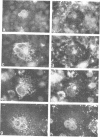
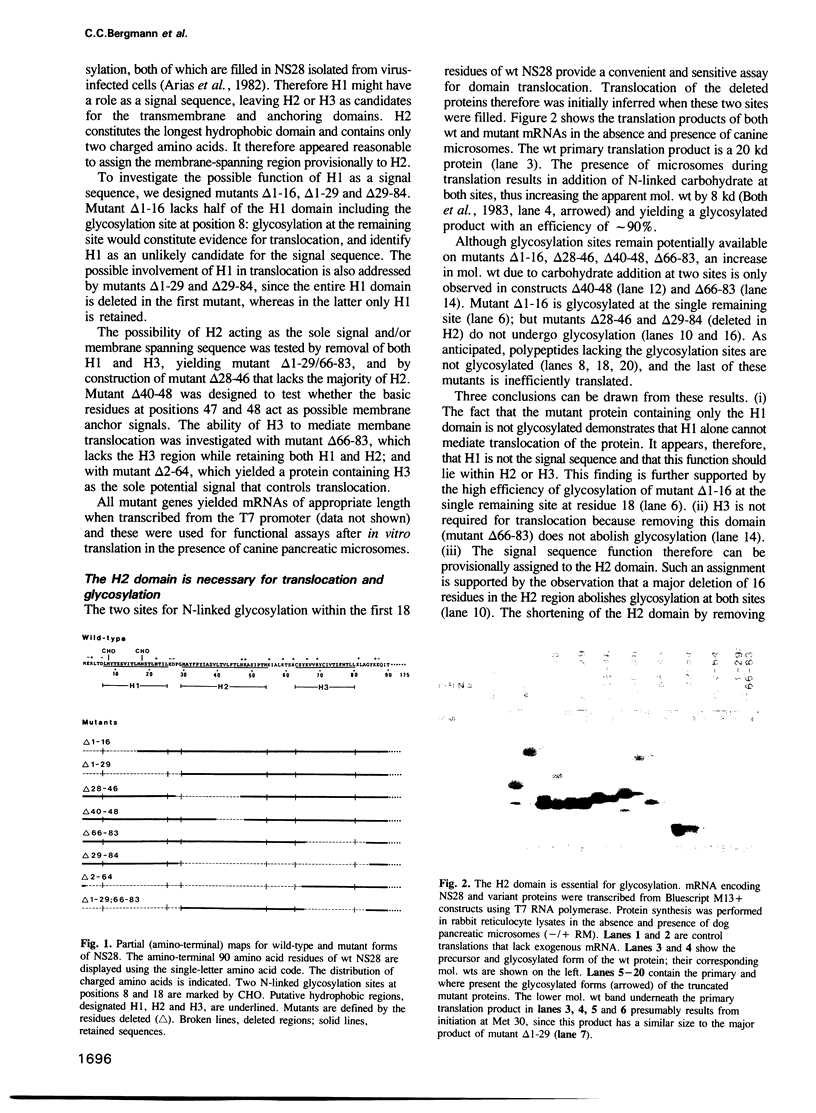
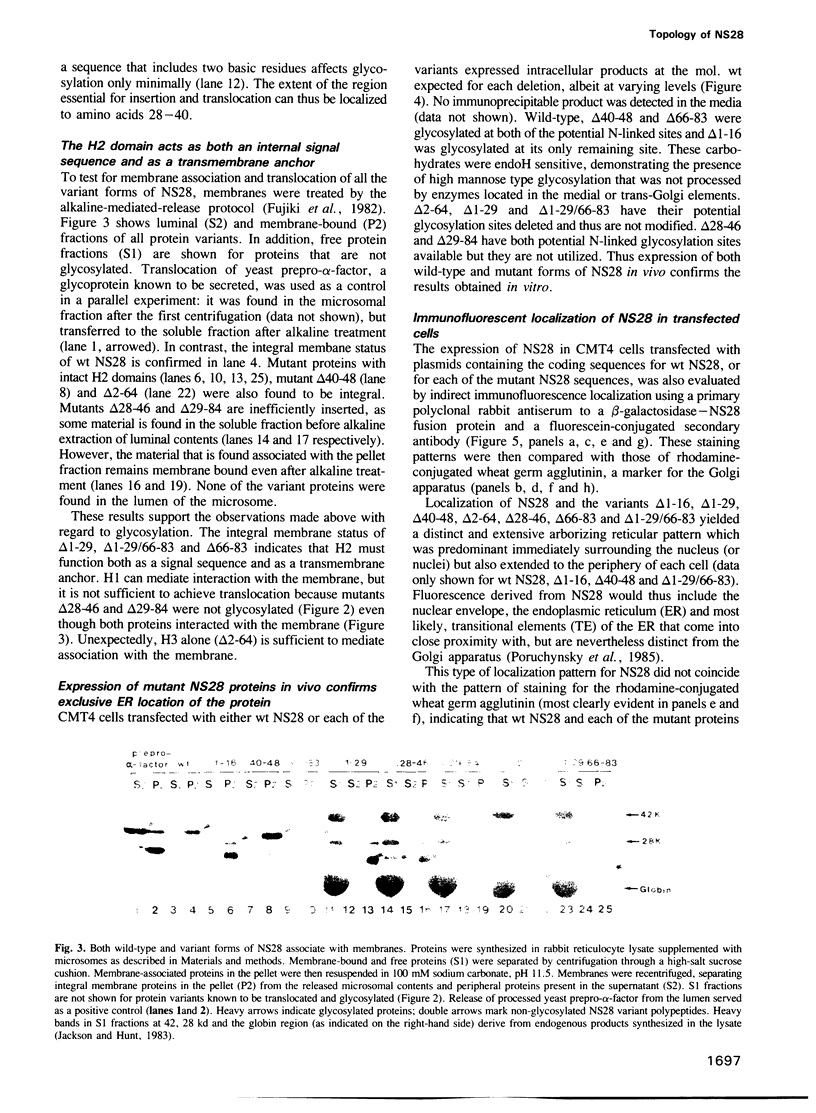
Abstract
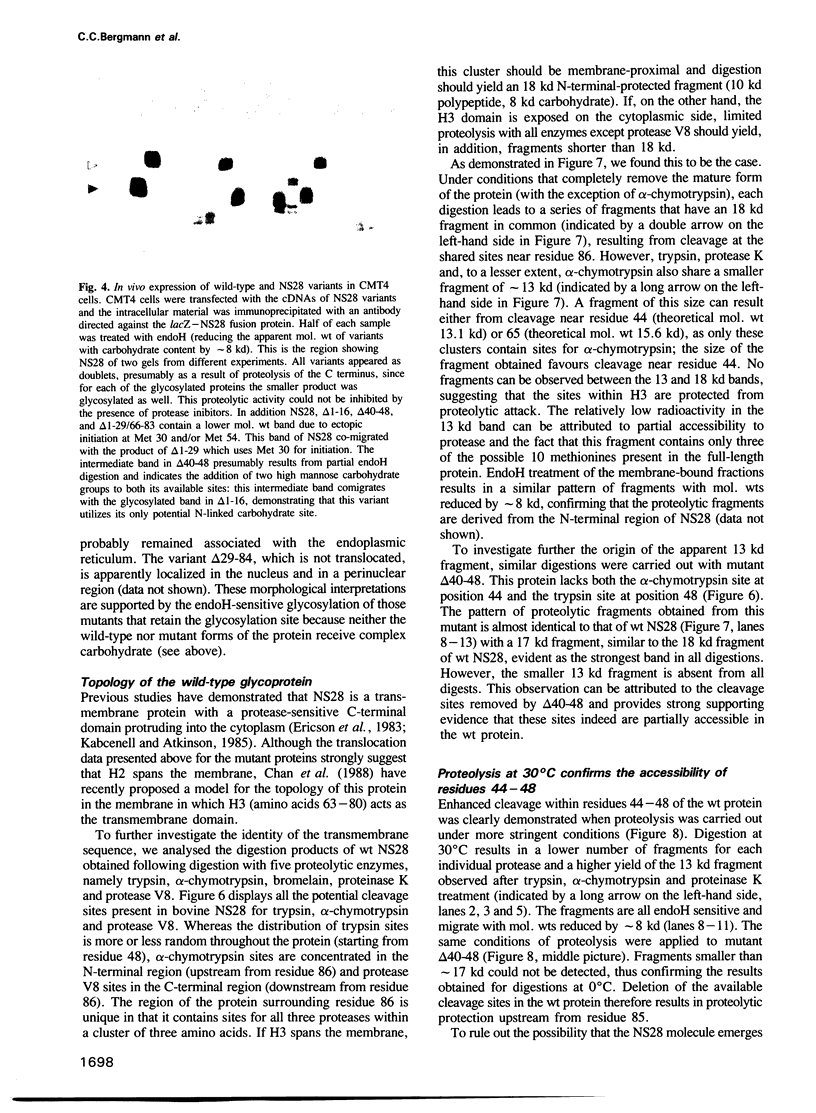
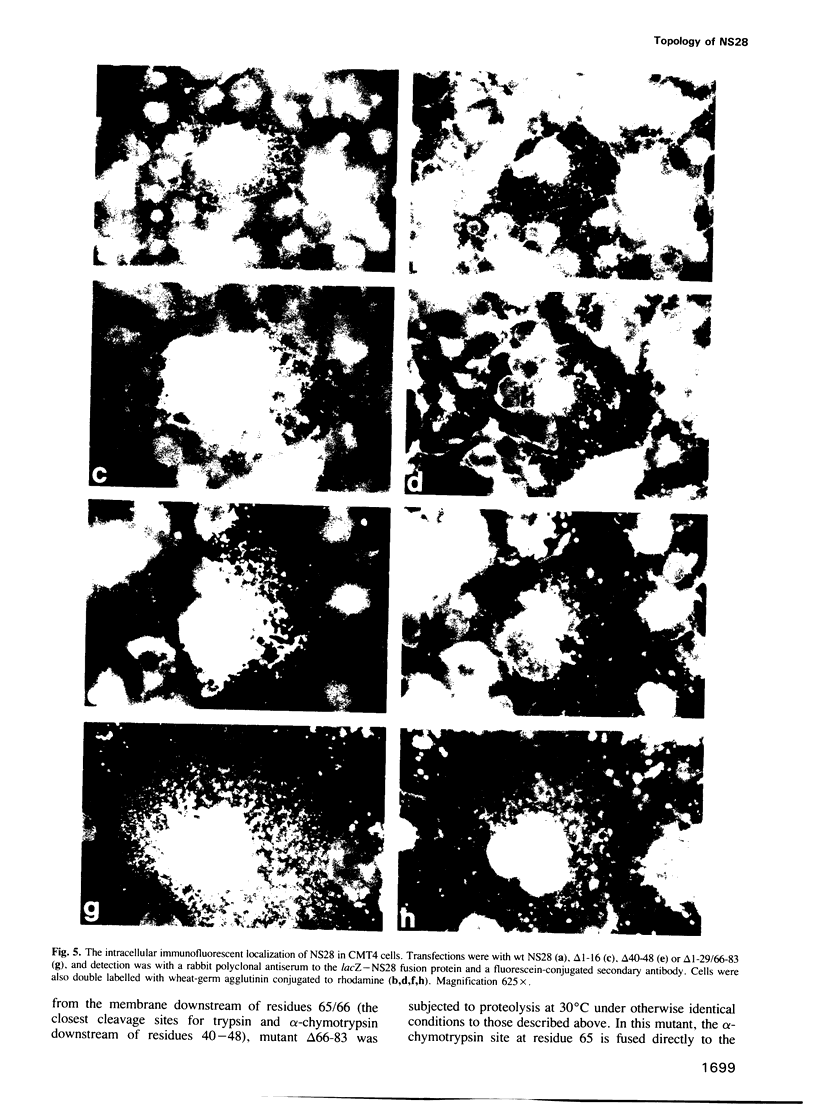
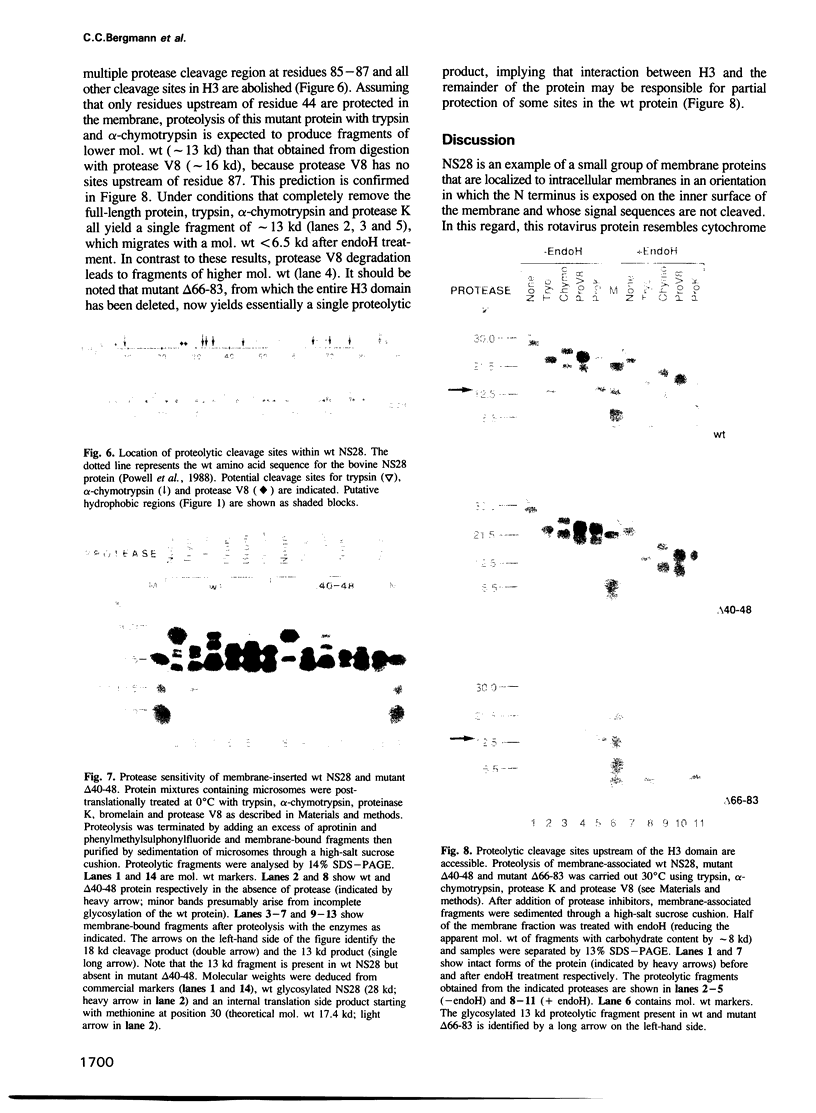
The rotavirus non-structural glycoprotein (NS28), the receptor for the virus core during budding into the lumen of the rough endoplasmic reticulum (RER), is 175 amino acids long and possesses an uncleaved signal sequence and two amino-terminal glycosylation sites. Utilizing one of three potential hydrophobic domains, the protein spans the membrane only once, with the glycosylated amino-terminal region oriented to the luminal side of the ER and the carboxy-terminal region to the cytoplasmic side. To localize sequences involved in translocation of NS28, we constructed a series of mutations in the coding regions for the hydrophobic domains of the protein. Mutant protein products were studied by in vitro translation and by transfection in vivo. In transfected cells, all mutant forms localize to the ER, and none are secreted. In vitro, each of the three hydrophobic domains is able to associate with microsomes. However, glycosylation and proteolysis of wild-type and mutant forms of NS28 indicates that the wild-type protein is anchored in the membrane only by the second hydrophobic domain, leaving approximately 131 residues exposed on the cytoplasmic side for receptor - ligand interaction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Rao J. K., Hargrave P. A. Structural prediction of membrane-bound proteins. Eur J Biochem. 1982 Nov 15;128(2-3):565–575. doi: 10.1111/j.1432-1033.1982.tb07002.x. [DOI] [PubMed] [Google Scholar]
- Arias C. F., López S., Espejo R. T. Gene protein products of SA11 simian rotavirus genome. J Virol. 1982 Jan;41(1):42–50. doi: 10.1128/jvi.41.1.42-50.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baybutt H. N., McCrae M. A. The molecular biology of rotaviruses. VII. Detailed structural analysis of gene 10 of bovine rotavirus. Virus Res. 1984 Oct;1(7):533–541. doi: 10.1016/0168-1702(84)90011-x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Siegman L. J., Bellamy A. R., Atkinson P. H. Coding assignment and nucleotide sequence of simian rotavirus SA11 gene segment 10: location of glycosylation sites suggests that the signal peptide is not cleaved. J Virol. 1983 Nov;48(2):335–339. doi: 10.1128/jvi.48.2.335-339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. K., Au K. S., Estes M. K. Topography of the simian rotavirus nonstructural glycoprotein (NS28) in the endoplasmic reticulum membrane. Virology. 1988 Jun;164(2):435–442. doi: 10.1016/0042-6822(88)90557-0. [DOI] [PubMed] [Google Scholar]
- Doyle C., Sambrook J., Gething M. J. Analysis of progressive deletions of the transmembrane and cytoplasmic domains of influenza hemagglutinin. J Cell Biol. 1986 Oct;103(4):1193–1204. doi: 10.1083/jcb.103.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson B. L., Graham D. Y., Mason B. B., Hanssen H. H., Estes M. K. Two types of glycoprotein precursors are produced by the simian rotavirus SA11. Virology. 1983 Jun;127(2):320–332. doi: 10.1016/0042-6822(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Rose J. K. Conversion of a secretory protein into a transmembrane protein results in its transport to the Golgi complex but not to the cell surface. Cell. 1984 Jul;37(3):779–787. doi: 10.1016/0092-8674(84)90413-6. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Kabcenell A. K., Atkinson P. H. Processing of the rough endoplasmic reticulum membrane glycoproteins of rotavirus SA11. J Cell Biol. 1985 Oct;101(4):1270–1280. doi: 10.1083/jcb.101.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabcenell A. K., Poruchynsky M. S., Bellamy A. R., Greenberg H. B., Atkinson P. H. Two forms of VP7 are involved in assembly of SA11 rotavirus in endoplasmic reticulum. J Virol. 1988 Aug;62(8):2929–2941. doi: 10.1128/jvi.62.8.2929-2941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. A specific transmembrane domain of a coronavirus E1 glycoprotein is required for its retention in the Golgi region. J Cell Biol. 1987 Sep;105(3):1205–1214. doi: 10.1083/jcb.105.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B. L., Estes M. K., Graham D. Y. Effects of tunicamycin on rotavirus morphogenesis and infectivity. J Virol. 1983 Apr;46(1):270–274. doi: 10.1128/jvi.46.1.270-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poruchynsky M. S., Tyndall C., Both G. W., Sato F., Bellamy A. R., Atkinson P. H. Deletions into an NH2-terminal hydrophobic domain result in secretion of rotavirus VP7, a resident endoplasmic reticulum membrane glycoprotein. J Cell Biol. 1985 Dec;101(6):2199–2209. doi: 10.1083/jcb.101.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. F., Gunn P. R., Bellamy A. R. Nucleotide sequence of bovine rotavirus genomic segment 10: an RNA encoding the viral nonstructural glycoprotein. Nucleic Acids Res. 1988 Jan 25;16(2):763–763. doi: 10.1093/nar/16.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi M., Mihara K., Sato R. A short amino-terminal segment of microsomal cytochrome P-450 functions both as an insertion signal and as a stop-transfer sequence. EMBO J. 1987 Aug;6(8):2425–2431. doi: 10.1002/j.1460-2075.1987.tb02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]