 | 10±3.5 | 0±0 | 6.3±2.4 | 30±6.8 | 1.3±1.3 | 3.8±2.4 |
| Day 5 0-NS | 16.3±9.9 | 2.5±1.4 | 0±0 | 3.8±2.4 | 36.3±8.8 | 0±0 | 1.3±1.3 |
| 0-PS | 20±2 | 10±7.1 | 0±0 | 3.8±1.3 | 11.3±5.5 | 0±0 | 15±8.9 |
| 0.5-NS | 31.3±10.7 | 3.8±2.4 | 0±0 | 1.3±1.3 | 23.8±12 | 0±0 | 0±0 |
| 0.5-PS | 10±7.1 | 6.3±3.8 | 0±0 | 7.5±6 | 18.8±8.3 | 0±0 | 17.5±12 |
| 2.5-NS | 0±0 | 10±6.8 | 0±0 | 5±3.5 | 32.5±6.3 | 2.5±1.4 | 10±8.4 |
| 2.5-PS | 1.3±1.3 | 6.3±4.7 | 1.3±1.3 | 10±2 | 27.5±11 | 0±0 | 13.8±12 |
= 0-NS, control; 0-PS, stress; 0.5-NS, 0.5 MeHg, no stress; 0.5-PS, 0.5 MeHg and PS; 2.5-NS, 2.5 MeHg, no stress; 2.5-PS, 2.5 MeHg and prenatal stress
Table 3.
Group Mean ± S.E.P.M. Counts of Maternal Behavior
| No contact; dam resting |
No contact; dam active |
Pup Licking/ Grooming |
Pup licking & arched back nursing |
Arched back nursing |
Blanket nursing |
Passive nursing |
|
|---|---|---|---|---|---|---|---|
| Day 1 0-NS1 | 11.3±8.3 | 22.5±12 | 1.3±1.3 | 10±3.5 | 15±8.7 | 0±0 | - |
| 0-PS | 2.5±1.4 | 20±10.6 | 1.3±1.3 | 8.8±3.1 | 27.5±8.3 | 0±0 | - |
| 0.5-NS | 7.5±3.2 | 32.5±12 | 1.3±1.3 | 3.8±2.4 | 15±7.9 | 0±0 | - |
| 0.5-PS | 3.8±2.4 | 36.3±13 | 0±0 | 2.5±1.4 | 17.5±13 | 0±0 | - |
| 2.5-NS | 13.8±4.3 | 22.5±7.5 | 0±0 | 5±2 | 13.85.5 | 6.3±3.8 | - |
| 2.5-PS | 1.3±1.3 | 18.8±6.3 | 1.3±1.3 | 7.5±4.3 | 28.810 | 2.5±2.5 | - |
| Day 2 0-NS | 16.3±14.6 | 13.8±7.5 | 0±0 | 8.8±4.3 | 16.3±9 | - | 5±5 |
| 0-PS | 7.5±4.3 | 28.8±8.5 | 2.5±2.5 | 3.8±2.4 | 17.5±11 | - | 0±0 |
| 0.5-NS | 16.3±8.3 | 37.5±6.3 | 0±0 | 2.5±2.5 | 3.8±3.8 | - | 0±0 |
| 0.5-PS | 17.5±8.5 | 11.3±5.2 | 0±0 | 3.8±2.4 | 27.5±9.7 | - | 0±0 |
| 2.5-NS | 12.5±9.5 | 30±7.9 | 0±0 | 0±0 | 16.3±9.9 | - | 1.3±1.3 |
| 2.5-PS | 3.8±3.8 | 40±13.5 | 1.3±1.3 | 2.5±1.4 | 12.5±9.2 | - | 0±0 |
| Day 3 0-NS | 25±9.1 | 15±5.4 | 0±0 | 5±3.5 | 15±9.8 | 0±0 | 0±0 |
| 0-PS | 15±3.5 | 11.3±4.7 | 0±0 | 8.8±2.4 | 25±5.4 | 0±0 | 0±0 |
| 0.5-NS | 23.8±10 | 21.3±3.8 | 1.3±1.3 | 2.5±1.4 | 10±5.8 | 1.3±1.3 | 0±0 |
| 0.5-PS | 10±4.6 | 16.3±3.1 | 0±0 | 8.8±4.3 | 23.8±4.7 | 0±0 | 1.3±1.3 |
| 2.5-NS | 21.3±8.3 | 22.5±3.2 | 1.3±1.3 | 1.3±1.3 | 12.5±6 | 0±0 | 1.3±1.3 |
| 2.5-PS | 13.8±1.3 | 33.8±3.1 | 0±0 | 6.3±2.4 | 3.8±2.4 | 0±0 | 2.5±1.4 |
| Day 4 0-NS | 12.5±3.2 | 27.5±7.8 | 1.3±1.3 | 2.5±1.4 | 16.3±9.9 | 0±0 | 0±0 |
| 0-PS | 3.8±2.4 | 26.3±5.2 | 0±0 | 5±2.9 | 25±4.1 | 0±0 | 0±0 |
| 0.5-NS | 18.8±3.8 | 21.3±6.6 | 0±0 | 7.5±1.4 | 12.5±6 | 0±0 | 0±0 |
| 0.5-PS | 12.5±5.2 | 17.5±7.5 | 2.5±1.4 | 5±2.9 | 10±8.4 | 3.8±3.8 | 8.8±8.8 |
| 2.5-NS | 2.5±1.4 | 27.5±7.8 | 2.5±2.5 | 1.3±1.3 | 20±7.4 | 0±0 | 6.3±3.8 |
| 2.5-PS | 10±4.6 | 31.3±4.3 | 1.3±1.3 | 2.5±1.4 | 11.3±6.6 | 0±0 | 3.8±3.8 |
| Day 5 0-NS | 23.8±5.2 | 27.5±3.2 | 1.3±1.3 | 3.8±2.4 | 3.8±2.4 | - | 0±0 |
| 0-PS | 10±6.1 | 18.8±10.3 | 0±0 | 6.3±3.1 | 20±7.4 | - | 5±5 |
| 0.5-NS | 28.8±9.4 | 15±6.1 | 0±0 | 3.8±2.4 | 12.5±6 | - | 0±0 |
| 0.5-PS | 10±3.5 | 23.8±9 | 0±0 | 6.3±3.1 | 12.5±4.8 | - | 7.5±7.5 |
| 2.5-NS | 11.3±5.5 | 28.8±5.5 | 0±0 | 5±3.5 | 8.8±4.3 | - | 6.3±6.3 |
| 2.5-PS | 15±3.5 | 36.3±9.4 | 1.3±1.3 | 5±5 | 2.5±2.5 | - | 0±0 |
= 0-NS, control; 0-PS, stress; 0.5-NS, 0.5 MeHg, no stress; 0.5-PS, 0.5 MeHg and PS; 2.5-NS, 2.5 MeHg, no stress; 2.5-PS, 2.5 MeHg and prenatal stress
3.4 Offspring Serum Corticosterone
Serum corticosterone was measured in a subset of offspring at 2 mos of age (just prior to initiation of behavioral testing in littermates) and in behaviorally and non-behaviorally tested offspring at the completion of behavioral testing (Figure 2). At 2 mos (top row), corticosterone levels were significantly higher in females, as expected, but no significant effects of MeHg ± PS were found in either males or females.
Figure 2.
Group mean ± SE corticosterone levels (ng/ml) in relation to MeHg exposure concentration in female (left column) and male (right column) at 2 mos of age (top row) and in animals following the completion of behavioral testing (bottom row). NS=non stress; PS=prenatal stress; Behav=underwent operant behavioral testing; NonBehav=did not undergo operant behavioral testing; MeHg=indicates statistically significant effect of MeHg; Behav vs NonBehav=indicates significant effect of behavioral testing. n=8–11/group.
At the completion of behavioral testing (bottom row), significant effects of sex were similarly present in the overall analyses, as were interactions of sex by behavioral vs. non-behavioral testing conditions, hence each sex was further analyzed separately. Corticosterone levels were significantly lower in behaviorally tested groups in both males, (F(1,116)=25.87, p<0.001) and females (F(1,114)=35.97, p<0.001) and reduced by MeHg independently of behavioral testing condition only in males (F(2,116)=3.2, p=0.045). Post-hoc tests indicated significant reductions at 2.5ppm relative to controls.
3.5 Offspring Brain Monoamines
3.5.1 PS-Related Changes
Three types of changes in monoamines were examined. Figure 3 depicts all statistically significant PS-related changes (main effect of PS in statistical analyses) in brain monoamines in both sexes. Regions and neurotransmitters affected by PS showed no overlap in males and females. In males, the broadest range of effects was seen in nucleus accumbens, where PS significantly increased levels of DA (F(1,35)=4.6, p=0.039), DOPAC (F(1,48)=4.51, p=0.039) and HVA (F(1,47)=4.95, p=0.031) relative to NS conditions. PS also significantly increased levels of striatal DOPAC (F(1,41)=4.432, p=0.041) and NE (F(1,37)=6.1, p=0.018), and, marginally, DA TO (F(1,47)=3.84, p=0.056) in olfactory bulb, and hypothalamic 5HT (F(1,30=5.22, p=0.03) in males.
Figure 3.
Group mean ± SE neurotransmitter values in relation to MeHg exposure concentration and PS (NS=non stress; PS=prenatal stress) for males (top box) and females (bottom box) in which statistically significant main effects of PS were found in the two-factor ANOVAs. n=4–10/group for males and n=6–10/group for females. * = significant difference from 0-NS; $ = significant difference from 0.5-NS.
In contrast, the broadest range of PS-related alterations in in females was observed in midbrain, where PS reduced levels of NE (F(1,44)=4.18, p=0.047), 5HT (F(1,44)=5.47, p=0.024) and HIAA (F(1,41)=4.29, p=0.044). PS also significantly reduced levels of nucleus accumbens 5HT (F(1,42)=9.15, p=0.004) and HIAA (F(1,42)=4.94, p=0.032), but increased frontal cortex levels of both DA (marginally, F(1,40)=3.88, p=0.056) and 5HT (F(1,43)=4.85, p=0.033). Interestingly, except for changes in frontal cortex in females, which were primarily seen only at the 2.5ppm concentration, PS-related changes in monoamines were in opposite directions in males as compared to females.
3.5.2 MeHg Related Changes
Statistically significant MeHg-induced alterations (main effect of MeHg in statistical analyses) in brain monoamines are presented in Figure 4. As with PS-induced changes, regions and neurotransmitters altered by MeHg were completely different in males vs. females. In the case of males, effects were relatively non-systematic, comprised of non-monotonic increases in frontal cortex HIAA (F(1,47)=3.61, p=0.035), marginal increases in nucleus accumbens HIAA (F(1,47)=3.00, p=0.059) at the 0.5 ppm, but not at the 2.5 ppm MeHg exposure level, and reductions in hypothalamic DOPAC (F(1,46)=4.22, p=0.021) at the 0.5 ppm MeHg exposure level, but not at the 2.5 MeHg exposure levels.
Figure 4.
Group mean ± SE neurotransmitter values in relation to MeHg exposure concentration and PS (NS=non stress; PS=prenatal stress) for males (top box) and females (bottom box) in which statistically significant main effects of MeHg were found in the two-factor ANOVAs. * =significantly different from 0-NS; +=significantly different from 0.5ppm; #=significantly different from 2.5ppm; ~=marginally significant. n=6–10/group for both male and female.
MeHg-related changes occurred in multiple regions in females, almost all of which also involved non-monotonic changes. These consisted of reductions in frontal cortex DOPAC levels (F(1,42)=3.37, p=0.044), in hypothalamic DA TO (F(1,43)=3.39, p=0.043) and marginally in olfactory bulb 5HT (F(1,44)=2.81, p=0.071) and similar marginally significant effects for olfactory bulb HIAA at the 2.5 MeHg exposure level (F91,44)=3.04, p=0.058), but increases in striatal 5HT (F(1,41)=5.76, p=0.006; all post hoc p values <0.05) at the 0.5 but not the 2.5 MeHg exposure level.
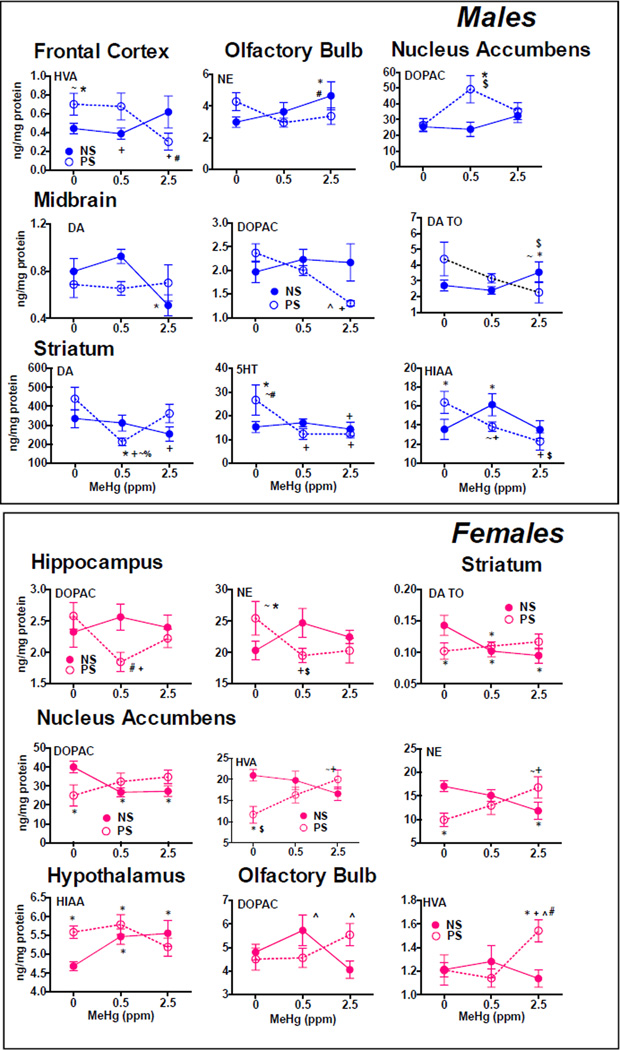
3.5.3 MeHg by PS Interactions
All statistically significant interactions of MeHg by PS are presented in Figure 5. While all significant comparisons are indicated in the figure, the results here are described primarily as the differences in MeHg exposure effects in PS vs non-PS conditions. As with effects of MeHg and of PS, there were generally no overlapping regions/neurotransmitter effects in males vs. females with the exception of nucleus accumbens DOPAC.
Figure 5.
Group mean ± SE neurotransmitter values in relation to MeHg exposure concentration and PS (NS=non stress; PS=prenatal stress) for males (top box) and females (bottom box) in which statistically significant interactions of MeHg by of PS were found in the two-factor ANOVAs. * = significantly different from 0-NS control; + = significantly different from 0-PS; $=significantly different from 0.5-NS; #=significantly different from 0.5-PS; ^= significantly different from 2.5-NS; %=significantly different from 2.5-PS; ~=marginally significant. n=3–10/group for males and n=6– 10/group for females.
In males, several examples of effects of MeHg that only occurred in the presence of PS were observed. For example, levels of midbrain DOPAC (F(2,43)=3.18, p=0.051) and striatal 5HT (F(2,31)=8.66, p=0.001) and were reduced only in 0.5-PS and/or 2.5-PS conditions. Further, significant reductions in frontal cortex levels of HVA (F(2,35)=4.04, p=0.026) were seen only under PS conditions and at the 2.5 MeHg exposure levels, whereas no changes occurred in non-PS conditions. Non-monotonic alternations of MeHg effects by PS were seen for striatal DA (F(2,33)=3.89, p=0.031) and for nucleus accumbens DOPAC (F(2,46)=3.21, p=0.0495), where effects were only observed in 0.5-PS groups and not in 2.5-PS groups. In the case of olfactory bulb NE (F(2,47)=3.11, p=0.054), and marginally for both midbrain DA (F(2,32)=2.83, p=0.074) and DA TO (F(2,35)=2.97, p=0.064), significant changes were restricted to the 2.5-NS group. Different directions of effects of MeHg vs. PS were seen for levels of striatal HIAA (F(2,44)=7.41, p=0.0017), with increases only at the 0.5 MeHg exposure level under NS conditions, but with marginal reductions at 0.5 and significant reductions at 2.5 under non-PS conditions.
Examples of effects of MeHg-related alterations in neurotransmitter levels only in the presence of PS were also evident in females, including changes in hippocampal DOPAC (F(2,42)=3.16, p=0.053) and NE (F(2,43)=4.21, p=0.021), nucleus accumbens HVA (F(2,42)=5.15, p=0.010), and olfactory bulb HVA (F(2,44)=3.73, p=0.032). In the case of hippocampal DOPAC and NE, these effects of MeHg only under conditions of PS were non-monotonic, being confirmed at 0.5 but not at 2.5ppm. Some neurochemical changes, in particular striatal DA TO (F(2,43)=3.56, p=0.037) and nucleus accumbens DOPAC (F(2,42)=5.51, p=0.0075) reductions, as well as hypothalamic 5HIAA increases (F(2,43)=3.73, p=0.032), were seen only in non-PS conditions. Changes that occurred in opposite directions included reductions in nucleus accumbens NE values in non-PS conditions, but increases under PS conditions, both at the 2.5ppm MeHg exposure concentration (F(2,42)=6.39, p=0.004). Both opposite and non-monotonic effects were seen with DOPAC levels in olfactory bulb (F(2,44)=3.85, p=0.029), where levels were increased only at the 0.5ppm MeHg exposure level under non-PS conditions relative to the 2.5 MeHg exposure, and increased only at the 2.5ppm MeHg exposure level relative to 2.5–NS under PS conditions.
Tables 4 and 5 contain all neurochemical data for comparative purposes.
Table 4.
Group Mean (S.E). Male Neurotransmitter Levels (ng/mg protein)
| REGION1 | DA | DOPAC | HVA | DA TO | NE | 5HT | HIAA | |
|---|---|---|---|---|---|---|---|---|
| Frontal Cortex | 0-NS | .87 (.11) | 2.23 (.11) | .44 (.055) | .39 (.046) | 24.1 (6.1) | 31.85 (3.5) | 2.4 (.17) * ~# |
| 0-PS | .78 ([21) | 2.24 (.14) | .7 (.116) | .37 (.10) | 25.77 (6.3) | 28.87 (1.9) | 2.2 (.18) | |
| 0.5-NS | .65 (.12) | 2.59 (.12) | .39 (.06) | .246 (.04) | 32 (4.36) | 34.84 (3.6) | 2.75 (.19) | |
| 0.5-PS | 1.17 (.29) | 2.36 (.21) | .68 (.14) | .537 (.16) | 19.3 (6.66) | 32.39 (3.4) | 3.1 (.3) | |
| 2.5-NS | .84 (.12) | 2.6 (.16) | .62 (.17) | .35 (.06) | 34.4 (8.16) | 28.43 (4.4) | 2.9 (.36) | |
| 2.5-PS | .76 (.18) | 2.2 (.11) | .30 (.09) | .354 (.084) | 23.3 (10.2) | 30.45 (5.57) | 2.07 (.11) | |
| Nucleus Accumb | 0-NS | 198.1 (31.4) + | 25.5 (2.6) +# | 17.2 (1.8) + | .093 (.004) | 16.3 (2.67) | 18.5 (1.62) | 11.46 (1.2) ~* |
| 0-PS | 220.7 (44.6) | 26.45 (4.2) | 20.54 (3.1) | .112 (.012) | 26.07 (4) | 18.73 (2.5) | 10.65 (1.55) | |
| 0.5-NS | 199 (36.6) | 23.82 (4.6) | 16.9 (2.08) | .133 (.024) | 21.7 (4.85) | 16.72 (2.59) | 12.71 (1.5) | |
| 0.5-PS | 354.5 (50.6) | 49.3 (8.7) | 25.9 (2.38) | .11 (.012) | 28 (5.27) | 18.6 (1.42) | 16.9 (1.94) | |
| 2.5-NS | 255.5 (42.7) | 32.4 (4.4) | 17.1 (2) | .138 (.022) | 22.25 (6.05) | 16.84 (3.6) | 11.57 (2.24) | |
| 2.5-PS | 340.5 (82.9) | 35.3 (5.3) | 18.18 (2.9) | .105 (.014) | 17.25 (2.93) | 15.1 (4.3) | 10.38 (3) | |
| Striatum | 0-NS | 335. (47.7) *# | 30.16 (3.3)+ | 21.56 (1.69) | .087 (.004) | 19.85 (1.6) * | 15.3 (2.4) *# | 12.5 (.31) # |
| 0-PS | 440.4 (61.1) | 50.53 (7.2) | 25 (1.45) | .126 (.013) | 40.94 (3.33) | 31.54 (4.93) | 16.38 (1.15) | |
| 0.5-NS | 312 (42[3) | 35.39 (4.7) | 23.2 (2.19) | .138 (.032) | 22.4 (2.92) | 16.99 (1.57) | 16.1 (1.16) | |
| 0.5-PS | 211.3 (20) | 33.68 (5.66) | 20.3 (1.45) | .15 (.025) | 29.17 (6.29) | 12.28 (2.25) | 13.83 (.49) | |
| 2.5-NS | 253[5 (37.9) | 37.65 (5.75) | 19.52 (2.27) | .144 (.018) | 22.77 (5.5) | 14.34 (2.92) | 13.51 (.93) | |
| 2.5-PS | 362 (49.6) | 49.6 (9.92) | 21 (1.27) | .104 (.024) | 25.36 (7.18) | 12.33 (1.75) | 12.28 (.91) | |
| Hippo | 0-NS | .226 (.17) | 2.47 (.22) | .052 (.005) | 30.25 (5.81) | 29.22 (4.9) | 5.72 (.57) | |
| 0-PS | .15 (.027) | 2.86 (.34) | .038 (.006) | 41.42 (6.52) | 23.76 (7.17) | 5.86 (.3) | ||
| 0.5-NS | .12 (.02) | 2.74 (.34) | .057 (.01) | 40.74 (8.58) | 29.39 (6.23) | 6.4 (.52) | ||
| 0.5-PS | .098 (.028) | 2.61 (.12) | .032 (.000) | 34.76 (5.21) | 25 (5.44) | 6 (.24) | ||
| 2.5-NS | .158 (.01) | 2.3 (.29) | .032 (.014) | 31.09 (4.12) | 20.77 (5.73) | 5.17 (.23) | ||
| 2.5-PS | .08 (.042) | 2.21 (.155) | .041 (.015) | 27 (3.27) | 26.97 (6.66) | 4.98 (.34) | ||
| Midbrain | 0-NS | .798 (.11) ~# | 2 (.25) ~# | 2.7 (.3) ~# | 45.57 (12.8) | 37.38 (4.9) | 5.77 (.52) | |
| 0-PS | .688 (.111) | 2.44 (.178) | 4.39 (1.07) | 59.41 (16) | 44.43 (6.53) | 6 (.56) | ||
| 0.5-NS | .925 (.061) | 2.13 (.252) | 2.4 (.24) | 49.6 (14.3) | 33.44 (5.79) | 5.82 (.82) | ||
| 0.5-PS | .654 (.058) | 2.06 (.112) | 3.16 (.30) | 77.37 (21.3) | 36 (5.58) | 6.56 (.55) | ||
| 2.5-NS | .511 (.089) | 2.17 (.39) | 3.55 (.66) | 66.84 (19.8) | 38.37 (7.18) | 5 (.4) | ||
| 2.5-PS | .7 (.153) | 1.31 (.056) | 2.27 (.67) | 60.46 (23.9) | 27.27 (7.02) | 5.76 (.96) | ||
| Hypothal | 0-NS | 6.61 (1.02) | 2.7 (.177) * | .46 (.05) ~+ | 95.7 (7.56) | 64.1 (2.71) + | 5 (.327) | |
| 0-PS | 5.53 (.65) | 2.9 (.188) | .495 (.068) | 93.52 (10.3) | 67.4 (3.76) | 5.39 (.297) | ||
| 0.5-NS | 6.28 (1.14) | 2.68 (.15) | .454 (.03) | 97.7 (10) | 54.9 (3.58) | 5 (.254) | ||
| 0.5-PS | 7.35 (1.14) | 2.45 (.112) | .343 (.031) | 92.7 (9.73) | 64.5 (5.3) | 5.69 (.254) | ||
| 2.5-NS | 5.47 (.91) | 3 (.164) | .483 (.033) | 109.8 (14.5) | 62.49 (5.13) | 5.38 (.236) | ||
| 2.5-PS | 8.06 (.271) | 3.05 (.108) | .363 (.018) | 114.8 (14.4) | 73.76 (4.86) | 5.18 (.20) | ||
| Olfactory Bulb | 0-NS | 1.32 (.135) | 5 (.516) | 1.8a (.27) | 3.9 (.28) ~+ | 2.99 (.326) # | 12.1 (1.76) | 1.71 (.16) |
| 0-PS | 1.18 (.17) | 4.84 (.41) | 1.89 (.17) | 4.57 (.54) | 4.29 (.575) | 10 (2.48) | 2.33 (.31) | |
| 0.5-NS | 1.3 (.137) | 4.51 (.75) | 1.8 (.142) | 3.3 (.398) | 3.64 (.6) | 11.75 (1.5) | 1.65 (.12) | |
| 0.5-PS | 1.26 (.2) | 4.94 (.37) | 1.58 (.198) | 4.58 (.63) | 2.95 (.28) | 11.7 (2.58) | 1.88 (.148) | |
| 2.5-NS | 1.68 (.27) | 5.76 (.69) | 1.93 (.18) | 3.64 (.28) | 4.66 (.89) | 11.8 (2.36) | 2.02 (.16) | |
| 2.5-PS | 1.47 (.3) | 5.19 (.64) | 1.92 (.257) | 4 (.56) | 3.37 (.52) | 15 (2.24) | 1.78 (.12) |
Regions: Frontal Cortex, Nucleus Accumb=Nucleus Accumbens, Striatum, Hippo=Hippocampus, Midbrain, Hypothal=Hypothalamus, Olfactory Bulb
= significant effect of MeHg;
= significant effect of PS;
significant interaction of MeHg×PS;
= marginal effect
Table 5.
Group Mean (S.E.) Female Neurotransmitter Levels (ng/mg protein)
| REGION1 | DA | DOPAC | HVA | DA TO | NE | 5HT | HIAA | |
|---|---|---|---|---|---|---|---|---|
| Frontal Cortex | 0-NS | 1.04 (.13) + | 2.6 (.22) * | .66 (.11) # | .41 (.05) | 16.4 (3.6) | 41.8 (3.6) *+ | 2.6 (.12) |
| 0-PS | 1.13 (.17) | 2.48 (.26) | .42 (.1) | .49 (.09) | 22 (3.9) | 48.3 (5.3) | 2.6 (.15) | |
| 0.5-NS | .95 (.11) | 2.02 (.08) | .51 (.13) | .47 (.05) | 14.7 (3.5) | 35.2 (2.3) | 2.3 (.18) | |
| 0.5-PS | 1.05 (.14) | 2.04 (.14) | .76 (.24) | .47 (.05) | 15.8 (4.1) | 39.3 (2.1) | 2.6 (.08) | |
| 2.5-NS | .8 (.16) | 2.0 (.32) | .5 (.09) | .49 (.1) | 14.8 (3.4) | 32.7 (3) | 2.3 (.18) | |
| 2.5-PS | 2.04 (.83) | 2.43 (.18) | .46 (.08) | .82 (.33) | 20.3 (4) | 40.7 (3) | 2.5 (.19) | |
| Nucleus Accumb | 0-NS | 294.1 (20.2) | 40.1 (3.1) # | 21 (1.4) +# | .14 (.01) | 17.1 (1.2) # | 28 (4) + | 15.5 (1.2) +~# |
| 0-PS | 207.2 (31.9) | 25 (5.6) | 11.7 (2) | .12 (.02) | 9.9 (1.4) | 10.9 (2) | 8.3 (1.5) | |
| 0.5-NS | 246.4 (30.1) | 26.7 (2.4) | 19.8 (2.2) | .12 (.01) | 15.1 (1.2) | 22.4 (3.7) | 12.8 (1.6) | |
| 0.5-PS | 261 (37.3) | 32.4 (4.5) | 16.3 (1.9) | .13 (.01) | 12.9 (1.9) | 14.3 (1.7) | 12.2 (1.8) | |
| 2.5-NS | 282.2 (34.6) | 27.3 (2.8) | 16.7 (1.6) | .1 (.007) | 11.8 (1.8) | 18.4 (1.9) | 14.3 (2) | |
| 2.5-PS | 290.5 (30.5) | 34.9 (3.6) | 20 (2.2) | .13 (.02) | 16.8 (2.3) | 14.1 (1.4) | 13.1 (1.6) | |
| Striatum | 0-NS | 296 (27.5) | 40.6 (4.2) | 19.6 (1.3) | .14 (.02) # | 20.9 (3.3) | 16.4 (3.4) * | 13.8 (1.2) |
| 0-PS | 319 (26.2) | 30.6 (2.2) | 19.5 (0.6) | .1 (.01) | 16.3 (1.3) | 13.3 (1.5) | 12.6 (.63) | |
| 0.5-NS | 347 (27.8) | 35.7 (5) | 19.5 (1.5) | .1 (.009) | 18.3 (2.2) | 21.1 (3.6) | 15 (.89) | |
| 0.5-PS | 341 (27.6) | 37.8 (4.4) | 21.9 (1.4) | .11 (.007) | 19.1 (1.7) | 21.2 (2.5) | 15.7 (1.1) | |
| 2.5-NS | 333 (32.3) | 30.8 (4.4) | 18.7 (1.1) | .1 (.012) | 15.9 (.81) | 13.7 (1.8) | 15.7 (1.6) | |
| 2.5-PS | 265 (30) | 29.6 (1.8) | 17.3 (0.7) | .12 (.01) | 16.8 (1.18) | 10.1 (.69) | 13.9 (1.1) | |
| Hipp | 0-NS | .23 (.17) | 2.33 (.24) # | .04 (.02) | 20.3 (1.5) # | 37.6 (3.3) | 5.3 (.31) | |
| 0-PS | .15 (.03) | 2.6 (.21) | .07 (.02) | 25.5 (2.7) | 44.8 (5) | 5.7 (.24) | ||
| 0.5-NS | .12 (.02) | 2.56 (.21) | .05 (.01) | 24.7 (2.3) | 35.9 (4.7) | 5.9 (.35) | ||
| 0.5-PS | .1 (.03) | 1.85 (.15) | .046 (.01) | 19.5 (1.15) | 35.2 (4.3) | 5.5 (.46) | ||
| 2.5-NS | .16 (.01) | 2.4 (.2) | .066 (.01) | 22.5 (1.03) | 34.9 (3.4) | 5.8 (.23) | ||
| 2.5-PS | .08 (.04) | 2.23 (.15) | .03 (.01) | 20.3 (2) | 30.9 (24) | 5.1 (.29) | ||
| Midbrain | 0-NS | .85 (.08) | 2.2 (.18) | 2.72 (.23) | 27.3 (1.2) + | 41 (2.1) +~# | 5.9 (.27) +~# | |
| 0-PS | .83 (.08) | 1.7 (.24) | 2.33 (.41) | 23.3 (1.8) | 44.2 (3.7) | 6.17 (.4) | ||
| 0.5-NS | .93 (.1) | 1.9 (.25) | 2.3 (.31) | 26.1 (2) | 46.2 (4.6) | 7.3 (.53) | ||
| 0.5-PS | .8 (.1) | 2 (.17) | 2.9 (.39) | 23.8 (1.6) | 34.7 (2.4) | 5.6 (.37) | ||
| 2.5-NS | .91 (.1) | 2.1 (.38) | 2.7 (.4) | 29.1 (3.4) | 48.2 (5.7) | 6.2 (.7) | ||
| 2.5-PS | .75 (.04) | 1.7 (.36) | 1.7 (.19) | 24.9 (3) | 34.6 (5.2) | 5.2 (.45) | ||
| Hypothal | 0-NS | 5.5 (.81) | 2.49 (.12) | .51 (.05) * | 66.1 (1.17) | 68.1 (4) +~# | 4.7 (.12) # | |
| 0-PS | 7.5 (.82) | 2.75 (.15) | .4 (.038) | 83.2 (8.1) | 74.3 (2.5) | 5.6 (.16) | ||
| 0.5-NS | 8.2 (1.2) | 2.64 (.09) | .38 (.05) | 83.1 (7.3) | 62.1 (3.2) | 5.5 (.21) | ||
| 0.5-PS | 7.5 (.83) | 2.6 (.1) | .35 (.038) | 75.6 (6.3) | 67.7 (4.3) | 5.8 (.26) | ||
| 2.5-NS | 5.6 (.63) | 2.68 (.15) | .51 (.05) | 74.4 (7.8) | 69.9 (3.5) | 5.6 (.34) | ||
| 2.5-PS | 6.2 (.93) | 2.63 (.21) | .46 (.03) | 70.4 (6.3) | 59.8 (5.1) | 5.2 (.24) | ||
| Olfactory Bulb | 0-NS | 1.14 (.1) ~# | 4.81 (.34) # | 1.21 (.06) # | 4.36 (.3) | 2.78 (.15) | 18.1 (1.9) ~* | 1.74 (.16) ~* |
| 0-PS | 1.03 (.13) | 4.5 (.47) | 1.21 (.13) | 4.6 (.55) | 2.48 (.18) | 16.4 (1.74) | 1.72 (.11) | |
| 0.5-NS | 1.36 (.26) | 5.7 (.65) | 1.28 (.14) | 4.6 (.41) | 2.58 (.25) | 13.2 (.74) | 1.52 (.1) | |
| 0.5-PS | .95 (.09) | 4.56 (.41) | 1.14 (.08) | 4.9 (.27) | 2.33 (.10) | 14.7 (1.34) | 1.5 (.08) | |
| 2.5-NS | 1.0 (.07) | 4.06 (.37) | 1.14 (.07) | 4.13 (.32) | 2.31 (.16) | 17.1 (1.62) | 1.86 (.12) | |
| 2.5-PS | 1.3 (.13) | 5.55 (.48) | 1.54 (.09) | 4.33 (.28) | 2.61 (.15) | 15.38 (.93) | 1.69 (.07) |
Regions: Frontal Cortex, Nucleus Accumb=Nucleus Accumbens, Striatum, Hippo=Hippocampus, Midbrain, Hypothal=Hypothalamus, Olfactory Bulb
= significant effect of MeHg;
= significant effect of PS;
significant interaction of MeHg×PS;
= marginal effect
3.6 Offspring Behavior
3.6.1 Locomotor Activity
Significant MeHg ± PS-induced changes in locomotor activity were seen only in males and only in rearing-related activity (Figure 6). Statistical analyses confirmed MeHg by PS interactions for both vertical counts (F(2,43)=3.94, p=0.027) and for vertical time (F(2,43)=3.43, p=0.042). In both cases, this primarily reflected the differential change at the 2.5ppm MeHg dose, where vertical activity was reduced under non-PS conditions, but increased under PS conditions.
Figure 6.
Group mean ± SE total vertical counts (left) and vertical time (right) relative to MeHg exposure concentration and PS (NS=non stress; PS=prenatal stress) for males. * = significantly different from 0-NS control; +=significantly different from 0-PS; #=significantly different from 0.5-NS. n=7–9/group.
3.6.2 Novel Object Recognition
Statistical analyses of NOR performance revealed MeHg × PS interactions only in females (Figure 7), as shown for average duration of novel object approaches and maximum duration of contact with novel object indices. Post-hoc analyses collapsed across 1 min intervals, revealed that these interactions stemmed from the lower duration index scores of the 0.5-PS and 2.5-PS groups (F(2,232)=5.45, p=0.007 and F(1,184)=3.37, p=0.043, respectively), consistent with enhanced effects of MeHg + PS. Both the 0.5-PS and 2.5-PS groups evidenced reduced index scores for both average duration and maximum duration relative to 0-NS control as well as 0-PS. In addition, each group showed lower overall duration indices than did their corresponding MeHg-NS group. For both indices, 0.5-PS indices were also significantly lower than those of the 2.5NS group, and in the case of the 2.5-PS group were significantly lower than the 0.5-NS group (all p values <0.05).
Figure 7.
Group mean ± SE index scores for duration of time in contact with novel object (top row) and maximal duration of time in contact with novel object (bottom row) by minute intervals of the 5 min test for groups as indicated for females (left column) and males (right column). NS=non stress; PS=prenatal stress; MeHg × PS = indicates significant interaction of MeHg by prenatal stress in the repeated measures ANOVA. * = significantly different from 0-NS control; + =significantly different from 0-PS; $=significantly different from 0.5-NS; #=significantly different from 0.5-PS; ^= significantly different from 2.5-NS; %= significantly different from 2.5PS; n=10– 11/group for males and for females.
3.6.3 Fixed Interval Performance
As with NOR, the impact of MeHg ± PS on FI 1 min schedule-controlled behavior was only seen in females. Figure 8 depicts MeHg ± PS-induced alterations in overall rate, run rate, postreinforcement pause time and interresponse time as indicated. Larger plots show both MeHg alone and MeHg+PS groups, while insets in each plot depict data for MeHg only groups to facilitate visualization of non-monotomic MeHg effects.
Figure 8.
Group mean ± SE overall rates (top left), run rates (top right), postreinforcement pause times (bottom left) and mean interresponse time values (bottom right) by block of 5 sessions in females for indicated treatment groups. Inset within each larger plot shows same data for MeHg-treated only groups. NS=non stress; PS=prenatal stress; PS = main effect of PS in the repeated measures ANOVA; PS × Session = interaction of prenatal stress by session repeated measures ANOVA; MeHg × PS = indicates significant interaction of MeHg by prenatal stress in the repeated measures ANOVA; MeHg × PS × session= indicates significant 3 way interaction of MeHg by prenatal stress by session repeated measures ANOVA. * = significantly different from 0-NS control; + = significantly different from 0-PS; $=significantly different from 0.5-NS; #=significantly different from 0.5-PS; ^= significantly different from 2.5-NS; %= significantly different from 2.5PS; ~=marginally significant. n=10–11/group for males and for females n=8–11/group.
As expected, FI overall response rates (top left) increased across sessions (Figure 8; MeHg × PS × sessions; F(76,2242)=1.32, P=0.036). MeHg-associated reductions were non-monotonic, with the lowest response rates in the 0.5-NS group (Figure 8 top left inset) that differed significantly from the 0-NS group and from the 2.5-NS group. These non-monotonic effects were also differentially influenced by PS. Specifically, FI response rates of the 2.5-NS group were further reduced by PS, such that they were statistically significantly lower than 0-NS and 2.5-NS rates. In contrast, the significantly lower FI response rates of the 0.5-NS group were mitigated under PS conditions (i.e., 0.5-PS), such that they were significantly greater than 0.5-NS. Thus, an overall pattern again consistent with both monotonic effects, with reductions at one MeHg exposure level (2.5) further enhanced by PS.
Changes in FI overall response rate appeared to reflect a combination of more modest changes in both run rate (top right; MeHg × PS, F(70,1995)=2.85, p=0.066) and postreinforcement pause time (bottom left; PS, F(1,2065)=4.08, p=0.048; PS × sessions (F(35,2065)=1.95, p=0.0007; MeHg × PS (F(2,2065)=2.64, p=0.0797). Specifically, MeHg-related reductions in FI overall rate appeared to be due to reductions in run rate in particular, given the significant reductions in run rate in the 0.5-NS group relative to 0-NS and 0.5-PS groups (p=0.068). Similarly, the 2.5-PS but not the 2.5-NS group run rates differed significantly from 0-NS control. This was consistent with the significantly longer interresponse time (IRT) values of the 0.5NS group relative to 0-NS control (MeHg × PS × session, F(70,1995)=1.664, p=0.0006) and of the 2.5-PS group, where IRTs were significantly greater than those of the 0-NS, 0-PS, 0.5-NS and 2.5-NS groups. The further reductions in FI overall rates at 2.5ppm produced by PS (2.5-PS) appear to primarily reflect significantly longer postreinforcement pause times of this group that were observed relative to all other groups.
Data from individual females within each of the treatment groups are shown for each of the 4 measures in Figure 9 from block 12, the final block of FI testing. As expected, there appear to be individual differences in susceptibility relative to control of MeHg ± PS subjects. Of particular interest, however, are the postreinforcement pause time and interresponse time values that tend to show a type of increasing effect across MeHg +PS. Specifically, the number of subjects in the higher range for postreinforcement pause and interresponse time values increased in the 0-PS and further in the 0.5-NS groups relative to 0-NS, and both the range of decisecond values and the number of subjects in the higher end of the range increased further in the 0.5-PS and 2.5-NS groups with essentially the greatest number of subject in the high range at 2.5-PS.
Figure 9.
Individual values of female subjects for overall rate, run rate, postreinforcement pause and mean interresponse time for groups as indicated. Data from the final block of FI testing (block 12). Dashed line for each group represents median value.
In contrast to the effects observed in females, males exhibited no significant effects of MeHg ± PS on any measure of FI performance (Figure 10). 4.
Figure 10.
Group mean ± SE overall rates (top left), run rates (top right), postreinforcement pause times (bottom left) and mean IRT values (bottom right) by block of 5 sessions in males for groups as indicated. n=10–11/group.
4. Discussion
Several findings emerged from this study, which was premised on the hypothesis that, given shared biological substrates and common adverse outcomes, developmental MeHg and PS could yield enhanced toxicity. For these reasons, the study focused on brain monoamine systems and behaviors known to be impacted by MeHg (Ceccatelli et al., 2013; Liang et al., 2009; Montgomery et al., 2008; Newland et al., 2013; Newland et al., 2004; Onishchenko et al., 2007; Peters et al., 2010; Yoshida et al., 2011) and/or mediated by brain monoamine systems (Carratu et al., 2006; Castoldi et al., 2006; Coccini et al., 2011; Farina et al., 2011). First, multiple examples of the effects of MeHg effects that were enhanced by PS, or occurring only under conditions of PS, i.e., being unmasked by PS, were observed, including changes in brain neurochemistry and in behavior, particularly in females. Secondly, MeHg was associated with significant non-monotonic functions both in terms of neurochemical and behavioral outcomes, where greater effects occurred at 0.5ppm than at 2.5ppm, and, again, particularly in females. Thirdly, as the above implies, both neurochemical and behavioral effects of MeHg ± PS were highly sex-dependent, an observation consistent with previous reports of sex differences in response to MeHg, although such differences have not been particularly consistent (Llop et al., 2013).
Alterations of MeHg effects by PS, either unmasking or enhancing MeHg effects, were observed in both sexes, but predominantly in females, and occurred at both the 0.5ppm and 2.5ppm MeHg exposure concentrations. Consistent with unmasking effects of PS, for example, reductions in males in frontal cortex HVA as well as midbrain DOPAC levels were only observed under conditions of both 2.5ppm + PS (Figure 5). Further, both striatal 5HT and 5HIAA were reduced in males by 0.5ppm+PS and 2.5ppm+PS, but no other conditions. In females, reductions in hippocampal DOPAC and NE were seen only under conditions of 0.5ppm +PS, consistent with non-monotonic unmasking effects. Additionally, increases in nucleus accumbens HVA and in olfactory bulb HVA were only found under conditions of 2.5ppm +PS. Collectively, these outcomes are consistent with prior reports of sex and brain-region dependent effects of MeHg on brain dopamine and serotonergic systems (Castoldi et al., 2006; Coccini et al., 2011).
As these findings also demonstrate, concentration-effect functions for MeHg-induced neurochemical changes can be significantly modified by PS and in some cases, neurochemical changes only occur under joint exposure conditions, underscoring the critical need to evaluate MeHg-induced changes within contexts relevant to human exposures. This is further evidenced by the fact that PS alone markedly changed neurotransmitter levels in some cases even in 0ppmtreated groups, as with striatal 5HT and 5HIAA in males and hippocampal NE, nucleus accumbens DOPAC and HVA, and in striatal DA TO in females. Further, it is clear that these PS-induced alterations of MeHg related neurochemical changes do not reflect differences in toxicokinetics, since no PS by MeHg interactions were found for either blood or brain Hg levels (Figure 1) nor in differential HPA axis stress response at least as indicated by corticosterone measurements (Figure 2).
PS also enhanced some adverse behavioral consequences of MeHg exposures or unmasked such effects in females. Duration of time in contact with a novel object in the novel object recognition task was only significantly impaired in the 0.5ppm-PS and 2.5ppm-PS groups, and not in response to PS alone or to MeHg alone, effects that only emerged during the final 3 min of testing (Figure 7). While some similarity in trends can be seen in males, these did not approach statistical significance. Thus, MeHg may affect learning/short term memory in females at lower exposure levels than typically appreciated in an environment that also includes stress.
PS also modified the effects of MeHg on FI performance of females (Figures 8–9). In this study, MeHg resulted in non-monotonic reductions in FI response rate in females (Figure 8), unlike a previous study that reported FI response rate increases in females at a higher level of MeHg (5 ppm), but not at a lower level as used here, 0.5 ppm (Reed and Newland, 2007a). The differences in MeHg effect could reflect differences in any of the several differences between the studies, such as ages of animals at testing and/or differences in the schedules (a simple FI here vs. a multiple clocked and unclocked FI in Reed et al. (Reed and Newland, 2007a). Notably, in this study, further reductions in FI overall rates occurred at 2.5ppm-PS, with rates lower than those of control, while neither PS alone (0-PS) nor 2.5ppm-NS produced significant rate decreases relative to control. Contributing to these reductions were the increased postreinforcement pause times, seen only in 2.5ppm-PS females as well as increases in mean IRT values. Interestingly, increases in nucleus accumbens HVA and NE were significantly enhanced at 2.5-PS only and nucleus accumbens dopamine is known to be critical to mediation of FI performance (Cory-Slechta et al., 1997).
These enhanced effects of MeHg in the presence of PS differ considerably from those noted in previous studies, where interactive effects of gestational MeHg were found to be enhanced by PS only at doses of MeHg associated with dam toxicity (Colomina et al., 1995; 1997; Domingo et al., 2004). While those studies involved mice, they also utilized quite different and shorter MeHg exposure regimens as well as PS paradigms, which may contribute to the difference in outcomes. Further, no evidence of maternal toxicity, particularly in maternal weight gain or behavior, appeared to influence the current results.
Some studies have suggested that MeHg-treated offspring might be more susceptible to stressors as adults. It has been shown that male offspring born to rat dams exposed to 8 mg/kg MeHg on gestational day 15 were significantly impaired in learning an active avoidance task in adulthood, and this was accompanied by markedly elevated corticosterone levels (Carratu et al., 2008a), findings consistent with an enhanced stress response. In our study, we followed the FI 1 min schedule with 3 sessions of extinction (withholding food reward) as a stressor (data not shown), but found no significant group differences in FI performance in either sex. Further, at the completion of behavioral testing, MeHg-induced reductions rather than increases in corticosterone were seen in males. While the differential direction of changes in corticosterone are not necessarily incompatible and both suggest dysfunction of the HPA axis, it may be that extinction was not a sufficient stressor to alter the behavioral response to extinction, but studies to address later stress responsivity are warranted.
Another notable finding from this study was the observation of non-monotonic concentration effect functions for multiple neurochemical effects of MeHg in both sexes (Figure 4), as well as for FI performance in females (Figure 8). In the case of neurochemical changes, these non-monotonic outcomes were observed in multiple brain regions, but in a sex-dependent capacity, specifically they tended to be more pronounced in females, with significant MeHginduced changes at 0.5ppm MeHg dosing level, but not at 2.5ppm. Additionally, these nonmonotonic functions correspond to the MeHg-induced pattern of FI performance in females, where 0.5ppm, but not 2.5ppm MeHg alone, reduced overall FI rates as well as run rates, while significantly increasing mean IRT values. In contrast, reductions in locomotor activity, seen only in males, occurred in a concentration-related fashion, with significant reductions in response to the higher MeHg exposure of 2.5ppm.
While non-monotonic effects of MeHg have been previously noted (Helmcke and Aschner, 2010; Karagas et al., 2012), it has been in a hormetic context, in which lower levels of exposure produce beneficial effects, with toxicity at higher doses. However, more pronounced effects of lower levels of MeHg have been noted in other contexts. For example, non-monotonic effects of MeHg were suggested in a study of the association between blood pressure and whole blood methylmercury among Intuit in Greenland. That study noted that in analyses based on quintiles of blood mercury levels, the effect on blood pressure was significant only for some quintiles (Nielsen et al., 2012). While the latter could reflect sample size issues, the non-monotonic functions found here do not appear to be an anomaly given that they were observed both for neurochemical and behavioral changes.
Sex-dependent differences in the consequences of PS are well known (Weinstock, 2007; 2011). The extent to which the neurotoxic effects of MeHg are sex-dependent have been less well characterized, although consistent with the findings here, locomotor activity was reported to be impacted by prenatal MeHg in male but not female rats (Rossi et al., 1997), although this has not been consistent across studies (Cauli et al., 2013). Sex-differences in MeHg toxicokinetics have repeatedly been reported (Nielsen, 1992; Nielsen et al., 1992; Nielsen and Andersen, 1991a; b). While this may have contributed to the differential neurotoxicity seen here, no differences in total brain Hg were seen at least at the one time point measured here. It is possible that brain Hg differed at other time points, and a time course study would be of interest in this regard. Of additional interest to consider in future studies is the possible impact of MeHg on sex hormones and corresponding receptors in brain, as MeHg has been reported to influence both estrogen and testosterone functions and to correspondingly alter brain Hg levels (Fossato da Silva et al., 2011; Hirayama et al., 1987; Jayasena et al., 2011; Oliveira et al., 2006). Of particular note, it was recently reported that estradiol could protect against methylmercury-induced neurotoxicity in cultured rat hippocampal slices (Yamazaki et al., 2013).
In conclusion, these data demonstrate that prenatal stress has the potential to both unmask and to enhance the neurotoxic effects of developmental MeHg exposure, in a sex-dependent manner. The nature of the behavioral deficits observed in females, i.e., reduced duration of contact with a novel object in the novel object recognition test, as well as reduced FI rates and increased pause time, are consistent with deficits in learning/short-term memory. Moreover, it appears that some neurotoxic effects of MeHg may be non-monotonic in nature, particularly in females, with greater effects at lower levels than at higher levels, findings reminiscent of the neurotoxicity of lead-associated IQ reductions. Importantly, these effects were observed in the absence of any significant MeHg ± PS-related changes in reproductive outcomes or maternal behavior. Collectively, the findings underscore the import of studying neurotoxic chemical effects in contexts that are relevant to the human environment, given that other pertinent factors in the environment may modify effect levels of MeHg and thereby alter risk profiles.
Highlights.
This study demonstrates enhanced and unmasked behavioral toxicity and neurochemical changes in rats when developmental exposure to methylmercury is combined with prenatal stress, both in terms of behavioral changes and brain neurochemistry, particularly in females.
Acknowledgements
These findings were supported in part by NCER EPA Grant RD-83457801-0 (D. Cory-Slechta, PI) and P30 ES001247 (T. Gasiewicz, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res. 2007;40:285–291. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus-accumbens information flow. J Neurosci. 2008;28:9797–9805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MA, Barros VG, Sarchi MI, Tarazi FI, Antonelli MC. Long-term effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Neurochem Res. 2002;27:1525–1533. doi: 10.1023/a:1021656607278. [DOI] [PubMed] [Google Scholar]
- Betts KS, Williams GM, Najman JM, Bor W, Alati R. Pre-trauma verbal ability at five years of age and the risk of post-traumatic stress disorder in adult males and females. J Psychiatr Res. 2012;46:933–939. doi: 10.1016/j.jpsychires.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Boucher O, Jacobson SW, Plusquellec P, Dewailly E, Ayotte P, Forget-Dubois N, et al. Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Quebec. Environ Health Perspect. 2012;120:1456–1461. doi: 10.1289/ehp.1204976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdineaud JP, Marumoto M, Yasutake A, Fujimura M. Dietary mercury exposure resulted in behavioral differences in mice contaminated with fish-associated methylmercury compared to methylmercury chloride added to diet. J Biomed Biotechnol. 2012;2012:681016. doi: 10.1155/2012/681016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carratu MR, Borracci P, Coluccia A, Giustino A, Renna G, Tomasini MC, et al. Acute exposure to methylmercury at two developmental windows: focus on neurobehavioral and neurochemical effects in rat offspring. Neuroscience. 2006;141:1619–1629. doi: 10.1016/j.neuroscience.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Carratu MR, Coluccia A, Modafferi AM, Borracci P, Scaccianoce S, Sakamoto M, et al. Prenatal methylmercury exposure: effects on stress response during active learning. Bull Environ Contam Toxicol. 2008a;81:539–542. doi: 10.1007/s00128-008-9557-8. [DOI] [PubMed] [Google Scholar]
- Carratu MR, Coluccia A, Modafferi AM, Borracci P, Scaccianoce S, Sakamoto M, et al. Prenatal methylmercury exposure: effects on stress response during active learning. Bull Environ Contam Toxicol. 2008b;81:539–542. doi: 10.1007/s00128-008-9557-8. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Blandini F, Randine G, Samuele A, Manzo L, Coccini T. Brain monoaminergic neurotransmission parameters in weanling rats after perinatal exposure to methylmercury and 2,2',4,4',5,5'-hexachlorobiphenyl (PCB153) Brain Res. 2006;1112:91–98. doi: 10.1016/j.brainres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Onishchenko N, Johansson C, Coccini T, Roda E, Vahter M, et al. Neurodevelopmental toxicity of methylmercury: Laboratory animal data and their contribution to human risk assessment. Regul Toxicol Pharmacol. 2008;51:215–229. doi: 10.1016/j.yrtph.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Cauli O, Piedrafita B, Llansola M, Felipo V. Gender differential effects of developmental exposure to methyl-mercury, polychlorinated biphenyls 126 or 153, or its combinations on motor activity and coordination. Toxicology. 2013;311:61–68. doi: 10.1016/j.tox.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Bose R, Edoff K, Onishchenko N, Spulber S. Long-lasting neurotoxic effects of exposure to methylmercury during development. J Intern Med. 2013;273:490–497. doi: 10.1111/joim.12045. [DOI] [PubMed] [Google Scholar]
- Clougherty JE, Rossi CA, Lawrence J, Long MS, Diaz EA, Lim RH, et al. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environ Health Perspect. 2010;118:769–775. doi: 10.1289/ehp.0901631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccini T, Roda E, Castoldi AF, Poli D, Goldoni M, Vettori MV, et al. Developmental exposure to methylmercury and 2,2',4,4',5,5'-hexachlorobiphenyl (PCB153) affects cerebral dopamine D1-like and D2-like receptors of weanling and pubertal rats. Arch Toxicol. 2011;85:1281–1294. doi: 10.1007/s00204-011-0660-y. [DOI] [PubMed] [Google Scholar]
- Colomina MT, Albina ML, Domingo JL, Corbella J. Effects of maternal stress on methylmercury-induced developmental toxicity in mice. Physiol Behav. 1995;58:979–983. doi: 10.1016/0031-9384(95)00140-e. [DOI] [PubMed] [Google Scholar]
- Colomina MT, Albina ML, Domingo JL, Corbella J. Influence of maternal stress on the effects of prenatal exposure to methylmercury and arsenic on postnatal development and behavior in mice: a preliminary evaluation. Physiol Behav. 1997;61:455–459. doi: 10.1016/s0031-9384(96)00462-3. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Brockel BJ, O'Mara DJ. Lead exposure and dorsomedial striatum mediation of fixed interval schedule-controlled behavior. Neurotoxicology. 2002;23:313–327. doi: 10.1016/s0161-813x(02)00059-1. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, O'Mara DJ, Brockel BJ. Nucleus accumbens dopaminergic mediation of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J Pharmacol Exp Ther. 1998;286:794–805. [PubMed] [Google Scholar]
- Cory-Slechta DA, Pazmino R, Bare C. The critical role of the nucleus accumbens dopamine systems in the mediation of fixed interval schedule-controlled operant behavior. Brain Res. 1997;764:253–256. doi: 10.1016/s0006-8993(97)00591-x. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Rossi-George A, Weston D, Thiruchelvam M. Experimental manipulations blunt time-induced changes in brain monoamine levels and completely reverse stress, but not Pb+/−stress-related modifications to these trajectories. Behav Brain Res. 2009;205:76–87. doi: 10.1016/j.bbr.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Weiss B, Cox C. Performance and exposure indices of rats exposed to low concentrations of lead. Toxicol Appl Pharmacol. 1985;78:291–299. doi: 10.1016/0041-008x(85)90292-3. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Weston D, Liu S, Allen JL. Brain hemispheric differences in the neurochemical effects of lead, prenatal stress, and the combination and their amelioration by behavioral experience. Toxicol Sci. 2013;132:419–430. doi: 10.1093/toxsci/kft015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PW, Cory-Slechta DA, Thurston SW, Huang LS, Shamlaye CF, Gunzler D, et al. Fish consumption and prenatal methylmercury exposure: cognitive and behavioral outcomes in the main cohort at 17 years from the Seychelles child development study. Neurotoxicology. 2011;32:711–717. doi: 10.1016/j.neuro.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R, Sokoloff P, Fuxe K. Codistribution of the dopamine D3 receptor and glucocorticoid receptor mRNAs during striatal prenatal development in the rat. Neurosci Lett. 1997;227:119–122. doi: 10.1016/s0304-3940(97)00316-9. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Domingo A, Colomina MT. Influence of maternal stress on metal-induced pre- and postnatal effects in mammals: a review. Biol Trace Elem Res. 2004;98:193–208. doi: 10.1385/bter:98:3:193. [DOI] [PubMed] [Google Scholar]
- Emack J, Matthews SG. Effects of chronic maternal stress on hypothalamo-pituitary-adrenal (HPA) function and behavior: no reversal by environmental enrichment. Horm Behav. 2011;60:589–598. doi: 10.1016/j.yhbeh.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Farina M, Rocha JB, Aschner M. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci. 2011;89:555–563. doi: 10.1016/j.lfs.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faro LR, Rodrigues KJ, Santana MB, Vidal L, Alfonso M, Duran R. Comparative effects of organic and inorganic mercury on in vivo dopamine release in freely moving rats. Braz J Med Biol Res. 2007;40:1361–1365. doi: 10.1590/s0100-879x2006005000157. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Aschner M. The importance of glutamate, glycine, and gamma-aminobutyric acid transport and regulation in manganese, mercury and lead neurotoxicity. Toxicol Appl Pharmacol. 2005;204:343–354. doi: 10.1016/j.taap.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Fossato da Silva DA, Teixeira CT, Scarano WR, Favareto AP, Fernandez CD, Grotto D, et al. Effects of methylmercury on male reproductive functions in Wistar rats. Reprod Toxicol. 2011;31:431–439. doi: 10.1016/j.reprotox.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Franco JL, Teixeira A, Meotti FC, Ribas CM, Stringari J, Garcia Pomblum SC, et al. Cerebellar thiol status and motor deficit after lactational exposure to methylmercury. Environ Res. 2006;102:22–28. doi: 10.1016/j.envres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Franks B, Curley JP, Champagne FA. Measuring variations in maternal behavior: Relevance for studies of mood and anxiety. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. Volume II. Springer; 2009. pp. 209–224. [Google Scholar]
- Fumagalli F, Bedogni F, Perez J, Racagni G, Riva MA. Corticostriatal brain-derived neurotrophic factor dysregulation in adult rats following prenatal stress. Eur J Neurosci. 2004;20:1348–1354. doi: 10.1111/j.1460-9568.2004.03592.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Pasini M, Frasca A, Drago F, Racagni G, Riva MA. Prenatal stress alters glutamatergic system responsiveness in adult rat prefrontal cortex. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06088.x. [DOI] [PubMed] [Google Scholar]
- Gharaei A, Ghaffari M, Keyvanshokooh S, Akrami R. Changes in metabolic enzymes, cortisol and glucose concentrations of Beluga (Huso huso) exposed to dietary methylmercury. Fish Physiol Biochem. 2011;37:485–493. doi: 10.1007/s10695-010-9450-3. [DOI] [PubMed] [Google Scholar]
- Goulet S, Dore FY, Mirault ME. Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol Teratol. 2003;25:335–347. doi: 10.1016/s0892-0362(03)00007-2. [DOI] [PubMed] [Google Scholar]
- Helmcke KJ, Aschner M. Hormetic effect of methylmercury on Caenorhabditis elegans. Toxicol Appl Pharmacol. 2010;248:156–164. doi: 10.1016/j.taap.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Guegant G, Cador M, Arnauld E, Arsaut J, Le Moal M, et al. Prenatal stress in rats facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine receptors in the nucleus accumbens. Brain Res. 1995;685:179–186. doi: 10.1016/0006-8993(95)00430-x. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45:292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama K, Yasutake A, Inoue M. Effect of sex hormones on the fate of methylmercury and on glutathione metabolism in mice. Biochem Pharmacol. 1987;36:1919–1924. doi: 10.1016/0006-2952(87)90489-8. [DOI] [PubMed] [Google Scholar]
- Jayasena N, Frederick PC, Larkin IL. Endocrine disruption in white ibises (Eudocimus albus) caused by exposure to environmentally relevant levels of methylmercury. Aquat Toxicol. 2011;105:321–327. doi: 10.1016/j.aquatox.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Perera F, Jankowski J, Rauh V, Flak E, Caldwell KL, et al. Fish consumption in pregnancy, cord blood mercury level and cognitive and psychomotor development of infants followed over the first three years of life: Krakow epidemiologic study. Environ Int. 2007;33:1057–1062. doi: 10.1016/j.envint.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, et al. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012;120:799–806. doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the behavioral effects of drugs. In: Tedeschi R, editor. Importance of Fundamental Principles in Drug Evaluation. New York: Raven Press; 1969. pp. 383–405. [Google Scholar]
- Kim CY, Nakai K, Kasanuma Y, Satoh H. Comparison of neurobehavioral changes in three inbred strains of mice prenatally exposed to methylmercury. Neurotoxicol Teratol. 2000;22:397–403. doi: 10.1016/s0892-0362(99)00077-x. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, et al. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 2004;56:400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Lederman SA, Jones RL, Caldwell KL, Rauh V, Sheets SE, Tang D, et al. Relation between cord blood mercury levels and early child development in a World Trade Center cohort. Environ Health Perspect. 2008;116:1085–1091. doi: 10.1289/ehp.10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Inskip M, Newhook D, Messier C. Neurobehavioral effect of chronic and bolus doses of methylmercury following prenatal exposure in C57BL/6 weanling mice. Neurotoxicol Teratol. 2009;31:372–381. doi: 10.1016/j.ntt.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Lin CY, Liou SH, Hsiech CM, Ku MC, Tsai SY. Dose-response relationship between cumulative mercury exposure index and specific uptake ratio in the striatum on Tc-99m TRODAT SPECT. Clin Nucl Med. 2011;36:689–693. doi: 10.1097/RLU.0b013e3181e9fa93. [DOI] [PubMed] [Google Scholar]
- Llop S, Lopez-Espinosa MJ, Rebagliato M, Ballester F. Gender differences in the neurotoxicity of metals in children. Toxicology. 2013;311:3–12. doi: 10.1016/j.tox.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009 doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Magos L. Selective atomic-absorption determination of inorganic mercury and methylmercury in undigested biological samples. Analyst. 1971;96:847–853. doi: 10.1039/an9719600847. [DOI] [PubMed] [Google Scholar]
- Magos L, Clarkson TW. Atomic absorption determination of total, inorganic, and organic mercury in blood. J Assoc Off Anal Chem. 1972;55:966–971. [PubMed] [Google Scholar]
- Mairesse J, Viltart O, Salome N, Giuliani A, Catalani A, Casolini P, et al. Prenatal stress alters the negative correlation between neuronal activation in limbic regions and behavioral responses in rats exposed to high and low anxiogenic environments. Psychoneuroendocrinology. 2007;32:765–776. doi: 10.1016/j.psyneuen.2007.03.013. [DOI] [PubMed] [Google Scholar]
- McClure WO, Ishtoyan A, Lyon M. Very mild stress of pregnant rats reduces volume and cell number in nucleus accumbens of adult offspring: some parallels to schizophrenia. Brain Res Dev Brain Res. 2004;149:21–28. doi: 10.1016/j.devbrainres.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Montgomery KS, Mackey J, Thuett K, Ginestra S, Bizon JL, Abbott LC. Chronic, low-dose prenatal exposure to methylmercury impairs motor and mnemonic function in adult C57/B6 mice. Behav Brain Res. 2008;191:55–61. doi: 10.1016/j.bbr.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Newland MC, Hoffman D, Heath JC, Donlin WD. Response inhibition is impaired by developmental methylmercury exposure: Acquisition of low-rate lever-pressing. Behav Brain Res. 2013 doi: 10.1016/j.bbr.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Paletz EM, Reed MN. Methylmercury and nutrition: adult effects of fetal exposure in experimental models. Neurotoxicology. 2008;29:783–801. doi: 10.1016/j.neuro.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicol Teratol. 2004;26:179–194. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Nielsen AB, Davidsen M, Bjerregaard P. The association between blood pressure and whole blood methylmercury in a cross-sectional study among Inuit in Greenland. Environ Health. 2012;11:44. doi: 10.1186/1476-069X-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB. Toxicokinetics of mercuric chloride and methylmercuric chloride in mice. J Toxicol Environ Health. 1992;37:85–122. doi: 10.1080/15287399209531659. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Andersen HL, Sorensen JA, Andersen O. Localization of gastrointestinal deposition of mercuric chloride studied in vivo. Pharmacol Toxicol. 1992;70:262–267. doi: 10.1111/j.1600-0773.1992.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Andersen O. Methyl mercuric chloride toxicokinetics in mice. I: Effects of strain, sex, route of administration and dose. Pharmacol Toxicol. 1991a;68:201–207. doi: 10.1111/j.1600-0773.1991.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Andersen O. Methyl mercuric chloride toxicokinetics in mice. II: Sexual differences in whole-body retention and deposition in blood, hair, skin, muscles and fat. Pharmacol Toxicol. 1991b;68:208–211. doi: 10.1111/j.1600-0773.1991.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, et al. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol. 2008;167:1171–1181. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, et al. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ Health Perspect. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira FR, Ferreira JR, dos Santos CM, Macedo LE, de Oliveira RB, Rodrigues JA, et al. Estradiol reduces cumulative mercury and associated disturbances in the hypothalamus-pituitary axis of ovariectomized rats. Ecotoxicol Environ Saf. 2006;63:488–493. doi: 10.1016/j.ecoenv.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Tamm C, Vahter M, Hokfelt T, Johnson JA, Johnson DA, et al. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol Sci. 2007;97:428–437. doi: 10.1093/toxsci/kfl199. [DOI] [PubMed] [Google Scholar]
- Ortega HG, Lopez M, Takaki A, Huang QH, Arimura A, Salvaggio JE. Neuroimmunological effects of exposure to methylmercury forms in the Sprague-Dawley rats. Activation of the hypothalamic-pituitary-adrenal axis and lymphocyte responsiveness. Toxicol Ind Health. 1997;13:57–66. doi: 10.1177/074823379701300105. [DOI] [PubMed] [Google Scholar]
- Patin V, Vincent A, Lordi B, Caston J. Does prenatal stress affect the motoric development of rat pups? Brain Res Dev Brain Res. 2004;149:85–92. doi: 10.1016/j.devbrainres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Peters JL, Weisskopf MG, Spiro A, 3rd, Schwartz J, Sparrow D, Nie H, et al. Interaction of stress, lead burden, and age on cognition in older men: the VA Normative Aging Study. Environ Health Perspect. 2010;118:505–510. doi: 10.1289/ehp.0901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MN, Newland MC. Prenatal methylmercury exposure increases responding under clocked and unclocked fixed interval schedules of reinforcement. Neurotoxicol Teratol. 2007a;29:492–502. doi: 10.1016/j.ntt.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Reed MN, Newland MC. Prenatal methylmercury exposure increases responding under clocked and unclocked fixed interval schedules of reinforcement. Neurotoxicol Teratol. 2007b;29:492–502. doi: 10.1016/j.ntt.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Rossi AD, Ahlbom E, Ogren SO, Nicotera P, Ceccatelli S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Exp Brain Res. 1997;117:428–436. doi: 10.1007/s002210050237. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Amarasiriwardena C, Korrick SA. Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children. Arch Pediatr Adolesc Med. 2012;166:1123–1131. doi: 10.1001/archpediatrics.2012.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SW, Meiller JC, Mahaffey KR. The endocrine effects of mercury in humans and wildlife. Crit Rev Toxicol. 2009;39:228–269. doi: 10.1080/10408440802233259. [DOI] [PubMed] [Google Scholar]
- Wada H, Cristol DA, McNabb FA, Hopkins WA. Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environmental Science and Technology. 2009;43:6031–6038. doi: 10.1021/es803707f. [DOI] [PubMed] [Google Scholar]
- Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114:1635–1644. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32:1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Sex-dependent changes induced by prenatal stress in cortical and hippocampal morphology and behaviour in rats: an update. Stress. 2011;14:604–613. doi: 10.3109/10253890.2011.588294. [DOI] [PubMed] [Google Scholar]
- Xu F, Farkas S, Kortbeek S, Zhang FX, Chen L, Zamponi GW, et al. Mercury-induced toxicity of rat cortical neurons is mediated through N-Methyl-D-Aspartate receptors. Mol Brain. 2012;5:30. doi: 10.1186/1756-6606-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Yamamoto M, Ishihara Y, Komatsu S, Munetsuna E, Onizaki M, et al. De novo synthesized estradiol protects against methylmercury-induced neurotoxicity in cultured rat hippocampal slices. PLoS One. 2013;8:e55559. doi: 10.1371/journal.pone.0055559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SJ, Masters JN, Baram TZ. Glucocorticoid receptor mRNA ontogeny in the fetal and postnatal rat forebrain. Mol Cell Neurosci. 1994;5:385–393. doi: 10.1006/mcne.1994.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Suzuki M, Satoh M, Yasutake A, Watanabe C. Neurobehavioral effects of combined prenatal exposure to low-level mercury vapor and methylmercury. J Toxicol Sci. 2011;36:73–80. doi: 10.2131/jts.36.73. [DOI] [PubMed] [Google Scholar]