SUMMARY
Many psychiatric and neurological disorders are characterized by learning and memory deficits, for which cognitive enhancement is considered a valid treatment strategy. The N-methyl-D-aspartate receptor (NMDAR) is a prime target for the development of cognitive enhancers due to its fundamental role in learning and memory. In particular, the NMDAR subunit NR2B improves synaptic plasticity and memory when over-expressed in neurons. However, NR2B regulation is not well understood and no therapies potentiating NMDAR function have been developed. Here, we show that serine 1116 of NR2B is phosphorylated by cyclin-dependent kinase 5 (Cdk5). Cdk5-dependent NR2B phosphorylation is regulated by neuronal activity and controls the receptor’s cell surface expression. Disrupting NR2B-Cdk5 interaction using a small interfering peptide (siP) increases NR2B surface levels, facilitates synaptic transmission, and improves memory formation in vivo. Our results reveal a novel regulatory mechanism critical to NR2B function that can be targeted for the development of cognitive enhancers.
INTRODUCTION
N-methyl-D-aspartate receptors (NMDARs) are ionotropic glutamate receptors that exhibit broad expression within the nervous system and are critically involved in neuronal processes such as synaptic plasticity and learning & memory (Bliss and Collingridge, 1993; Martin et al., 2000). Functional NMDARs are obligate heterotetrameric complexes formed by two glycine-binding GluN1 subunits assembled with two of several isoforms of the glutamate-binding GluN2 (A, B, C or D subtypes) or glycine-binding GluN3 (A or B subtypes) (Cull-Candy and Leszkiewicz, 2004; Dingledine et al., 1999). The biophysical properties of NMDARs are dependent on their subunit composition (Morrisett, 1997). Indeed, NMDAR subunit composition varies greatly between different synapses and neurons, as well as during neuronal development (Monyer et al., 1994; Sheng et al., 1994). Therefore, precise spatio-temporal regulation of NMDAR subunit expression, composition, trafficking and localization is critical for proper neuronal function.
Brain regions involved in mnemonic functions rely predominantly upon GluN2A (NR2A) and GluN2B (NR2B) subunit-containing NMDARs. During postnatal development, NR2B expression steadily decreases, while NR2A levels rise. Compared to NR2A-containing NMDARs, receptors that include NR2B inactivate more slowly and, consequently, have been associated with increased levels of synaptic plasticity (Cull-Candy and Leszkiewicz, 2004; Lau and Zukin, 2007). Consistently, up-regulation of NR2B expression in mice improves synaptic plasticity and memory formation (Crair and Malenka, 1995; Tang et al., 1999). Numerous animal models that feature elevated NR2B levels via altered synthesis, transport, or degradation exhibit improved synaptic plasticity and memory (Lee and Silva, 2009). Hence, targeting NR2B and its regulatory machinery has been singled out as an attractive approach for cognitive enhancement (Bibb et al., 2010; Collingridge et al., 2013).
One possible strategy to increase NR2B levels involves induction of its de novo synthesis, for example, via cAMP response element (CRE)-mediated gene expression or chromatin remodeling (Fujita et al., 2012; Jiang et al., 2010; Myers et al., 1999). Alternative targets include transport or degradation of NR2B via its coupling to distinct intracellular signaling pathways (Hawasli et al., 2007; Yin et al., 2011). The molecular machinery regulating trafficking, sub-cellular localization and degradation of NMDAR subunits is not yet well understood, but it has been recognized that phosphorylation of NMDAR subunits including NR2B is important for the regulation of such processes (Chen and Roche, 2007; Ma and Jan, 2002; Wenthold et al., 2003). One protein kinase implicated in the metabolism of NR2B is Cdk5 (Cyclin-dependent kinase 5), albeit no direct phosphorylation of NR2B by Cdk5 has been reported (Hawasli et al., 2007). Cdk5 is a proline-directed serine/threonine kinase that is activated upon interaction with the neuron-specific co-factors p35 or p39 (Dhavan and Tsai, 2001). Cdk5 has been implicated in numerous CNS processes, including cortical layer formation, neurotransmission, and mnemonic functions (Angelo et al., 2006). Accordingly, a previous study showed that Cdk5 conditional knockout (cKO) mice have improved synaptic plasticity and learning and memory via regulation of NR2B degradation by calpain (Hawasli et al., 2007). Interestingly, transgenic mice overexpressing the truncated Cdk5 activator p25 also exhibit enhanced plasticity and memory formation (Angelo et al., 2006; Angelo et al., 2003; Fischer et al., 2005), suggesting that reduced Cdk5 expression or displacement from physiological substrates potentiates synaptic remodeling processes. Despite these results indicating a close mechanistic relationship between NR2B and Cdk5 in the control of mnemonic functions, the underlying molecular processes remain unknown.
Here we show that Cdk5 directly phosphorylates NR2B at Ser1116 and that the phosphorylation state of this serine residue is specifically regulated by physiological neuronal activity. Cdk5-dependent Ser1116 phosphorylation prevents cell surface expression of NR2B-containing NMDAR and thus attenuates synaptic transmission. Disrupting the Cdk5-mediated regulation of NR2B using small interfering peptides (siPs) to block protein-protein interactions reduces this phosphorylation, increases NR2B surface levels, facilitates neurotransmission, and improves hippocampus-dependent learning and memory, suggesting that this novel regulatory mechanism may indeed be a suitable target for the development of cognitive enhancers.
RESULTS
Cdk5 phosphorylates NR2B at Ser1116 and controls surface level expression
Cdk5 has been previously shown to contribute to synaptic plasticity and memory formation through its control of NR2B (GluR2B) levels via calpain-mediated degradation (Hawasli et al., 2007). As Cdk5 directly associates with this receptor, we investigated whether it could phosphorylate NR2B. To identify novel Cdk5 phosphorylation sites, we employed a PhosphoScan approach utilizing antibody-based enrichment of phospho-peptides from mouse brain with a phospho-specific Cdk5 substrate antibody followed by tandem mass spectrometry (Rush et al., 2005). This approach identified Ser1116 within the cytoplasmic carboxy-terminal of NR2B as a novel Cdk5 phosphorylation site (Figure 1A and B). To monitor physiological changes in Ser1116 NR2B phosphorylation, a phosphorylation state-specific antibody was generated. The phospho-Ser1116 (P-S1116) NR2B antibody detected time-dependent in vitro phosphorylation of recombinant NR2B at Ser1116 by Cdk5 (Figure 1C). Analysis of various mouse brain regions, namely cortex, hippocampus, cerebellum and striatum, further revealed that NR2B is phosphorylated at Ser1116 in vivo (Figure S1A and B), indicating that this NR2B phosphorylation functions broadly throughout the brain. Treatment of acute mouse hippocampal slices with the specific Cdk5 inhibitor CP681301 caused dose-dependent P-S1116 NR2B reduction (Figure 1D). Moreover, Cdk5 inhibitor-induced reduction of NR2B phosphorylation at Ser1116 followed a similar time-course as the reduction of phospho-Thr75 DARPP-32, a well-established Cdk5 substrate (Bibb et al., 1999) (Figure S1C). Finally, P-S1116 NR2B levels were markedly reduced in hippocampal lysate of Cdk5 cKO mice (Hawasli et al., 2007) compared to wild type (WT) controls (Figure 1E). Together, these results demonstrate that Ser1116 of NR2B is a physiological Cdk5 phosphorylation site and raise the question of how this novel posttranslational modification might affect NR2B function.
Figure 1. Cdk5 phosphorylates the NR2B subunit of the NMDAR at Ser1116 and regulates its cell surface expression.
(A) Schematic of NR2B indicating domains and motifs. (B) Positive identification of serine residue 1116 of NR2B as a Cdk5 substrate by LC MS/MS analysis of phosphopeptides immunoprecipitated with phospho-Cdk substrate antibody. (C) Time-dependent Ser1116 NR2B phosphorylation (P-S1116) by Cdk5 in vitro detected by quantitative immunoblottng. Quantitated values were normalized to total NR2B levels. (D) Dose-dependent reduction of P-S1116 in hippocampal slices incubated with the Cdk5 inhibitor, CP681301 (n = 4; *p < 0.05, **p < 0.01, ***p < 0.001; ANOVA). (E) Quantitative immunoblot analysis of P-S1116 and total NR2B in hippocampal lysates from Cdk5 cKO and WT mice (n = 4). Loss of Cdk5 in cKO is also shown (bottom panel). (F) Inhibition of Cdk5 increases NR2B cell surface levels in hippocampal neurons. Top panels show immunofluorescence staining of cell surface GFP-NR2B (green) and post-synaptic, intra-neuronal Homer-1 (red) in hippocampal neurons treated with vehicle (control) or the Cdk5 inhibitor CP681301. Bottom panels depict high magnification of stained dendrites. (G) Quantification of cell surface GFP-NR2B fluorescence within dendritic processes (n = 22–26 cells). Data are represented as mean ± SEM. See also Figure S1.
NMDAR are assembled in the endoplasmic reticulum, trafficked along the secretory pathway and delivered to the post-synaptic plasma membrane (Lau and Zukin, 2007; Wenthold et al., 2003). The molecular machinery underlying NMDAR trafficking and sub-cellular localization is not yet well characterized, but it has been recognized that phosphorylation of NMDAR subunits including NR2B is important for the regulation of such processes (Chen and Roche, 2007; Ma and Jan, 2002; Wenthold et al., 2003). We noted that Ser1116 of NR2B occurs adjacent to a putative ER retention signal, RXR, (Ma and Jan, 2002) (i.e., NH2-…RRRPPRS*PDHK…COOH) and, therefore, investigated whether Cdk5-dependent phosphorylation of NR2B at Ser1116 affected its subcellular localization. The effect of Cdk5 inhibition on NR2B cell surface levels was assessed in cultured hippocampal neurons overexpressing NR2B tagged with extracellular N-terminal green-fluorescent protein (GFP-NR2B). Immunofluorescence staining using anti-GFP antibody thus enabled visualization of cell surface GFP-NR2B. Surface-stained GFP-NR2B exhibited a punctate pattern within dendritic processes (Figure 1F). Counter-labeling with Homer-1, a marker of post-synaptic densities (PSDs), demonstrated that the cell surface fraction of GFP-NR2B localized in proximity to PSDs. Treatment of cultured hippocampal neurons with the Cdk5 inhibitor CP681301 increased surface expression of NR2B in dendritic processes approximately 1.3-fold (Figure 1F and G) suggesting that NR2B phosphorylation by Cdk5 modulates NR2B surface levels.
Cdk5-dependent regulation of NR2B function
The abundance of postsynaptic NMDAR is a major determinant of synaptic plasticity and memory formation (Lee and Silva, 2009; Tang et al., 1999). Thus blocking Cdk5-dependent retention of NR2B from the cell surface could be important in regulating its contribution to NMDAR function. To assess this directly, mouse hippocampal brain slices were either treated with CP681301 or vehicle (Figure 2A). Cdk5 inhibition increased cell surface expression of NR2B 3.5-fold with concomitant reduction in P-S1116 levels to approximately 30% of untreated controls. The effect appeared specific for NR2B as no changes in surface levels of NR2A or the AMPA receptor subunit GluR1 were observed (Figure S2A and B).
Figure 2. NR2B phosphorylation at Ser1116 regulates NMDAR function.
(A) Increased cell surface NR2B correlates with reduced P-S1116 in hippocampal slices. Levels of cell surface-biotinylated NR2B pulled-down from lysates of hippocampal slices incubated in the absence (−) or presence (+) of the Cdk5 inhibitor CP681301 are shown with blots and quantitation (n = 4). Prior to pull-down, lysates were tested for NR2B Load and P-S1116 (bottom panel). (B) Effect of Cdk5 inhibition on the NR2B component of NMDAR-EPSC. Voltage-clamp EPSC recordings of total NMDAR-EPSC (INMDA) in the absence or presence of the specific NR2B inhibitor ifenprodil from pyramidal neurons within the hippocampal area CA1 pretreated with vehicle (control) or the Cdk5 inhibitor CP681301 are shown. (C and D) Quantitation of NMDAR EPSC recordings showing an increase of ifenprodil-sensitive NR2B component (C) and prolonged decay kinetics (D) of NMDAR EPSC in cells incubated in the absence (−) or presence (+) of CP681301 (n = 6–7). Data are represented as mean ± SEM. See also Figure S2A and B.
To evaluate the impact of Cdk5-dependent regulation of NR2B surface levels on physiological function, whole-cell voltage-clamp recording of synaptically evoked NMDAR-mediated excitatory postsynaptic currents (EPSC) were conducted in CA1 pyramidal neurons of hippocampal slices treated with the Cdk5 inhibitor CP681301 (Figure 2B–D). Administration of CP681301 increased the NMDAR-EPSC amplitude by approximately 1.5-fold. Furthermore, application of the NR2B-specific inhibitor, ifenprodil, had a greater attenuating effect on NMDAR-EPSC in CP681301-treated slices (33% reduction) in comparison to controls (20% reduction) (Figure 2C), suggesting that the NR2B component of NMDAR-EPSC was increased by Cdk5 inhibition. The deactivation kinetics of NMDAR EPSC was increased in CP681301-treated slices (170.7 ± 19.4 ms) as compared to untreated slices (112.1 ± 10.5 ms) (Figure 2D). This result is consistent with increased NR2B function in response to CP681301 treatment, because NR2B-containing NMDAR inactivate more slowly than receptors composed of other subunits (Cull-Candy and Leszkiewicz, 2004).
Together, these results indicate that Cdk5 regulates the subcellular localization of NMDAR via NR2B phosphorylation at Ser1116, thereby controlling the level of functional NR2B-containing NMDAR within the synaptic membrane and modulating NMDAR-mediated synaptic currents.
Regulation of Phospho-Ser1116 NR2B by glutamatergic neurotransmission, synaptic plasticity and memory formation
NMDAR are central to glutamatergic neurotransmission (Bliss and Collingridge, 1993) and are controlled by complex molecular machinery integrating various postsynaptic signaling cascades (Husi et al., 2000; Newpher and Ehlers, 2008). To better understand how Cdk5-dependent NR2B phosphorylation might be regulated and contribute to NR2B function in plasticity and memory formation, the effect of glutamatergic neurotransmission on P-S1116 NR2B was assessed ex vivo and in vivo. Treatment of acute mouse hippocampus slices with NMDA dose-dependently attenuated P-S1116, when normalized to total NR2B levels (Figure 3A). NMDA treatment also reduced total NR2B levels, consistent with previous reports of NMDA-induced, calpain-mediated NR2B degradation (Guttmann et al., 2001; Hawasli et al., 2007). The ability of NMDA to reduce P-S1116 in hippocampal slices was attenuated by treatment with the PP2A/PP1 inhibitor okadaic acid (Figure S2C), indicating that some of the NMDAR-induced effect on P-S1116 is mediated via serine/threonine phosphatase activity.
Figure 3. Phosphorylation of Ser1116 NR2B is modulated by glutamatergic neurotransmission, synaptic plasticity, and memory formation.
(A) Immunoblots of lysates from NMDA-treated mouse hippocampal slices probed for P-S1116 and total NR2B (n = 4; *p < 0.05; ANOVA). (B) Induction of in vivo CA1 LTP in mouse. Sample traces of 5 min periods pre- (a) and post- (b) high frequency stimulation (HFS; two trains of 50 pulses at 100 Hz; 30 s inter-train interval) and LTP induction are shown. (C) Quantitative immunoblotting of CA1-specific lysates from non-induced (−) and LTP-induced (+) mice for P-S1116 and total NR2B (n = 5). (D) Induction of CA1 LTD in mouse hippocampal slices with sample traces of 5 min periods pre- (a) and post- (b) low frequency stimulation (LFS; 900 stimuli at 1 Hz). (E) Quantitative immunoblotting of non-induced (−) and LTD-induced (+) CA1-specific lysates probed for P-S1116 and total NR2B (n = 5). (F) Quantitative immunoblots of CA1-specific lysates from contextual fear conditioned (FC) and control (Context and Foot Shock) mice obtained 1 h post-training for P-S1116 and total NR2B levels (n = 4–6). Data are represented as mean ± SEM. See also Figure S2C.
In agreement with the attenuating effect of NMDA on P-S1116, induction of in vivo long-term potentiation (LTP) in the hippocampal area CA1 also reduced P-S1116. Tetanic stimulation of mouse Schaffer commissural-CA1 pyramidal cell synapses in vivo caused a significant increase in fEPSP slope (1.4-fold as compared to baseline) that was maintained for 1 h post-stimulus (Figure 3B). One hour after LTP induction, P-S1116 was reduced to approximately 40% of the level in control CA1 lysates (Figure 3C), demonstrating the physiological regulation of P-S1116 in synaptic plasticity. In contrast, induction of long-term depression (LTD) did not affect P-S1116 levels (Figure 3D and E). As excitatory activation of hippocampal NMDAR is integral to learning and memory (Nakazawa et al., 2004), we also assessed the involvement of Cdk5-dependent NR2B phosphorylation in mnemonic processes by evaluating P-S1116 levels following contextual fear conditioning. One hour post-training, P-S1116 was reduced in CA1 lysates of fear-conditioned mice to 80% of the levels in non-shocked context-exposed controls (Figure 3F). Foot shock in the absence of context had no effect on P-S1116. Taken together, these results show that NR2B phosphorylation at Ser1116 is regulated by glutamatergic neurotransmission, synaptic plasticity, and learning and memory, suggesting an important role for this signaling mechanism in cognition.
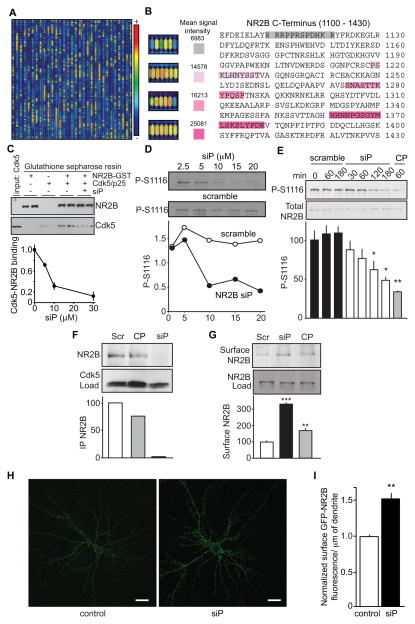
The protein-protein interaction between NR2B and Cdk5 can be selectively disrupted by small-interfering peptides
NR2B and Cdk5 form a complex both in vitro and in vivo (Hawasli et al., 2007). To identify physiological protein-protein interaction sites between NR2B and Cdk5, overlapping eight amino acid peptide cassettes of the NR2B carboxy-terminal domain were synthesized on a peptide microarray microchip (Gao et al., 2004) that was then probed for Cdk5 binding using recombinant Cdk5 followed by immunodetection with anti-Cdk5 antibody (Figure 4A). Four motifs exhibiting high Cdk5-binding were identified by this approach (Figure 4B). To determine if these motifs were actual NR2B-Cdk5 interaction sites, peptides corresponding to these motifs were synthesized and tested for their ability to interfere with NR2B-Cdk5 binding. In this screen, the peptide corresponding to amino acid residues 1111–1127 [RRPPRSPDHKRYFRDKE; NR2B small interfering peptide (NR2B siP)] most potently blocked the pull-down of Cdk5 by recombinant GST-tagged NR2B in vitro (Figure 4C). Moreover, the NR2B siP dose-dependently inhibited in vitro phosphorylation of Ser1116 NR2B by Cdk5 (Figure 4D). In contrast, a scrambled control peptide (RRRSYFHKEDRPPRDK) did not attenuate Ser1116 phosphorylation in vitro. Interestingly, the NR2B-siP did not inhibit the in vitro phosphorylation of inhibitor-1 (Figure S3A), a well-defined Cdk5 substrate (Nguyen et al., 2007), indicating that the inhibition of Ser1116 NR2B phosphorylation by NR2B siP was specific.
Figure 4. Identification of NR2B-Cdk5 interaction motifs and development of a small-interfering peptide (siP).
(A) Peptide micro-array of the NR2B C-terminal domain showing high (+, red) to low (−, blue) Cdk5 binding regions. (B) The cytoplasmic C-terminus amino acid sequence of NR2B with potential Cdk5 binding sites highlighted with their corresponding peptide array tiles. (C) NR2B siP blocks pull-down of Cdk5 with recombinant NR2B immobilized on GST beads, demonstrating disruption of NR2B-Cdk5 interaction in vitro. Immunoblots show relative levels of NR2B and Cdk5 under conditions indicated. NR2B siP dose-dependently disrupts NR2B-Cdk5 binding in vitro as depicted in plot (n = 3). (D) Immunoblots showing that NR2B siP dose-dependently inhibits in vitro phosphorylation of Ser1116 NR2B by Cdk5, whereas the control peptide (scramble) has no effect.
(E) Time-dependent reduction of P-S1116 in hippocampal slices treated with NR2B siP (25 μM) or the Cdk5 inhibitor CP681301 (CP; 50 μM), but not scrambled (Scr) control peptide (n = 4; *p < 0.05, **p < 0.01; ANOVA). (F) NR2B siP, but not the scrambled peptide, nor CP681301 (CP) disrupts NR2B-Cdk5 interaction in hippocampal slices (4 pooled slices for each sample) as evaluated by co-immunoprecipitation. Cdk5 Load is also shown. (G) NR2B cell surface levels are increased in hippocampal slices treated with NR2B siP (1 h, 100 μM) or the Cdk5 inhibitor CP681301 (1 h, 50 μM), but not with control peptide (Scramble; 1 h, 100 μM) (n = 4; **p < 0.01; ***p < 0.001; ANOVA). NR2B Load is also indicated. (H) Immunofluorescence staining of cultured hippocampal neurons for surface GFP-NR2B after treatment with control peptide or NR2B-siP. (I) Quantification of dendritic cell surface GFP-NR2B (n = 49–64 cells). Scale bar = 20 μm. Data are represented as mean ± SEM. See also Figure S3.
To further assess the efficacy and consequences of targeting Cdk5-NR2B protein interactions by small interfering peptides ex vivo and in vivo, cell-permeabilizing polyarginine or penetratin N-terminal tags were included in the NR2B siP and control peptides. These tags are commonly used to deliver peptides across the cell membrane into the cytosol of neurons (Joliot and Prochiantz, 2004).
Consistent with the effects observed in vitro, application of the NR2B siP to hippocampal slices decreased P-S1116 in a time- and dose-dependent manner (Figures 4E and S3B). Treatment with the NR2B siP did not alter levels of NR2B or Cdk5 expression (Figures 4E and S3B). The specificity of NR2B siP observed in vitro was retained in vivo, as it had no effect on the phosphorylation state of the well-characterized Cdk5 site Thr75 DARPP-32 (Bibb et al., 1999), but it time-dependently reduced P-S1116 in acute mouse striatal slices (Fig. S3C and D). These data show selective modulation of P-S1116 by siP, and also that this mechanism may be targeted in multiple limbic structures mediating learning and memory. Furthermore, NR2B siP treatment of hippocampal slices effectively blocked subsequent co-immunoprecipitation of Cdk5 and NR2B (Figure 4F) indicating that siP disrupts Cdk5-NR2B protein-protein interactions. In contrast, treatment with scrambled control or the Cdk5 inhibitor CP681301, did not affect Cdk5-NR2B interactions.
Consistent with our data that Cdk5 regulates NR2B cell surface expression, the siP induced a 3.3-fold increase in NR2B cell surface levels in hippocampal slices (Figure 4G). Finally, cell surface GFP-NR2B levels increased 1.5-fold following 30 min treatment with NR2B siP as compared to control peptide (S1116A) in primary hippocampal cultures (Figure 4H and I). Thus, Cdk5-NR2B protein-protein interactions can be targeted in intact brain tissue with NR2B siP, resulting in reduced Cdk5-dependent phosphorylation of Ser1116 NR2B and appreciable increases in cell surface expression of the receptor.
Disruption of NR2B-Cdk5 interaction by NR2B siP increases NR2B function and facilitates synaptic transmission
Given the efficacy of the NR2B siP in targeting Cdk5-NR2B interactions and increasing NR2B cell surface levels, its impact on NMDAR-mediated EPSC was assessed. Treatment of cortical slices with the NR2B siP produced a 1.8-fold increase in the NMDA/AMPA EPSC ratio as compared to a scrambled control peptide (Figure 5A). Moreover, slices incubated with NR2B siP exhibited a 1.5-fold increase in the ifenprodil-sensitive NR2B component of NMDAR EPSC (Figure 5B and C), suggesting that the NR2B siP-induced increase in NMDAR EPSC is due to recruitment of additional NR2B-containing NMDAR to the cell surface.
Figure 5. Disruption of NR2B-Cdk5 interaction by NR2B-siP facilitates synaptic transmission.
(A) Recordings and quantification of NMDAR/AMPAR EPSC ratio from cortical pyramidal neurons treated with NR2B siP or scrambled (Scr) control (n = 12–16). (B and C) NMDAR EPSC sensitivity to the NR2B inhibitor, ifenprodil, in slices incubated with NR2B siP or scramble control (n = 6–7). (D and E) Extracellular CA3-CA1 field recordings in hippocampal slices treated with NR2B siP or control peptide (30 min, 2 μM). Sample traces of 2 min before (a) and at end (b) of siP application and Quantitation of fEPSP slope (n = 4). (F and G) Extracellular field recordings in hippocampal slices incubated with the specific NR2B inhibitor Ro 25–6981 (3 μM) or from Cdk5 cKO were treated with NR2B siP (30 min, 2 μM) and quantification of fEPSP slope (n = 3) did not show a significant effect of NR2B siP with NR2B inhibition or in absence of Cdk5 (cKO). Data are represented as mean ± SEM.
Given the importance of NR2B in mediating the potentiation of synaptic transmission during plasticity, we assessed the effects of NR2B siP on extracellularly recorded field excitatory postsynaptic potentials (fEPSP) in the hippocampal CA3-CA1 pathway. Treatment of hippocampal slices with NR2B siP rapidly induced a 1.9-fold increase in fEPSP slope compared to slices infused with a control peptide (Figure 5D and E). Upon washout of the NR2B siP, fEPSP slopes progressively decreased until they reached steady-state levels with fEPSP slopes that were 1.6-fold greater than control treated slices.
To determine whether the NR2B siP-induced changes in hippocampal neurotransmission were due to NR2B, the effects of the NR2B siP on basal fEPSPs were assessed in slices co-incubated with the specific NR2B antagonist Ro25–6981 (Figure 5F and G). Under these conditions the NR2B siP-mediated increase was blocked. Cdk5 cKO mice lack approximately 70% of hippocampal Cdk5 and exhibit a reduced threshold for induction of long-term potentiation (LTP), but normal baselines for fEPSP fiber volley amplitude (Hawasli et al., 2007). Interestingly, the ability of the NR2B siP to raise CA1-CA3 fEPSPs was almost absent in Cdk5 cKO mice with a small (1.2-fold) but still significant increase in fEPSP slopes (Figure 5F and G). Thus the effect of the peptide on synaptic transmission is mediated via NR2B and Cdk5. Together these results show that NR2B siP selectively disrupts NR2B-Cdk5 interaction, reduces P-S1116, increases cell surface levels of NR2B, and facilitates neurotransmission by enhancing NR2B function.
NR2B siP improves learning and memory
The data presented so far indicate that the subcellular localization of NR2B is regulated by Cdk5. Reduction in Cdk5 activity, as well as disruption of Cdk5-NR2B interactions consistently increased NR2B surface levels and facilitated NMDA-mediated synaptic function. As previous research has demonstrated that elevated NR2B function enhances cognitive performance (Lee and Silva, 2009; Tang et al., 1999), we investigated whether administration of NR2B siP can improve mnemonic function. For this purpose, we first established robust in vivo protocols to deliver cell-penetrating NR2B siP to the appropriate brain regions and test their effect on learning and memory. NMDAR are required for hippocampus-dependent learning and memory, such as acquisition of contextual fear memory (Barkus et al., 2010; Bast et al., 2003). Accordingly, infusion of the selective NR2B inhibitors Ro25–6981 or ifenprodil into dorsal hippocampus was previously found to impair contextual fear learning, but not locomotor or anxiety-related behavior (Zhang et al., 2008). Hence, NR2B siP was infused bilaterally into the CA1 subfield of dorsal hippocampus continuously for 72 h using mini-osmotic pumps. Due to the size of the osmotic pump and the robust behavioral performance these initial experiments were performed in rat. Spatio-temporal tracking of fluorescein-tagged NR2B siP (FITC siP) confirmed correct targeting, continuity of the delivery, and neuronal uptake of the peptide. FITC siP was specifically detected in dorsal hippocampus at 12, 24, and 72 h after initiation of pumping. The FITC siP was present in hippocampal area CA1 and labeled the cytoplasm of pyramidal neurons (Figure 6A). After pumping was stopped, intracellular FITC siP signal was markedly reduced by 6 h and had largely disappeared by 24 h (Figure S4A).
Figure 6. Enhancement of fear memory by NR2B siP.
(A) Immunostain of rat dorsal hippocampus infused for 24 h with FITC NR2B siP (green) and counterstained with MAP2 (red). Inset shows FITC-positive CA1 pyramidal neurons. Scale bars = 250 μm and 50 μm (inset). (B and C) Immunoblots showing infused NR2B siP vs. scramble control reduces P-S1116 (n = 4) (B) and increases NR2B cell surface expression (4 pooled tissue punches for each condition) (C) in epifluorescent hippocampal area CA1. (D) Experimental design and quantitation of context- and tone-elicited fear memory in two-month-old rats chronically infused with scrambled or NR2B siP into dorsal hippocampus (n = 20) showing that NR2B siP improved contextual fear memory. (E) Experimental design and quantitation of fear memory in acutely infused three-month-old mice. Ninety minutes prior to training mice were acutely infused with NR2B siP or control peptide into dorsal hippocampus. Twenty-four hours later, NR2B siP treated mice exhibited improved context freezing, but not freezing to tone (n = 6–10). Data are represented as mean ± SEM. See also Figure S4 and S5.
(F) Model of NR2B regulation via Ser1116 phosphorylation by Cdk5. At basal state, Cdk5 constitutively phosphorylates NR2B at Ser1116, withholding it from the cell surface. Glutamatergic neurotransmission reduces Cdk5-dependent NR2B phosphorylation enabling translocation of NR2B to the cell surface and thereby contributing to memory formation. Addition of NR2B siP disrupts NR2B-Cdk5 interactions, reduces Ser1116 phosphorylation, and more NR2B translocates to the cell surface, thereby enhancing memory formation.
Within the FITC siP-infused region, P-S1116 was reduced by approximately 40% compared to FITC-scrambled peptide infused controls (Figure 6B). Infusion of FITC siP also caused a 3-fold increase in NR2B surface expression (Figure 6C). Together, these results validate this approach for the delivery of NR2B siP into pyramidal neurons of dorsal hippocampus and show that the NR2B siP induces biochemical and cellular changes in vivo comparable to our in vitro and ex vivo observations.
With these parameters established, the effect of NR2B siP infusion on fear memory was evaluated. Continuous bilateral NR2B siP infusion into rat dorsal hippocampus 24 h prior to fear conditioning increased hippocampus-dependent contextual fear memory 1.7-fold compared to scrambled peptide controls (Figure 6D). In agreement with the hippocampal-independent nature of cue-associated fear learning (Phillips and LeDoux, 1992), no effect was observed on tone-induced freezing. Administration of neither NR2B siP, nor scrambled control affected nociception, pain threshold, or motor reflexes (Figure S4B and C). During extinction trials, contextual memory enhancement persisted in NR2B siP-treated rats at 48 and 72 h after initial training (Figure S4D and E). The conditioned fear response was extinguished by 96 h for both groups (Figure S4E). Following extinction and expiration of the mini-osmotic pump, rats were re-trained. Twenty-four hours after re-training, the contextual fear memory was increased in rats previously infused with NR2B siP during the initial acquisition phase, but not scrambled controls (Figure S4F) suggesting that behavioral effects persist even after NR2B siP clearance. These data also indicate that the siP-improved memory may have been more retained or consolidated.
The learning and memory improvements associated with NR2B siP infusion correlated directly with reduced P-S1116 NR2B. NR2B siP infusion and fear conditioning, both decreased P-S1116 NR2B in CA1 one hour post-training (Figure S5A). Animals that had been infused with NR2B siP and undergone fear conditioning exhibited the lowest phosho-Ser1116 levels.
Previous studies found that NMDAR inhibition during the acquisition phase impaired fear memory (Bast et al., 2003; Zhang et al., 2008; Zhao et al., 2005). Therefore, we evaluated whether an acute NR2B siP infusion shortly before the training session could also improve fear memory. For this purpose, 3 month-old mice were acutely surgerized under isofluorane anesthesia and NR2B siP was infused into dorsal hippocampus 90 min prior to fear conditioning. Acute NR2B siP infusion resulted in significantly elevated freezing behavior in the context test as compared to controls (Figure 6E). The freezing levels during the context test of the acute paradigm were, in fact, comparable to the levels observed with chronic infusion. The tone test did not reveal any significant changes between acute peptide-infused groups. Finally, to evaluate whether NR2B siP administration may be a valid treatment for age-related cognitive decline, 14 month-old mice were acutely infused with NR2B siP and tested for fear memory (Figure S5B). As observed for 3 month-old mice, acute NR2B siP infusion 90 min prior to training increased contextual fear memory 1.7-fold compared to control peptide or non-infused cannulated controls. Thus, increasing NR2B surface levels may be a suitable memory enhancement strategy even for later stages in life. These results are also consistent with studies showing that NR2B over-expression in forebrain neurons improves learning and memory in aged mice (Brim et al., 2013; Cao et al., 2007).
Taken together, these findings show that selective disruption of NR2B-Cdk5 interactions via NR2B siP enhances hippocampus-dependent learning and memory through potentiation of a regulatory mechanism that is involved in memory formation. Thus, it is possible that this novel mechanism regulating NR2B function via Cdk5 may be a suitable target for the development of memory enhancers as therapeutics for cognitive impairment as well as age-dependent cognitive decline.
DISCUSSION
In this study, we present a novel molecular mechanism regulating the subcellular localization of NR2B-containing NMDAR. This mechanism appears to be central to synaptic transmission and mnemonic functions and can be targeted to enhance memory. A model of this mechanism and the effects of targeting it may be suggested (Figure 6F). Upon glutamatergic neurotransmission, such as that occurring during synaptic plasticity induction or memory formation, Cdk5-dependent Ser1116 phosphorylation is reduced and subsequently leads to increased cell surface levels of NR2B-containing NMDAR. Consequently, selective disruption of NR2B-Cdk5 interactions via the NR2B siP increases NR2B surface levels, thereby facilitating synaptic transmission. Intra-hippocampal infusion of the NR2B siP improved fear memory suggesting that the regulation of NR2B by Cdk5 may be a suitable target for the development of cognitive enhancers.
The molecular basis of cognition is thought to involve activity-dependent changes in intracellular signaling, gene expression, protein synthesis, remodeling of synapses and neurotransmission (Bibb et al., 2010). In particular, excitatory glutamatergic neurotransmission, as well as its regulatory machinery and downstream targets have been singled out as central molecular mechanisms. Signaling cascades that modulate expression, activity, trafficking, and degradation of neurotransmitter receptors are critical to the formation of memories (Chen and Roche, 2007; Lau and Zukin, 2007; Wenthold et al., 2003). Accordingly, enhanced synaptic plasticity, learning and memory has been observed in several animal models that feature increased NR2B surface levels as a result of manipulating various molecular processes including NR2B expression, trafficking, and degradation (Crair and Malenka, 1995; Hawasli et al., 2007; Lee and Silva, 2009; Tang et al., 1999; Wong et al., 2002; Yin et al., 2011). Thus a robust body of data singles out NR2B as a prominent factor for cognitive function.
NR2B has been shown to serve as a substrate of various protein kinases (Wenthold et al., 2003). In particular, tyrosine phosphorylation of its extreme C-terminus modulates its anchoring to the postsynaptic density and may prevent endocytosis. Furthermore, NR2B has been suggested to traffic dynamically from extra- to intra-synaptic locations on the cell surface, possibly through association of its extreme C-terminus with different members of the membrane-associated guanylate kinase (MAGUK) family. In contrast, Ser1116 appears to reside within a region of the cytoplasmic terminus that interacts with early components of receptor trafficking in the postsynaptic endoplasmic reticulum (Vandenberghe and Bredt, 2004).
Previously Cdk5 cKO was shown to increase total NR2B levels by disrupting the NR2B-Cdk5-calpain complex, thereby attenuating activity-dependent degradation of NR2B by calpain (Hawasli et al., 2007). Here, pharmacological inhibition of Cdk5 revealed that the protein kinase also phosphorylates NR2B, thereby modulating the activity-dependent trafficking of the receptor to the cell surface. Thus Cdk5 appears to have two roles, regulating NR2B trafficking as well as metabolism. Both these roles are likely important for synaptic plasticity and memory, although the temporal sequence and molecular integration of these two activity-dependent events requires further study.
It also remains to be determined if dephosphorylation of Ser1116 targets NR2B selectively to the synapse or to both synaptic and extrasynaptic compartments. As the increases in cell surface NR2B observed here were greater than the potentiation of NR2B-mediated current, it is possible that not all NR2B driven to the surface was functional. Interestingly, other NMDAR subunits possess endoplasmic reticulum retention motifs similar to the one indicated here in NR2B. Furthermore, some of these are also regulated via phosphorylation (Scott et al., 2001) and in response to neuronal activity (Mu et al., 2003), suggesting a common overall process in synaptic remodeling or homeostasis.
Numerous neurodevelopmental, neurological, and neuropsychiatric disorders, such as Alzheimer’s disease, depression, and schizophrenia manifest cognitive deficits. Interestingly, deficiencies in NMDAR trafficking have been implicated in the pathophysiology of many of these diseases [e.g., see (Snyder et al., 2005)]. Moreover, age-related cognitive decline occurs progressively in all aging humans, albeit at variable rates. While behavioral interventions are being advanced as one group of approaches, effective treatments to counter cognitive deficits or memory decline are extremely limited.
Peptides are now an integral component of CNS research, and protein-protein interactions and intracellular signaling mechanisms provide a very large base of new therapeutic targets (Jubb et al., 2012). Until recently, CNS drug discovery has focused on developing synthetic small molecules that act primarily upon targets involved in neurotransmission including receptors and ion channels. Drug target pool algorithms such as the “rule of five” (Lipinski, 2000) and “the druggable genome” (Hopkins and Groom, 2002) have been used in an effort to facilitate the discovery of small molecules of high specificity for oral delivery. However, these restrictions have often had the unintended consequences of producing molecules with poor selectivity and unwanted side effects. Larger molecules including peptides and peptidomemetics that interfere with protein-protein interactions provide more contact with targets and greater selectivity but have been limited by low bioavailability, poor membrane permeability, and metabolic instability. These obstacles are being circumvented by a number of creative means and protein- or peptide-based drugs are now roughly 10% of the pharmaceutical market and growing (Craik et al., 2013). As an example of this potential, peptides that target NR2B interactions with the postsynaptic density have been shown to neuroprotect from ischemic injury in primates (Cook et al., 2012).
Here we demonstrate memory enhancement by selectively targeting a single phosphorylation site and NR2B-Cdk5 protein-protein interactions with a peptide injected intracranially. It will be interesting to find out, if further optimization in the targeting of this and other synaptic mechanisms will lead to more practical delivery modes and clinically applicable therapies.
EXPERIMENTAL PROCEDURES
Please see the Supplemental Experimental Procedures for detailed methods on Phosphorylation-site Identification, Phosphorylation State-specific Antibody Generation, In vitro Phosphorylation, Binding Assays, Peptide Array Analysis, In vivo LTP, Whole-cell Recordings, Hippocampal cannulation and siP infusion
Antibodies and Reagents
Cdk5 (C-8), and NR2A antibodies (Ab) were obtained from Santa Cruz; P-Thr75 DARPP-32, DARPP-32, and Inhibitor-1 Ab from Cell Signaling Technology; EGFP, FITC and α-tubulin Ab from Abcam; NR2B Ab from PhosphoSolutions; Homer-1 Ab from Synaptic System; MAP-2 and NeuN Ab from Millipore; Secondary anti-mouse, anti-rabbit and anti-goat IgG Ab from Pierce; Cy3-conjugated anti-rabbit IgG secondary Ab from Jackson Immunoresearch; and Alexa647-conjugated anti-mouse IgG secondary Ab from Molecular Probes. P-Ser6 inhibitor-1 Ab was previously described (Nguyen et al., 2007). The specific Cdk5 inhibitor, CP681301, was provided by Pfizer Pharmaceutical. Ifenprodil and Ro 25–6981 were from Tocris. All peptides were synthesized by the UT Southwestern Protein Chemistry Technology Center utilizing the Perseptive Biosystems Pioneer and Applied Biosystems 433 synthesizers. Peptides were verified by mass spectrometry analysis and reversed-phase HPLC chromatography. The sequences of the small interfering peptides (siP) were NR2B siP, RRPPRSPDHKRYFRDKE; scrambled, RRRSYFHKEDRPPRDK; R7 NR2B siP, RRRRRRRRPPRSPDHKRYFRDKE; R7 scramble, RRRRRRRRRSYFHKEDRPPRDK; Pen NR2B siP, RQIKIWFQNRRMKWKK-PPRSPDHKRYFRDKE and Pen control, RQIKIWFQNRRMKWKK-PPRAPDHKRYFRDKE. The FITC tag was attached at the C-terminus of the siP.
Animals
Animals were housed in a 12 h light/dark cycle (lights on from 6:00 a.m. to 6:00 p.m.), with food and water available ad libitum. Mice in the C57/BL6 background were obtained from Jackson and were housed 3–4 per cage. Cdk5 cKO mice were bred and maintained as previously specified (Hawasli et al., 2007). For all experiments, aged-matched male littermates were used, unless otherwise noted. Sprague-Dawley rats were obtained from Charles River Laboratories and bred and maintained at the UT Southwestern animal facility. All behavioral experiments were conducted in the UTSW Rodent Behavior Core. All experimental procedures were reviewed and approved by the University of Texas Southwestern Institutional Animal Care and Use Committee (IACUC).
Slice Pharmacology, Immunoprecipitation and Cell Surface Labeling
Hippocampal, striatal and cortical slices were prepared as previously described (Hawasli et al., 2007; Sahin et al., 2006; Yuen et al., 2012). Pharmacological treatments were performed in Mg2+-free Kreb’s buffer containing NMDA (50 or 100 μM, 15 min), CP-681301 (5–100 μM, 60 min) or siP (0–100 μM, 0–180 min). The R7-tagged peptides were used in slice experiments assessing substrate phosphorylation levels. Cell surface biotinylation assays were performed using Pierce® Cell Surface Protein Isolation Kit (Thermo Scientific) according to manufacturer’s instruction. In brief, hippocampal slices were equilibrated in ice-cold oxygenated ACSF for 5 min and then incubated for 30 min with Sulfo-NHS-SS-Biotin (0.5 mg/ml in ACSF; Pierce). Biotinylation reaction was quenched with ACSF containing 50mM NH4Cl and 50mM glycine and tissue dissected in ice-cold dissection buffer containing protease and phosphatase inhibitors (Plattner et al., 2006). Immunoprecipitation (IP) was performed as previously described (Plattner et al., 2006).
Lysate Preparation and Immunoblotting
Brain dissection, tissue removal and lysate preparation were performed with specific emphasis on maintaining protein integrity and phosphorylation levels by using protease and phosphatase inhibitors as described (Bibb et al., 1999; Plattner et al., 2006). Graphs of all quantitative immunoblot analyses of P-S1116 NR2B represent the ratio of P-S1116/Total NR2B. Hippocampal subfield CA1 tissue including stratum radiatum, stratum pyramidale and stratum oriens was microdissected in ice-cold dissection buffer containing protease and phosphatase inhibitors (Plattner et al., 2006) using a dissecting microscope. After excision of the intact hippocampus from the brain, the dentate gyrus and CA3 were removed longitudinally using the basal vein and the hippocampal fissure as guidance clues.
Extracellular Field Recordings
Hippocampal slices were prepared from 3 month-old C57BL/6 mice as described (Hawasli et al., 2007) and field excitatory postsynaptic potentials (fEPSP) from CA1 were evoked by square current pulses (0.1 ms at 0.033 Hz) with a bipolar stimulation electrode placed at the Schaffer collaterals. Results were obtained using a stimulus intensity to induce 50% of the maximal fEPSP slope. Once the baseline was stable, Pen siP or Pen control peptides were added to the perfusion buffer for 30 min. For recordings performed in presence of the NR2B selective blocker Ro 25–6981, the Pen siP peptide was added 10 min after starting Ro 25–6981 (3 μM) bath perfusion. The effects of the siPs were evaluated as percent change from baseline fEPSP slope.
LTD analysis was performed in hippocampal slices from 16–17 day-old C57BL/6 mice as previously reported (Billard, 2010; Milner et al., 2004) using modified ACSF (in mM: 130 NaCl, 26 NaHCO3, 3 KCl, 2 MgCl2, 1.25 NaH2PO4, 1 CaCl2, 10 Glucose, pH 7.4, 300 mOsm). After 15 minutes of stable baseline recording a low frequency stimulation protocol (900 stimuli at 1 Hz) was applied to induce LTD that was maintained for at least one hour. At the end of the LTD recording the CA1 was micro-dissected and homogenized in lysate buffer containing protease and phosphatase inhibitors.
Dissociated neuronal cultures, transfection, drug treatments and live-labeling of cell surface receptors
Dissociated hippocampal neurons were prepared from P0 rats as described in (Goda and Colicos, 2006) and plated onto glass coverslips coated with poly-D-lysine (10 μg/ml) and laminin (2 μg/ml). Cultures were maintained in Neurobasal media (Invitrogen) supplemented with 6 mg/ml glucose, 0.1% Mito™ serum extender, 2.5% B27, and 2 mM Glutamax. Hippocampal neurons were transfected at DIV 7–8 using the calcium-phosphate method and used at DIV 14–18. Constructs include pRK5-EGFP-NR2B that encodes a N-terminally EGFP-tagged NR2B subunit (GFP-NR2B).
To evaluate the effect of Cdk5 inhibition on cell surface expression of NR2B, neuronal cultures were treated with 25 μM CP68130 for 1 h or with 2 μM NR2B-siP for 30 min, 6–10 days after transfection with pRK5-EGFP-NR2B. DMSO or control peptides were used as controls. Immunofluorescence staining of cell surface GFP-NR2B was performed by incubating non-permeabilized, live neuronal cultures with rabbit polyclonal EGFP ab (1:500, Abcam, Ab6556) for 15 min at 37°C (Cingolani et al., 2008; Pozo et al., 2012). Neurons were fixed in PBS containing 4% paraformaldehyde and 4% sucrose for 10 min at 25°C and then permeabilized and blocked with PBS containing 10% fetal bovine serum, 0.2% gelatine and 0.02% Triton X-100 for 1h at 25°C. Following incubation with mouse monoclonal Homer-1 ab (1:1000, SYSY, 160011) for 1h at 25°C, neurons were washed and incubated with Cy3-conjugated anti-rabbit IgG and Alexa 647-conjugated anti-mouse IgG for 1h at 25°C. Cells were washed and mounted onto microscope slides for confocal imaging. Z-stacks were acquired with an inverted 510 Meta Zeiss confocal microscope using a 63X water objective, sequential mode and 4 frames average. Baseline microscopy settings were established using control cultures and maintained constant throughout the entire imaging session. ImageJ was used to project Z-stacks and measure dendritic fluorescence. Dendritic length was assessed by tracing processes with NeuronJ and used to normalize dendritic fluorescence.
Animal Behavior
All behavior was carried out with the experimenter blind to treatment groups. For cued fear conditioning in rats, animals were left to explore the conditioning box (Med Associates) for 2 min, followed by a 30 s period with tone representation (90 dB, 10 kHz), which co-terminated with a mild foot shock (2 s, 0.7 mA, constant current). Rats remained in the chamber 2 min after shocking before returning to their home cage. Conditioning boxes were thoroughly cleaned after each animal. To test context-dependent fear memory, rats were re-introduced 24 h post-shock into the conditioning box for 5 min. Freezing responses (motionless except respirations) were recorded using a 5-s interval time-sampling method. Twenty-seven hours after conditioning, tone-recall was assessed in a novel context with novel odor (vanilla). The rats were left exploring the novel context for 3 min without tone followed by a 3 min period with tone representation. Extinction of fear memory was evaluated by re-exposing rats to the conditioning box at 24, 48, 72, and 96 h post-shock for 5 min. Following fear memory extinction, rats were re-trained with a 0.7 mA foot shock and contextual learning was analyzed 24 h after re-training. Nociceptive response and motor reflex were evaluated by measuring the stimulus threshold to elicit flinching, jumping, and vocalizing in the fear conditioning paradigm and tail flick test.
For mice, cued fear conditioning was conducted in an automated system (Med Associates) using a two conditioned stimulus (CS)–unconditioned stimulus (US) pairings paradigm of 4 min with 60 s inter-trial-interval. The CS was a tone (75 dB, 8 kHz) coterminating with a mild foot shock US (2 s, 0.7 mA, constant current). Context-dependent conditioning was tested 24 h later by exposing the mice to the conditioning box for 5 min and tone-dependent freezing was measured at 27 h by placing the mice in a novel context with novel scent (vanilla) for 3 min pre-CS followed by 3 min CS. Freezing responses were assessed by computer-aided scoring, as well as, offline manual scoring. For biochemistry and cell surface biotinylation animals were sacrificed 1 h after fear conditioning.
Statistical analyses
All data are represented as mean ± SEM unless stated otherwise. Statistical analysis was performed using the Student t-test unless stated otherwise. For all experiments *p < 0.05; **p < 0.01; ***p < 0.001 were considered significant.
Supplementary Material
HIGHLIGHTS.
The NR2B subunit of the NMDA receptor is phosphorylated at Ser1116 by Cdk5.
Synaptic plasticity and memory formation alter NR2B Ser1116 phosphorylation.
Disruption of NR2B-Cdk5 complex increases surface NR2B and synaptic transmission.
Hippocampal infusion of NR2B-Cdk5 small interfering peptide improves memory.
Acknowledgments
We thank J. Rush (Cell Signaling Technologies) for mass spectroscopy analysis, A. Mussachio and M. Mapelli (European Institute of Oncology, Milan, Italy) for recombinant Cdk5/p25, U. Bayer (University of Colorado Denver) for the NR2B plasmid, H. Ball (UTSW Protein Technology Center) for peptide synthesis, P. Sykes (Charles River) for cannulated rats, S. Birnbaum for help with behavioral experiments, K. Richter and Pfizer, Inc. for CP68130, C. Hebel (LC Sciences) for peptide array analyses, S. Vicini (Georgetown University) for the GFP-NR2B plasmid, C. Castro and S. Saldana for technical assistance and T.V.P. Bliss for support. This work was supported by basic science training program T32-DA7290 in drug abuse (T.M.K.); National Institutes of Health grants to Z.H. (MH084233, MH085774) and J.A.B. (MH079710, MH083711, DA016672, DA033485, NS073855).
Footnotes
AUTHOR CONTRIBUTIONS
F.P., A.H., T.M.K., K.P., A.N., T.W., Z.Y. and J.A.B. designed experiments; F.P., A.H., T.M.K., K.P., P.Z., E.Y.Y., A.H.H., S.F.C., A.N. and A.G. performed and analyzed experiments; F.P., Z.Y. and J.A.B. supervised and interpreted experiments; F.P. and J.A.B. wrote manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angelo M, Plattner F, Giese KP. Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. J Neurochem. 2006;99:353–370. doi: 10.1111/j.1471-4159.2006.04040.x. [DOI] [PubMed] [Google Scholar]
- Angelo M, Plattner F, Irvine EE, Giese KP. Improved reversal learning and altered fear conditioning in transgenic mice with regionally restricted p25 expression. Eur J Neurosci. 2003;18:423–431. doi: 10.1046/j.1460-9568.2003.02746.x. [DOI] [PubMed] [Google Scholar]
- Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 2010;626:49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Mayford MR, Tsien JZ, Alberini CM. Cognition Enhancement Strategies. J Neurosci. 2010;30:14987–14992. doi: 10.1523/JNEUROSCI.4419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signaling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Billard JM. Long-term depression in the hippocampal CA1 area of aged rats, revisited: contribution of temporal constraints related to slice preparation. PLoS One. 2010;5:e9843. doi: 10.1371/journal.pone.0009843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Brim BL, Haskell R, Awedikian R, Ellinwood NM, Jin L, Kumar A, Foster TC, Magnusson KR. Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behav Brain Res. 2013;238:211–226. doi: 10.1016/j.bbr.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Cui Z, Feng R, Tang YP, Qin Z, Mei B, Tsien JZ. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci. 2007;25:1815–1822. doi: 10.1111/j.1460-9568.2007.05431.x. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, Goda Y. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Volianskis A, Bannister N, France G, Hanna L, Mercier M, Tidball P, Fang G, Irvine MW, Costa BM, et al. The NMDA receptor as a target for cognitive enhancement. Neuropharmacology. 2013;64:13–26. doi: 10.1016/j.neuropharm.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483:213–217. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des. 2013;81:136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Morinobu S, Takei S, Fuchikami M, Matsumoto T, Yamamoto S, Yamawaki S. Vorinostat, a histone deacetylase inhibitor, facilitates fear extinction and enhances expression of the hippocampal NR2B-containing NMDA receptor gene. J Psychiatr Res. 2012;46:635–643. doi: 10.1016/j.jpsychires.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Gao XB, Pellois JP, Na Y, Kim Y, Gulari E, Zhou X. High density peptide microarrays. In situ synthesis and applications. Mol Divers. 2004;8:177–187. doi: 10.1023/b:modi.0000036233.58271.25. [DOI] [PubMed] [Google Scholar]
- Goda Y, Colicos MA. Photoconductive stimulation of neurons cultured on silicon wafers. Nat Protoc. 2006;1:461–467. doi: 10.1038/nprot.2006.67. [DOI] [PubMed] [Google Scholar]
- Guttmann RP, Baker DL, Seifert KM, Cohen AS, Coulter DA, Lynch DR. Specific proteolysis of the NR2 subunit at multiple sites by calpain. J Neurochem. 2001;78:1083–1093. doi: 10.1046/j.1471-4159.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jakovcevski M, Bharadwaj R, Connor C, Schroeder FA, Lin CL, Straubhaar J, Martin G, Akbarian S. Setdb1 histone methyltransferase regulates mood-related behaviors and expression of the NMDA receptor subunit NR2B. J Neurosci. 2010;30:7152–7167. doi: 10.1523/JNEUROSCI.1314-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6:189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- Jubb H, Higueruelo AP, Winter A, Blundell TL. Structural biology and drug discovery for protein-protein interactions. Trends Pharmacol Sci. 2012;33:241–248. doi: 10.1016/j.tips.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Ma D, Jan LY. ER transport signals and trafficking of potassium channels and receptors. Curr Opin Neurobiol. 2002;12:287–292. doi: 10.1016/s0959-4388(02)00319-7. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Milner AJ, Cummings DM, Spencer JP, Murphy KP. Bi-directional plasticity and age-dependent long-term depression at mouse CA3-CA1 hippocampal synapses. Neurosci Lett. 2004;367:1–5. doi: 10.1016/j.neulet.2004.04.056. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morrisett R. Electrophysiologic characteristics of heteromeric recombinant NMDA receptors. In: Monaghan DT, Wenthold RJ, editors. The Ionotropic Glutamate Receptors. Totowa, New Jersey, USA: Humana Press; 1997. [Google Scholar]
- Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron. 2003;40:581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- Myers SJ, Dingledine R, Borges K. Genetic regulation of glutamate receptor ion channels. Annu Rev Pharmacol Toxicol. 1999;39:221–241. doi: 10.1146/annurev.pharmtox.39.1.221. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C, Nishi A, Kansy JW, Fernandez J, Gillardon F, Allen PB, Hemmings HC, Jr, Nairn AC, Bibb JA. Regulation of protein phosphatase inhibitor-1 by cyclin-dependent kinase 5. J Biol Chem. 2007;282:16511–16520. doi: 10.1074/jbc.M701046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–228. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Plattner F, Angelo M, Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem. 2006;281:25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- Pozo K, Cingolani LA, Bassani S, Laurent F, Passafaro M, Goda Y. beta3 integrin interacts directly with GluA2 AMPA receptor subunit and regulates AMPA receptor expression in hippocampal neurons. Proc Natl Acad Sci U S A. 2012;109:1323–1328. doi: 10.1073/pnas.1113736109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol. 2005;23:94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- Sahin B, Shu H, Fernandez J, El-Armouche A, Molkentin JD, Nairn AC, Bibb JA. Phosphorylation of protein phosphatase inhibitor-1 by protein kinase C. J Biol Chem. 2006;281:24322–24335. doi: 10.1074/jbc.M603282200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Vandenberghe W, Bredt DS. Early events in glutamate receptor trafficking. Curr Opin Cell Biol. 2004;16:134–139. doi: 10.1016/j.ceb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Wong RW, Setou M, Teng J, Takei Y, Hirokawa N. Overexpression of motor protein KIF17 enhances spatial and working memory in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:14500–14505. doi: 10.1073/pnas.222371099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Takei Y, Kido MA, Hirokawa N. Molecular motor KIF17 is fundamental for memory and learning via differential support of synaptic NR2A/2B levels. Neuron. 2011;70:310–325. doi: 10.1016/j.neuron.2011.02.049. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Wu LJ, Gong B, Ren M, Li BM, Zhuo M. Induction- and conditioning-protocol dependent involvement of NR2B-containing NMDA receptors in synaptic potentiation and contextual fear memory in the hippocampal CA1 region of rats. Mol Brain. 2008;1:9. doi: 10.1186/1756-6606-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.