Abstract
Background
Binge drinking represents the major form of excessive alcohol (EtOH) consumption in the US. Episodic (such as binge) drinking results in blood alcohol levels (BAL) of 18–80 mM, and leads to alcohol-induced cerebral artery constriction (AICAC). AICAC was shown to arise from EtOH-induced inhibition of large-conductance, calcium/voltage-gated potassium (BK) channels in the vascular smooth muscle. Factors that modulate BK channel-mediated AICAC remain largely unknown.
Methods
Male Sprague-Dawley rats were placed on high-cholesterol (2% of cholesterol) diet for 18–23 weeks. Their littermates were placed on control iso-caloric diet. AICAC was evaluated both in vivo and in vitro, by means of pial arteriole diameter monitoring through a closed cranial window and diameter measurements of isolated, pressurized cerebral arteries. Cholesterol level in the cerebral artery tissue was manipulated by methyl-β-cyclodextrin to reverse dietary-induced accumulation of cholesterol. BK channel surface presence on the plasma membrane of cerebral artery myocytes was evaluated by immunofluorescence staining. BK channel function in pressurized cerebral artery was assessed using selective BK channel blocker paxilline.
Results
Within 5 minutes of 50 mM EtOH injection into carotid artery in vivo, arteriole diameter decreased by 20% in control group. Pial arteriole constriction was significantly reduced in rats on high-cholesterol diet, resulting in only 10% reduction of diameter. BAL in both groups, however, remained the same. Significant reduction of AICAC in group on high-cholesterol diet compared to control was also observed after middle cerebral artery dissection and in vitro pressurization at 60 mmHg, this reduction remaining after endothelium removal. Cholesterol level in de-endothelialized cerebral arteries was significantly increased in rats on high-cholesterol diet. Removal of excessive cholesterol content restored AICAC to the level, observed in cerebral arteries of rats on normal diet. Immunofluorescence staining of BK channel- forming and accessory, smooth muscle-specific β1 subunit in freshly isolated cerebral artery myocyte showed that high-cholesterol diet did not down-regulate surface presence of BK protein. However, paxilline-induced cerebral artery constriction was diminished in arteries from rats on high-cholesterol diet.
Conclusions
Our data indicate that dietary cholesterol protects against AICAC. This protection is caused by cholesterol buildup in the arterial tissue and diminished function (but not surface presence) of EtOH target – BK channel.
Keywords: ethanol, MaxiK channel, cerebral artery, vascular smooth muscle, closed cranial window
INTRODUCTION
Episodic, moderate-to-heavy ethyl alcohol (ethanol, EtOH) intake is a primary form of excessive alcohol consumption in the US (www.cdc.gov; www.nih.gov). Recent data report that 28.8% of adult females and 43.1% males in the US incur in binge drinking, a most common form of episodic drinking (www.nih.gov). Binge drinking affects wide range of age groups: although binge drinking is more common among young adults, binge drinkers aged 65 and older report binge drinking more often (www.cdc.gov). Besides being associated with numerous health complications, episodic drinking that results in blood alcohol levels (BAL) of 35–80 mM (0.16–0.37% ethanol in the blood) is also associated with an increased risk for cerebral ischemia, stroke and death from ischemic stroke (Puddey et al., 1999; Reynolds et al., 2003). Cerebral ischemia may result from, or be exacerbated by enhanced constriction of cerebral arteries.
In a rat model widely used to mimic human cerebral artery reactivity, it has been demonstrated that alcohol-induced cerebrovascular constriction (AICAC) is caused by ethanol inhibition of calcium/voltage-gated potassium channels of large conductance (BK) in vascular smooth muscle (Liu et al., 2004). Located on the plasma membrane, BK channels generate outward potassium currents, tend to hyperpolarize the membrane and diminish smooth muscle cell (myocyte) contractility (Brayden and Nelson, 1992; Orio et al., 2002). Therefore, BK channel inhibition by EtOH results in arterial constriction. Physiological factors that modulate BK channel-mediated AICAC remain largely unknown.
In the present work we studied effect of dietary cholesterol intake on AICAC in rat model. Using in vivo pial arteriole diameter monitoring through a closed cranial window, and in vitro diameter measurements of pressurized cerebral artery we demonstrate a protective effect of cholesterol buildup in cerebral artery tissue against AICAC. Removal of accumulated cholesterol from de-endothelialized cerebral artery restores AICAC back to the level observed in control group. Immunofluorescence detection of BK channel-forming (α) and accessory, smooth muscle-characteristic β1 subunit on the surface of cerebral artery myocyte rules out down-regulation BK channel surface presence during high-cholesterol diet. Yet, using selective BK channel blocker paxilline on de-endothelialized cerebral arteries we show that BK channel function in high-cholesterol diet group is diminished. We conclude that dietary cholesterol protects against AICAC by cholesterol buildup in the arterial tissue and diminished function (but not surface presence) of EtOH target – BK channel.
MATERIALS AND METHODS
Ethical Aspects of Research
The care of animals and experimental protocols were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, which is an Association for Assessment and Accreditation of Laboratory Animal Care-accredited institution.
High-cholesterol diet
A group of 25 day-old male Sprague-Dawley rats was placed on a high-cholesterol diet (2% cholesterol) in standard rodent food by Harland-Teklad (Indianapolis, IN). Another group of the same age was fed control, isocaloric to high-cholesterol, diet from the same supplier. Rats were used for experimentation after 18–23 weeks on control or high- cholesterol diet.
Determination of plasma lipids
Plasma cholesterol, low- and high-density lipoproteins were determined using Cobas Mira biochemistry analyzer (Roche, Basel, Switzerland).
Pial Arteriolar Diameter Measurements
The measurements were performed as previously reported (Bukiya et al., 2013), and now described in on-line Supplementary Material and Methods. Pial arterioles of 50–100 µm in external diameters were used to test vascular reactivity. Basal (pre-EtOH) PAD values were measured over a 10-min period under basal conditions. EtOH (ultra pure, American Bioanalytical, Natick, MA) was diluted in sodium saline solution (SSS) to a final concentration of 50 mM. One mL of this solution was infused into the cerebral circulation via the carotid artery, with 1 mL of SSS being used as negative control infusion. PAD was monitored for up to 20 min following infusion of EtOH-containing or control solutions.
Blood alcohol level (BAL) measurements
100 µL blood was collected through a femoral artery catheter every 2 min during the first 7 min following in vivo infusion of 1 mL saline containing 50 mM EtOH into circulation. Blood alcohol levels were detected using the Nicotinamide Adenine Dinucleotide-Alcohol Dehydrogenase Reagent kit following manufacturer instructions (Sigma-Aldrich, Saint Louis, MO).
Cerebral Artery Diameter Measurement
The measurements were performed as previously validated and reported by our group (Bukiya et al., 2007; Vaithianathan et al., 2008; Bukiya et al., 2011; Bukiya et al., 2013), and now described in on-line Supplementary Material and Methods. The effect of drug applications was evaluated at the time it reached a maximal and steady level, which was usually achieved within 10–20 min of drug application. For testing alcohol action on cerebral vessels, we used 50 mM EtOH, which corresponds to 0.23% BAL. This is the half-maximal concentration for AICAC in our rat model (Liu et al, 2004). This EtOH level does not exceed 0.3% BAL, a superior limit that is rarely reached during alcohol intoxication in humans (Lange and Voas, 2000) and results in life-threatening alcohol poisoning (Celik et al., 2013). Single dose of ethanol was only tested on EtOH-naïve animals to avoid development of desensitization, which has been reported in our model and results from repeated ethanol exposures (Bukiya et al., 2011).
Cholesterol and protein determinations
Free cholesterol and total protein levels in de-endothelialized arteries were determined using the Amplex Red Cholesterol Assay kit (Molecular Probes, Carlsbad, CA) and the Pierce BCA protein assay kit (Thermo Scientific, Waltham, MA) following manufacturers’ instructions.
Removal of elevated cholesterol from de-endothelialized arteries
Pressurized de-endothelialized arteries were perfused with 5 mM methyl-β-cyclodextrin (MβCD) - containing bath solution (PSS) for 10 min. Using cholesterol and protein determination post-experiment, we confirmed that this treatment resulted in tissue cholesterol≈60 µg/mg. This value is similar to cholesterol level in control group.
Immunocytochemistry and confocal fluorescence imaging
Freshly dispersed myocytes were fixed in 4% paraformaldehyde and treated with blocking solution containing 20% goat serum, 2% bovine serum albumin, and 0.1% Triton-100 for 30 min. Slips were incubated with mouse monoclonal anti-BK α (channel-forming) subunit antibody ab99046 (Abcam, Cambridge, MA) and rabbit polyclonal anti-BK β1 subunit antibody (Thermo Scientific, Waltham, MA) at 4°C overnight. Two negative controls were used. To determine any possible background fluorescence caused by tissue natural fluorescence and by non-specific binding of secondary antibody to the specimen, we omitted addition of primary antibodies (Figs. S1–2). To confirm specificity of binding for anti-BK β1 antibody, we pre-incubated antibody with immunogenic peptide corresponding to the BK β1 subunit sequence (Waltham, MA) (Fig. S2).
The day following overnight incubation with anti-BK subunit antibodies, slips were washed and incubated with pre-absorbed Alexa-488-conjugated anti-rabbit and Cy5-conjugated anti-mouse secondary antibodies at room temperature in the dark for 2 hours. Slips were mounted using ProLong AntiFade kit (Invitrogen, Carlsbad, CA) and sealed using clear nail polish. Immunofluorescence images were obtained sequentially using 488 and 635 laser lines of Olympus FV-1000 laser scanning confocal system (Center Valley, PA).
Chemicals
Ultra-pure alcohol was purchased from American Bioanalytical (Natick, MA). All other chemicals were purchased from Sigma (St. Louis, MO).
Data analysis
Artery diameter data were analyzed using IonWizard 4.4 software (IonOptics). The arterial diameter before drug application was obtained by averaging diameter values during 3 minutes of recording immediately before drug application. A drug-induced change (delta) in arterial diameter was determined as difference (in %) between diameter before drug application and diameter at the peak of drug effect. The latter was obtained during drug application. Myogenic (arterial) tone was calculated according to the formula: myogenic tone (%)=(1-active diameter/passive diameter)×100 (Adebiyi et al., 2007).
Fluorescence was quantified using built-in function in FV10-ASW 3.1 software (Olympus American Inc., Center Valley, PA). For detection of BK channel protein-associated fluorescence signal in plasmalemmal region was used. Fluorescence was quantified by measuring the intensity of pixels above a set threshold defined as the mean fluorescence intensity outside the cells (i.e., background) plus four times its standard (Amberg and Santana, 2003).
Final plotting, fitting and statistical analysis of the data were conducted using Origin 8.5.1 (OriginLab, Northampton, MA) and InStat 3.0 (GraphPad, La Jolla, CA). Statistical analysis was conducted using either one-way ANOVA and Bonferroni’s multiple comparison test or paired Student’s t-test, according to experimental design. Significance was set at P<0.05. Data are expressed as mean±SEM; n=number of pial arterioles/arteries, each pial arteriole or artery diameter data being obtained from a separate rat.
RESULTS
Dietary cholesterol protects against alcohol-induced cerebral arteriole constriction in vivo
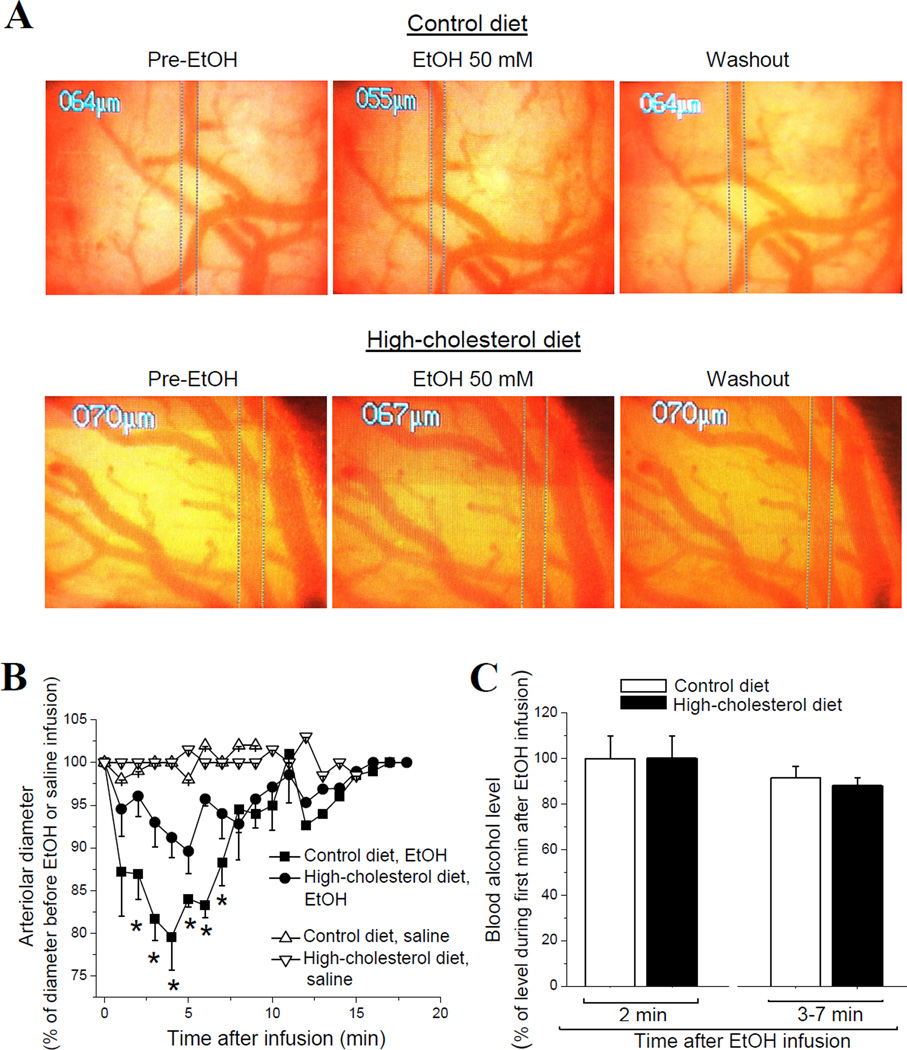
We first evaluated the blood lipid profile of rats on a high-cholesterol diet. As expected, high-cholesterol diet resulted in significant increase of plasma cholesterol and low-density lipoproteins (LDL, “bad cholesterol”) (Aydin et al., 2009; El-Sayyad et al., 2012; Asemi et al., 2013). High-density lipoproteins (HDL, “good cholesterol”) level, however, remained unchanged (Table 1). To determine the impact of high-cholesterol diet on AICAC, we used a closed cranial window on anesthetized rats (Baumbach and Heistad, 1985). Control SS- or EtOH-containing solutions were infused into the cerebral circulation via a catheter in the carotid artery of rat on high-cholesterol vs. control diet. Infusion of sodium saline solution (SSS) did not cause any significant changes in pial arteriole diameter (PAD) in either group. In contrast, infusion of 50 mM EtOH into cerebral circulation of control rats rendered a significant, 20% decrease in PAD (Fig. 1A) (p<0.01). This EtOH-induced pial arteriole constriction was sustained throughout 7 minutes of diameter monitoring (Fig. 1B). In contrast to the control group, infusion of 50 mM EtOH into cerebral circulation of rats on high-cholesterol diet rendered no more than 10% decrease in PAD. This constriction represents only half of that observed in the control group and is significantly less than the constriction observed in control group during 3–7 minutes after EtOH infusion (Fig. 1B) (p<0.05). Therefore, dietary cholesterol seems to protect against alcohol-induced pial arteriole in vivo constriction.
Table 1. Blood lipid measurement of control and high-cholesterol diet group of rats.
Rats fed high-cholesterol diet for 18–23 weeks show a distinct profile of blood lipids. Averaged level of cholesterol and low-density lipoproteins (LDL) is significantly increased, while high-density lipoproteins (HDL) remain unchanged. Blood lipid measurements represent averaged from 9 rats on control and 11 rats on high-cholesterol diet.
| Blood lipid | Control diet | High-cholesterol diet |
|---|---|---|
| Cholesterol (mg/dL) | 103.22±6.78 | 134.18±9.57* |
| LDL (mg/dL) | 10.88±1.09 | 32.25±6.04** |
| HDL (mg/dL) | 80.33±4.23 | 80.36±4.21 |
p<0.05,
p<0.01, when compared to control diet.
Figure 1.
Dietary cholesterol protects against alcohol-induced pial arteriole in vivo constriction independently of EtOH elimination rate from the systemic compartment. (A) Screenshots of brain surface obtained through a closed cranial window. Vertical dashed lines highlight external diameter of pial arteriole. Pial arteriole diameter was monitored before (left) and after (middle) a 50 mM EtOH infusion into cerebral circulation via catheter in carotid artery. Washout of EtOH was achieved by infusion of sodium saline (right). (B) Averaged data show a significant decrease in pial arteriole diameter (PAD) in response to EtOH in rats on high-cholesterol diet (n=4) compared to control group (n=4). *p<0.05, when compared to EtOH-induced arteriole constriction in control group. (C) Averaged blood alcohol levels after infusion of 50 mM EtOH into cerebral circulation of rats on control vs. high-cholesterol diet. Data show lack of significant differences between the groups throughout 7 min following EtOH infusion. Each data point was obtained from ≥4 animals.
BAL measurements did not reveal any significant difference between control and high-cholesterol diet groups within first 7 minutes after EtOH infusion into circulation (Fig. 1C). Thus, protective effect of dietary cholesterol against the AICAC observed throughout 3–7 min following EtOH infusion into circulation (Fig. 1B) cannot be attributed to a different EtOH elimination rate from the blood of rats on control vs. high-cholesterol diet.
Dietary cholesterol-driven protection against AICAC does not require presence of humoral circulating factors, or an intact endothelium
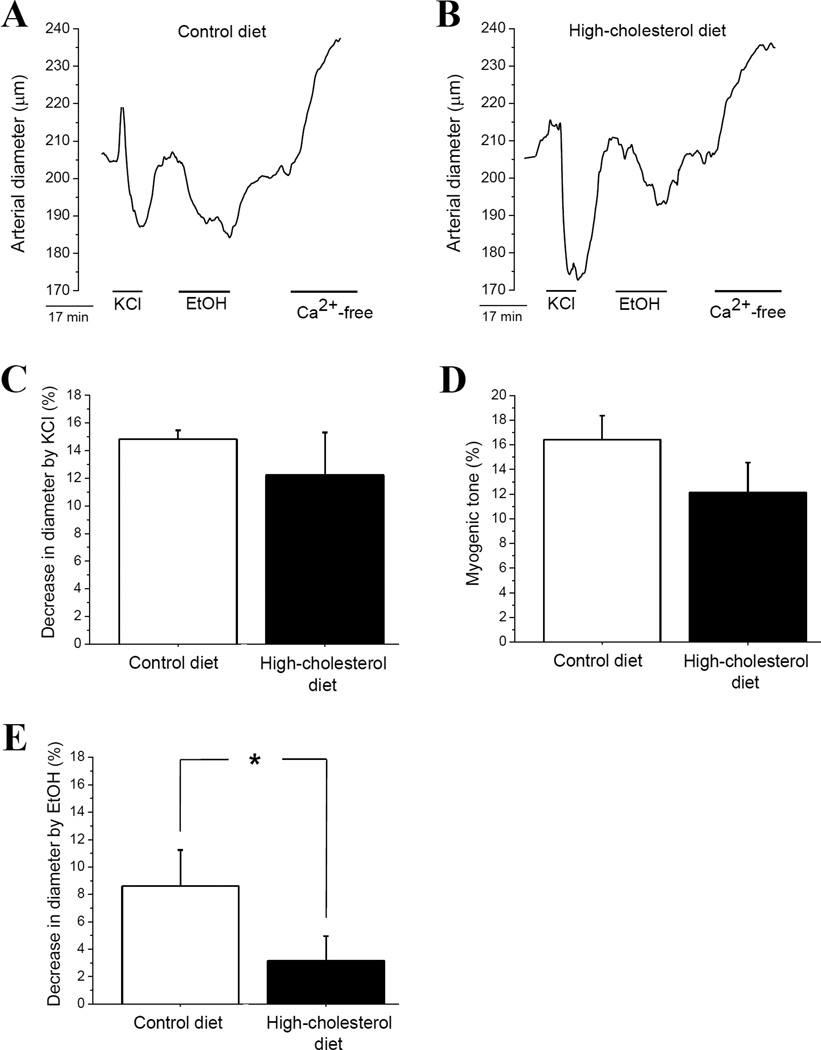
To rule out the contribution of circulating factors in observed protective effect of high-cholesterol diet, we used isolated, in vitro pressurized middle cerebral arteries of rats. After myogenic tone development at 60 mmHg, arteries were probed with 60 mM KCl to evaluate their contractile response to depolarization. KCl-induced cerebral artery constriction was not affected by high-cholesterol diet: KCl caused 10–15% decrease in arterial diameter in both groups (Fig. 2A, B, C). At the end of each experiment, the artery was perfused with Ca2+-free Physiological Saline Solution (PSS) to confirm presence of myogenic tone. We did not detect significant difference in myogenic tone of arteries from rats on control vs. high-cholesterol (Fig. 2D).
Figure 2.
Dietary cholesterol protects against alcohol-induced cerebral artery constriction (AICAC) in absence of complex neuronal integrity and factors from systemic blood circulation. Diameter traces from isolated, in vitro pressurized middle cerebral artery of rats on control (A) vs. high-cholesterol (B) diet. Isolated arterial segments (1–2 mm long) possessed an intact, functional endothelium. However, they were deprived of physiologically active circulating factors. (C) Averaged data showing similar degree of KCl-induced constriction in cerebral arteries from rats on control vs. high-cholesterol diet. (D) Averaged data showing similar degree of myogenic tone in cerebral arteries from rats on control vs. high-cholesterol diet. (E) Averaged data showing protective effect of dietary cholesterol against AICAC when evaluated in isolated arterial segments. In (C-E), each point represents the average of ≥5 arteries, each artery harvested from a different rat. *p<0.05, when compared to arteries from rats on control diet.
As observed with pial arterioles in vivo, application of 50 mM EtOH to pressurized middle cerebral arteries from control rats resulted in up to 12% decrease in arterial diameter. This effect disappeared upon washout with EtOH-free PSS (Fig. 2A). In contrast, application of 50 mM EtOH to pressurized middle cerebral arteries from rats on high-cholesterol diet resulted in only 6–7% decrease in arterial diameter (Fig. 2B). This constriction is significantly smaller than that found in control group (Fig. 2E). Therefore, the protective effect of dietary cholesterol 1) does not require presence of physiologically active factors in systemic circulation, 2) operates at the level of the isolated cerebral vessel, and 3) cannot be explained by overall decrease in arterial contractility during high-cholesterol feeding.
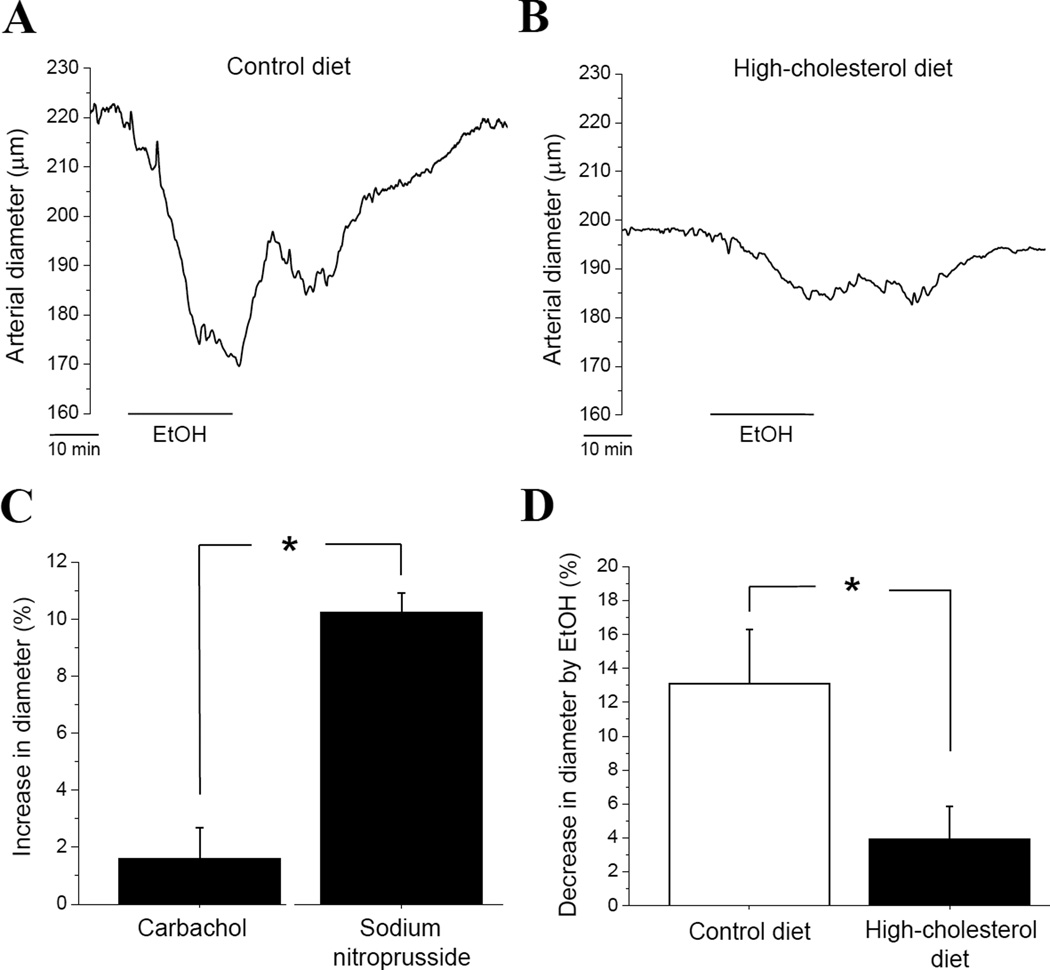
To determine the role of endothelium in cholesterol-driven protection against AICAC, we evaluated EtOH-induced vasoconstriction in pressurized middle cerebral arteries with denuded endothelium (Fig. 3A). Application of 50 mM EtOH to the de-endothelialized arteries from rats subjected to control diet resulted in up to 20% decrease of arterial diameter (p<0.01) (Fig. 3A, D). In contrast, application of 50 mM EtOH to pressurized middle cerebral arteries from rats on high-cholesterol diet only led to 6–7% decrease in arterial diameter (p<0.05) (Fig. 3B, D). This constriction is significantly smaller than that found in control group (p<0.05) (Fig. 3D). Remarkably, protective effect of high-cholesterol diet against AICAC constriction is similar in arteries with intact endothelium (Fig. 2E) and in de-endothelialized vessels (Fig. 3D). Therefore, dietary cholesterol-driven protection against AICAC does not require presence of functional endothelium and/or endothelium-derived vasoactive factors.
Figure 3.
Dietary cholesterol protects against alcohol-induced cerebral artery constriction (AICAC) in absence of a functional endothelium. Diameter traces from isolated, de-endothelialized and in vitro pressurized middle cerebral artery of rat on control (A) vs. high-cholesterol (B) diet. (C) Averaged diameter data from de-endothelialized arteries in response to endothelium-dependent (1 µM carbachol) vs. endothelium-independent (1 µM sodium nitroprusside) vasodilators. Here and in (D), each point represents the average of ≥3 arteries, each artery harvested from a different rat. *p<0.05, when compared to sodium nistroprusside. (D) Averaged data show a protective effect of dietary cholesterol against AICAC in isolated de-endothelialized arteries. *p<0.05, when compared to arteries from rats on control diet.
Protective effect of dietary cholesterol against AICAC is caused by accumulation of cholesterol within arterial tissue
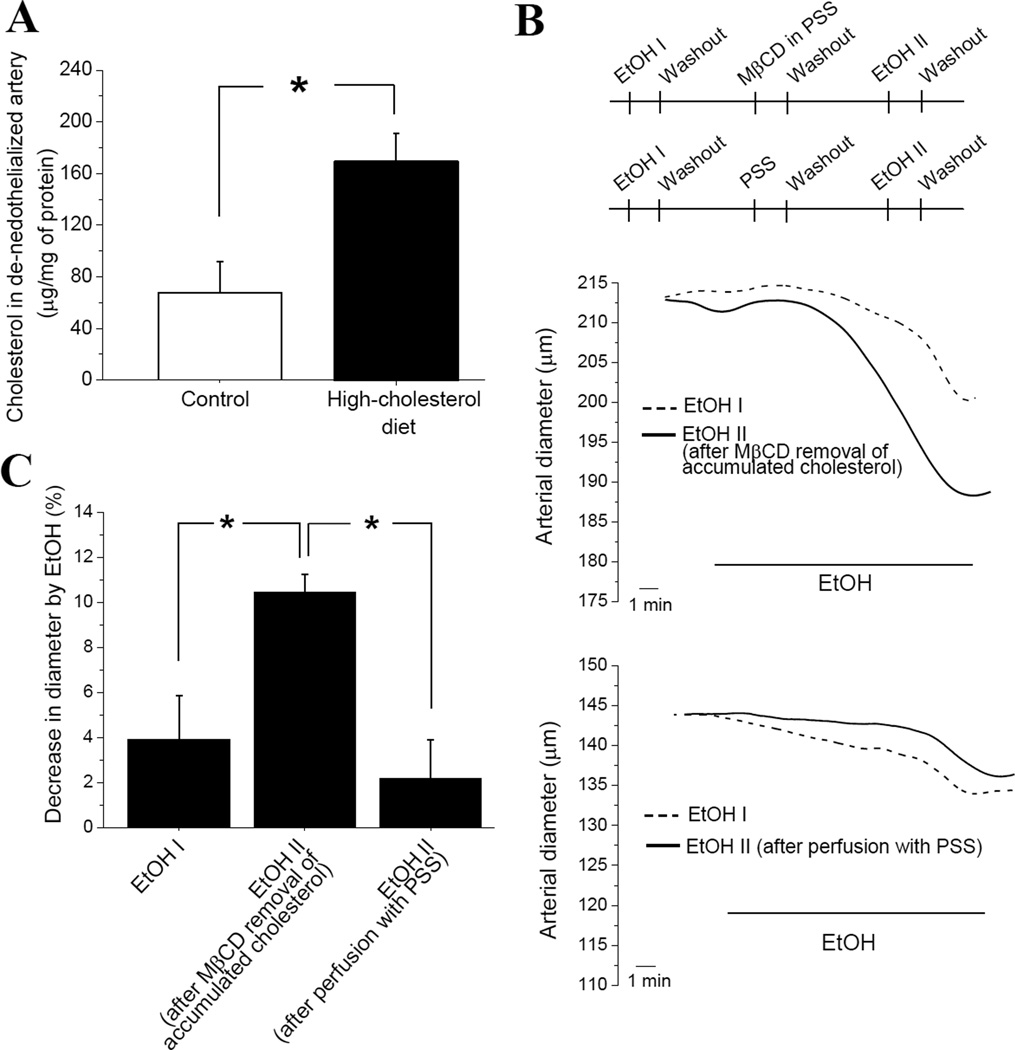
We recently showed that AICAC is dependent on cholesterol level in the tissue. Therefore, we decided to measure cholesterol content in de-endothelialized cerebral arteries from rats on high-cholesterol diet. This diet significantly increased cholesterol level in de-endothelialized cerebral arteries from ≈65 µg/mg of protein to over 160 µg/mg (p<0.05) (Fig. 4A).
Figure 4.
Dietary cholesterol protects against AICAC via accumulation of cholesterol in arterial tissue. (A) Free cholesterol content (in µg per mg of total tissue protein) in de-endothelialized arteries of rats receiving control (n=6) vs. high-cholesterol diet (n=6). (B) Outline of experimental design aimed to regain AICAC by removal of accumulated cholesterol from de-endothelialized, pressurized cerebral arteries of rats on high-cholesterol diet. MβCD: methyl-beta-cyclodextrin, PSS: physiologic saline solution. Representative trace of cerebral artery diameter (top) shows augmented AICAC after removal of accumulated cholesterol using MβCD-containing PSS. Representative trace of cerebral artery diameter (bottom) shows lack of augmentation of AICAC after artery perfusion with MβCD-free PSS. (C) Averaged data comparing AICAC by EtOH I (n=6) with AICAC after MβCD removal of accumulated cholesterol (n=3), and after artery perfusion with MβCD-free PSS (n=3). *p<0.05, when compared to AICAC following removal of accumulated cholesterol from de-endothelialized, pressurized cerebral arteries of rats on high-cholesterol diet.
We set to determine whether the increase in tissue cholesterol content could contribute to reduced AICAC during high-cholesterol diet. For this purpose, de-endothelialized pressurized cerebral arteries from rats on high-cholesterol diet were first tested with 50 mM EtOH (EtOH I in Fig. 4B, top scheme), then briefly perfused with cholesterol carrier methyl-beta-cyclodextrin (MβCD), and probed by 50 mM EtOH again (EtOH II, Fig. 4B, top scheme). Second application of ethanol (EtOH II) to the same vessel usually is not larger than first one (EtOH I) (Fig. 4B, top diameter trace). However, after MβCD removal of accumulated cholesterol from the artery, AICAC (caused by EtOH II) was significantly larger than AICAC before the removal (EtOH I) or when compared to corresponding ethanol application after prolonged incubation of the vessel in MβCD-free PSS (Fig. 4C). Notably, AICAC in group on high-cholesterol diet after removal of accumulated cholesterol was similar to AICAC detected in de-endothelialized arteries of group on control diet (Fig. 4C vs. Fig. 3D). Therefore, dietary cholesterol protects against AICAC via accumulation of cholesterol in arterial tissue.
High-cholesterol diet does not down-regulate membrane surface presence of BK channel protein in cerebral artery myocytes
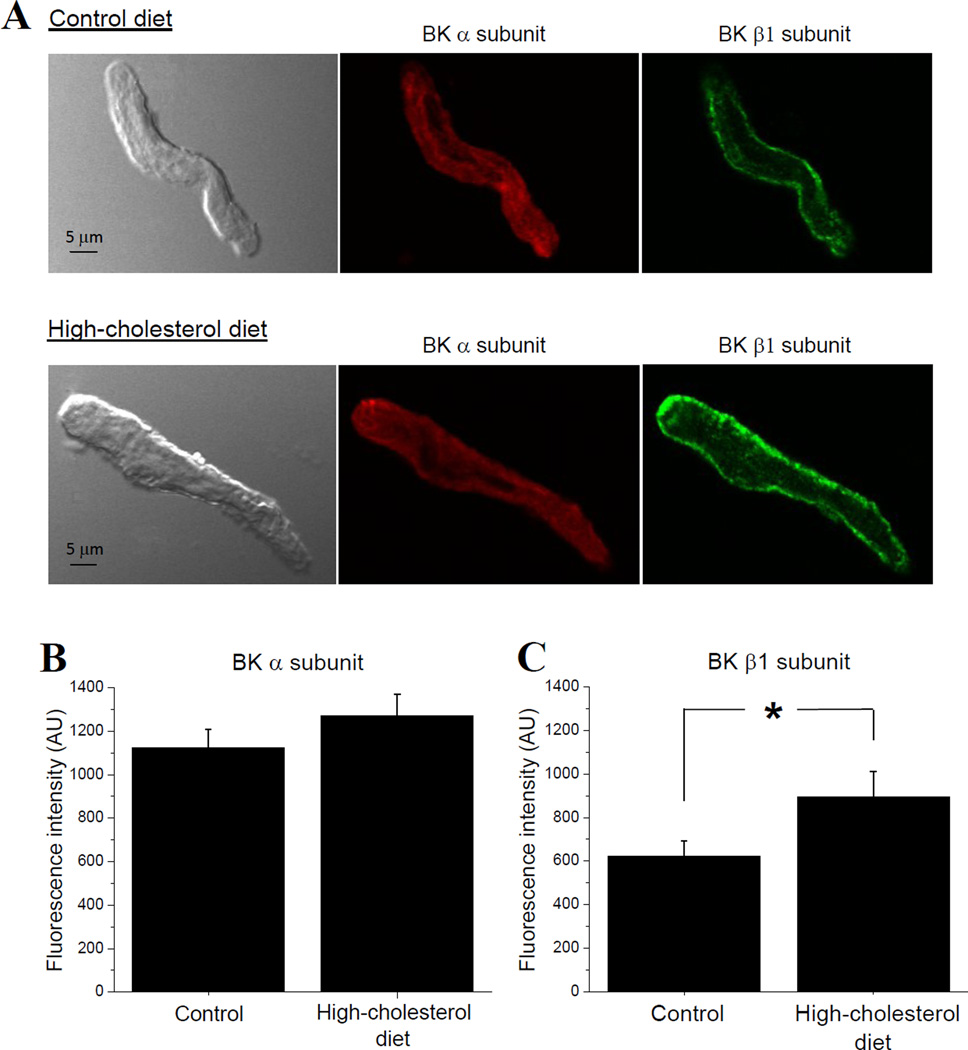
AICAC is caused by ethanol inhibition of calcium/voltage-gated potassium channels of large conductance (BK) in vascular smooth muscle (Liu et al., 2004). To test the possibility that ablated AICAC during high-cholesterol diet could arise from diet-induced down-regulation of BK surface presence, we used immunofluorescence staining of BK channel-forming (α) and smooth-muscle characteristic BK β1 subunits in freshly isolated cerebral artery myocytes. We did not detect significant differences between BK α subunit-associated fluorescence signal in myocytes from rats on control vs. high-cholesterol diet (Fig. 5A, B). BK β1 subunit-associated fluorescence was significantly increased in myocytes from rats on high-cholesterol diet when compared to control diet group (Fig. 5A, C). Therefore, ablated AICAC during high-cholesterol diet could not be attributed to down-regulation of BK protein surface presence on myocyte membranes. Alternatively, it may arise from diminished functional properties of BK channel.
Figure 5.
High-cholesterol diet does not down-regulate BK protein surface presence on cerebral artery myocytes. (A) Representative images of isolated myocytes subjected to immunofluorescence staining against BK channel-forming α (red) and smooth muscle characteristic BK β1 (green) subunits. Averaged fluorescence intensity (in arbitrary units) of BK α (B) and BK β1 (C) subunit-associated signal in cerebral artery myocytes from rats on control (28 myocytes isolated from 4 separate rats) vs. high-cholesterol diet (25 myocytes isolated from 4 separate rats). *p<0.05, when compared to control diet.
Function of BK channel in de-endothelialized cerebral arteries is diminished during high-cholesterol diet
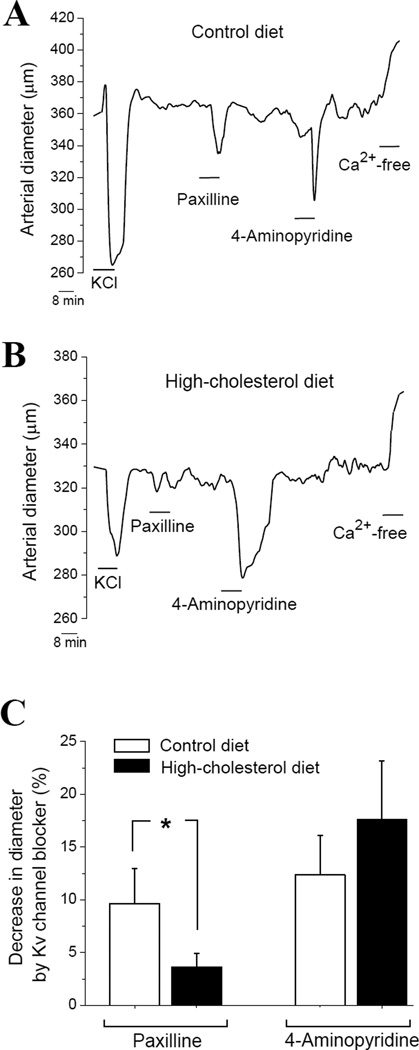
To assess functional integrity of BK channels, we tested arterial constriction in response to selective BK channel blocker paxilline (Strøbaek et al., 1996). In control group, paxilline-induced cerebral artery constriction reached ≈ 10% (Fig. 6A, C). This result is consistent with previously reported data (Bukiya et al., 2011). In contrast, paxilline-induced cerebral artery constriction during high-cholesterol diet was significantly smaller, reaching only 4% (p<0.05) (Fig. 6B, C). Reduced constriction by BK channel blocker is indicative of decreased contribution of BK channel into arterial diameter regulation and decreased activity of BK channel (Brenner et al., 2000). Therefore, our data document that during high-cholesterol diet function of relevant ethanol target – BK channel – is diminished.
Figure 6.
High-cholesterol diet selectively impairs BK channel function. Reprsentative traces of arterial diameter of isolated, de-endothelialized and in vitro pressurized middle cerebral artery of rat on control (A) vs. high-cholesterol (B) diet. (C) Averaged constriction of de-endothelialized arteries in response to 1 µM paxilline and 0.8 mM 4-aminopirydine in control (n=5) vs. high-cholesterol diet group (n=4).*p<0.05, when compared to paxilline-induced constriction of the arteries from rats on control diet.
Notably, constriction by 4-aminopiridyne, a blocker of KV channels other than BK (Nelson and Quayle, 1995), was similar in both groups, reaching ≈15–17% (Fig. 6). This fact suggests that impairment of BK channel-mediated regulation of arterial diameter during high-cholesterol diet is selective and does not extend to potassium channels other than BK-type.
DISCUSSION
In the present study we used a rat model of high-cholesterol diet to demonstrate a protective effect of dietary cholesterol against AICAC. For the first time we showed that the presence of two independent risk factors for cerebral ischemia, high blood alcohol level and elevated cholesterol, do not synergistically act on cerebral artery diameter. Moreover, elevated cholesterol caused decrease of AICAC. Our findings in a rat model (Fig. 1) are in agreement with earlier clinical observations: despite the undisputable fact that excessive alcohol consumption generally represents a risk factor for stroke and cerebrovascular disease, excessive alcohol consumption was ruled out as a risk factor for cerebral ischemia and ischemic stroke in a group of hypercholesterolemic patients (Uchiyama et al., 2009). Therefore, both earlier clinical observations and our present mechanistic work document that elevated cholesterol is protective against consequences of excessive alcohol drinking on cerebrovascular function.
Using isolated pressurized cerebral arteries we established that cholesterol-driven protection against AICAC did not require presence of complex neuronal network, biologically active factors circulating on the blood, or intact endothelium (Figs. 2–3). Dietary cholesterol did not down-regulate membrane surface presence of ethanol target – vascular smooth muscle BK channel (Fig. 5), but ablated BK channel function (Fig. 6). Thus, we suggest that high-cholesterol diet-driven increase in arterial tissue cholesterol level results in BK channel inhibition, reduced participation in vasomotion and, therefore, in vascular responses to EtOH.
Our data showed that EtOH at concentrations reached in the blood stream during binge drinking (50 mM or 0.23%) caused up to 20% decrease in arteriole and arterial diameter (Figs. 1A–B, 2A, E). This finding is consistent with previous reports describing in vivo and in vitro vasoconstrictive effect of alcohol (Altura and Altura, 1984; Gordon et al., 1995; Yang et al., 2001; Liu et al., 2004). AICAC constriction is expected to cause up to 50–60% reduction in cerebral blood flow, because changes in artery diameter are related to changes in cerebral blood flow by a factor of ~3 (Gourley and Heistad, 1984). Such a profound alteration in cerebral blood flow would have devastating consequences on neuronal health, as it was shown that drop in cerebral blood flow by 50% leads to extracellular accumulation of neurotoxic glutamate (Hossmann, 1994). Cerebral blood flow reduction over 50% attenuates ATP synthesis and decreases neuronal ability for action potential firing (Hossmann, 1994).
Cholesterol-rich diet critically controls blood lipid levels in several species, including humans (Howell et al., 1997, El-Sayyad et al., 2012; Asemi et al., 2013). However, we showed for the first time that high-cholesterol diet not only significantly increased blood cholesterol and LDL levels, but also doubled the level of free cholesterol in de-endothelialized cerebral artery tissue (Fig. 4A). Moreover, removal of accumulated cholesterol from arterial tissue restored AICAC to the value detected in control group (Fig. 3D vs. 4C). These data complement our earlier finding showing that depletion of cholesterol below normal level in arterial tissue also protects against AICAC (Bukiya et al., 2011). Therefore, it seems that AICAC is tightly controlled by cholesterol levels: too high or too low cholesterol being protective against AICAC. While mechanisms which ablate AICAC in cholesterol-depleted arteries remain unknown, in the present work we were able to unveil cellular players and processes that enable protection against AICAC during high-cholesterol diet.
Our data show that high-cholesterol diet attenuates AICAC in the absence of endothelium (Fig. 3D). This fact makes our findings applicable to conditions in which endothelial function is compromised (such as during most common neurological and vascular disorders, ageing) (Brown and Thore, 2011; Grammas et al., 2011; Toda, 2012). After de-endothelialization, the remaining vessel includes the tunica media layer formed by vascular smooth muscle, and tunica adventitia that contains nerve fibers, fibroblasts, and few pacemaker cells (Lee, 1995). Considering that smooth muscle cells account for the vast majority of tissue content in de-endothelialized cerebral arteries (Walmsley, 1983a; 1983b; Lee, 1995), we hypothesize that protective effect of elevated cholesterol against AICACA operates at the level of arterial smooth muscle.
One of the most relevant molecular targets of EtOH in vascular system is vascular smooth muscle BK channel (Liu et al., 2004; Bukiya et al., 2009). Using a rat model, it has been demonstrated that AICAC is caused by EtOH inhibition of arterial myocyte BK channels (Liu et al., 2004). High-cholesterol diet could potentially affect amount of BK channel protein. For instance, a down-regulated expression of the BK channel β1 subunit was reported in the sphincter of Oddi cells from rabbits fed with a high-cholesterol diet (Du et al., 2006). However, we did not detect significant changes in BK channel-forming (α) protein on the surface of cerebral artery myocyte during high-cholesterol diet. BK β1 subunit, in contrast, was significantly up-regulated by diet. Yet, function of BK channel in cerebral arteries from rats on high-cholesterol diet was selectively impaired (Fig. 6).
It should be noted that smooth muscle BK channels are not only mediators of AICAC (Liu et al., 2004), but also participate in regulating basal myogenic tone of cerebral arteries (Nelson and Quayle, 1995) and its modification by several vasoconstrictors (Toro et al., 2013). Down-regulation of BK channel function by the high-cholesterol may explain multitude of abnormalities observed in basal function of cerebral arteries during hypercholesterolemia (Kitayama et al., 2007) as cerebral artery responses to BK channel-dependent stimuli other than EtOH are expected to be also reduced by the diet.
Impairment in BK channel function during high-cholesterol intake could arise from direct inhibition of vascular smooth muscle BK channel activity by cholesterol. Moreover we have recently identified distinct amino acids that form several cholesterol-sensing sites on BK channel-forming (α) subunit and are responsible for cholesterol-induced BK channel inhibition (Singh et al., 2012). Conceivably, given that accessory β1 subunits enhance BK channel opening, an up-regulation of BK β1 subunits in the membrane surface might represent an attempt to compensate for reduced activity of BK channel-forming protein due to presence of cholesterol.
In addition to inhibition of BK channel function, cholesterol accumulation in arterial tissue may interfere with EtOH partitioning into lipid membranes and reaching the sites of action. The rigid steroid ring system in cholesterol molecule results in increased packing density of the phospholipids and decreased acyl chain mobility (Seelig and Seelig, 1980). This effect may inhibit diffusion of EtOH molecules across the membrane and negatively reflect on EtOH ability to reach relative molecular targets. Notably, peak of alcohol-induced constriction of pial arterioles during high-cholesterol diet was observed after 5 min of EtOH infusion into cerebral circulation. This is ≈1 min longer than time at which peak of alcohol-induced pial arteriole constriction was reached in control group (Fig. 1B).
Cholesterol may also counteract effect of EtOH in the membrane. For instance, insertion of ethanol molecules into the membranes is associated with increased acyl chain mobility (Sun and Sun, 1985). These opposite effects of cholesterol vs. ethanol on the membrane lipid order correlate with the opposite effects of cholesterol vs. ethanol on the stability of closed state of BK channel (Crowley et al., 2003). Therefore, it may be hypothesized that accumulated cholesterol in arterial tissue “cancels” consequences of ethanol partitioning into the membrane and interference with BK channel function. Another intriguing possibility would be that excessive cholesterol directly interferes with EtOH-sensing site(s) on BK channel protein precluding EtOH-induced BK channel inhibition.
Future identification of intimate molecular mechanisms which underlie cholesterol-driven protection against AICAC will unveil points of intervention for therapeutic countermeasures to combat alcohol-driven cerebrovascular pathology.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by Alcoholic Beverage Medical Research Foundation grant for New Investigator, NIH Support Opportunity for Addiction Research for New Investigators R0-AA020184 (AB) and NIH Merit Award R37 AA11560 (AD). We deeply thank Maria Asuncion- Chin (University of Tennessee HSC) for excellent technical assistance.
REFERENCES
- Adebiyi A, Zhao G, Cheranov SY, Ahmed A, Jaggar JH. Caveolin-1 abolishment attenuates the myogenic response in murine cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1584–H1592. doi: 10.1152/ajpheart.00584.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura BM, Altura BT. Alcohol, the cerebral circulation and strokes. Alcohol. 1984;1:325–331. doi: 10.1016/0741-8329(84)90056-9. 1984. [DOI] [PubMed] [Google Scholar]
- Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- Asemi Z, Tabassi Z, Samimi M, Fahiminejad T, Esmaillzadeh A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial. Br J Nutr. 2013;109:2024–2030. doi: 10.1017/S0007114512004242. [DOI] [PubMed] [Google Scholar]
- Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–645. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- Aydin S, Uzun H, Sozer V, Altug T. Effects of atorvastatin therapy on protein oxidation and oxidative DNA damage in hypercholesterolemic rabbits. Pharmacol Res. 2009;59:242–247. doi: 10.1016/j.phrs.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Heistad DD. Regional, segmental, and temporal heterogeneity of cerebral vascular autoregulation. Ann Biomed Eng. 1985;13:303–310. doi: 10.1007/BF02584248. [DOI] [PubMed] [Google Scholar]
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407(6806):870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Dopico AM. The BK channel accessory beta1 subunit determines alcohol-induced cerebrovascular constriction. FEBS Lett. 2009;583:2779–2784. doi: 10.1016/j.febslet.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Toro L, Dopico AM. Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol Pharmacol. 2007;72:359–369. doi: 10.1124/mol.107.034330. [DOI] [PubMed] [Google Scholar]
- Bukiya AN, McMillan JE, Fedinec AL, Patil SA, Miller DD, Leffler CW, Parrill AL, Dopico AM. Cerebrovascular dilation via selective targeting of the cholane steroid-recognition site in the BK channel β1-subunit by a novel nonsteroidal agent. Mol Pharmacol. 2013;83:1030–1044. doi: 10.1124/mol.112.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Vaithianathan T, Kuntamallappanavar G, Asuncion-Chin M, Dopico AM. Smooth muscle cholesterol enables BK β1 subunit-mediated channel inhibition and subsequent vasoconstriction evoked by alcohol. Arterioscler Thromb Vasc Biol. 2011;31:2410–2423. doi: 10.1161/ATVBAHA.111.233965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik S, Karapirli M, Kandemir E, Ucar F, Kantarci MN, Gurler M, Akyol O. Fatal ethyl and methyl alcohol-related poisoning in Ankara: A retrospective analysis of 10,720 cases between 2001 and 2011. J Forensic Leg Med. 2013;20:151–154. doi: 10.1016/j.jflm.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Treistman SN, Dopico AM. Cholesterol antagonizes ethanol potentiation of human brain BKCa channels reconstituted into phospholipid bilayers. Mol Pharmacol. 2003;64:365–372. doi: 10.1124/mol.64.2.365. [DOI] [PubMed] [Google Scholar]
- Du P, Cui GB, Wang YR, Zhang XY, Ma KJ, Wei JG. Down regulated expression of the beta1 subunit of the big-conductance Ca2+ sensitive K+ channel in sphincter of Oddi cells from rabbits fed with a high cholesterol diet. Acta Biochim Biophys Sin (Shanghai) 2006;38:893–899. doi: 10.1111/j.1745-7270.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- El-Sayyad HI, Khalifa SA, Fouda YA, Yonis AS. Effects of diabetes and/or hypercholesterolemia on skin development of rat fetuses. Nutrition. 2012;28:698–706. doi: 10.1016/j.nut.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Gordon EL, Nguyen TS, Ngai AC, Winn HR. Differential effects of alcohols on intracerebral arterioles. Ethanol alone causes vasoconstriction. J Cereb Blood Flow Metab. 1995;15:532–538. doi: 10.1038/jcbfm.1995.66. [DOI] [PubMed] [Google Scholar]
- Gourley JK, Heistad DD. Characteristics of reactive hyperemia in the cerebral circulation. Am J Physiol. 1984;246:H52–H58. doi: 10.1152/ajpheart.1984.246.1.H52. [DOI] [PubMed] [Google Scholar]
- Grammas P, Martinez J, Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med. 2011;13:e19. doi: 10.1017/S1462399411001918. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- Howell WH, McNamara DJ, Tosca MA, Smith BT, Gaines JA. Plasma lipid and lipoprotein responses to dietary fat and cholesterol: a meta-analysis. Am J Clin Nutr. 1997;65:1747–1764. doi: 10.1093/ajcn/65.6.1747. [DOI] [PubMed] [Google Scholar]
- Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. 2007;38:2136–2141. doi: 10.1161/STROKEAHA.107.481879. [DOI] [PubMed] [Google Scholar]
- Lange JE, Voas RB. Defining binge drinking quantities through resulting BACs. Annu Proc Assoc Adv Automot Med. 2000;44:389–404. [PMC free article] [PubMed] [Google Scholar]
- Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995;66:149–173. doi: 10.1016/0163-7258(94)00071-a. [DOI] [PubMed] [Google Scholar]
- Liu P, Xi Q, Ahmed A, Jaggar JH, Dopico AM. Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proc Natl Acad Sci U S A. 2004;101:18217–18222. doi: 10.1073/pnas.0406096102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- Puddey IB, Rakic V, Dimmitt SB, Beilin LJ. Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors--a review. Addiction. 1999;94:649–663. doi: 10.1046/j.1360-0443.1999.9456493.x. [DOI] [PubMed] [Google Scholar]
- Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA. 2003;289:579–588. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- Seelig J, Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980;13:19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Singh AK, McMillan J, Bukiya AN, Burton B, Parrill AL, Dopico AM. Multiple cholesterol recognition/interaction amino acid consensus (CRAC) motifs in cytosolic C tail of Slo1 subunit determine cholesterol sensitivity of Ca2+- and voltage-gated K+ (BK) channels. J Biol Chem. 2012;287:20509–20521. doi: 10.1074/jbc.M112.356261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøbaek D, Christophersen P, Holm NR, Moldt P, Ahring PK, Johansen TE, Olesen SP. Modulation of the Ca(2+)-dependent K+ channel, hslo, by the substituted diphenylurea NS 1608, paxilline and internal Ca2+ . Neuropharmacology. 1996;35(7):903–914. doi: 10.1016/0028-3908(96)00096-2. [DOI] [PubMed] [Google Scholar]
- Sun GY, Sun AY. Ethanol and membrane lipids. Alcohol Clin Exp Res. 1985;9:164–180. doi: 10.1111/j.1530-0277.1985.tb05543.x. [DOI] [PubMed] [Google Scholar]
- Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther. 2012;133:159–176. doi: 10.1016/j.pharmthera.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Toro L, Li M, Zhang Z, Singh H, Wu Y, Stefani E. MaxiK channel and cell signalling. Pflugers Arch. 2103 doi: 10.1007/s00424-013-1359-0. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama S, Nakaya N, Mizuno K, Ohashi Y, Tajima N, Kushiro T, Teramoto T, Nakamura H MEGA Study Group. Risk factors for stroke and lipid-lowering effect of pravastatin on the risk of stroke in Japanese patients with hypercholesterolemia: analysis of data from the MEGA Study, a large randomized controlled trial. J Neurol Sci. 2009;284:72–76. doi: 10.1016/j.jns.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Vaithianathan T, Bukiya A, Liu J, Liu P, Asuncion-Chin M, Fan Z, Dopico A. Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J Gen Physiol. 2008;132:13–28. doi: 10.1085/jgp.200709913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley JG. Vascular smooth muscle orientation in straight portions of human cerebral arteries. J Microsc. 1983a;131:361–375. doi: 10.1111/j.1365-2818.1983.tb04261.x. [DOI] [PubMed] [Google Scholar]
- Walmsley JG. Vascular smooth muscle orientation in curved branches and bifurcations of human cerebral arteries. J Microsc. 1983b;131:377–389. doi: 10.1111/j.1365-2818.1983.tb04262.x. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Wang J, Zheng T, Altura BT, Altura BM. Importance of PKC and PI3Ks in ethanol-induced contraction of cerebral arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2001;280:H2144–H2152. doi: 10.1152/ajpheart.2001.280.5.H2144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.