Abstract
Tick-borne encephalitis virus (TBEV), a member of the Flaviviridae family, is a leading cause of viral encephalitis in Europe and Asia. Dendritic cells (DCs), as early cellular targets of infection, provide an opportunity for flaviviruses to inhibit innate and adaptive immune responses. Flaviviruses modulate DC function, but the mechanisms underpinning this are not defined. We examined the maturation phenotype and function of murine bone marrow-derived DCs infected with LGTV, a naturally attenuated member of the TBEV serogroup. LGTV infection failed to induce DC maturation or a cytokine response. Treatment with LPS or LPS/IFNγ, strong inducers of inflammatory cytokines, resulted in enhanced TNFα and IL-6 production, but suppressed IL-12 production in infected DCs compared to uninfected "bystander" cells or mock-infected controls. LGTV-mediated antagonism of type I IFN (IFN-I) signaling contributed to inhibition of IL-12p40 mRNA expression at late time-points following stimulation. However, early suppression was still observed in DCs lacking the IFN-I receptor (Ifnar−/−) suggesting that additional mechanisms of antagonism exist. The early IFN-independent inhibition of IL-12p40 was nearly abolished in DCs deficient in interferon regulatory factor-1 (IRF-1), a key transcription factor required for IL12 production. LGTV infection did not affect Irf-1 mRNA expression, but rather diminished IRF-1 protein levels and nuclear localization. The effect on IRF-1 was also observed in DCs infected with the highly virulent Sofjin strain of TBEV. Thus, antagonism of IRF-1 is a novel mechanism that synergizes with the noted ability of flaviviruses to suppress IFNAR-dependent signaling, resulting in the orchestrated evasion of host innate immunity.
Introduction
TBEV, the causative agent of tick-borne encephalitis (TBE), is a single-stranded, positive-sense RNA virus belonging to the family Flaviviridae. Approximately one-third of TBEV infections cause an acute febrile illness that progresses to severe neurologic disease including meningitis and meningoencephalitis with mortality rates ranging from 0.5% to 40% depending on the TBEV subtype (1, 2). Endemic throughout Europe and Asia, TBEV is considered an emerging pathogen due to its recent expansion into new geographical areas and increased incidence of human infections (3). Approximately 10,000 clinical cases of TBE are reported annually. Other emerging tick-borne flaviviruses include Kyasanur Forest disease virus (KFDV) and Omsk hemorrhagic fever virus (OHFV) that cause hemorrhagic fever, also with relatively high mortality rates (4). There are currently no specific therapies for the treatment of flavivirus infections, underscoring the importance of identifying therapeutic targets for development of novel antivirals.
Dendritic cells (DCs) represent a fundamental bridge between innate and adaptive immunity. In peripheral tissues such as the skin, immature DCs recognize RNA virus infection through expression of pathogen recognition receptors (PRRs) in the cytosol, such as the RNA helicases retinoic acid inducible gene (RIG-I) and melanoma differentiation associated gene-5 (MDA5), and in the endosome, through Toll-like receptor (TLR)3, TLR7, and TLR8 (5). Following virus recognition, DCs migrate to local lymphoid tissues and undergo a process of maturation that involves cytokine production and antigen presentation to activate naive T cells and shape adaptive immunity. DCs also represent early targets of TBEV infection following the bite of an infected tick (6), providing the virus with opportunities to manipulate DC functions as a means of evading host immunity. Indeed, many flaviviruses including dengue virus (DENV) (7–9), West Nile virus (WNV) (10) and Japanese encephalitis virus (JEV) (11–13) infect DCs resulting in impaired DC maturation and T cell priming/proliferation. This manipulation of DC function is thought to be important in virus pathogenesis, although the molecular mechanisms are unknown.
DCs bridge innate and adaptive immunity in part through production of type I IFN (IFNα/β, or IFN-I). Type I IFNs are secreted following PRR ligation and bind to the IFN-α/β receptor (IFNAR) in an autocrine and paracrine fashion to activate Janus kinase – signal transducer and activator of transcription (JAK-STAT) signal transduction. In turn, this leads to the expression of a broad array of IFN-stimulated genes (ISGs). ISGs encode a variety of effector proteins responsible for establishing an antiviral state in cells by directly inhibiting virus replication (e.g., protein kinase R, viperin, IFIT, and TRIM proteins) (14–17) and by enhancing DC maturation through upregulation of co-stimulatory molecules (e.g., CD40, CD80 and CD86), MHC class I and II and cytokine production, most notably IL-12 (18). All flaviviruses, including TBEV, encode for IFN-I antagonists that suppress JAK-STAT signaling and ISG expression (19–23). However, the contribution of flavivirus-mediated inhibition of IFN-I to the suppression of DC maturation is unclear.
Ligation of PRRs stimulates activation of multiple transcription factors including interferon regulatory factors (IRFs) that regulate expression of target genes. Examples include IRF-3 and IRF-7 that regulate genes encoding IFN-I, and IRF-1, IRF-8 and IRF-4 that regulate genes encoding pro-inflammatory cytokines such as IL-12 (24). Recently, IRF-1 was identified as a potent inhibitor of a broad spectrum of viruses including flaviviruses (25, 26). Furthermore, IRF-1 is essential for host protection against lethal WNV infection, shaping both innate and adaptive responses to infection (27). Although IRF-1 is itself an ISG, it regulates a transcriptional profile of antiviral genes unique to that induced by IFN-I (26). Thus, IRF-1 and IFN-I responses appear to cooperatively promote an effective and essential antiviral program.
In this study, we analyzed the interaction between tick-borne flaviviruses and DCs to understand the effect of flaviviruses on DC function. Using Langat virus (LGTV), a naturally attenuated member of TBEV serogroup, we found that virus infection impairs DC maturation by suppressing costimulatory molecule expression and selectively inhibiting IL-12 production. This immature DC phenotype was associated with an impaired functional capacity to induce T cell proliferation. Further investigation of the inhibition of IL-12 expression revealed a coordinated impairment of IRF-1 and IFN-I signaling. LGTV and TBEV infection resulted in reduced levels of IRF-1 protein and nuclear localization without affecting Irf-1 mRNA expression. These data identify IRF-1 as a novel target of flavivirus-mediated antagonism of innate immunity.
Materials and Methods
Mice
C57Bl/6 (WT) mice and C57BL/6-Tg (TcraTcrb)425Cbn/J (called OTII) mice that express a T-cell receptor (TCR) specific for peptide 323–339 of OVA in the context of I-Ab were purchased from The Jackson Laboratory and housed in the animal facility at Rocky Mountain Laboratories (RML). Interferon α/β receptor 1-deficient mice on a C57Bl/6 background (Ifnar−/−) were kindly provided by Dr. G. Cheng of the University of California at Los Angeles and bred at RML. All animal procedures were performed in strict accordance with the regulations and guidelines of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Viruses
Vero cells were cultured in DMEM containing 10% fetal calf serum (FCS, Life Technologies, Grand Island, NY). Langat virus strain TP21 (LGTV) and TBEV (Sofjin strain) were propagated by infecting Vero cells at a multiplicity of infection (MOI) of 0.005. Virus stocks were prepared by centrifugation (100,000 × g, 4°C) of infected cell culture supernatants collected 72 h post infection (hpi) and stored at −80°C. Virus titers were determined by a focus-forming (FFU) assay in Vero cells (19). Work with TBEV was performed under BSL4 conditions.
Preparation of primary bone marrow-derived dendritic cells
For each experiment murine bone-marrow derived dendritic cells (DCs) were prepared from one 8- to 12-wk-old female mouse as previously described (28) with some modifications. Briefly, bone marrow cells were flushed from femurs and tibiae in IMDM media (Life Technologies, Grand Island, NY). Red blood cells were lysed with ACK lysis buffer (Life Technologies) and seeded into 6-well tissue culture plates (1–2 × 106 per well) in complete DC medium (IMDM containing 10% FCS, 200 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 50 nM 2-mercaptoethanol) supplemented with 20 ng/ml of murine GM-CSF (R&D Systems, Minneapolis, MN). Fresh complete DC media containing GM-CSF was added every other day and non-adherent, immature dendritic cells were harvested on day 8 for all experiments. DC cultures ranged from 85–95% pure (CD11b+/CD11c+) as determined by flow cytometry with >95% viability based on trypan blue exclusion. During the course of an experiment (24–48 hpi) viability dropped slightly (~85%), but there was no difference in viability in infected or stimulated cultures compared to mock-infected controls.
Infection and stimulation of DCs
Immature DCs were seeded at 4–6 × 105 cells per well in a 24-well plate, and were mock-infected or infected with LGTV (MOI of 30) or TBEV (MOI of 10). For UV-inactivated virus controls, DCs were exposed to and MOI equivalent of irradiated LGTV (360 mJ/cm2, UV-crosslinker). At 28 hpi, cells were stimulated with LPS (1 µg/ml), LPS plus IFNγ (20 ng/ml) (Peprotech, Rocky Hill, NJ), polyriboinosinic-polyribocytoidylic acid (PIC) (20 µg/ml), or loxoribine (1 mM) (all TLR ligands were purchased from InvivoGen, San Diego, CA). For intracellular cytokine detection, brefeldin A (10 µg/ml) (Sigma-Aldrich, St. Louis, MO) was added to the cultures 6 h prior to collecting cells for staining. Surface phenotype was assessed at 20–24 h post-stimulation and intracellular staining was assessed at 8 h post-stimulation. For IL-10R blocking experiments, 10ug/ml anti-CD210 (1B1.3a) or RtIgG (BD Biosciences) was added to the cultures one hour after infection and maintained throughout stimulation(29).
Flow cytometry
The surface phenotype of DCs was analyzed by flow cytometry using antibodies against CD11c-PE-Cy7 (clone HL3, BD Biosciences, San Diego, CA), CD80-FITC (clone 16-10A1, BD Biosciences), CD86-PerCP-Cy5.5 (clone GL1, eBiosciences, San Diego, CA), I-A/I-E-PE (MHC class II) (clone M5/115.15.2), and CD40-PE (clone 1C10, eBiosciences). For intracellular staining, cells were first stained with anti-CD11c, fixed, permeabilized and then stained using antibodies against TNF-α (clone MP6-XT22, eBiosciences) and IL-12p40 (clone C15.6, BD Biosciences). LGTV envelope protein was detected by intracellular staining using a mouse monoclonal antibody (clone 11H12) kindly provided by Dr. C. Schmaljohn at the United States Army Medical Research Institute for Infectious Diseases (USAMRIID). Data were obtained on a LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo version 8.3.3 (Tree Star, Inc., Ashland, OR). For all flow data analyses, debris and dead cells were excluded with initial gates based on forward- and side-scatter parameters and positive staining with live/dead yellow fixable dye (Life Technologies). A CD11c positive gate was included in all analyses to confirm DC phenotype.
Cytokine analysis by BioPlex assay and quantitative RT-PCR
DC culture supernatants were collected at the indicated time points following stimulation and cytokines were quantified using Bioplex Pro Assay according to the manufacturer's instructions (BioRad, Hercules, CA). Total RNA was isolated from cultured DCs using a Qiagen RNeasy kit (Qiagen, Valencia, CA) with DNase I digestion. RNA was reverse transcribed using a SuperScript VILO cDNA synthesis kit (Life Technologies). cDNA was then used as template in TaqMan-PCR reactions according to the manufacturer's instructions. The Taqman primer and probes sets included IL-12p35 (assay ID: Mm00434165), IL-12p40 (assay ID: Mm99999067), IRF-1 (assay ID: Mm01288580), and HPRT (assay ID: Mm01545399) (Life Technologies). Reactions were performed in triplicate and analyzed using Applied Biosystems 7900HT real-time PCR system (Life Technologies). The specificity of RT-PCR was confirmed by analysis of RNA samples that had not been reverse transcribed. Results were normalized to HPRT mRNA levels and expressed as fold change relative to RNA samples from mock-infected, unstimulated DCs using the comparative threshold cycle method.
T cell proliferation assays
OVA-specific CD4+ T cells were enriched from spleens of OTII mice by magnetic negative selection using the mouse CD4+ T cell purification kit II (Miltenyi, Auburn, CA) and labeled with 5 µM of the vital dye 5,6-carboxyfluorescein succinimidyl ester (CFSE; Life Technologies). DCs (105 cells per well) plated in triplicate in a 96-well round-bottom were infected with LGTV for 24 h and stimulated as described above. At 24 h post stimulation, DCs were loaded with 1µg/ml of chicken ovalbumin 323–339 peptide (InvivoGen) for 2 h and washed to remove unbound peptide. Antigen-loaded DCs were then co-cultured with CFSE-labeled OVA-specific CD4+ T cells (5 × 105) in complete DC media at a DC to lymphocyte ratio of 1:5. After 4 days, cells were collected and stained with anti-CD3-eFluor 450 (eBiosciences). Proliferation was determined by measuring CFSE dilution using an LSRII flow cytometer (BD Biosciences). Live/dead yellow fixable stain (Invitrogen) was used to exclude dead cells prior to subsequent flow analyses.
Immunoblotting
Protein lysates were prepared from 4–6 × 105 DC using lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1 % SDS, 1% NP-40, 0.5% Na-deoxycholate and DNase I) containing protease inhibitor cocktail (Roche, Indianapolis, IN). Total lysates from approximately 1 × 105 cells were resolved on a 10% Tris-glycine gel (Life Technologies), and transferred to a nitrocellulose membrane (BioRad). Membranes were probed with rabbit anti-mouse IRF-1 (clone D5E4; Cell Signaling Technology, Boston, MA), goat anti-mouse IRF-8 (clone T-14; Santa Cruz Biotechnology, Santa Cruz, CA) or mouse anti-β-actin (Sigma-Aldrich). Bound antibodies were detected with HRP-conjugated species-specific anti-IgG antibodies (Dako, Carpinteria, CA). Blots were developed using ECL Plus chemiluminescence reagents (GE Healthcare, Piscataway, NJ).
Luciferase reporter assays
Human hepatoma cell line (Huh7) (30) was co-transfected with pTK-Renilla and either IRF-1 or guanylate-binding protein 2 (Gbp-2) firefly luciferase reporter plasmids (31). At 24 h post-transfection, cells were mock-infected or infected with LGTV (MOI 3) and either left untreated or treated with IFNγ (100 µg/ml) at 24 hpi for 6 h. Cell extracts were prepared for measurement of luciferase activity using a Dual-Luciferase Reporter Assay System per the manufacturer's instructions (Promega, Madison, WI). The reporter activity of each sample was normalized to the constitutive luciferase activity of pTK-Renilla and expressed as fold change over the luciferase activity in the mock-infected, unstimulated control.
Immunofluorescence assay
DCs (1–2 × 105 cells per well) were plated on 8-well Lab-Tek chamber slides (Thermo Scientific, Waltham, MA) in complete DC medium and infected with LGTV for 24 h. Cells were then stimulated with LPS (1 µg/ml) plus IFNγ (20 ng/ml) for 3.0 h, fixed with 4% formaldehyde/PBS, and stored in PBS at 4°C. For detection of IRF-1 and viral protein, slides were incubated with 100% methanol for 8 min at −20°C, followed by incubation with blocking buffer (1% bovine serum albumin [Sigma], 2% normal goat serum [Life Technologies], and 0.01M glycine/Dulbecco's PBS) for 1–2 h at RT. Slides were stained using rabbit anti-IRF-1 antibody (clone D5E4) and mouse anti-envelope protein (clone 11H12). Bound antibodies were detected with Alexa Fluor 594-conjugated anti-rabbit IgG and Alexa Fluor 488-conjugated anti-mouse IgG, respectively (Invitrogen). Nuclei were stained using Prolong Gold + DAPI mounting media (Invitrogen). Immunofluorescent images were obtained using a Zeiss LSM710 confocal microscope.
Statistical Analyses
The Student's t test was used for statistical analysis using Prism software (GraphPad Prism5, San Diego, CA).
Results
LGTV infection inhibits DC maturation in response to TLR ligand stimulation
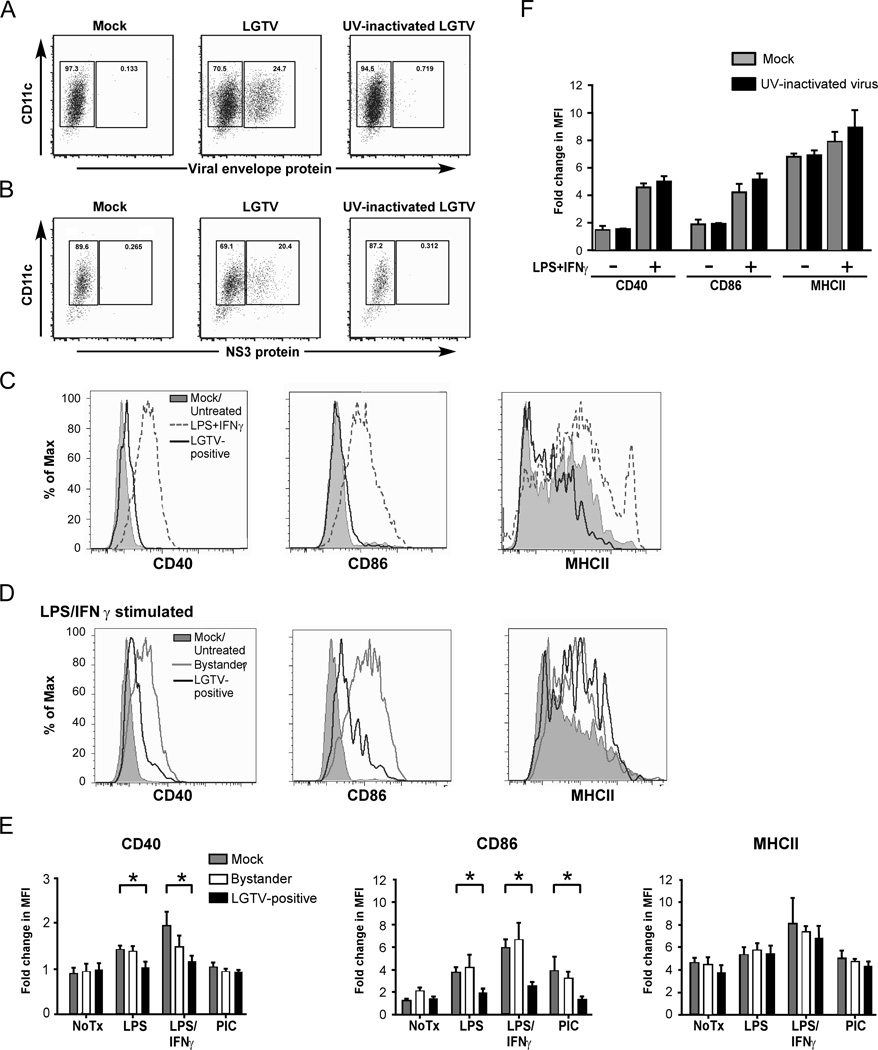
Upon recognition of a pathogen, immature DCs in the periphery up-regulate surface expression of MHCII and co-stimulatory molecules necessary for optimal development of adaptive immune responses. To investigate the effect of tick-borne flaviviruses on DC maturation, DCs were infected with LGTV, and analyzed by flow cytometry for expression of the DC maturation markers CD40, CD86 and MHCII. Intracellular staining for viral envelope protein was used to distinguish viral antigen positive cells (infected) from viral antigen negative cells (bystander) in the same culture. The percentage of DCs expressing LGTV envelope protein at 24 hpi was 15–25% (Fig. 1A). A similar percentage of cells positive for virus were found by intracellular staining for the nonstructural protein, NS3, that is only detectable during virus replication, confirming that these cells contain replicating virus (Fig. 1B). As shown in Fig. 1C, LGTV-positive DCs showed negligible up-regulation of CD40, CD86 and MHCII surface expression compared to the positive control of DCs treated with LPS plus IFNγ, a strong stimulus for DC maturation (Fig. 1C). Thus, DCs fail to upregulate maturation markers following LGTV infection and therefore exhibit an immature phenotype.
Figure 1.
LGTV replication suppresses DC maturation. DCs were mock-infected, infected with LGTV strain TP21 or exposed to UV-inactivated LGTV. At 40 hpi, cells were stained for surface CD11c and intracellular viral protein and analyzed by flow cytometry. A and B, Representative dot plots of CD11c and viral envelope protein (A) or NS3 protein (B) shows the gating and percentage of LGTV-positive and LGTV-negative bystander populations. C, Expression of CD40, CD86, and MHCII on mock-infected (filled gray lines) and gated LGTV-positive cells (black line) are shown. DCs treated with LPS/IFNγ (gray dashed line) for 24 h served as a positive control for maturation. D, At 28 hpi, mock- and LGTV-infected DCs were cultured in the presence of various activation stimuli and changes in surface phenotype were assessed. Expression of CD40, CD86, and MHCII on LGTV-negative bystander (gray line) and LGTV-positive DCs (black line) following stimulation with LPS/IFNγ compared to mock-infected, untreated (filled gray lines) cells. E, Fold change in the geometric mean fluorescence intensity (MFI) of surface markers relative to isotype controls following no treatment or stimulation with LPS, LPS/IFNγ or poly I:C (PIC). Results are presented as the mean±SEM of three independent experiments. *P<0.05. F, The fold change in MFI of CD40, CD86 and MHCII following LPS/ IFNγ stimulation of DC exposed to UV-inactivated virus and mock-infected controls. Results from 1 of 2 independent experiments are presented as the mean±SD of triplicate samples.
To determine whether the lack of DC maturation was due to a virus-mediated block in signal transduction, or simply an inability of DCs to recognize LGTV infection, the response of infected DCs to maturation stimuli was tested. At 28 hpi, DCs were left untreated or treated with LPS/IFNγ for 24 h prior to staining for viral antigen and DC maturation markers. Bystander cells responded to LPS/IFNγ stimulation by up-regulating CD40 and CD86 to similar levels as those observed in stimulated, mock-infected DCs (compare Fig. 1D with 1E). In contrast, LGTV-positive DCs in the same culture maintained low expression of maturation markers except for MHCII that was comparably upregulated in both LGTV-infected and bystander DCs (Fig. 1D and E). Similarly, LGTV-infected DCs did not upregulate expression of CD40 and CD86 surface expression in response to LPS alone (Fig. 1D). Although treatment with PIC, a TLR3 ligand, did not induce CD40 or MHCII expression, upregulation of CD86 by this stimulus was also suppressed in LGTV infected DCs (Fig. 1E). Incubation of DCs with UV-inactivated virus did not result in suppression of LPS/IFNγ stimulated CD40 and CD86 expression (Fig. 1F). Taken together, these results indicate that LGTV replication inhibits DC maturation induced by multiple TLR-signaling pathways.
LGTV suppresses production of IL-12 and induction of T cell proliferation by DCs
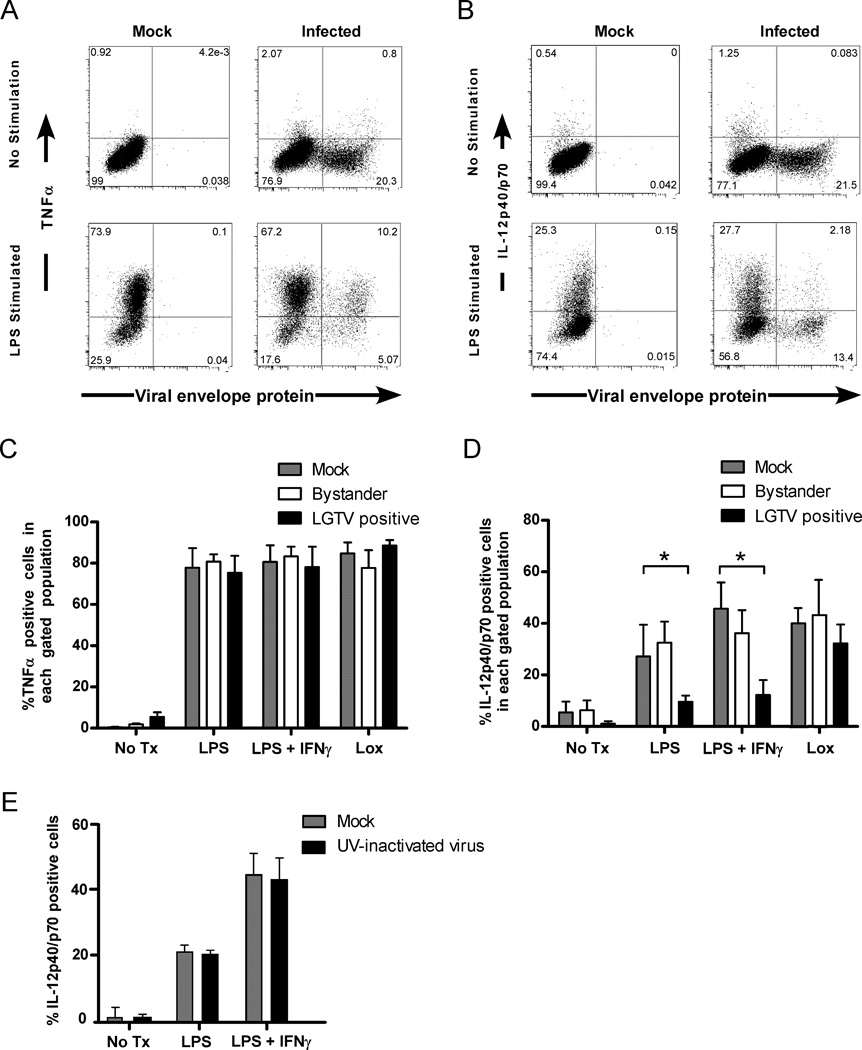
In addition to maturation phenotype, activated DCs produce cytokines required for optimal immune responses. To determine the effect of LGTV infection on cytokine production, DCs were stained for intracellular IL-12p40/p70, TNF-α, or IL-6, in conjunction with viral envelope protein, and analyzed by flow cytometry. Cytokine expression was notably weak in LGTV infected DCs with a similar low percentage (5–10%) of both infected and bystander DCs expressing TNF-α (Fig. 2A), IL-12 (Fig. 2B) and IL-6 (data not shown). To determine whether infection actively inhibited cytokine expression, DCs were left untreated or treated with LPS, LPS/IFNγ, or loxoribine (a TLR7 ligand) at 28 hpi and analyzed at 8 h post-stimulation. A high percentage (~80%) of infected, bystander, or mock-infected DCs expressed TNF-α (Fig. 2A and C), indicating that infection had no effect on induction of this cytokine. However, a lower percentage of infected DCs produced IL-12p40/p70 upon stimulation with LPS or LPS/IFNγ compared to bystander or mock-infected DCs (Fig. 2B and D). LGTV infection did not affect IL-12 production following stimulation with loxoribine, suggesting that a lack of IL-12p40/p70 was due to a specific block in signal transduction and not to a general inability of infected cells to produce this cytokine. The suppressive effect also required virus replication as DCs exposed to UV-irradiated virus showed no defect in IL-12 expression following stimulation (Fig. 2E).
Figure 2.
Production of IL-12p40/p70, but not TNF-α, is suppressed in LGTV-infected DCs following TLR stimulation. DCs were mock-infected, or infected with LGTV for 28 h and then stimulated with LPS, LPS/IFNγ, or loxoribine for 8 h and analyzed by flow cytometry. Representative dot plots for viral protein and TNF-α (A) or IL-12p40/p70 (B) in CD11c-positive cells are shown. The percentage of cells expressing TNF-α (C) or IL-12p40/p70 (D) for each population as indicated is shown. *P<0.05. E, The percentage of DCs, mock-infected or exposed to UV-inactivated virus, that express IL-12p40/p70 following stimulation with LPS, or LPS/IFNγ. Results are presented as the mean±SD of three independent experiments.
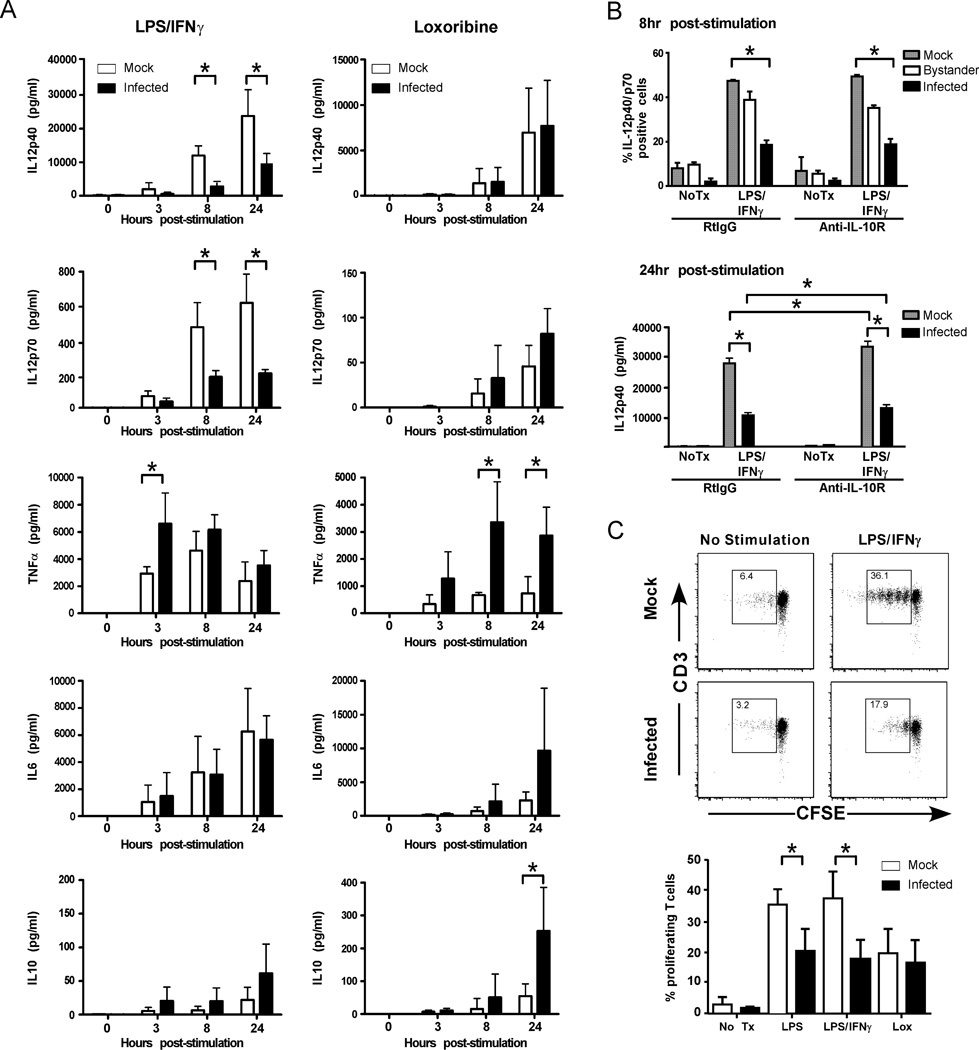
To further investigate these findings, we measured IL-12p40, IL-12p70, TNF-α, IL-6, and IL-10 in culture supernatants using a multiplex cytokine bead assay (Fig. 3A). LPS/IFNγ stimulated high levels of IL-12p40 and IL-12p70 in mock-infected DC cultures that were significantly reduced in LGTV-infected cultures. This effect of infection was not observed following stimulation with loxoribine (Fig. 3A). Furthermore, LGTV infection resulted in similar IL-6 production throughout the time course and enhanced TNF-α secretion at early time points (3 and 8 h) post LPS/IFNγ or loxoribine treatment compared to mock-infected cultures. Thus, LGTV infected DCs exhibit a specific defect in IL-12 production that is not associated with a general inability to produce and secrete cytokines.
Figure 3.
LGTV infection of DCs inhibits IL-12 production and T cell proliferative capacity. DCs were mock-infected or infected with LGTV for 28 h and then treated with medium alone, or medium containing LPS/IFNγ or loxoribine. A, Culture supernatants were harvested at 0, 3, 8, and 24 h following stimulation and levels of IL-12p40, IL-12p70, TNF-α, IL-6, and IL-10 were determined by Bio-plex immunoassay. Data are compiled from three independent experiments and expressed as the mean±SD. *P<0.05. B, DC infection and LPS/IFNγ stimulation was performed in the presence of anti-IL10R or RtIgG control antibodies. Cells were stained for intracellular IL-12p40 at 8 h post-stimulation and analyzed by flow cytometry (upper graph) or supernatants were collected at 24 h post-stimulation and secreted cytokines were measured by Bioplex (lower graph). The graphs represent the mean ± SD of cells expressing IL-12p40 in each population or of IL-12p40 production. Data is representative of 2 independent experiments performed in triplicate. *P<0.05. C, At 24 h post-stimulation, DCs were loaded with Ova323 peptide and cocultured with CFSE-labeled, Ova-specific CD4+ T cells at a ratio of 1:5 (DCs to T cells). After 4 days, cells were harvested and analyzed by flow cytometry. The dot plots (CFSE versus CD3) show the loss of CFSE fluorescence in T cells (identified by CD3 positive staining) cocultured with untreated or LPS/IFNγ-stimulated DCs. Representative dot blots show the percentage of proliferating cells, as indicated by the boxed gate, among total gated live T cells. The graph represents the mean ± SD of proliferating cells from three independent experiments. *P<0.05.
A possible explanation for suppressed IL-12 expression is that LGTV infection induced IL-10, a well-characterized immunosuppressive cytokine that inhibits DC production of IL-12 (reviewed in (32)). Indeed, approximately a 3-fold greater concentration of IL-10 was detected in infected cultures compared to mock-infected cultures following LPS/IFNγ stimulation (Fig. 3A). To determine if suppression of IL-12 was mediated by IL-10, experiments were repeated in the presence of IL-10R blocking antibody. Blocking the IL-10R did not recover IL-12 expression in infected DCs at 6 hr post-stimulation as measured by flow cytometry (Fig. 3B, upper graph). Blocking the IL-10R did increase the overall levels of IL-12p40/p70 at 24 hr post-stimulation confirming that IL-10R signaling was blocked. However, it did not recover suppression of IL12p40/p70 by LGTV infection (Fig. 3B, lower graph). Thus, LGTV-mediated suppression of IL-12 secretion is not due to the inhibitory effects of IL-10, but rather appears to be a direct effect of virus replication.
A critical measure of DC function is their capacity to induce proliferation of CD4+ T cells. To examine this, mock- or LGTV-infected DCs were treated with various maturation stimuli as described above. At 24 h post-stimulation, DCs were loaded with OVA323 peptide and cocultured with CFSE-labeled OVA-specific CD4+ T cells. LGTV- and mock-infected DCs induced similar low levels of T cell proliferation as demonstrated by CFSE dilution (Fig. 3B). Although LPS/IFNγ stimulation increased the T cell stimulatory capacity of both DC cultures, the degree of T cell proliferation was significantly lower in LGTV-infected DCs. This suppressed T cell proliferative capacity of infected DCs was also observed with LPS stimulation, but not with loxoribine (Fig. 3B). Thus, impairment of costimulatory marker upregulation and IL-12 production in DCs by LGTV is associated with a compromised ability to drive antigen-specific T cell proliferation.
LGTV-mediated suppression of DC function involves both IFN-dependent and -independent pathways
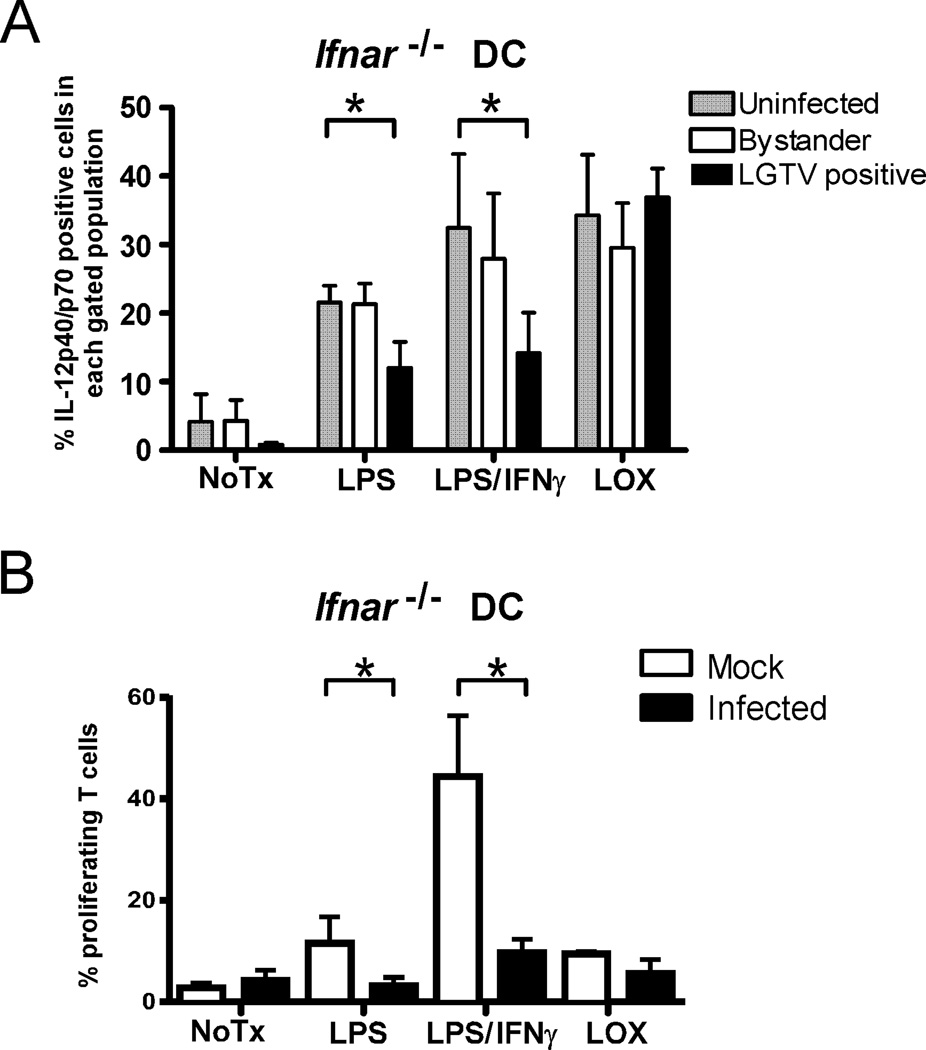
Optimal production of IL-12 by DCs requires cooperation between TLR and IFN-I signaling (29) suggesting that IFN-I antagonism by LGTV may be responsible for the loss of IL-12 expression. To examine this, we removed the influence of IFN-I by repeating our studies using DCs from IFN-I receptor deficient (Ifnar−/−) mice. The percentage of mock-infected and bystander cells expressing detectable levels of IL-12 was reduced in Ifnar−/− DCs compared to WT DCs (compare Fig. 4A to Fig. 2D) by approximately 25% indicating that maximum expression of IL-12 requires IFN-I signaling. However, this effect of IFN-I signaling on IL-12 expression was not observed in LGTV-infected DCs, confirming that viral inhibition of IFN-I signaling contributes to IL-12 suppression. Nevertheless, a significant inhibition of IL-12 expression (Fig. 4A) and an impaired capacity to stimulate OVA-specific T cell proliferation following stimulation (Fig. 4B) remained evident in LGTV-infected Ifnar−/− DCs (Fig. 4B). Thus, LGTV must encode a second mechanism that synergizes with IFN-I antagonism to inhibit IL-12 production and T cell proliferative capacity of infected DCs.
Figure 4.
LGTV impairment of DC function is partially independent of IFN-I signaling. DCs derived from Ifnar−/− mice were mock-infected or infected with LGTV for 28 h and then stimulated with LPS, LPS/IFNγ, or loxoribine. A, Percentages of mock-infected, LGTV-positive, and LGTV-negative bystander DCs expressing IL-12p40/p70 as determined by flow cytometry. Data are presented as the mean ± SD of three independent experiments. *P<0.05. B, At 24 h post-stimulation, Ifnar−/− DCs were loaded with Ova323 peptide and cocultured with CFSE-labeled, Ova-specific CD4+ T cells. After 4 days, cells were harvested and analyzed by flow cytometry. The graph represents the mean ± SD of proliferating cells from three independent experiments. *P<0.05.
LGTV suppresses IL-12p40 mRNA expression by inhibiting IFN-I signaling and IRF-1
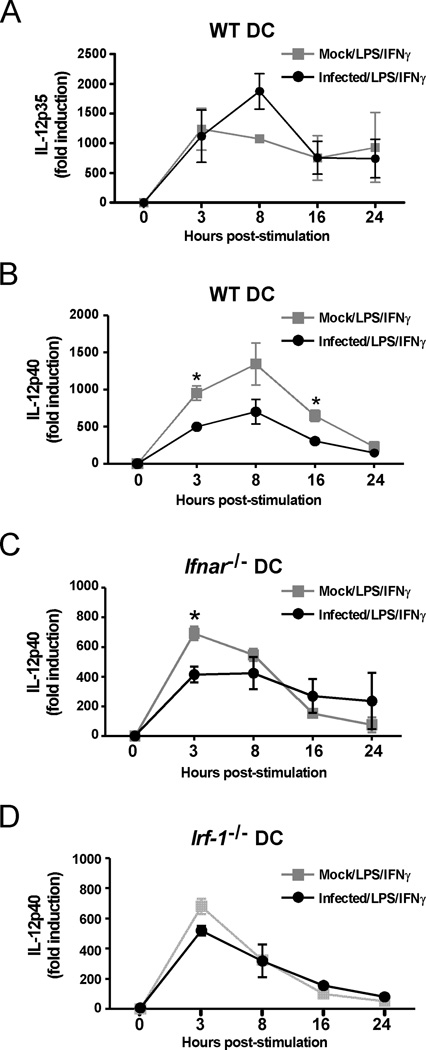
To determine the second mechanism of antagonism, we concentrated on IL-12 as a critical cytokine for T cell activation (reviewed in (32)). Biologically active IL-12 (IL-12p70) is composed of two subunits, IL-12p35 and IL-12p40. Examination of the kinetics of IL-12p35 and IL-12p40 mRNA expression in DC cultures revealed that LPS/IFNγ-induced transcription of IL-12p40, but not IL-12p35, mRNA was inhibited in LGTV-infected WT DCs (Fig. 5A and 5B). The inhibitory effect of LGTV infection on IL-12p40 mRNA expression was nearly eliminated in Ifnar−/− DCs at 8 and 16 h post-stimulation (Fig. 5C) suggesting a contribution of IFN-I antagonism at these time points. However, an IFN-I-independent block in IL-12p40 expression remained evident at 3 h post-stimulation. Expression of IL-12p40 mRNA is regulated by multiple transcription factors, predominantly NF-κB, IRF1, IRF2 and IRF8 (33–37). A block associated with NF-κB could be ruled out because flaviviruses are known to activate this transcription factor (38) and LGTV infection of DCs had no inhibitory effect on the expression of cytokines driven by NF-κB, such as TNF-α and IL-6. Therefore, we examined the role of IRF-1 (34, 35) by utilizing DCs derived from Irf-1−/− mice. LPS/IFNγ-stimulated IL-12p40 mRNA expression was not different between LGTV- and mock-infected Irf-1−/− DCs (Fig. 5D). Thus, LGTV suppression of IL-12p40 mRNA is dependent on IRF-1, particularly during early gene induction, identifying IRF-1 as a potential target of viral antagonism.
Figure 5.
LGTV suppresses IL-12p40 mRNA expression by a mechanism dependent on IFN-I signaling and IRF-1. DCs derived from WT, Ifnar−/− or Irf-1−/− mice were mock-infected or infected with LGTV for 28 h and then stimulated with LPS/IFNγ. IL-12p35 and IL-12p40 mRNA expression was determined by quantitative RT-PCR and the fold induction was calculated relative to mock-infected, untreated controls. The graphs show the kinetics of mRNA expression for IL-12p35 in WT DCs(A) or IL-12p40 in WT DCs (B), Ifnar−/− DCs (C) and Irf-1−/− DCs (D). Data represent mean fold induction ± SEM of at least 3 independent experiments. *P<0.05 compared to mock-infected, treated at the same time point.
LGTV infection reduces IRF-1 nuclear localization and transcriptional activity
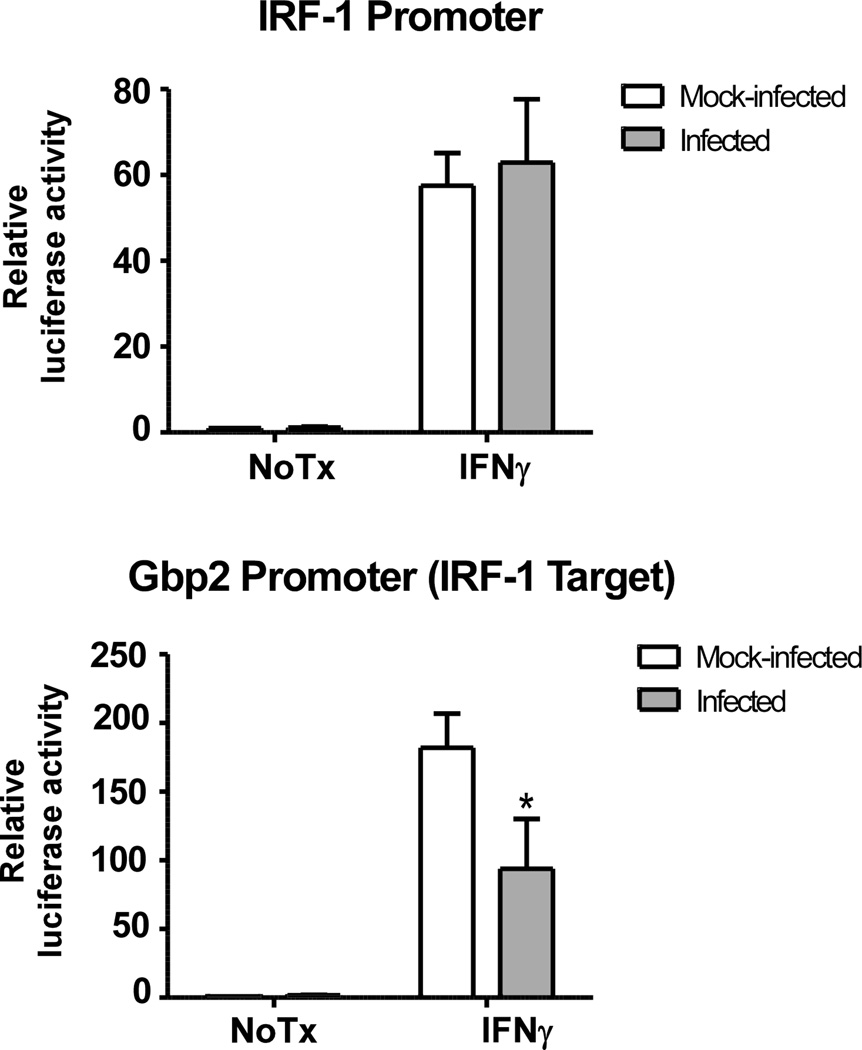
To examine whether LGTV directly affects IRF-1 transcriptional activity, luciferase reporter assays were performed in Huh7 cells known to express IRF-1 (39) using two plasmids: one containing an IRF-1 responsive Gbp-2 promoter and another containing the Irf-1 gene promoter (31, 40). IFNγ stimulation resulted in 60- to 80-fold induction of luciferase expression driven by the Irf-1 gene promoter in both mock- and LGTV-infected cells (Fig. 6). However, LGTV infection reduced reporter activity driven by the IRF-1 responsive Gbp2 promoter compared to mock-infected cells (Fig. 6) suggesting that LGTV can antagonize IRF-1 function.
Figure 6.
LGTV attenuates IRF-1 transcriptional activity. Huh7 cells were transfected with l IRF-1 or Gbp-2 luciferase reporter plasmids 24 h prior to infection with LGTV (MOI 3). At 24 hpi, cells were treated with or without IFNγ for 6 h and luciferase enzyme luminescent activity was measured. Luciferase activity was normalized to the constitutive luciferase activity of pTK-Renilla and expressed as fold change over the activity of mock-infected, unstimulated control. Data represent the mean ± SD of the fold change from three independent experiments. *P<0.05.
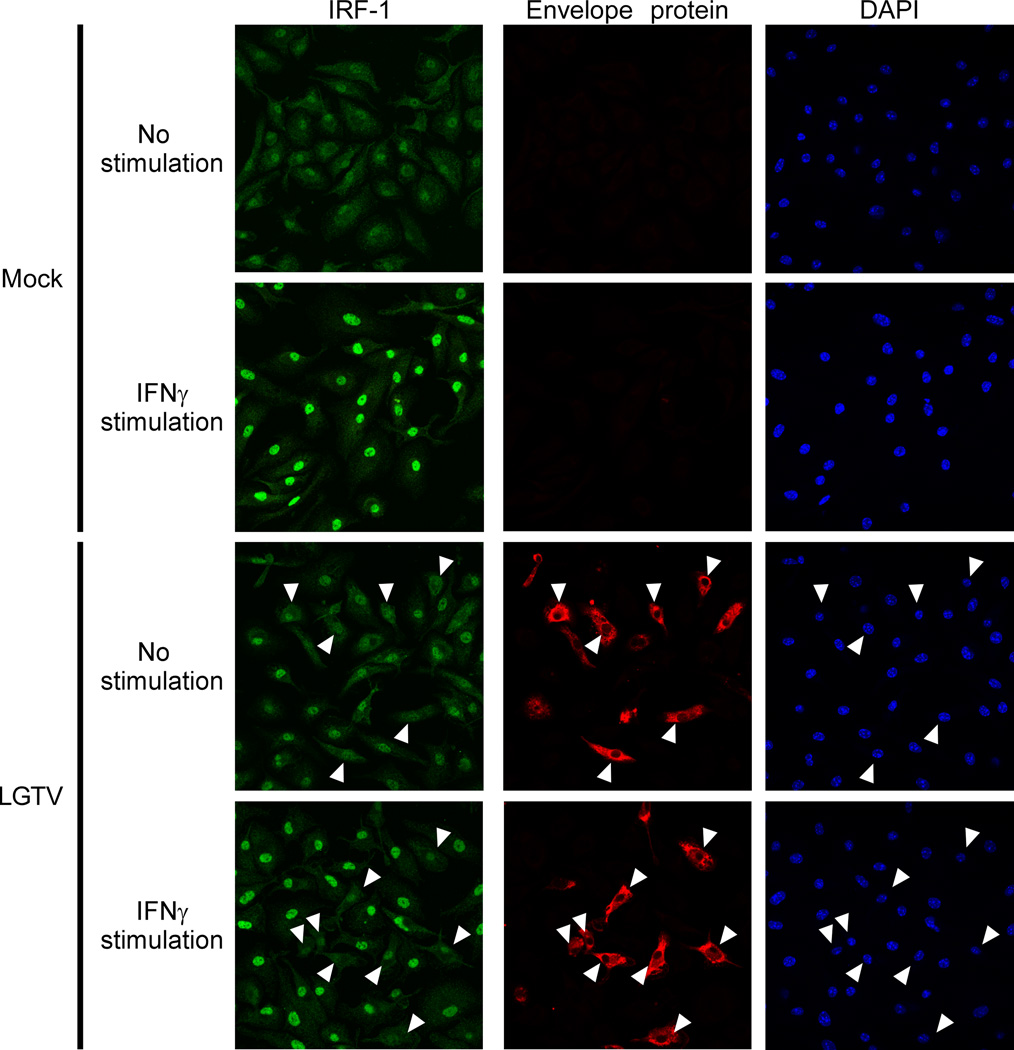
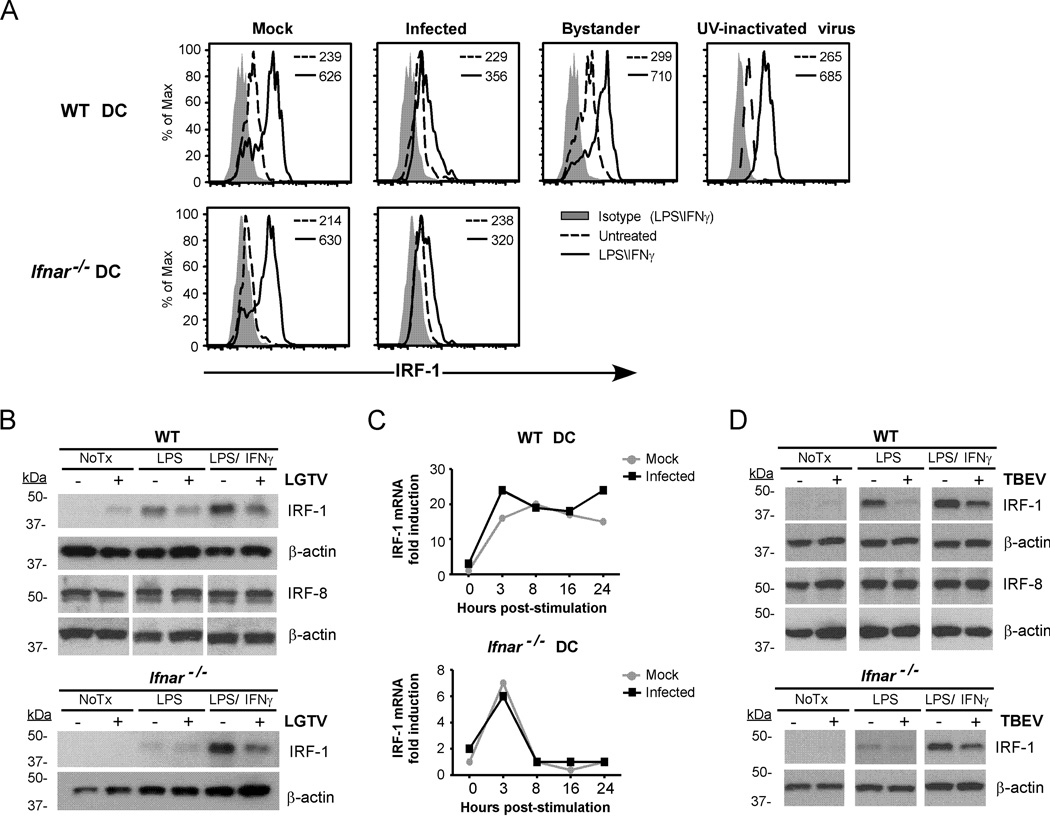
To examine this possibility further, nuclear localization of IRF-1 in infected WT DCs was assessed by IFA using antibodies specific for IRF-1 and viral envelope protein. IRF-1 clearly accumulated in the cell nucleus of LPS/IFNγ–stimulated mock-infected DCs compared to unstimulated controls (Fig. 7; upper panels). In stimulated LGTV-infected cultures, strong nuclear localization of IRF-1 was observed in bystander cells (Fig. 7; lower panels) but was obviously diminished in LGTV-positive DCs. This was associated with reduced levels of IRF-1 protein in LGTV-positive cells as shown by flow cytometry (Fig. 8A) and western blot (Fig. 8B). LGTV infection alone induced low levels of IRF-1 in WT DCs (Fig. 8B), but this occurred only in bystander cells as indicated by flow cytometry. Loss of IRF-1 protein was independent of viral IFN-I antagonism as it occurred in both WT and Ifnar−/− DCs, but dependent on virus replication as it was not evident in cells incubated with UV-inactivated virus (Fig. 8A). Expression of IRF-8, another positive regulator of IL-12-p40 (41), was unaffected by LGTV infection (Fig. 8B). Furthermore, consistent with the luciferase reporter experiments, LGTV had no effect on the induction of Irf-1 mRNA expression by LPS/IFNγ in either WT or Ifnar−/− DCs (Fig. 8C). Thus, LGTV infection specifically depletes cellular levels of IRF-1 protein without affecting Irf-1 mRNA expression. Finally, we extended our analysis to include the highly pathogenic Sofjin strain of TBEV. WT or Ifnar−/− DCs infected with TBEV and stimulated with LPS or LPS/IFNγ for 3h had reduced IRF-1 protein compared to mock-infected controls, whereas IRF-8 expression was not different (Fig. 8D). Taken together, these studies suggest that both LGTV and TBEV antagonize IRF-1 by reducing IRF-1 protein levels and nuclear localization.
Figure 7.
LGTV inhibits IRF-1 nuclear localization. At 28 h post-infection, mock- and LGTV-infected WT DCs were treated with LPS/IFNγ for 3 h and analyzed by immunofluorescence. Cells were stained for IRF-1 (green) and viral envelope protein (red). Nuclei were counterstained with DAPI (blue). Examples of cells that are positive for viral envelope protein and show diminished nuclear IRF-1 staining are indicated by the arrowheads in all viral envelope protein, IRF-1 and DAPI panels. Images are representative of three independent experiments.
Figure 8.
Flavivirus infection of DCs diminishes IRF-1 protein levels without affecting Irf-1 mRNA expression. WT or Ifnar−/− DCs were mock-infected, infected with LGTV or exposed to UV-inactivated virus for 28 h. Cells were treated with medium alone, or medium containing LPS/IFNγ. A, Histograms for intracellular IRF-1 expression are shown for mock-infected DC cultures, gated infected cells or bystander cells from infected cultures and DCs exposed to UV-inactivated virus as indicated. Most DCs (70–90%) were infected in Ifnar−/− cultures and therefore IRF-1 expression in bystander cells was not determined. MFI values of IRF-1 expression in untreated and stimulated samples are shown. B, Total protein lysates were assayed for IRF-1 and IRF-8 by immunoblot. Duplicate blots were probed for β-actin as a loading control. C, RNA was isolated at various times post-stimulation and Irf-1 mRNA expression was determined by quantitative RT-PCR. The data shown are representative of three independent experiments. D, WT and Ifnar−/− DCs were infected with TBEV (Sofjin strain) for 28h and left untreated, or stimulated with LPS or LPS/IFNγ for 3 h. IRF-1 and IRF-8 were detected by immunoblot. Blots were stripped and reprobed for β-actin. A representative immunoblot of three independent experiments for both LGTV and TBEV is shown.
Discussion
IRF-1 is emerging as an important regulator of early cellular responses to virus infection, responsible for induction of antiviral effector genes that partly overlap with, but do not require, IFN-I (25, 26). Hence, IRF-1 may provide cellular resistance to viral infection at a crucial time following infection before IFN-I can be expressed. Here, we demonstrate that tick-borne flaviviruses inhibit IRF-1 independently of their ability to antagonize IFN-I signaling. One outcome of IRF-1 antagonism in virus-infected DCs was a failure in early IL-12p40 gene induction resulting in reduced IL-12p40 and IL-12p70 protein expression. This suppressive effect was compounded by a requirement for signaling through IFNAR to amplify and sustain IL-12p40 expression, which is potently inhibited in LGTV or TBEV infected cells ((19, 22)). The cumulative effect was a disruption of DC function in driving T cell proliferation. Thus, our studies have revealed that the tick-borne flaviviruses, LGTV and TBEV, coordinate suppression of signals through both IRF-1 and IFNAR in a multifaceted strategy to avoid early antiviral immunity.
DCs are critical for adaptive immunity against flavivirus infections, particularly for generation of cytotoxic T lymphocyte (CTL) responses (42). Following virus infection, innate responses control the magnitude and quality of the adaptive immune response by modulating the function of DCs (reviewed in (5)). Specifically, both signaling through PRR and secreted IFN are important for phenotypic and functional maturation of DCs. LGTV infection inhibited upregulation of DC costimulatory molecules, thereby resulting in an immature and potentially tolerogenic phenotype. Furthermore, we previously showed that LGTV infection inhibits IFN-induced STAT1 phosphorylation in both human monocyte-derived DCs and mouse bone marrow-derived DCs (19). Therefore, we can speculate that the lack of DC maturation following LGTV infection involves IFN-I antagonism. Consistent with this hypothesis, inhibition of IFN-I signaling using an anti-IFNAR monoclonal antibody during WNV infection of mice resulted in decreased expression of CD86 on antigen presenting cells, lower levels of IL-12p40 production and elevated levels of IL-10, all factors that likely diminish optimal antigen presentation to CD8+ T cells (43). WNV also antagonizes IFN-I-dependent signaling (23, 44). Collectively, these data suggest that, similar to other models of virus infection (45, 46), a significant link exists between flavivirus IFN-I antagonism and efficient priming and development of adaptive immune responses. However, it is also possible that WNV suppresses IRF-1, as Scherbik et al. (47) reported delayed Irf1 mRNA transcription and decreased IRF-1 protein levels in WNV infected mouse embryonic fibroblasts although they did not explore this finding any further. Thus, it is possible that suppression of IRF-1 and IFN-I is a common feature of multiple flaviviruses.
To uncover flavivirus antagonism of IRF-1, our studies utilized LPS/IFNγ as a classic, potent stimulus of DC maturation, cytokine production and driver of T cell proliferation (48–50). Aspects of inhibition were also seen with TLR3 stimulation (poly I:C-stimulated CD86 expression) or with IFNγ alone (IRF-1-dependent luciferase expression) suggesting that viral antagonism is not restricted to TLR4 signaling. Interestingly, suppression was not observed following TLR7 stimulation (loxoribine), which may reflect the relative importance of IRF-1 and IFN-I in each pathway. Alternatively, TLR7 may not be a strong enough stimulus to reveal the block in IRF-1 signaling.
The regulation of biologically active IL-12 production is complex and involves the coordinated expression of each of its subunits, IL-12p35 and IL-12p40. We observed suppression of IL-12p40 expression, but not IL-12p35, suggesting that signaling events proximal to TLRs are not affected by infection. Because IRF-1 can regulate the expression of both IL-12p40 and IL-12p35, it was surprising that IL-12p35 was not negatively affected by LGTV. However, due to the complexity of the two promoters it is possible that, at least in the context of murine DCs, IL-12p35 is less dependent on IRF-1 or its expression is compensated by other transcription factors.
IL-12p40 forms additional complexes with IL-23p19 to produce IL-23, also expressed by macrophages and DCs (51). IL-23 is an important stimulator of memory T-cell proliferation, and can promote CD4+ T cells to differentiate into Th17 cells that have central roles in inflammation(51). Interestingly, IL-12p40 was critical for resistance to WNV-induced disease, necessary for IL-23-dependent homing of macrophages to the central nervous system (CNS) and control of virus replication (52). Thus, it is possible that flavivirus suppression of IL-12p40 expression promotes virus pathogenesis through suppression of both IL-12 and IL-23.
IL-12p40 is likely to be only one of many gene products affected by viral degradation of IRF-1. In support of this, IRF-1 restricts WNV replication in macrophages independently of IFN-I levels, suggesting that IRF-1 transcriptional targets include ISGs that directly inhibit virus replication (27). The relative role of IRF-1 in induction of specific genes is complex and depends on the cell type, the availability of other transcription factors (e.g., IRF-2), and the gene itself. For example, transcription of the gene encoding class II transactivator (CIITA), the major regulator of MHCII expression, is controlled by three independent promoters that have differential cell-type specific usage and requirements for IRF-1 (53). Non-hematopoietic cells (endothelial, fibroblasts, and astrocytes, etc) utilize a promoter that contains an IRF-1 binding site and involves IFNγ-induced expression of IRF-1, whereas professional antigen presenting cells, such as DCs, utilize a distinct promoter for constitutive CIITA expression that does not require IRF-1. Thus, virus modulation of IRF-1 may have specific and unique consequences to primary cellular targets of flavivirus infection, including DCs, endothelia, fibroblasts, and neurons, as has been suggested for WNV infection (27).
Our findings suggest that LGTV and TBEV interfere with IRF-1 function in a cell type- and species-independent manner. Virus infection had no effect on the expression of Irf1 mRNA in infected mouse DCs, or on the amplitude of reporter gene expression driven by the Irf1 promoter in human Huh7 cells following stimulation with IFNγ. This was surprising considering the ability of many flaviviruses including LGTV to inhibit IFNγ-dependent STAT1 phosphorylation (19). It is possible that virus antagonism of IFNγ signaling is cell-type dependent or that alternative transcription factors drive Irf1 expression. In reference to the latter, NF-κB can function as an alternative regulator of IRF-1 transcription, particularly in the absence of STAT1 (31). Nevertheless, IRF-1-dependent gene expression was suppressed in LGTV-infected Huh7 cells suggesting that IRF-1 function was compromised. Diminished levels of IRF-1 were observed in LGTV or TBEV infected DCs, although only apparent after stimulation. This observation, together with the fact that IRF-1 degradation was incomplete, raises the intriguing possibility that only the activated form of IRF-1 is modulated by flavivirus infection. An incomplete loss of IRF-1 may be sufficient to suppress gene expression because, in the absence of high levels of IRF-1, IRF-2 competes with IRF-1 for promoter binding and represses target gene transcription (54).
The finding that LGTV infection diminished IRF-1 protein levels without affecting Irf-1 mRNA expression suggests that the protein may be targeted for enhanced degradation. IRF-1 has a short half-life (~30 min) and is normally turned over in the cell via the ubiquitin-proteosome degradative pathway. Interestingly, treatment of LGTV-infected DCs with inhibitors of the proteasome or lysosome, another major cellular pathway of protein degradation, failed to recover IRF-1 protein levels in LGTV-infected DCs (S. Robertson and S. Best, unpublished observations). It is possible that IRF-1 is more directly targeted as a substrate for flavivirus-encoded proteases (i.e., NS2B/3) or a cytosolic cellular protease. Alternatively, tick-borne flaviviruses may have evolved a strategy to inhibit nuclear export or translation of Irf-1 mRNA. These possibilities will be the subject of future studies to define the molecular mechanism by which tick-borne flaviviruses inhibit IRF-1.
Other viral and bacterial pathogens also antagonize IRF-1 as a means of dampening host innate immunity. In contrast to LGTV and TBEV, suppression of IRF-1 by hepatitis C virus (HCV), a member of the Flaviviridae family, occurs at the level of transcription and is associated with capsid expression (55). HCV is also able to prevent IRF-1 activation in response to dsRNA stimulation via NS5A-mediated suppression of protein kinase R activation (39). Vaccinia virus, as well as other members of the poxvirus family, encodes two host-range genes (K1L and C7L) that suppress IRF-1-dependent restriction of virus replication(56). In this case, vaccinia virus appears to inhibit a critical IRF-1-inducible factor rather than directly targeting IRF-1. In bacteria, adenylate cyclase toxin (CyaA) of Bordetalla pertussis suppresses LPS/IFNγ-induced expression of IRF-1 and IRF-8 mRNA expression (57). In addition, lipids isolated from virulent Francisella tularensis strain (SchuS4) prevents binding of IRF-1 and IRF-8 to the human IL-12p40 promoter thereby inhibiting IL-12p40 production in primary human DCs (58). Thus, the broad spectrum of pathogens shown to interfere with IRF-1 function underscores the importance of this factor in the host-pathogen interface.
Because DCs are early cellular targets of flavivirus infection, virus modulation of IRF-1 and IFN-I signaling in DCs offers an opportunity to manipulate innate and adaptive immune responses. Important insight into the pathogenic role of flavivirus antagonism of IRF-1 will come from identifying the viral protein responsible and its host cell interacting partner(s). The pathogenesis of recombinant viruses that can no longer antagonize IRF-1 can then be studied. Elucidation of the IRF-1-specific transcriptional response as it relates to restriction of flavivirus replication will also be important for a complete understanding of both the anti-flavivirus innate immune response and the consequence of IRF-1 antagonism to viral pathogenesis.
Acknowledgements
We thank Anita Mora for assistance with graphics and Heinz Feldmann, Jeff Shannon and Catherine Bosio for critical review of the manuscript. The work with TBEV was conducted in compliance with regulation 42 CFR 73 under the Division of Select Agents and Toxins (Centers for Disease Control and Prevention).
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID).
References
- 1.Gritsun TS, Lashkevich VA, Gould EA. Tick-borne encephalitis. Antiviral Res. 2003;57:129–146. doi: 10.1016/s0166-3542(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 2.Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet. 2008;371:1861–1871. doi: 10.1016/S0140-6736(08)60800-4. [DOI] [PubMed] [Google Scholar]
- 3.Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, Solomon T. Tick-borne encephalitis virus - a review of an emerging zoonosis. J. Gen. Virol. 2009;90:1781–1794. doi: 10.1099/vir.0.011437-0. [DOI] [PubMed] [Google Scholar]
- 4.Dobler G. Zoonotic tick-borne flaviviruses. Vet. Microbiol. 2010;140:221–228. doi: 10.1016/j.vetmic.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 6.Labuda M, Austyn JM, Zuffova E, Kozuch O, Fuchsberger N, Lysy J, Nuttall PA. Importance of localized skin infection in tick-borne encephalitis virus transmission. Virology. 1996;219:357–366. doi: 10.1006/viro.1996.0261. [DOI] [PubMed] [Google Scholar]
- 7.Chase AJ, Medina FA, Muñoz-Jordán JL. Impairment of CD4+ T cell polarization by dengue virus-infected dendritic cells. J. Infect. Dis. 2011;203:1763–1774. doi: 10.1093/infdis/jir197. [DOI] [PubMed] [Google Scholar]
- 8.Palmer DR, Sun P, Celluzzi C, Bisbing J, Pang S, Sun W, Marovich MA, Burgess T. Differential effects of dengue virus on infected and bystander dendritic cells. J. Virol. 2005;79:2432–2439. doi: 10.1128/JVI.79.4.2432-2439.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nightingale ZD, Patkar C, Rothman AL. Viral replication and paracrine effects result in distinct, functional responses of dendritic cells following infection with dengue 2 virus. J. Leukoc. Biol. 2008;84:1028–1038. doi: 10.1189/jlb.0208105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian F, Wang X, Zhang L, Lin A, Zhao H, Fikrig E, Montgomery RR. Impaired interferon signaling in dendritic cells from older donors infected in vitro with west nile virus. J. Infect. Dis. 2011;203:1415–1424. doi: 10.1093/infdis/jir048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aleyas AG, George JA, Han YW, Rahman MM, Kim SJ, Han SB, Kim BS, Kim K, Eo SK. Functional modulation of dendritic cells and macrophages by Japanese encephalitis virus through MyD88 adaptor molecule-dependent and -independent pathways. J. Immunol. 2009;183:2462–2474. doi: 10.4049/jimmunol.0801952. [DOI] [PubMed] [Google Scholar]
- 12.Aleyas AG, Han YW, George JA, Kim B, Kim K, Lee C-K, Eo SK. Multifront assault on antigen presentation by Japanese encephalitis virus subverts CD8+ T cell responses. J. Immunol. 2010;185:1429–1441. doi: 10.4049/jimmunol.0902536. [DOI] [PubMed] [Google Scholar]
- 13.Cao S, Li Y, Ye J, Yang X, Chen L, Liu X, Chen H. Japanese encephalitis Virus wild strain infection suppresses dendritic cells maturation and function, and causes the expansion of regulatory T cells. Virol. J. 2011;8:39. doi: 10.1186/1743-422X-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuel MA, Whitby K, Keller BC, Marri A, Barchet W, Williams BRG, Silverman RH, Gale M, Diamond MS. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 2006;80:7009–7019. doi: 10.1128/JVI.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szretter KJ, Brien JD, Thackray LB, Virgin HW, Cresswell P, Diamond MS. The interferon-inducible gene viperin restricts West Nile virus pathogenesis. J. Virol. 2011;85:11557–11566. doi: 10.1128/JVI.05519-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daffis S, Szretter KJ, Schriewer J, Li J, Youn S, Errett J, Lin T-Y, Schneller S, Zust R, Dong H, Thiel V, Sen GC, Fensterl V, Klimstra WB, Pierson TC, Buller RM, Gale M, Shi P-Y, Diamond MS. 2'-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor RT, Lubick KJ, Robertson SJ, Broughton JP, Bloom ME, Bresnahan WA, Best SM. TRIM79α, an interferon-stimulated gene product, restricts tick-borne encephalitis virus replication by degrading the viral RNA polymerase. Cell Host Microbe. 2011;10:185–196. doi: 10.1016/j.chom.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 19.Best SM, Morris KL, Shannon JG, Robertson SJ, Mitzel DN, Park GS, Boer E, Wolfinbarger JB, Bloom ME. Inhibition of interferon-stimulated JAK-STAT signaling by a tick-borne flavivirus and identification of NS5 as an interferon antagonist. J. Virol. 2005;79:12828–12839. doi: 10.1128/JVI.79.20.12828-12839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones M, Davidson A, Hibbert L, Gruenwald P, Schlaak J, Ball S, Foster GR, Jacobs M. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 2005;79:5414–5420. doi: 10.1128/JVI.79.9.5414-5420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin R-J, Chang B-L, Yu H-P, Liao C-L, Lin Y-L. Blocking of interferon-induced Jak-Stat signaling by Japanese encephalitis virus NS5 through a protein tyrosine phosphatase-mediated mechanism. J. Virol. 2006;80:5908–5918. doi: 10.1128/JVI.02714-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werme K, Wigerius M, Johansson M. Tick-borne encephalitis virus NS5 associates with membrane protein scribble and impairs interferon-stimulated JAK-STAT signalling. Cell. Microbiol. 2008;10:696–712. doi: 10.1111/j.1462-5822.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- 23.Laurent-Rolle M, Boer EF, Lubick KJ, Wolfinbarger JB, Carmody AB, Rockx B, Liu W, Ashour J, Shupert WL, Holbrook MR, Barrett AD, Mason PW, Bloom ME, García-Sastre A, Khromykh AA, Best SM. The NS5 protein of the virulent West Nile virus NY99 strain is a potent antagonist of type I interferon-mediated JAK-STAT signaling. J. Virol. 2010;84:3503–3515. doi: 10.1128/JVI.01161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 25.Dixit E, Boulant S, Zhang Y, Lee ASY, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, Nibert ML, Superti-Furga G, Kagan JC. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141:668–681. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brien JD, Daffis S, Lazear HM, Cho H, Suthar MS, Gale M, Diamond MS. Interferon regulatory factor-1 (IRF-1) shapes both innate and CD8+ T cell immune responses against West Nile virus infection. PLoS Pathog. 2011;7:e1002230. doi: 10.1371/journal.ppat.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EEM, Trinchieri G, Caux C, Garrone P. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 31.Kumar A, Yang YL, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams BR. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-kappaB. The EMBO Journal. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 33.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol. Cell. Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taki S, Sato T, Ogasawara K, Fukuda T, Sato M, Hida S, Suzuki G, Mitsuyama M, Shin EH, Kojima S, Taniguchi T, Asano Y. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity. 1997;6:673–679. doi: 10.1016/s1074-7613(00)80443-4. [DOI] [PubMed] [Google Scholar]
- 35.Salkowski CA, Kopydlowski K, Blanco J, Cody MJ, McNally R, Vogel SN. IL-12 is dysregulated in macrophages from IRF-1 and IRF-2 knockout mice. J. Immunol. 1999;163:1529–1536. [PubMed] [Google Scholar]
- 36.Maruyama S, Sumita K, Shen H, Kanoh M, Xu X, Sato M, Matsumoto M, Shinomiya H, Asano Y. Identification of IFN regulatory factor-1 binding site in IL-12 p40 gene promoter. J. Immunol. 2003;170:997–1001. doi: 10.4049/jimmunol.170.2.997. [DOI] [PubMed] [Google Scholar]
- 37.Wang IM, Contursi C, Masumi A, Ma X, Trinchieri G, Ozato K. An IFN-gamma-inducible transcription factor, IFN consensus sequence binding protein (ICSBP), stimulates IL-12 p40 expression in macrophages. J. Immunol. 2000;165:271–279. doi: 10.4049/jimmunol.165.1.271. [DOI] [PubMed] [Google Scholar]
- 38.Chang T-H, Liao C-L, Lin Y-L. Flavivirus induces interferon-beta gene expression through a pathway involving RIG-I-dependent IRF-3 and PI3K-dependent NF-kappaB activation. Microbes Infect. 2006;8:157–171. doi: 10.1016/j.micinf.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Pflugheber J, Fredericksen B, Sumpter R, Wang C, Ware F, Sodora DL, Gale M. Regulation of PKR and IRF-1 during hepatitis C virus RNA replication. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4650–4655. doi: 10.1073/pnas.062055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briken V, Ruffner H, Schultz U, Schwarz A, Reis LF, Strehlow I, Decker T, Staeheli P. Interferon regulatory factor 1 is required for mouse Gbp gene activation by gamma interferon. Mol. Cell. Biol. 1995;15:975–982. doi: 10.1128/mcb.15.2.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. Interferon consensus sequence binding protein-deficient mice display impaired resistance to intracellular infection due to a primary defect in interleukin 12 p40 induction. J. Exp. Med. 1997;186:1523–1534. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto AK, Daffis S, Brien JD, Gainey MD, Yokoyama WM, Sheehan KCF, Murphy KM, Schreiber RD, Diamond MS. A temporal role of type I interferon signaling in CD8+ T cell maturation during acute West Nile virus infection. PLoS Pathog. 2011;7:e1002407. doi: 10.1371/journal.ppat.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo J-T, Hayashi J, Seeger C. West Nile virus inhibits the signal transduction pathway of alpha interferon. J. Virol. 2005;79:1343–1350. doi: 10.1128/JVI.79.3.1343-1350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munir S, Le Nouën C, Luongo C, Buchholz UJ, Collins PL, Bukreyev A. Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells. J. Virol. 2008;82:8780–8796. doi: 10.1128/JVI.00630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munir S, Hillyer P, Le Nouën C, Buchholz UJ, Rabin RL, Collins PL, Bukreyev A. Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLoS Pathog. 2011;7:e1001336. doi: 10.1371/journal.ppat.1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scherbik SV, Stockman BM, Brinton MA. Differential expression of interferon (IFN) regulatory factors and IFN-stimulated genes at early times after West Nile virus infection of mouse embryo fibroblasts. J. Virol. 2007;81:12005–12018. doi: 10.1128/JVI.01359-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snijders A, Kaliński P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 1998;10:1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 49.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kaliński P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 2000;164:4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Cao S, Herman LM, Ma X. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-gamma-primed IL-12 production by IFN regulatory factor 1. J. Exp. Med. 2003;198:1265–1276. doi: 10.1084/jem.20030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 52.Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, Anderson JF, Flavell RA, Fikrig E. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30:242–253. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reith W, LeibundGut-Landmann S, Waldburger J-M. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- 54.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 55.Ciccaglione AR, Stellacci E, Marcantonio C, Muto V, Equestre M, Marsili G, Rapicetta M, Battistini A. Repression of interferon regulatory factor 1 by hepatitis C virus core protein results in inhibition of antiviral and immunomodulatory genes. J. Virol. 2007;81:202–214. doi: 10.1128/JVI.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng X, Schoggins J, Rose L, Cao J, Ploss A, Rice CM, Xiang Y. C7L family of poxvirus host range genes inhibits antiviral activities induced by type I interferons and interferon regulatory factor 1. J. Virol. 2012;86:4538–4547. doi: 10.1128/JVI.06140-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hickey FB, Brereton CF, Mills KHG. Adenylate cycalse toxin of Bordetella pertussis inhibits TLR-induced IRF-1 and IRF-8 activation and IL-12 production and enhances IL-10 through MAPK activation in dendritic cells. J. Leukoc. Biol. 2008;84:234–243. doi: 10.1189/jlb.0208113. [DOI] [PubMed] [Google Scholar]
- 58.Ireland R, Wang R, Alinger JB, Small P, Bosio CM. Francisella tularensis SchuS4 and SchuS4 Lipids Inhibit IL-12p40 in Primary Human Dendritic Cells by Inhibition of IRF1 and IRF8. J. Immunol. 2013;191:1276–1286. doi: 10.4049/jimmunol.1300867. [DOI] [PMC free article] [PubMed] [Google Scholar]