Abstract
Purpose
Despite preclinical evidence supporting anti-cancer effects of cardiac glycosides, epidemiologic studies consistently show elevated breast cancer risk in digoxin users. We studied this association in the Nurses’ Health Study cohort to evaluate influences of screening mammography and lifestyle-related risk factors.
Methods
We followed 90,202 postmenopausal women from 1994 until 2010. Self-reported breast cancers were confirmed by medical record review. We fit Cox regression models to estimate associations between time-varying digoxin use and breast cancer incidence, overall and by tumor ER status, accounting for mammography screening and established breast cancer risk factors.
Results
There were 5,004 digoxin users over 1.05 million person-years of observation, among whom 144 breast cancer cases occurred. Digoxin users were more likely to undergo mammographic screening, to be former users of postmenopausal hormones, and to take other medications than never-users; the groups were similar on reproductive history and alcohol consumption. Current digoxin use of >4 years’ duration was associated with a 45% increased rate of breast cancer compared with never use (HRadj=1.45, 95% CI: 1.13, 1.86). The association appeared stronger for ER-positive disease (HRadj=1.46, 95% CI: 1.10, 1.95) than for ER-negative disease (HRadj=1.12, 95% CI: 0.52, 2.37). Associations were robust to restriction on regular mammography use and to adjustment for established breast cancer risk factors, including lifestyle-related exposures.
Conclusions
The positive association between digoxin use and breast cancer occurrence was not attenuated when lifestyle-related breast cancer risk factors and screening practices were accounted for. Digoxin, a common cardiac drug worldwide, may promote breast carcinogenesis.
Digoxin belongs to the cardiac glycosides (CGs), a family of naturally-derived steroid compounds which are used to treat heart failure and atrial fibrillation [1]. Despite the advent of novel medication classes to treat the same indications, CGs remain clinically prevalent worldwide, likely because of their favorable combination of effectiveness and economy. Therefore, any effect of CGs on breast cancer occurrence would have major public health implications.
Early evidence suggested an ameliorative effect of CGs on breast cancer. A 1979 letter reported less aggressive breast tumor phenotypes and a lower risk of metastases among women taking CGs, compared with unexposed women [2]. This cohort of 175 breast cancer survivors was followed for over 20 years, over which time the CG-exposed group had an 82% lower risk of breast cancer-specific mortality than the CG-unexposed group [3].
The earliest mechanistic hypothesis for these observations was that the steroid core of CGs attenuated estrogen receptor (ER) signaling, akin to the action of tamoxifen [2]. This hypothesis has both refuting and supporting evidence [4, 5]. A host of recent preclinical studies provide evidence for antineoplastic effects of CGs through non-hormonal pathways including signal transduction by the sodium/potassium ATPase, inhibition of topoisomerase II, regulation of fibroblast growth factor 2 (FGF-2), prevention of NF-κB activation, and inhibition of hypoxia inducible factor-1α (HIF-1α) [6–16].
Several studies have investigated the association between CG use and breast cancer incidence. Friedman and Haux independently reported 20% and 25% increased risks of breast cancer in CG users, respectively, though these were not described as positive associations due to a lack of statistical significance [17, 18]. A similar association is calculable from data reported by Stenkvist in 1980 [19]. Most recently, two Danish studies reported precisely-measured positive associations between digoxin use and invasive breast cancer occurrence [20, 21]. The first of these reported a 30% increased breast cancer risk among digoxin users compared with non-users (adjusted odds ratio=1.30; 95% CI: 1.14, 1.48) [20]. The second study showed a positive association that was slightly stronger for ER-positive than for ER-negative breast cancer (ER-positive adjusted hazard ratio [HR]=1.35, 95% CI: 1.26, 1.45; ER-negative adjusted HR=1.20, 95% CI: 1.03, 1.40) [21]. The Danish studies found no evidence of confounding by indication, and were conducted independently using the nation’s high-quality medical and prescription registries. Despite the many strengths of these studies and the close agreement of their associations, they were conducted in partially overlapping source populations, neither could address potential confounding by lifestyle-related risk factors, and data on screening mammography were limited.
We sought to strengthen the evidence for an effect of digoxin use on breast cancer incidence by measuring the association in the prospective Nurses’ Health Study (NHS) cohort. In addition to prescription drug histories and cancer diagnoses, the NHS collects high-quality longitudinal data on lifestyle, demographic, and reproductive risk factors for breast cancer.
Materials and Methods
This study was approved by the Committee on the Use of Human Subjects in Research at Brigham and Women’s Hospital.
Source population: Nurses’ Health Study
The NHS began in 1976 with the enrollment of 121,700 female U.S. registered nurses between the ages of 30 and 55. Participants returned baseline questionnaires to report medical history and reproductive information. New data are collected biennially to update prior exposures and ascertain new diagnoses, and the response rate at each questionnaire cycle has been approximately 90 percent. Use of prescription and over-the-counter medications has been ascertained since the cohort’s inception. The 1994 questionnaire was the first to inquire about use of digoxin or another anti-arrhythmic drug, and in 1996 digoxin and other anti-arrhythmic drugs were ascertained separately. The present study followed all NHS participants who in 1994 had returned a questionnaire, were postmenopausal, and were without a history of diagnosed cancer. Women who were premenopausal at baseline entered the study upon becoming postmenopausal.
Definitions of analytic variables
Incident breast cancers were reported on questionnaires and subsequently confirmed by physician review of medical records. Tumor ER status was abstracted from pathology reports, which yields classification accuracy similar to that of centralized testing [22].
Digoxin use was assessed biennially, with subjects reporting if they took digoxin in the past two years. If a participant answered yes, she was considered exposed for the two-year period covered by the questionnaire, otherwise she was considered unexposed. Using these dichotomous longitudinal variables, we constructed the following exposure categories: A) ever vs. never used digoxin; B) time-varying exposure categorized as current user, former user, or never used; and C) time-varying cumulative duration of digoxin exposure, categorized as current user for more than 4 years, current user for 4 years or less, former user, or never used. To address potential confounding by indication we conducted a sub-analysis using the same digoxin exposure definitions as above, but substituting a reference group of women who did not use digoxin but did use a beta blocker, ACE inhibitor, or calcium channel blocker in the same follow-up cycle.
Numerous breast cancer risk factors were candidate confounders in our analyses. Age at menarche was modeled in categories of <12, 12–13, or ≥14 years of age. Age at first birth (AFB) and parity were factored into a single variable with the following categories: nulliparous, 1 or 2 children and AFB≤30, 1 or 2 children and AFB>30, 3 or 4 children and AFB≤30, 3 or 4 children and AFB>30, 5 or more children and AFB≤30, and 5 or more children and AFB>30 [23]. Postmenopausal hormone (PMH) use was categorized as missing, never used, formerly used, currently using and for less than 5 years, and currently using and for 5 or more years [24]. Furthermore, we evaluated the type of PMH used (estrogen, estrogen plus progesterone, or other). History of breast cancer in a first-degree female biological relative and personal history of benign breast disease (BBD) were coded dichotomously [25]. Use of aspirin was categorized as <1 day/week, 1–6 days/week, and daily [26]. Regular use of ibuprofen, anti-lipidemic drugs, preventive tamoxifen therapy, and receipt of a screening mammogram in the preceding follow-up cycle were classified dichotomously [27–29]. Height in inches was modeled continuously. BMI was categorized as underweight (0≤BMI<18.5), normal (18.5≤BMI<25.0), overweight (25.0≤BMI<30), and obese (BMI≥30) [30, 31]. Alcohol intake was defined as the cumulative average daily consumption between 1980 (the first year of alcohol assessment by questionnaire) and the follow-up cycle in question, then summarized in the following categories: 0 g/day, 0.1–4.9 g/day, 5.0–9.9 g/day, 10–19.9 g/day, and ≥20 g/day [32].
Statistical analysis and meta-analysis
We tabulated summary statistics for key baseline characteristics among all subjects according to ever/never digoxin status over follow-up.
We fit age- and multivariable-adjusted Cox proportional hazards models to estimate the associations between digoxin use and incidence of any invasive, ER-positive, or ER-negative breast cancer. When modeling the ER-positive outcome, women with ER-negative disease were censored on their date of diagnosis, and vice-versa. Women were followed from baseline until the first of breast cancer incidence, death from any cause, diagnosis with another malignancy, or loss to follow-up. With the exception of static covariates (e.g., age at menarche), all independent variables were modeled as time-varying covariates. Polytomous variables were modeled with design variables to allow for nonlinear effects across coding levels. Proportionality of hazard functions by digoxin exposure status was evaluated with Wald tests for the interaction between main exposures and the logarithm of time. Age was used as the time axis in our models.
To summarize current evidence for an association between CG use and breast cancer incidence, we conducted a meta-analysis of epidemiologic studies. We searched the PubMed database (www.pubmed.gov) using the following search string: “((((digoxin[MeSH Terms]) OR digitalis[MeSH Terms]) OR digitalis glycosides[MeSH Terms]) OR cardiac glycosides[MeSH Terms]) AND breast neoplasms[MeSH Terms]”. From the 67 search results, we identified 4 papers reporting on the association between current or ever CG use and breast cancer incidence [17, 19–21]. One additional report was identified from the reference lists of papers identified by the PubMed search [18], and findings reported herein were included as well. Heterogeneity of associations was assessed by the Q-statistic, and estimates were pooled using a random effects model [33, 34].
Analyses were carried out with SAS v.9.3 (SAS Institute, Cary, NC). Statistical tests were two-sided with alpha=5%.
Results
Baseline cohort characteristics
Table 1 reports the distribution of key characteristics according to digoxin status among NHS participants who were postmenopausal in 1994 (n=74,970). Compared with non-users, digoxin users were older on average (mean age 62.2 years and 65.7 years, respectively), were more likely to be former users of PMH, were more likely to be taking diuretics, lipid-lowering drugs, beta blockers, calcium channel blockers, and ACE inhibitors, had a higher prevalence of CHF, AF, and diabetes, and were more likely to undergo mammographic screening. Among PMH users, women in the digoxin-exposed group were more likely to have used unopposed estrogens. Digoxin users and non-users were similar with respect to height, adiposity, ages at menarche and menopause, and reproductive history.
Table 1.
Characteristics of postmenopausal NHS participants at baseline (1994), according to digoxin statusover follow-up.
| Characteristics | Digoxin users (n=5,004) | Non-users (n=69,966) |
|---|---|---|
| Age at baseline, mean (sd) | 65.7 (5.4) | 62.2 (6.3) |
|
| ||
| Age at menarche, mean (sd) | 12.5 (1.9) | 12.5 (1.8) |
|
| ||
| Age at menopause, mean (sd) | 49.2 (5.0) | 49.0 (5.7) |
|
| ||
| Height (inches) in 1976, mean (sd) | 64.8 (2.6) | 64.3 (3.3) |
|
| ||
| Body mass index, n (%) | ||
| Underweight (<20.0) | 104 (2.1) | 976 (1.4) |
| Normal (20.0 to 24.9) | 2,110 (43) | 29,582 (43) |
| Overweight (25.0 to 29.9) | 1,535 (31) | 23,925 (35) |
| Obese (≥30.0) | 1,206 (24) | 14,459 (21) |
| (Missing) | 49 | 1,024 |
|
| ||
| Ethanol consumption, n (%) | ||
| Non-drinker | 1,288 (27) | 14,835 (23) |
| 0.1 to 4.9 grams/day | 1,962 (41) | 28,789 (44) |
| 5.0–9.9 grams/day | 626 (13) | 8,872 (13) |
| 10.0–19.9 grams/day | 541 (11) | 8,154 (12) |
| ≥20 grams/day | 399 (8.3) | 5,135 (7.8) |
| (Missing) | 188 | 4,181 |
|
| ||
| Parity/age at first birth, n (%) | ||
| Nulliparous | 315 (6.5) | 3,922 (5.7) |
| 1–2 births, age ≤30 | 1,259 (26) | 18,957 (28) |
| 1–2 births, age >30 | 277 (5.7) | 3,454 (5.0) |
| 3–4 births, age ≤30 | 2,024 (41) | 29,857 (43) |
| 3–4 births, age >30 | 149 (3.1) | 1,307 (1.9) |
| ≥5 births, age ≤30 | 844 (17) | 10,948 (16) |
| ≥5 births, age >30 | 19 (0.4) | 214 (0.3) |
| (Missing) | 117 | 1,307 |
|
| ||
| Postmenopausal hormone (PMH) use, n (%) | ||
| Never used hormones | 1,361 (31) | 21,159 (33) |
| Former user | 1,246 (28) | 13,834 (21) |
| Current user, duration <5 years | 441 (9.8) | 10,610 (16) |
| Current user, duration ≥5 years | 1,475 (33) | 18,782 (29) |
| (Missing) | 481 | 5,581 |
|
| ||
| PMH type (among current users), n (%) | ||
| Oral estrogen | 1,129 (59) | 14,828 (50) |
| Oral estrogen + progesterone | 643 (34) | 12,747 (43) |
| Other/unknown | 144 (7.5) | 1,817 (6.2) |
| (Missing) | 0 | 0 |
|
| ||
| Use of other medications, n (%) | ||
| Tamoxifen (preventive) | 3 (0.06) | 46 (0.07) |
| Lipid-lowering drugs | 682 (14) | 5,623 (8.0) |
| NSAIDs | 1,143 (23) | 15,182 (22) |
| Beta blockers | 1,062 (21) | 6,960 (10) |
| Calcium channel blockers | 994 (20) | 6,059 (8.7) |
| ACE inhibitors | 725 (14) | 5,174 (7.4) |
| Diuretics | 1,037 (21) | 6,292 (9.0) |
|
| ||
| Medical history, n (%) | ||
| Congestive heart failure | 735 (15) | 777 (1.1) |
| Atrial fibrillation | 2,133 (43) | 2,633 (3.8) |
| Diabetes | 570 (11) | 3,348 (4.8) |
| Regular screening mammograms | 3,911 (78) | 51,968 (74) |
Digoxin use and breast cancer risk
Table 2 reports associations estimated from regression of time to breast cancer diagnosis on longitudinal digoxin exposure and covariates. A total of 90,202 postmenopausal women, of whom 5,004 were exposed to digoxin, contributed ~1.05 million person-years of follow-up to the analysis. Models for any invasive disease, ER-positive disease, and ER-negative disease were based on 4,576 cases, 3,317 cases, and 713 cases, respectively. Associations are reported with reference to women who never used digoxin.
Table 2.
Associations between time-varying digoxin use and incidence of invasive breast cancer in the Nurses’ Health Study, 1994–2010.
| No. cases | Age-adjusted HR (95% CI) |
Fully adjusted** HR (95% CI) |
|
|---|---|---|---|
|
| |||
| All invasive cases (n=4,576) | |||
|
| |||
| Digoxin use | |||
| Never | 4,359 | 1. ref | 1. ref |
| Former | 73 | 1.03 (0.82, 1.30) | 1.03 (0.82, 1.31) |
| Current, ≤4 years | 79 | 1.23 (0.98, 1.54) | 1.22 (0.97, 1.53) |
| Current, >4 years | 65 | 1.50 (1.17, 1.92) | 1.45 (1.13, 1.86) |
|
| |||
| ER-positive cases (n=3,317) | |||
|
| |||
| Digoxin use | |||
| Never | 3,158 | 1. ref | 1. ref |
| Former | 51 | 1.01 (0.76, 1.34) | 1.03 (0.77, 1.36) |
| Current, ≤4 years | 59 | 1.31 (1.01, 1.69) | 1.28 (0.98, 1.66) |
| Current, >4 years | 49 | 1.55 (1.17, 2.06) | 1.46 (1.10, 1.95) |
|
| |||
| ER-negative cases (n=713) | |||
|
| |||
| Digoxin use | |||
| Never | 694 | 1. ref | 1. ref |
| Former | 5 | 0.48 (0.20, 1.17) | 0.48 (0.20, 1.17) |
| Current, ≤4 years | 7 | 0.67 (0.32, 1.42) | 0.65 (0.30, 1.37) |
| Current, >4 years | 7 | 1.22 (0.57, 2.57) | 1.12 (0.52, 2.37) |
Adjusted for age (time scale for the regression), height, body mass index, age at menarche, age at menopause, alcohol consumption, age at first birth and parity, use of postmenopausal hormones (and preparation used), family history of breast cancer, personal history of benign breast disease, receipt of screening mammogram in the past two years, and use of aspirin, ibuprofen, cholesterol-lowering drugs, and tamoxifen use for breast cancer prevention.
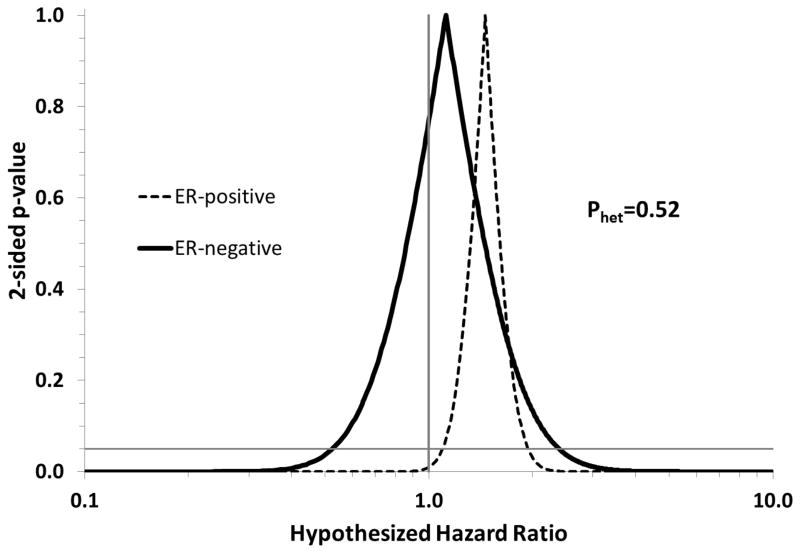
Current use of digoxin for more than four years was associated with a 45% increase in the rate of invasive breast cancer incidence (HRadj=1.45, 95% CI: 1.13, 1.86), and a 46% increase in the rate of ER-positive disease (HRadj=1.46, 95% CI: 1.10, 1.95). The association with ER-negative disease was weaker (HRadj=1.12, 95% CI: 0.52, 2.37), but measured with much lower precision. Figure 1 graphically compares associations between longer duration of use (>4 years) and incidence of ER-positive and ER-negative disease using confidence interval functions [35]. These curves show that the two estimates are not readily distinguishable, owing to the poor precision with which the ER-negative association was measured.
Figure 1.
Confidence interval functions depicting the associations between digoxin use (current users with more than 4 years of exposure compared with never users) and the incidence of ER-positive and ER-negative breast cancer. Postmenopausal participants in the Nurses’ Health Study, 1994–2010.
Current use of digoxin for ≤4 years was less strongly associated with incidence of invasive breast cancer (HRadj=1.22, 95% CI: 0.97, 1.53) and ER-positive breast cancer (HRadj=1.28, 95% CI: 0.98, 1.66). The corresponding association with ER-negative breast cancer was protective but imprecisely measured (HRadj=0.65, 95% CI: 0.30, 1.37).
Former use of digoxin was not associated with a higher rate of invasive, ER-positive, or ER-negative breast cancer (Table 2). When never use, current use of shorter duration, and current use of longer duration were modeled with a single ordinal variable to assess linear trend, we observed a positive slope in the log-hazard (fully adjusted slope=0.188, 95% CI: 0.078, 0.299; P=0.0008), indicating a monotonic increase in the breast cancer hazard across these exposure categories.
In all cases, estimates from fully-adjusted models were very similar to estimates from age-adjusted models.
When we compared digoxin users with an alternative reference group of women unexposed to digoxin but exposed either to a beta blocker, calcium channel blocker, or ACE inhibitor, we continued to observe positive associations between current digoxin use and breast cancer incidence (Table 3). This provides evidence that the observed digoxin/breast cancer associations are not attributable to the medical indications for digoxin and other cardiac drugs, or to some common cause of the indications for digoxin therapy and incident breast cancer.
Table 3.
Assessment of potential confounding by indication. Associations between time-varying digoxin use and incidence of invasive breast cancer, based on comparison of digoxin users with non-users of digoxin who had used other cardiac drugs. Nurses’ Health Study, 1994–2010. See statistical analysis section for details and rationale.
| No. cases | Age-adjusted HR (95% CI) |
Fully adjusted** HR (95% CI) |
|
|---|---|---|---|
|
| |||
| All invasive cases (n=1,474) | |||
|
| |||
| Digoxin use | |||
| Never/other cardiac | 1,257 | 1.ref | 1. ref |
| Former | 73 | 0.95 (0.75, 1.22) | 0.95 (0.74, 1.22) |
| Current, ≤4 years | 79 | 1.20 (0.94, 1.51) | 1.19 (0.94, 1.51) |
| Current, >4 years | 65 | 1.45 (1.13, 1.88) | 1.45 (1.12, 1.88) |
|
| |||
| ER-positive cases (n=1,111) | |||
|
| |||
| Digoxin use | |||
| Never/other cardiac | 952 | 1. ref | 1. ref |
| Former | 51 | 0.88 (0.66, 1.18) | 0.89 (0.66, 1.20) |
| Current, ≤4 years | 59 | 1.22 (0.93, 1.60) | 1.25 (0.95, 1.64) |
| Current, >4 years | 49 | 1.44 (1.07, 1.93) | 1.47 (1.09, 1.97) |
|
| |||
| ER-negative cases (n=222) | |||
|
| |||
| Digoxin use | Model did not converge | ||
| Never/other cardiac | 203 | 1. ref | |
| Former | 5 | 0.42 (0.17, 1.03) | |
| Current, ≤4 years | 7 | 0.64 (0.29, 1.38) | |
| Current, >4 years | 7 | 1.07 (0.50, 2.32) | |
Adjusted for age (time scale for the regression), height, body mass index, age at menarche, age at menopause, alcohol consumption, age at first birth and parity, use of postmenopausal hormones (and preparation used), family history of breast cancer, personal history of benign breast disease, receipt of screening mammogram in the past two years, and use of aspirin, ibuprofen, cholesterol-lowering drugs, and tamoxifen use for breast cancer prevention.
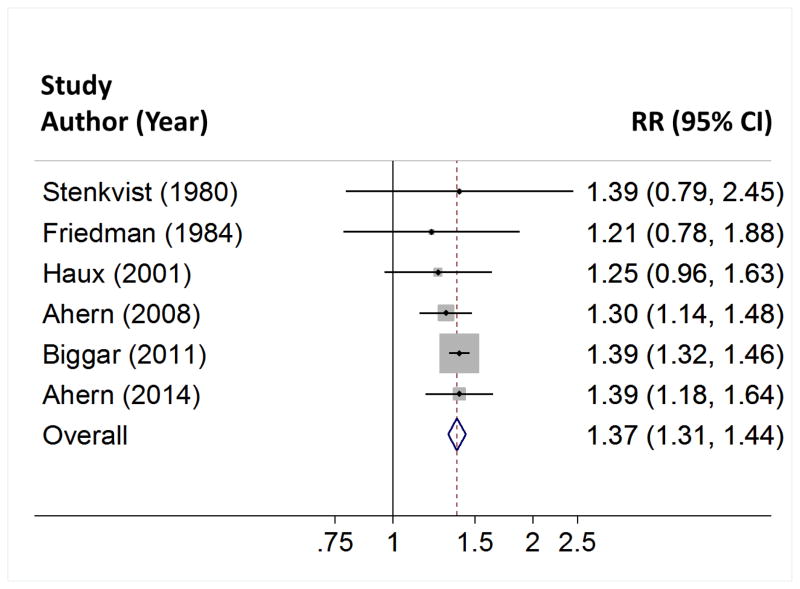
Table 4 summarizes the associations reported by 6 studies of digoxin use and breast cancer incidence. The studies found risks of breast cancer to be elevated by 21% to 40% in digoxin users compared with non-users (Pheterogeneity=0.89). Pooling the results in a random effects meta-analysis showed a 37% increase in breast cancer risk (95% CI: 31% to 44%) among digoxin users, compared with non-users. These results are depicted graphically in Figure 2.
Table 4.
Summary and random effects meta-analysis of published associations between cardiac glycoside use and breast cancer incidence, 1980–2013.
| First author | Country | Year published | Relative risk (95% CI) |
|---|---|---|---|
| Stenkvist | Sweden | 1980 | 1.39 (0.79, 2.45)* |
| Friedman | United States | 1984 | 1.21 (0.78, 1.88)** |
| Haux | Norway | 2001 | 1.25 (0.95, 1.62) |
| Ahern | Denmark | 2008 | 1.30 (1.14, 1.48) |
| Biggar | Denmark | 2011 | 1.39 (1.32, 1.46) |
| Ahern | United States | 2014 | 1.40 (1.18, 1.65) |
| Pooled: | 1.37 (1.31, 1.44) | ||
| P for homogeneity (Q statistic) | 0.89 | ||
Calculated from data reported in Table 1 of the Stenkvist paper (reference 20), with variance calculated as the sum of the inverse values of the cell frequencies.
The Friedman paper did not report a confidence interval or variance for the standardized incidence ratio they reported. We estimated the variance as the inverse of the number of observed cases reported (n=20) and used this to calculate the confidence limits.
Figure 2.
Forest plot depicting associations between digoxin use and breast cancer incidence. Random effects meta-analysis of 6 studies.
Discussion
We observed a positive association between current use of digoxin and the incidence of invasive breast cancer, which strengthened with increasing duration of use. Former users of digoxin did not have an elevated rate of breast cancer incidence compared with never-users. Associations were somewhat stronger for ER-positive disease than for ER-negative disease, which is consistent with the hypothesis that digoxin acts through estrogen signaling pathways to promote breast tumor growth and survival. However, we had relatively limited numbers of digoxin-exposed ER-negative cases and there was no graphical or statistical evidence of heterogeneity (Figure 1; Pheterogeneity=0.52). Other ER-negative associations appeared protective but were based on 5 to 7 exposed cases, and most likely represent chance findings in sparse data [36].
Our findings agree with two earlier Danish studies of digoxin use and breast cancer incidence. The first of these could not examine heterogeneity by tumor ER status, but nonetheless reported a 30% increase in the odds of breast cancer occurrence among digoxin users (ORadj=1.30, 95% CI: 1.14, 1.48), with a stronger association observed among the longest-duration users (7–18 years of digoxin use: ORadj=1.39, 95% CI: 1.10, 1.74) [20]. The second study included more than 25 million person-years of observation, over which time more than 2,000 breast cancers occurred among digoxin users [21]. That study reported a slightly stronger association with ER-positive than with ER-negative disease (ER-positive: IRRadj=1.35, 95% CI: 1.26, 1.45; ER negative: IRRadj=1.20, 95% CI: 1.03, 1.40), and a null association among former users, all in agreement with our findings. As noted by the authors of the earlier Danish studies [20, 37], three earlier studies found similar associations, but these were not emphasized for lack of statistical significance [17–19].
Beyond the somewhat stronger association observed with ER-positive breast cancer, other compelling evidence supports an estrogen-mimetic action of digoxin [37]. First, digitalis (a related compound) interacts with human estrogen receptors in vitro [5]. Second, the digoxin/breast cancer associations follow the same pattern as observed with exogenous hormone use: there is an elevated breast cancer risk among current users that wanes upon discontinuation [24, 38, 39]. Third, it has long been known that prolonged cardiac glycoside therapy is associated with gynecomastia in men [40], which reverses upon discontinuation of therapy [41]. Furthermore, digoxin is associated with higher incidence of male breast cancer [42, 43], and lower incidence of prostate cancer [44], both of which are consistent with estrogenic effects on the respective tissues of origin [45, 46]. Perhaps the most compelling evidence comes from another Danish study, which showed a positive association between digoxin use and incidence of uterine cancer (an estrogen-sensitive malignancy), but null associations with incident cancers of the ovary and cervix (which are not strongly affected by estrogen) [47].
Lack of an association with ovarian and cervical cancers does not preclude a role of digoxin in the promotion of some breast cancer subtypes through non-estrogenic mechanisms. Current evidence does not rule out a positive association between digoxin use and the incidence of ER-negative disease, which speaks to the potential importance of non-hormonal effects of digoxin. These mechanisms include interference with signaling cascades tied to the sodium-potassium ATPase [6–10, 12, 13, 48, 49], attenuation of p53 [16], and inhibition of HIF-1α [15]. However, current literature concerning these mechanisms largely supports an anti-neoplastic effect of cardiac glycosides.
The major strength of this study was its ability to account for lifestyle risk factors and screening practices that previous studies could not address. Prior evidence was weakened by potential residual confounding or differential breast cancer classification accuracy by these factors. For example, the positive association between BMI and postmenopausal breast cancer [50] means that a skew toward higher BMI among digoxin users, compared with non-users, could have explained the positive associations observed in earlier studies. Similar arguments can be made for PMH use, alcohol consumption, ages at menarche and menopause, and reproductive history. Digoxin users may also be more compliant with mammographic screening recommendations, which appeared to be the case in our cohort. This could lead to higher sensitivity of breast cancer classification among exposed women, which could readily account for the positive associations we and others have observed. We found that none of these factors substantially biased associations between digoxin use and breast cancer incidence, which strengthens the overall evidence for causality.
Other key strengths include our use of prospectively-ascertained medication history, so that any exposure measurement error is expected to be non-differential between cases and non-cases. Breast cancer diagnoses were confirmed by medical record review, so exposure measurement error should be uncorrelated with errors in outcome classification. Furthermore, we found no evidence of confounding by indication when we compared breast cancer rates in digoxin users to rates in users of other cardiac drugs.
There are limitations to bear in mind when evaluating our results. First, digoxin data were left-truncated, meaning that use of the drug before 1992 was unknown. It is therefore possible that some women classified as never-users could have been past users who discontinued before 1992. Furthermore, this truncation means that our categories of digoxin exposure duration reflect only the minimum cumulative duration. Another limitation is that a non-trivial proportion of women were missing data on PMH use (8.9% of digoxin users and 11% of non-users). Our regression models used a missing indicator to maximize the number of digoxin-exposed breast cancer cases available for analysis. The missing indicator method can lead to substantial bias if the variable in question is strongly related to both the exposure and outcome [51]. However, regression estimates were quite similar when we restricted analyses to women with complete PMH data (data not shown). We are therefore confident that use of the missing indicator method has not left our associations residually confounded by PMH status.
In summary, we observed a positive association between current digoxin use and the incidence of breast cancer, which appeared somewhat stronger for ER-positive disease. Breast cancer risk in former users was similar to that in never users. Our meta-analysis showed strong consistency among published studies of this association. Future studies should evaluate associations according to digoxin therapy duration with greater precision, and focus on maximizing the number of ER-negative cases studied. Mechanistic studies explaining a positive effect of digoxin on breast carcinogenesis are also sparse, and would strengthen the evidence for a causal association.
Acknowledgments
This work was supported by P01 CA 87969, and T32 CA 009001. We would like to thank the participants and staff of the NHS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. In addition, this study was approved by the Connecticut Department of Public Health (DPH) Human Investigations Committee. Certain data used in this publication were obtained from the DPH. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
Conflict of interest:
No author declared a potential conflict of interest.
References
- 1.Harrison T, Fauci A. Harrison’s principles of internal medicine. 1998:1264–1295. [Google Scholar]
- 2.Stenkvist B, Bengtsson E, Eriksson O, et al. Cardiac glycosides and breast cancer. Lancet. 1979;1(8115):563. doi: 10.1016/s0140-6736(79)90996-6. [DOI] [PubMed] [Google Scholar]
- 3.Stenkvist B. Is digitalis a therapy for breast carcinoma? Oncology Reports. 1999;6(3):493–496. [PubMed] [Google Scholar]
- 4.Cove D. Digoxin and hormone receptors. Lancet. 1979;314(8135):204. doi: 10.1016/s0140-6736(79)91475-2. [DOI] [PubMed] [Google Scholar]
- 5.Rifka S, Pita J, Vigersky R, et al. Interaction of digitalis and spironolactone with human sex steroid receptors. The Journal of Clinical Endocrinology and Metabolism. 1978;46(2):338–344. doi: 10.1210/jcem-46-2-338. [DOI] [PubMed] [Google Scholar]
- 6.Prassas I, Diamandis E. Novel therapeutic applications of cardiac glycosides. Nature Reviews Drug Discovery. 2008;7(11):926–935. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- 7.Newman R, Yang P, Pawlus A, et al. Cardiac glycosides as novel cancer therapeutic agents. Molecular Interventions. 2008;8(1):36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Aizman O, Aperia A. Na,K-ATPase as a signal transducer. Ann N Y Acad Sci. 2003;986:489–496. doi: 10.1111/j.1749-6632.2003.tb07233.x. [DOI] [PubMed] [Google Scholar]
- 9.Nesher M, Shpolansky U, Rosen H, et al. The digitalis-like steroid hormones: New mechanisms of action and biological significance. Life Sciences. 2007;80(23):2093–2107. doi: 10.1016/j.lfs.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Riganti C, Campia I, Kopecka J, et al. Pleiotropic effects of cardioactive glycosides. Current Medicinal Chemistry. 2011;18(6):872–885. doi: 10.2174/092986711794927685. [DOI] [PubMed] [Google Scholar]
- 11.Chen J-Q, Contreras R, Wang R, et al. Sodium/potasium ATPase (Na+, K+-ATPase) and ouabain/related cardiac glycosides: a new paradigm for development of anti- breast cancer drugs? Breast Cancer Research and Treatment. 2005;96(1):1–15. doi: 10.1007/s10549-005-9053-3. [DOI] [PubMed] [Google Scholar]
- 12.Kometiani P, Liu L, Askari A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Molecular Pharmacology. 2005;67(3):929–936. doi: 10.1124/mol.104.007302. [DOI] [PubMed] [Google Scholar]
- 13.Winnicka K, Bielawski K, Bielawska A. Cardiac glycosides in cancer research and cancer therapy. Acta Poloniae Pharmaceutica. 2006;63(2):109–115. [PubMed] [Google Scholar]
- 14.Falconer I, Beresford A, Jones A, et al. Effect of digoxin on DNA synthesis and cell viability in human breast tumour tissue in organ culture. Chemotherapy. 1983;29(5):368–372. doi: 10.1159/000238221. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Qian D, Tan Y, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proceedings of the National Academy of Sciences. 2008;105(50):19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Zheng M, Li Z, et al. Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Research. 2009;69(16):6556–6564. doi: 10.1158/0008-5472.CAN-09-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman G. Digitalis and breast cancer. Lancet. 1984;2(8407):875. doi: 10.1016/s0140-6736(84)90915-2. [DOI] [PubMed] [Google Scholar]
- 18.Haux J, Klepp O, Spigset O, et al. Digitoxin medication and cancer; case control and internal dose-response studies. BMC Cancer. 2001;1(1):11. doi: 10.1186/1471-2407-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenkvist B, Bengtsson E, Eklund G, et al. Evidence of a modifying influence of heart glucosides on the development of breast cancer. Analytical and Quantitative Cytology. 1980;2(1):49–54. [PubMed] [Google Scholar]
- 20.Ahern T, Lash T, Sørensen H, et al. Digoxin treatment is associated with an increased incidence of breast cancer: a population-based case-control study. Breast Cancer Research. 2008;10(6):R102. doi: 10.1186/bcr2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biggar R, Wohlfahrt J, Oudin A, et al. Digoxin use and the risk of breast cancer in women. Journal of Clinical Oncology. 2011;29(16):2165–2170. doi: 10.1200/JCO.2010.32.8146. [DOI] [PubMed] [Google Scholar]
- 22.Collins L, Marotti J, Baer H, et al. Comparison of estrogen receptor results from pathology reports with results from central laboratory testing. Journal of the National Cancer Institute. 2008;100(3):218–221. doi: 10.1093/jnci/djm270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo J, Moral R, Balogh G, et al. The protective role of pregnancy in breast cancer. Breast Cancer Research. 2005;7(3):131–142. doi: 10.1186/bcr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuzick J. Hormone replacement therapy and the risk of breast cancer. European Journal of Cancer. 2008;44(16):2344–2349. doi: 10.1016/j.ejca.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 25.Cook N, Rosner B, Hankinson S, et al. Mammographic screening and risk factors for breast cancer. American Journal of Epidemiology. 2009;170(11):1422–1432. doi: 10.1093/aje/kwp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terry M, Gammon M, Zhang F, et al. Association of Frequency and Duration of Aspirin Use and Hormone Receptor Status With Breast Cancer Risk. Journal of the American Medical Association. 2004;291(20):2433–2440. doi: 10.1001/jama.291.20.2433. [DOI] [PubMed] [Google Scholar]
- 27.Cuzick J, Warwick J, Pinney E, et al. Tamoxifen-Induced Reduction in Mammographic Density and Breast Cancer Risk Reduction: A Nested Case-Control Study. Journal of the National Cancer Institute. 2011 doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 28.Moysich K, Beehler G, Zirpoli G, et al. Use of common medications and breast cancer risk. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(7):1564–1595. doi: 10.1158/1055-9965.EPI-07-2828. [DOI] [PubMed] [Google Scholar]
- 29.Tria Tirona M. Breast cancer screening update. American Family Physician. 2013;87(4):274–278. [PubMed] [Google Scholar]
- 30.Hjartaker A, Langseth H, Weiderpass E. Obesity and diabetes epidemics: cancer repercussions. Advances in Experimental Medicine and Biology. 2008;630:72–93. doi: 10.1007/978-0-387-78818-0_6. [DOI] [PubMed] [Google Scholar]
- 31.Zalesin K, Franklin B, Miller W, et al. Impact of obesity on cardiovascular disease. Endocrinology and Metabolism Clinics of North America. 2008;37(3):663–84. ix. doi: 10.1016/j.ecl.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Rosner B, Hankinson S, et al. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. Journal of the American Medical Association. 2011;306(17):1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takkouche B, Cadarso-Suarez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. American Journal of Epidemiology. 1999;150(2):206–15. doi: 10.1093/oxfordjournals.aje.a009981. [DOI] [PubMed] [Google Scholar]
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan K, Foster D. Use of the confidence interval function. Epidemiology. 1990;1(1):39–42. doi: 10.1097/00001648-199001000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Greenland S, Schwartzbaum J, Finkle W. Problems due to small samples and sparse data in conditional logistic regression analysis. American Journal of Epidemiology. 2000;151(5):531–539. doi: 10.1093/oxfordjournals.aje.a010240. [DOI] [PubMed] [Google Scholar]
- 37.Biggar R. Molecular pathways: digoxin use and estrogen-sensitive cancers--risks and possible therapeutic implications. Clinical Cancer Research. 2012;18(8):2133–2137. doi: 10.1158/1078-0432.CCR-11-1389. [DOI] [PubMed] [Google Scholar]
- 38.Beral V Million Women Study C. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 39.Colditz G, Hankinson S, Hunter D, et al. The Use of Estrogens and Progestins and the Risk of Breast Cancer in Postmenopausal Women. New England Journal of Medicine. 1995;332(24):1589–1593. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 40.Braunstein G. Gynecomastia. New England Journal of Medicine. 1993;328(7):490–495. doi: 10.1056/NEJM199302183280708. [DOI] [PubMed] [Google Scholar]
- 41.Lewinn E. Gynecomastia during digitalis therapy; report of eight additional cases with liver-function studies. New England Journal of Medicine. 1953;248(8):316–320. doi: 10.1056/NEJM195302192480802. [DOI] [PubMed] [Google Scholar]
- 42.Ewertz M, Holmberg L, Tretli S, et al. Risk factors for male breast cancer--a case-control study from Scandinavia. Acta Oncologica. 2001;40(4):467–471. doi: 10.1080/028418601750288181. [DOI] [PubMed] [Google Scholar]
- 43.Lenfant-Pejovic M, Mlika-Cabanne N, Bouchardy C, et al. Risk factors for male breast cancer: a Franco-Swiss case-control study. International Journal of Cancer. 1990;45(4):661–665. doi: 10.1002/ijc.2910450415. [DOI] [PubMed] [Google Scholar]
- 44.Platz E, Yegnasubramanian S, Liu J, et al. A novel two-stage, transdisciplinary study identifies digoxin as a possible drug for prostate cancer treatment. Cancer Discovery. 2011;1(1):68–77. doi: 10.1158/2159-8274.CD-10-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh W. The evolving role of estrogen therapy in prostate cancer. Clinical Prostate Cancer. 2002;1(2):81–89. doi: 10.3816/cgc.2002.n.009. [DOI] [PubMed] [Google Scholar]
- 46.Weiss J, Moysich K, Swede H. Epidemiology of male breast cancer. Cancer Epidemiology, Biomarkers & Prevention. 2005;14(1):20–26. [PubMed] [Google Scholar]
- 47.Biggar R, Wohlfahrt J, Melbye M. Digoxin use and the risk of cancers of the corpus uteri, ovary and cervix. International Journal of Cancer. 2012;131(3):716–721. doi: 10.1002/ijc.26424. [DOI] [PubMed] [Google Scholar]
- 48.Tian J, Li X, Liang M, et al. Changes in sodium pump expression dictate the effects of ouabain on cell growth. Journal of Biological Chemistry. 2009;284(22):14921–14929. doi: 10.1074/jbc.M808355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Contreras R, Wang R, et al. Sodium/potassium ATPase (Na+, K+-ATPase) and ouabain/related cardiac glycosides: A new paradigm for development of anti- breast cancer drugs? Breast Cancer Research and Treatment. 2006;96(1):1–15. doi: 10.1007/s10549-005-9053-3. [DOI] [PubMed] [Google Scholar]
- 50.Ritte R, Lukanova A, Berrino F, et al. Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: a large prospective cohort study. Breast Cancer Research. 2012;14(3):R76. doi: 10.1186/bcr3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenland S, Finkle W. A critical look at methods for handling missing covariates in epidemiologic regression analyses. American Journal of Epidemiology. 1995;142(12):1255–1264. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]