Abstract
Purpose
To update eligibility and outcome measures in trials that evaluate systemic treatment for patients with progressive prostate cancer and castrate levels of testosterone.
Methods
A committee of investigators experienced in conducting trials for prostate cancer defined new consensus criteria by reviewing previous criteria, Response Evaluation Criteria in Solid Tumors (RECIST), and emerging trial data.
Results
The Prostate Cancer Clinical Trials Working Group (PCWG2) recommends a two-objective paradigm: (1) controlling, relieving, or eliminating disease manifestations that are present when treatment is initiated and (2) preventing or delaying disease manifestations expected to occur. Prostate cancers progressing despite castrate levels of testosterone are considered castration resistant and not hormone refractory. Eligibility is defined using standard disease assessments to authenticate disease progression, prior treatment, distinct clinical subtypes, and predictive models. Outcomes are reported independently for prostate-specific antigen (PSA), imaging, and clinical measures, avoiding grouped categorizations such as complete or partial response. In most trials, early changes in PSA and/or pain are not acted on without other evidence of disease progression, and treatment should be continued for at least 12 weeks to ensure adequate drug exposure. Bone scans are reported as “new lesions” or “no new lesions,” changes in soft-tissue disease assessed by RECIST, and pain using validated scales. Defining eligibility for prevent/delay end points requires attention to estimated event frequency and/or random assignment to a control group.
Conclusion
PCWG2 recommends increasing emphasis on time-to-event end points (ie, failure to progress) as decision aids in proceeding from phase II to phase III trials. Recommendations will evolve as data are generated on the utility of intermediate end points to predict clinical benefit.
INTRODUCTION
Evaluating drugs to treat prostate cancer poses unique challenges. Measurable disease occurs infrequently, the natural history may be prolonged over decades, and because the treatment population is elderly, pursuing aggressive therapies may cause more harm than good. In 1999, the Prostate-Specific Antigen Working Group (PCWG1) addressed these challenges in their consensus recommendations for the conduct of clinical trials.1 They focused on trial development for patients with metastatic prostate cancer whose disease was progressing despite castrate levels of testosterone and defined eligibility and outcome measures based on clinically relevant end points, and proposed standards for the use of prostate-specific antigen (PSA). In 2000, a broader collective of cancer researchers introduced New Guidelines to Evaluate the Response to Treatment in Solid Tumors (Response Evaluation Criteria in Solid Tumors [RECIST]).2 This international initiative sought to standardize criteria to assess tumor response in trials for all solid tumors. Although RECIST served some cancer swell, its metrics did not capture some key characteristics of prostate cancer.3 For example, post-therapy changes in PSA, a routinely reported outcome in prostate cancer clinical trials and the primary focus of PCWG1, were not addressed by RECIST. In fact, none of the approved treatments for patients with prostate cancer would be available if trial outcomes were based solely on either the PCWG1 criteria or RECIST.
Since these two initiatives were introduced, the biology and natural history of prostate cancer have become better understood, and diverse new therapies, including bone-targeted agents and signaling inhibitors, have become available for clinical testing. In 2004, the US Food and Drug Administration (FDA) challenged the prostate cancer clinical trials community to rework the eligibility and outcome measures from PCWG1 so they could be applied across the clinical spectrum of the disease. The subsequent process prompted the formation of the Prostate Cancer Clinical Trials Working Group (PCWG2), a collective of international investigators who developed this report through meetings and electronic communication.
This article addresses clinical trials for patients with progressive prostate cancer despite castrate levels of testosterone and frames clinical trial questions for agents that act by diverse mechanisms. The consensus is that researchers should adopt a paradigm in which trial objectives are defined on the basis of controlling, relieving, or eliminating disease manifestations that are present when treatment is initiated, and/or of preventing or delaying disease manifestations expected to occur. This new paradigm expands the focus of prostate cancer clinical trials from traditional outcome measures such as early changes in PSA to time-to-event end points that capture the impact of treatment on important clinical manifestations and indicate when a drug should be stopped as the measure of antitumor effect. It also recommends standardized criteria for assessing patients. A goal of these recommendations is to ensure that a drug is not discontinued because of inappropriate outcome measures before it has had a chance to work.
Although the intent of these guidelines is to maximize the ability of phase II trials to screen or select promising therapies, the eligibility and outcome measures have broad applicability and are relevant to the design and conduct of phase III trials. Incorporation of similar parameters into phase III trials assessing overall survival is encouraged to generate the databases that will allow validation or refinement of the intermediate end points proposed herein.
I. CONCEPTUALIZING THE DISEASE
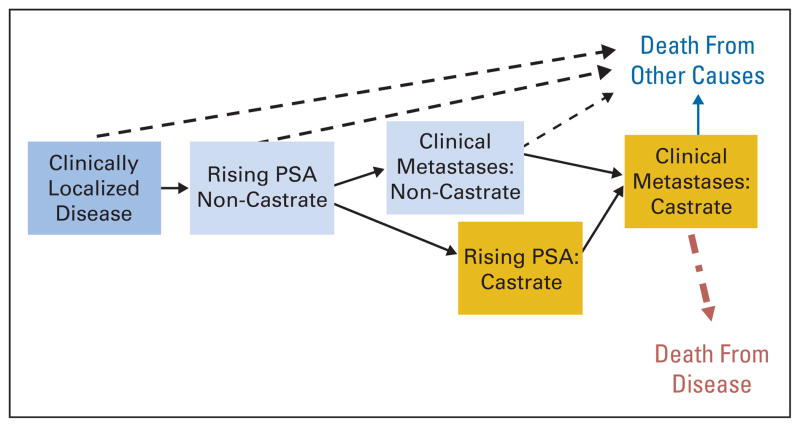
Investigators need to adopt a common language to categorize the clinical spectrum of prostate cancer from diagnosis to metastasis. When PCWG1 was published, no common vocabulary was broadly accepted. PCWG2 categorizes the disease continuum of prostate cancer on the basis of whether metastases are detectable (clinically or by imaging) and whether the serum testosterone level is in the castrate range by a surgical orchiectomy or medical therapy (Fig 1).3,4 Each state on this continuum represents a scenario encountered routinely in clinical practice.
Fig. 1.
Prostate cancer clinical-states model, a framework for patient management and drug development. Modified from Scher et al3,4 with permission. PSA, prostate-specific antigen.
The clinical-states model identifies patients with distinct prognoses who might benefit (or not) from specific therapeutic approaches. The rising PSA states (castrate and noncastrate) signify that no detectable metastatic disease was found in the past or is now present. The clinical metastases states (castrate and noncastrate) signify that disease was detectable at some point in the past, regardless of whether it is detectable now. Along this disease continuum, a patient can only advance. For example, a patient with radiographically evident bone metastases at diagnosis would be assigned to the clinical metastases–noncastrate disease state. If that patient is treated with androgen depletion, no longer has radiographically evident disease, and has a PSA level that is not rising, he remains categorized in the clinical metastases–noncastrate state.
II. DEFINING THERAPEUTIC OBJECTIVES
Since the publication of PCWG1 criteria, clinical investigators have used them to define the primary end points for phase II trials for prostate cancer patients with progressive, castration-resistant disease. These trials are designed to demonstrate whether the therapeutic effects observed justify further evaluation in large-scale phase III trials. Phase III trials characterize the risk/benefit profile of the treatment in relation to either a placebo or established standards, such as time to clinically relevant progression, survival, or quality of life. The clinical-states model offers investigators a framework to standardize phase II end points to appropriately inform phase III end points.
PCWG2 distinguishes two types of phase II trial objectives: (1) those based on controlling, relieving, or eliminating disease manifestations that are present when treatment is initiated, and (2) those based on preventing or delaying future disease manifestations. Traditional measures of response reflect when a treatment is working; measures of progression indicate when a drug should be stopped. Because of the uncertainties associated with assessing response in bone and the controversy surrounding the clinical significance of post-therapy changes in PSA, PCWG2 recommends expanding the focus of phase II trials from measures of response to measures of progression. For most agents, a reliably determined, clinically relevant improvement in time to progression provides the most useful way to assess whether to proceed from a phase II to a phase III trial and may, if reproduced in a randomized, controlled trial, be evidence of clinical benefit from a regulatory perspective.
The drug evaluation pathways for cytotoxic and noncytotoxic agents need to be developed separately. Cytotoxic drugs typically produce a decline in PSA and regression of target lesions, whereas agents that act to slow tumor growth, inhibit destruction of bone, or inhibit angiogenesis may not. For example, a bone-directed therapy may prevent disease-related complications in the skeleton without influencing the growth of soft-tissue disease. Depending on the agent and the study, PCWG2 recommends that the effects of cytotoxic drugs be assessed with both control/relieve/eliminate or prevent/delay end points, and noncytotoxic drugs with prevent/delay end points.
Changes in existing manifestations of disease provide signals whether or not a treatment has produced an antitumor effect at an early stage, even though such changes may not necessarily signify clinical benefit. For example, a declining PSA level may be useful to screen for the activity of a cytotoxic agent, even though it does not mean that the patient will live longer. However, when designing trials with control, relieve, or eliminate end points for patients with symptoms, it is often difficult to distinguish whether a symptom is related to the cancer, prior treatment, comorbidities, or a combination of factors.
Patients who lack discernible disease manifestations (eg, symptomatic bone pain), may be enrolled onto trials with prevent or delay end points that seek to prevent symptoms from occurring in the future. Manifestations that may occur in the future include growth at an existing site of disease, spread to additional sites, an increase in markers, new disease-related symptoms (eg, pain or other skeletal events), and death resulting from disease. The success of trials evaluating prevent or delay end points depends on the ability to define a patient cohort with a defined probability of developing the manifestations that the treatment is designed to prevent and in what time frame. Biases in interpreting the significance of time-to-event end points in phase II trials have been well described and support the case for randomized trial designs.5 Regardless of the end point, it is essential that the trial be designed in a way that does not allow a drug to be discontinued prematurely on the basis of criteria that do not reflect that the treatment was ineffective or failed to benefit the patient.
III. ESTABLISHING ELIGIBILITY FOR ENROLLMENT
After defining the primary end points of efficacy (either control/relieve/eliminate or prevent/delay), investigators can effectively set eligibility criteria. PCWG1 restricted enrollment in trials to patients with progressive disease despite castrate levels of testosterone, based on changes in PSA, measurable disease, and bone scan, while controlling for antiandrogen withdrawal responses to avoid the potential erroneous misattribution of response to a study agent. PCWG2 modifies these eligibility criteria by authenticating disease with standardized assessments, considering the prior treatment history in more detail, defining distinct clinical subtypes, and highlighting the importance of predictive models for future clinical events.
The demonstration of a survival benefit in a phase III trial and a confirmatory trial7 led to the approval of docetaxel in 2004.6 Since then, clinical trials for patients with castrate metastatic disease are being designed in three contexts: before receiving treatment with docetaxel, with agents in combination with docetaxel to improve first-line outcomes, and as second-line treatment for patients with disease that has progressed despite docetaxel. Independent of the context, PCWG2 recommends defining therapeutic objectives in relation to the mechanism of action of the agent under study, documenting disease manifestations at the time treatment is started (Table 1), and serially evaluating patients post-treatment using standard assessments that relate to the objectives of the trial.
Table 1.
Clinical Manifestations of Progressive, Castration-Resistant Prostate Cancer (%)
| Manifestation | MSKCC3 (N = 124) | SWOG-99167 (N = 770) | TAX-3276 (N = 1,106) |
|---|---|---|---|
| Rising PSA | 94 | 90 | 87 |
|
| |||
| Bone | 84 | 88 | 93 |
|
| |||
| Substantive pain | 35 | 36 | 36 |
|
| |||
| Soft-tissue lesions | |||
| Lung/liver | 16 | 19 | 22 |
| Lymph nodes | 24 | 24 | 18 |
|
| |||
| Prostate/prostate bed | 2 | Not specified | Not specified |
Abbreviations: MSKCC, Memorial Sloan-Kettering Cancer Center; SWOG, Southwest Oncology Group; PSA, prostate-specific antigen.
Authenticating Disease Progression
Authenticating disease progression is achieved by establishing standard pretreatment assessments and identifying standard criteria for disease progression for entry.
Pretreatment assessments
PCWG1 did not define a standard pretreatment evaluation, so PCWG2 builds on the standards for base-line evaluations recommended by RECIST and provides guidelines for imaging and symptom assessment.
Baseline evaluations
Baseline evaluations should be tailored both to target outcome measures and to contribute to the development of prognostic factors or other research questions. For the baseline evaluation, PCWG2 recommends documenting patient demographics, including age and performance status, clinical stage, PSA and Gleason score at the time of diagnosis, details and dates of the primary therapy (eg, pathologic stage and/or dose and type of radiation therapy as appropriate), and post-treatment PSA nadir. Details and dates of prior hormonal and nonhormonal therapies should be recorded, along with additional PSA measurements that can be used to estimate PSA doubling times (PSA-DTs). The presence or absence of disease in the primary site should also be documented.
Imaging
PCWG2 pretreatment evaluations include imaging of the chest by plain radiograph or computed tomography (CT), a CT scan or magnetic resonance imaging (MRI) of the abdomen/pelvis, and radionuclide bone scan. To assess local disease, PCWG2 suggests an endorectal MRI or ultrasound of the prostate or prostate bed. For those with symptoms of neurologic compromise, PCWG2 recommends MRI of the spine and base of the skull. PCWG2 also recognizes that detecting metastases will improve as more sensitive imaging tests become standard, but does not recommend positron emission tomography (PET) using fluorodeoxyglucose or other tracers and ProstaScint (Cytogen Corp, Princeton, NJ) scanning because they are considered investigational at this time.
Symptoms and health-related quality of life
When enrolling a patient onto a clinical trial that incorporates symptoms and health-related quality of life, symptoms of disease should be characterized at baseline using validated instruments according to standards defined by the FDA in its guidance for patient-reported outcomes.8 The evaluation should include confirmation that patient input was included during development of the measure(s); that the content and the construct were validated; and that the measure(s) were reliable for the population being studied. A lead-in period of observation is advised to ensure adequate baseline assessments. Potentially relevant domains include pain, fatigue, anorexia/weight loss, constipation, and urinary symptoms.6,8
The use of pain relief as a trial end point may be particularly valuable because the presence of pain is a known prognostic factor for survival,9 and palliation of symptoms is a therapeutic goal. An acceptable criterion for trial enrollment is new pain in an area of radiographically evident disease. Pain measures in particular should include assessments of intensity, frequency, and duration quantified (eg, on a five-point scale such as the McGill-Melzack Pain Questionnaire or IMMPACT [Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials] recommendations).10 Level of bother, location(s), likely relationship to prostate cancer (v prior therapy or comorbidities), and analgesic requirements should also be recorded.
Health-related quality of life can also be evaluated through patient self-reported instruments that have been developed in keeping with the FDA guidance. In addition to assessing selected symptoms, these instruments may emphasize the effects of disease on physical, social, psychological/emotional, and cognitive functioning. New instruments should also be developed, recognizing that validation is laborious and is generally outside the realm of a phase II trial.
Criteria for disease progression
PCWG1 defined progression criteria for enrollment on the basis of changes in PSA, bone metastases, and measurable disease. PCWG2 retains most of the original recommendations with modifications (Table 2).
Table 2.
Criteria of Progression for Trial Eligibility By Disease Manifestation
| Variable | PCWG1 (1999)1 | PCWG2 (2007) |
|---|---|---|
| PSA | Obtain sequence of rising values at ≥ 1-week intervals 5.0 ng/mL minimum level for entry |
Obtain sequence of rising values at a minimum of 1-week intervals 2.0 ng/mL minimum starting value Estimate pretherapy PSA-DT if 3 or more values available 4 or more weeks apart |
| Target lesions | Nodal or visceral site progression sufficient for trial entry independent of PSA Measurable disease not required for trial entry |
Nodal or visceral progression sufficient for trial entry independent of PSA Measurable lesions not required for entry Use RECIST to record soft-tissue (nodal and visceral) lesions as target or nontarget Only lymph nodes ≥ 2 cm in diameter should be used to assess for a change in size Record presence of nodal and/or visceral disease separately |
| Prostate/prostate bed (primary site) | Not addressed | Record prior treatment of primary tumor Perform directed pelvic imaging (CT, MRI, PET/CT, endorectal MRI, transrectal ultrasound) to document presence or absence of disease |
| Bone | Not defined | Progression = appearance of 2 or more new lesions Confirm ambiguous results by other imaging modalities (eg, CT or MRI) |
| Other sites of disease | Not addressed | Patients with treated epidural lesions and no other epidural progression are eligible |
Abbreviations: PCWG1, Prostate-Specific Antigen Working Group 1; PCWG2, Prostate Cancer Clinical Trials Working Group 2; PSA, prostate-specific antigen; PSA-DT, PSA doubling time; RECIST, Response Evaluation Criteria in Solid Tumors; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
PSA
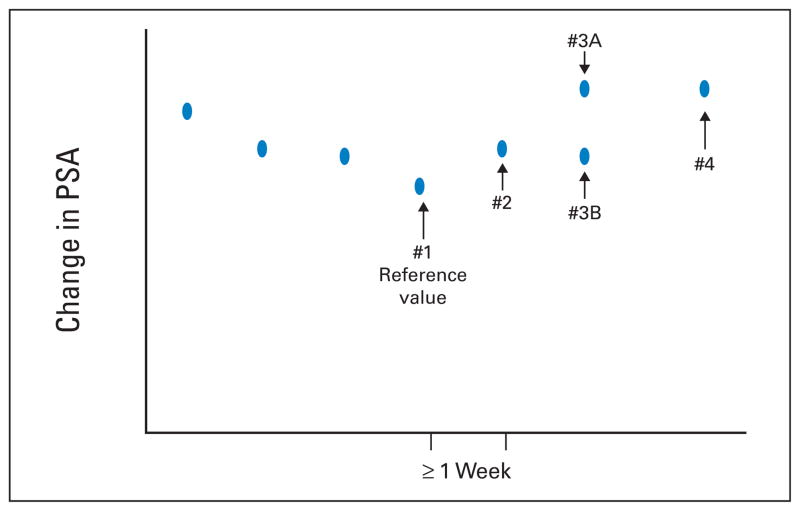
For patients who manifested disease progression solely as a rising PSA level, PCWG1 required obtaining a sequence of rising values at least 1 week apart and made 5.0 ng/mL the minimum starting level for trial entry (Table 2).1 PCWG2 keeps the timing of PSA testing at a minimum of 1-week intervals and recommends reducing the threshold PSA level from 5.0 ng/mL to 2.0 ng/mL because of the availability of more sensitive assays (Fig 2). Given the prognostic significance of the rate of rise in PSA,11 PCWG2 advises estimating a pretreatment PSA-DT11a if at least three values are available, but does not recommend delaying either treatment or enrollment onto a trial simply to estimate PSA-DT.11a
Fig. 2.
Eligibility based on prostate-specific antigen (PSA) changes. The reference value (#1) is the last PSA measured before increases are documented, with subsequent values obtained a minimum of 1 week apart. If the PSA at time point 3 (value #3A) is greater than that at point 2, then eligibility has been met. If the PSA is not greater than point 2 (value #3B), but value #4 is, the patient is eligible assuming that other criteria are met, if values 3A or #4 are 2 ng/mL or higher, a reduction from the 5 ng/mL specified in the previous guidelines.1 Reprinted from Bubley et al.1
Target (nodal and visceral) lesions or measurable disease
A requirement of measurable lesions (target lesions as defined by RECIST) for trial entry is not recommended by PCWG2 because it shifts the emphasis from bone metastases, which develop in upwards of 90% of patients, to lymph nodes, which occur in only 20% to 25% of patients with prostate cancer and contribute less to morbidity than do other sites of metastases.3 The result is that much energy might be wasted on an end point of lesser clinical significance. That said, however, trials that are collecting data on measurable lesions should follow RECIST, and progression in a nodal or visceral site is sufficient to document disease progression. PCWG2 advises recording the presence or absence of nodal and visceral disease (ie, liver and lung) pretreatment and outcomes post-treatment separately. Up to 10 visceral and nodal lesions in total should be recorded (with a maximum of five in any one organ),2 although, in one series, the median number of target lesions was three.3 Because small lymph nodes are difficult to measure accurately and may not be malignant, PCWG2 recommends that the greatest diameter of a lymph node must measure at least 2 cm by spiral CT to be considered a target lesion.3
The prostate (primary site)
PCWG2 recommends both recording the treatments that targeted the primary tumor and performing directed pelvic imaging to determine whether disease is present in this area at the time of enrollment. This should include a CT scan or MRI at a minimum, and, in centers with the relevant expertise, endorectal MRI or transrectal ultrasound. A prostate mass or recurrence in the prostate bed is not considered metastatic disease; many develop in cases where the surgical margins were positive or there was extracapsular extension.
Bone
Even with the improved imaging modalities that have developed since the publication of PCWG1, it is difficult to interpret the clinical significance of changes in size or intensity of bone metastases on bone scan. When the bone scan is the sole indicator of progression, PCWG2 defines progression in bone when at least two or more new lesions are seen on bone scan compared with a prior scan for trial entry. In situations where the scan findings are suggestive of a flare reaction, or apparent new lesion(s) may represent trauma, it may prove useful to confirm these results with other imaging modalities such as MRI or fine-cut CT. Confirmation is generally not necessary if multiple new areas of uptake are observed. Consistent criteria for progression of disease in bone using PET and MRI are still under investigation.
Other sites of disease
PCWG2 recommends that patients who meet other trial criteria may be enrolled if they have epidural disease that has been treated and there is no progression in the treated area. Aside from epidural lesions, other nontarget lesions considered by RECIST (including leptomeningeal spread, ascites, pleural/pericardial effusions, and abdominal masses not confirmed) are rare in prostate cancer.
Evaluating hormonal status and prior systemic therapies
Equally important as pretreatment assessments and in defining disease progression is to evaluate and record historical factors that may affect sensitivity to treatment.
Prior hormonal interventions
PCWG2 considers a “hormonal intervention” the addition or discontinuation of a hormonal therapy with therapeutic intent because of disease progression. Androgen depletion that is discontinued and restarted as part of a planned intermittent or cycling approach is considered a single intervention independent of the number of cycles. PCWG2 recommends that investigators record the prior hormonal interventions received by a patient by documenting the number, type, and duration of administration when available. Although most patients treated with gonadotropin-releasing hormone (GnRH) analog therapy initially or subsequently receive an antiandrogen, many do not. The duration of administration may be as short as 1 month in some and continuous in others. Because a range of outcomes have been reported for secondary hormonal manipulations. PCWG2 advises classifying tumors that are progressing with castrate levels of testosterone as “castration resistant”12; and not “hormone refractory” because many patients respond to second- and third-line hormonal therapies. Patients who are receiving antiandrogens as monotherapy and have noncastrate testosterone levels should receive testosterone-lowering therapy before being considered for trial.
Serum testosterone levels
PCWG1 defined castrate status as a serum testosterone level of less than 50 ng/dL (< 1.7 nmol/L). It is now recognized that the measured level of testosterone in the blood may not accurately reflect intratumoral androgen levels,13,14,14a which are often sufficient to stimulate tumor growth. Sources include the adrenal glands or the tumor itself as a result of the upregulation of the genes involved in androgen synthesis.15,16 Recognizing that some patients have higher testosterone levels despite continued androgen depletion with GnRH analog therapy, and that total testosterone levels do not reflect bioavailable testosterone because of the variation in the levels of sex hormone-binding globulin and the laboratory-to-laboratory variation in the measurement of hormone levels, PCWG2 retains the maximal serum testosterone level of 50 ng/dL (1.7 nmol/L) for entry. PCWG2 also reaffirms that castrate status be maintained for any patient who has not undergone surgical orchiectomy by continued GnRH analog administration, recognizing that, in some patients, testosterone levels might remain suppressed if GnRH analog therapy were discontinued.
Antiandrogen discontinuation responses
To avoid misattributing benefit to a study agent, PCWG1 required documenting progressive disease after discontinuing antiandrogen treatment. However, because the time to completely withdraw from the treatment can range from 4 to 8 weeks, depending on the half-life of the antiandrogen, many otherwise eligible patients were not included in clinical trials. Withdrawal responses typically occur in patients who are treated with combined androgen blockade (a GnRH analog or orchiectomy in combination with continuous antiandrogen) as initial therapy for a prolonged period of time, or who have responded to adding a peripheral antiandrogen as second-line therapy.17 For these situations, PCWG2 recommends evaluating patients for withdrawal responses. PCWG2 advises investigators not to wait to assess for a withdrawal response in patients who did not respond or who showed a decline in PSA for 3 months or less after an antiandrogen was administered as a second-line or later intervention.
Prior nonhormonal therapies
All of the treatments for local disease (surgery, radiation therapy, and so on) should be recorded. Other systemic therapies (eg, first-line docetaxel and/or biologic agents) should also be described in detail, including the response, the reason for discontinuation, and the interval off treatment.18
Clinical Subtypes Based on Patterns of Spread
Investigators can enhance eligibility guidelines by defining clinical subtypes based on the patterns of spread. PCWG1 defined four patient cohorts: (1) progressive measurable disease; (2) progressive bone metastases; (3) increasing PSA and stable metastases; and (4) increasing PSA and no metastatic disease.1 This classification does not reflect the pattern of spread of disease, nor does it separate patients on the basis of prognosis, more favorable for patients in the rising-PSA–castrate state with no documented metastatic disease at present or in the past, and worse for patients with visceral disease. In response, PCWG2 defines five patient cohorts (Table 3) that include a poor prognostic group with visceral disease, a group with bone metastases with or without nodal metastases but no visceral organ disease, a group with nodal disease and no visceral or bone disease, and a group with locally recurrent or progressing tumors and no metastases. Depending on the question, specific phase II trials can be considered for particular subtypes.
Table 3.
Clinical Subtypes Based on Patterns of Spread in Prostate Cancer
| Subtype | Pattern of Spread |
|---|---|
| 1 | Locally progressing tumors and no metastatic disease |
| 2 | Rising PSA and no detectable metastatic disease (rising PSA–castrate) |
| 3 | Nodal spread and no evident bone or visceral (liver or lung) disease |
| 4 | Bone disease with or without nodal disease and no evident visceral spread |
| 5 | Visceral metastases with or without spread at other sites |
Abbreviation: PSA, prostate-specific antigen.
Assessing Prognosis
Understanding and determining patient prognosis at the time of enrollment on a trial is particularly important for studies using delay/prevent end points as the primary objective. Toward this aim are reports on the median time to radiographic19,20 and symptomatic progression after first-line androgen depletion.19 Another estimated the time interval between first-, second- and third-line chemotherapy in those fit to receive it, as well as the overall survival for each group.21 Nomograms based on baseline clinical and biologic determinants (eg, PSA and lactate dehydrogenase) to estimate overall survival have also been reported.22,23,23a The literature contains references to a variety of tumor markers (eg, measures of bone turnover) in addition to PSA, but their routine assessment in phase II trials is unproven. Because these models are undergoing prospective validation and new ones are being developed, PCWG2 does not have a formal recommendation regarding their use. In the future, prognostic models may help to select patients for specific trials, evaluate therapies that delay progression of disease, and calculate time-to-event measures in asymptomatic patients.
IV. TREATMENT DURATION: A STANDARDIZED WINDOW OF EXPOSURE
A successful phase II trial should provide sufficient information not only to justify evaluating a treatment in a definitive phase III trial but also to ensure that the activity of a potentially useful agent is not missed. To this end, the trial design and outcomes should be based on the anticipated effect of the drug on the malignant process including PSA and other tumor markers, the experience to date with the drug in prostate cancer and in other tumor types, and an estimate of how long the drug must be administered before a favorable effect might be seen. The underpinnings of PCWG1 have been retained, including reporting biochemical, radiographic, and clinical outcome measures independently. Major changes include considering cytotoxic and noncytotoxic agents separately when designing trials, and emphasizing clinically significant time-to-event end points. To do so, PCWG2 advises that, in the absence of clinically compelling indicators of disease progression, early changes (within 12 weeks) in indicators such as serum PSA, patient-reported pain, and radionuclide bone scan be ignored. To ensure that time to progression is measured consistently and reliably, PCWG2 recommends that disease assessments be performed at fixed intervals, and at the time of removal from study, using the same methods of assessment used for enrollment (Table 4).
Table 4.
Suggested Frequency of Assessment for Commonly Used Measures in Prostate Cancer Clinical Trials
| Measure | Frequency |
|---|---|
| PSA | By cycle (every 3 or 4 weeks) |
| Alkaline phosphatase, LDH | By cycle (every 3 or 4 weeks) |
| Bone scans | Every 12 weeks |
| CT/MRI | Every 12 weeks |
| Symptoms | Every cycle |
NOTE. With planned overall disease assessments every 12 weeks.
Abbreviations: PSA, prostate-specific antigen; LDH, lactate dehydrogenase; CT, computed tomography; MRI, magnetic resonance imaging.
Ensuring a Sufficient Window of Drug Exposure and Reducing the Reliance on Early Changes in PSA
An issue for the evaluation of both cytotoxic and noncytotoxic agents is to ensure that a drug is administered for a sufficient period of time to work and for a favorable effect on the disease to be demonstrated using the available methods of disease assessment. Even with an effective therapy, serum PSA levels may continue to rise for a period of time before declining, a tumor may continue to increase in size before it regresses,24,25 and symptoms may worsen before they improve. The continued rise in PSA that can occur before a decline is observed could lead to the conclusion that a drug has had an unfavorable, favorable, or no effect depending on the timeframe for assessment. Trials of low-dose therapies administered on a more chronic basis and those of biologic agents that are designed to induce a host immune response may be particularly susceptible to this type of bias. As such, patients with disease-related symptoms and/or rapidly growing lesions, such as those being treated in the postchemotherapy setting, may not be appropriate candidates for treatments known to work slowly or be delayed. In contrast, patients who are asymptomatic and who have disease that is progressing more slowly, such as those in the pre- or first-line chemotherapy setting may be more appropriate candidates for trials of these types of agents.
The method of disease assessment can also affect the outcome. Bone scans in particular may appear worse before they improve, resulting in the erroneous conclusion that the treatment has failed when, in fact, it was too early to assess the effect of the drug. This may have occurred in the evaluation of the endothelin-1 antagonist atrasentan.26
With the caveat that patient safety is tantamount, and that a therapy should be discontinued if rapid disease progression is documented or the patient develops worsening symptoms of disease or toxicity, PCWG2 recommends a protocol-specified minimum exposure of 12 weeks for trials in the prechemotherapy or first-line chemotherapy setting. PCWG2, recognizing that declines in serum PSA, if they occur, may not do so for several weeks, and that a robust PSA-based surrogate for clinical benefit has not yet been identified,27 recommends that PSA measurements obtained during the first 12 weeks not be used as the sole criterion for clinical decision making. Further, to avoid discontinuing a treatment prematurely, PCWG2 encourages investigators to err on the side of continuing treatment in equivocal cases where there is no clear evidence of progression or clinical deterioration, and where patient safety is not compromised. PCWG2 also recommends repeating all of the disease assessments performed at entry when treatments are stopped.
Confirmation of Time-to-Event Outcomes
Therapy may be prematurely discontinued if outcome measures do not reflect disease status accurately. As noted, bone scans in particular, are relatively insensitive in the early follow-up period. Consideration of drug pharmacodynamics is also important. For example, a successful vaccination might produce a lymphocytic infiltrate in a tumor mass that would transiently increase its size giving the false impression of worsening disease. To avoid misinterpreting these early results, PCWG2 asserts that any post-treatment change in disease status, be it favorable or unfavorable, be confirmed using a second assessment at a later time point.
Progression-free survival (PFS) is a composite end point defined as the time from study entry or random assignment to disease progression in bone or soft-tissue, symptoms, or death. The conventional method used in the analysis of a PFS end point is based on the time to the first progression observed, using a right-censored approach, because in most clinical trials, assessments of all the components of the composite end point are not measured at the same time. This simplifies the analysis, but may lead to difficulties in interpretation. In general, the exact time of treatment failure (with the exception of death) is not known; rather, the failure is known to have occurred during an interval of time. A more appropriate method to analyze the PFS end point is to use an interval-censored approach in which all assessments of the composite PFS end point (PSA, bone, CT scans, and symptom assessments) are performed at the same time points. PCWG2 recommends that, where possible, all assessments of disease be collected at the same time interval (bone scan, CT scan, and PSA at 12-week intervals). In addition to PSA, it is also important to confirm post-treatment changes in measurable target lesions, radionuclide bone scans, and symptoms.
V. MEASURING OUTCOMES AND REPORTING
PCWG1 recommended that trial outcomes based on post-therapy changes in PSA, bone, soft-tissue (visceral and nodal) disease, or symptoms be reported separately. PCWG2 recommends that investigators measure early response outcomes by the changes in the individual disease manifestations that were present initially for both cytotoxic and noncytotoxic drugs with the same methods that were used at enrollment (Table 5). Delay/prevent end point trials require knowledge of the probability that an event might occur and when. If a protocol defines a composite end point for progression, the specified progression in any measure (with the exception of early changes in PSA or pain as explained earlier herein) overrides a change or improvement in other measures.
Table 5.
Suggested Outcome Measures for Phase II Clinical Trials in Prostate Cancer
| Variable | PCWG1 (1999)1 | PCWG2 (2007) |
|---|---|---|
| PSA | Monitor PSA ≥ 1/month | Recognize that a favorable effect on PSA may be delayed for 12 weeks or more, even for a cytotoxic drug |
| Monitor PSA by cycle but plan to continue through early rises for a minimum of 12 weeks unless other evidence of progression | ||
| Ignore early rises (prior to 12 weeks) in determining PSA response | ||
| PSA response: | For control/relieve/eliminate end points: | |
| Defined a PSA partial response as a > 50% decline from baseline (measured twice 3 to 4 weeks apart) | Record the percent change from baseline (rise or fall) at 12 weeks, and separately, the maximal change (rise or fall) at any time using a waterfall plot32* | |
| Progression: | Progression: | |
| After decline from baseline: progression = 50% increase from nadir and an increase of at least 5 ng/mL, or back to baseline, whichever was lowest | Decline from baseline: record time from start of therapy to first PSA increase that is ≥ 25% and ≥ 2 ng/mL above the nadir, and which is confirmed by a second value 3 or more weeks later (ie, a confirmed rising trend)† | |
| The requirement of an increase of 5 ng/mL is decreased to 2 ng/mL, and the requirement for a 50% increase is reduced to 25% | ||
| Record duration of PSA decline | Recording the duration of PSA decline of little value | |
| No decline from baseline: | ||
| PSA progression ≥ 25% and ≥ 2 ng/mL after 12 weeks | ||
| Soft-tissue lesions | Change in size of lymph nodes or parenchymal masses on physical exam or x-ray | For control/relieve/eliminate end points: |
| Use RECIST with caveats | ||
| Only report changes in lymph nodes that were ≥ 2 cm in diameter at baseline | ||
| Record changes in nodal and visceral soft tissue sites separately | ||
| Record complete elimination of disease at any site separately | ||
| Confirm favorable change with second scan | ||
| Record changes using waterfall plot | ||
| For delay/prevent end points: | ||
| Use RECIST criteria for progression, with additional requirement that progression at first assessment be confirmed by a second scan 6 or more weeks later‡ | ||
| Note that for some treatments, a lesion may increase in size before it decreases | ||
| Bone | No definition for response provided | For control/relieve eliminate end points: |
| Record outcome as new lesions or no new lesions | ||
| First scheduled reassessment: | ||
| No new lesions: continue therapy | ||
| New lesions: perform a confirmatory scan 6 or more weeks later | ||
| Confirmatory scan: | ||
| No new lesions: continue therapy | ||
| Additional new lesions: progression | ||
| Subsequent scheduled reassessments: | ||
| No new lesions: continue | ||
| New lesions: progression | ||
| Progression: | For prevent/delay end points (progression): | |
| > 1 new lesion | The appearance of ≥ 2 new lesions, and, for the first reassessment only, a confirmatory scan performed 6 or more weeks later that shows a minimum of 2 or more additional new lesions§ | |
| Worsening scan = progressive disease, regardless of PSA | The date of progression is the date of the first scan that shows the change | |
| Symptoms | Not addressed | Consider independently of other outcome measures |
| Document pain and analgesia at entry with a lead in period and measure repeatedly at 3- to 4-week intervals | ||
| Perform serial assessments of global changes in HRQOL, urinary or bowel compromise, pain management, additional anticancer therapy | ||
| Ignore early changes (≤ 12 weeks) in pain or HRQOL in absence of compelling evidence of disease progression | ||
| Confirm response or progression of pain or HRQOL end points ≥3 weeks later |
Abbreviations: PCWG1, Prostate-Specific Antigen Working Group 1; PCWG2, Prostate Cancer Clinical Trials Working Group 2; PSA, prostate-specific antigen; HRQOL, health-related quality of life.
See Figure 3.
See Figure 4.
Particularly important when anticipated effect on PSA is delayed or for biologic therapies.
See Figure 5.
PCWG2 also recommends that grouped categorizations of response such as complete, partial, or stable not be used, and that time to progression and time to treatment failure, which includes discontinuing therapy because of disease progression, toxicity, or patient withdrawal, be recorded separately.
PSA
For control/relieve/eliminate end points, PCWG1 emphasized the importance of understanding the effect of an agent on PSA,1 and that some drugs (especially noncytotoxic agents) may modulate PSA expression independent of an effect on tumor cell growth or survival (Table 5).28,29 Despite this recommendation, PCWG2 recognizes that the utility of assays that are currently available to measure the in vitro effects of a drug on PSA have not been tested prospectively.30
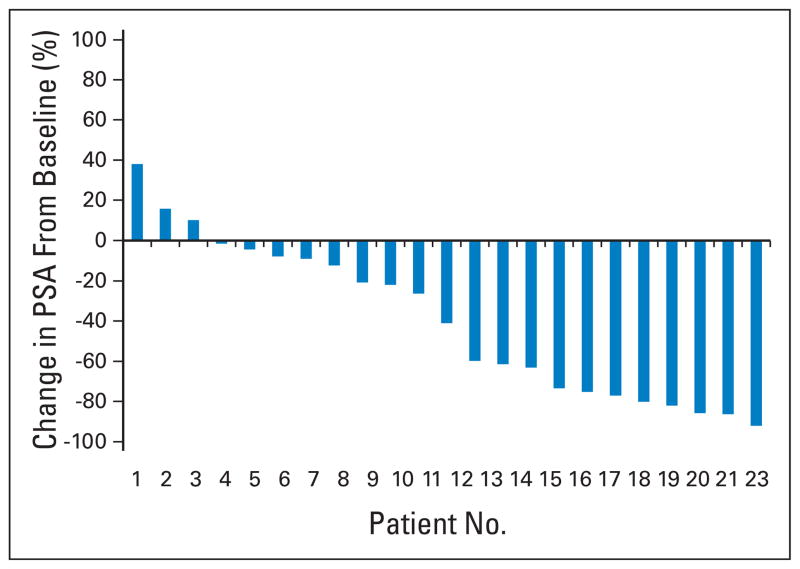
PCWG1 suggested that investigators report PSA response rates. PCWG2 advises against reporting PSA response rates because these are of little value given the uncertain significance of a defined degree of decline from baseline, be it 50% or 30%, and no criterion has been shown prospectively to be a surrogate of clinical benefit.27 To report PSA-based outcomes, PCWG2 recommends that the percentage of change in PSA from baseline to 12 weeks (or earlier for those who discontinue therapy), as well as the maximum decline in PSA that occurs at any point after treatment be reported for each patient using a waterfall plot (Fig 3).31 Waterfall plots provide a broader and more sensitive display of data, and are more informative until a validated surrogate of clinical benefit is available. PCWG2 recommends that the same waterfall plot be used to illustrate outcomes for noncytotoxic agents. PCWG2 discourages the use of changes in PSA-DT or PSA slope as a primary end point, because their clinical significance is uncertain. It also recommends eliminating reports of the “duration of PSA control” as described in PCWG1 guidelines because its interpretation varies between investigators.
Fig. 3.
Waterfall plot showing the maximal (at 12 weeks or at any time point) prostate-specific antigen (PSA) post-therapy change from baseline. Note that the proportion of patients showing any defined degree of decline is readily assessable.
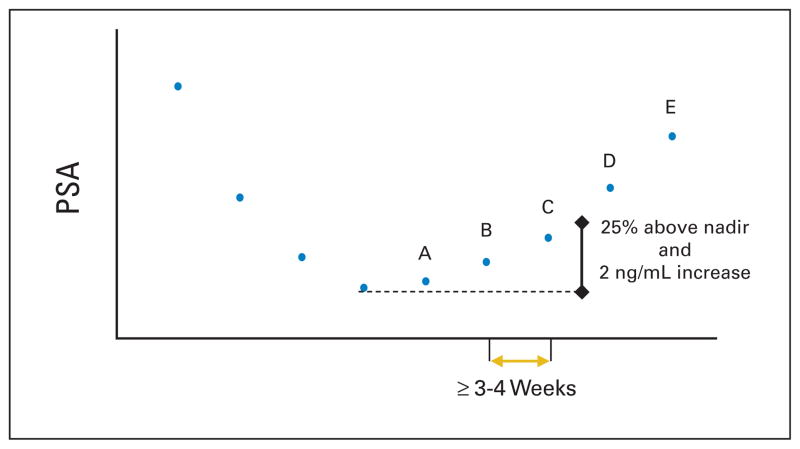
For delay/prevent end points, PCWG1 defined PSA progression in different ways on the basis of whether a decline from baseline was observed (Table 5). Asserting again that early changes in PSA should not be used for clinical decision making, PCWG2 defines PSA progression as the date that a 25% or greater increase and an absolute increase of 2 ng/mL or more from the nadir is documented, which is confirmed by a second value obtained 3 or more weeks later (Fig. 4). This recommendation recognizes that variations in progression times might occur simply on the basis of the rate of PSA rise. PCWG2 considers the requirement of an increase of 5 ng/mL from the nadir too high, particularly for those in whom the PSA is below 5 ng/mL at the time treatment is started, for those with high-grade tumors that do not produce large amounts of PSA, and for those who achieve post-treatment nadir values that fall below 5 ng/mL. Where no decline from baseline is documented, PCWG2 defines PSA progression as a 25% increase from the baseline value along with an increase in absolute value of 2 ng/mL or more (rather than 5 ng/mL) after 12 weeks of treatment (Fig. 4).
Fig. 4.
Prostate-specific antigen (PSA) progression. An increase of 25% and absolute increase of 2 ng/mL or more above the nadir. Values A, B, and C show rising PSA values that do not meet the criteria. Value D is the first PSA value that is greater than 25% and more than 2 ng/mL above the nadir, confirmed with a further rise in PSA shown by value E. For reporting purposes, PSA progression would be recorded on the date value D was obtained.
Trials for patients in the rising-PSA–castrate state raise the most questions about the significance of PSA changes to assess prognosis at entry, as well as the clinical significance of PSA elevations when used as the sole end point to define progression. This is because the prognosis for this group of men is not well defined32,33 and it is difficult to maintain patients on a clinical trial until radiographic or symptomatic progression is documented while PSA levels are rising.
Despite this, PCWG2 emphasizes the importance of keeping patients on trial until radiographic or symptomatic progression, which better reflects a change in clinical status, is documented and that an effort is made not to discontinue therapy solely on the basis of a rise in PSA in the absence of other indicators of disease progression. This is particularly relevant for patients with low PSA values at entry and those with a slow rate of rise in PSA at progression. PCWG2 recognizes that this may be difficult in practice. In this regard, it is important that both the protocol and the consent form specify that PSA elevations may not reflect overall disease status. In this setting, random assignment to a placebo control should be considered, stopping early on the basis of prospectively defined futility criteria.
Measurable Soft-Tissue Lesions
For control/relieve/eliminate end points, PCWG2 accepts with modifications RECIST criteria for evaluating drugs or approaches anticipated to produce tumor regression. The modifications are that changes in nodal and visceral sites be recorded and reported separately, and lymph nodes in the pelvis must measure at least 2 cm in greatest diameter to be considered target lesions. PCWG2 also recommends that the complete elimination of disease at a particular site be recorded separately. PCWG2 reinforces the recommendation in RECIST that any favorable change should be confirmed using a second follow-up scan. As with changes in PSA, PCWG2 suggests that changes in the size of the target lesions be reported as a waterfall plot to facilitate comparison between studies.
For prevent/delay end points, progression in a nodal or visceral site should also be defined using RECIST, with the recognition that, for some therapies, early unfavorable changes may not accurately reflect disease status (Table 5). As noted, a lymphocytic infiltration of a tumor mass after successful immunization may result in an enlarged soft-tissue lesion that could be an early indication that the treatment is working. Further, because the effects of some agents (noncytotoxic) may be delayed, the degree of increase in tumor size at the first 12-week assessment should also be confirmed before it is considered a treatment failure.
Bone
Given the frequency of bone involvement in patients with progressive, castration-resistant disease, the decreased emphasis of early changes in PSA, and the increased availability of cytostatic agents, reliable methods to assess changes in bone are of increasing importance. PCWG2 recognizes that standards for using MRI and PET to assess bone metastases are under active investigation, so only radionuclide bone scans are considered here. PCWG2 also recognizes that there are no validated criteria for response on radionuclide bone scan.
For control/relieve/eliminate end points, the PCWG2 recommends that post-treatment changes be recorded simply as either “no new lesions” or “new lesions.” However, progression at the first scheduled assessment should be confirmed on a second scan performed 6 or more weeks later, in the absence of clearly worsening soft-tissue(nodal and visceral) disease or disease-related symptoms. In the rare case where visible lesions disappear, this too should be confirmed at the next scheduled assessment.
For prevent/delay end points, progressing disease on bone scan is considered when a minimum of two new lesions is observed. PCWG1 made the provision that a worsening bone scan on the first follow-up manifests tumor “flare”34; PCWG2 does not recommend performing a follow-up bone scan before 12 weeks of treatment unless clinically indicated. At the first 12-week reassessment, defining disease progression requires a confirmatory scan (which shows additional new lesions compared with the first follow-up scan) performed 6 or more weeks later, because lesions visible at the first 12-week assessment may represent disease that was not detected on the pretreatment scan.35 When further progression is documented on the confirmatory scan, the date of progression recorded for the trial, is the date of the first scan that shows the change(Fig 5).
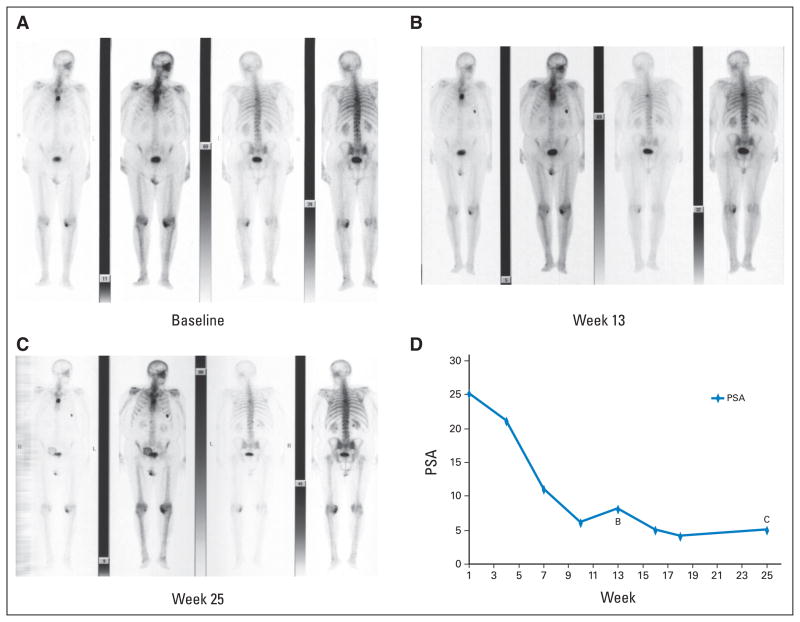
Fig. 5.
Serial bone scans and prostate-specific antigen (PSA) values from a patient treated with cytotoxic treatment showing (B) two new areas of tracer uptake at week 13 relative to (A) the pretreatment baseline with (C) no additional new lesions on the week-25 scan. The patient did not meet the criteria for progression because no additional new lesions were documented on the week 25 scan. (D) Note that the PSA values continued to decrease through this interval.
Symptoms
PCWG2 considers symptoms and health-related quality of life independently from other outcome measures, on the basis of the importance of symptoms for clinical benefit, the limited correlations between pain response and post-therapy PSA decline, and the independent status of pain and PSA as predictors of survival.9 If pain or other symptomatic outcome measures are used to assess the effects of an investigational agent, levels of pain, analgesia, and fatigue should be documented at trial entry using well-validated self-report questionnaires (as described in Establishing Eligibility in Enrollment), and measured repeatedly at 3- to 4-week intervals during treatment (Table 4). Other dimensions of pain including frequency, duration, level of bother, location(s), and likelihood of relationship to prostate cancer (versus prior therapy or comorbidities) should also be recorded as supportive information. (A reasonable definition used in the TAX-327 study,6 for instance, was an increase in the 5-point present intensity scale and/or an increase by 50% in the analgesic score, each averaged over the previous week and confirmed at a subsequent assessment 3 to 4 weeks later.)
Transient increases in pain may occur before improvement, and those occurring in the first 12 weeks should be ignored in the absence of other compelling evidence of disease progression. Changes in symptoms should be confirmed as for other outcome measures. In contrast to assessing pain relief, the assessment of pain progression is more difficult because of the subjectivity as to what constitutes a “clinically significant increase” from baseline or from the point of maximal response to an intervention. Other domains to monitor that may help determine whether the disease is progressing include worsening in global quality of life, developing urinary or bowel compromise, or needing to change anticancer therapy (most commonly, needing to administer radiation therapy for palliating an osseous or epidural lesion). However, the prognostic significance of changes in these parameters has not been demonstrated.
The evaluation of symptoms and health-related quality of life requires a significant commitment of patient and staff resources to ensure compliance, and adds considerable cost to a trial. A clear hypothesis is therefore essential, as is an understanding of the clinical relevance of score changes in the selected measures for the population being studied. It should not be assumed that a particular measure meets these standards or is appropriate for a given population, even if it has been used in prior published studies. For registration-track drugs, the FDA recommends that sponsors discuss any planned patient-reported outcomes with the agency during phase II, and that any such measures be incorporated in phase II research to explore measurement properties before use in phase III trials. Randomized trial designs are particularly useful to address these questions.
VI. DEVELOPING PHASE III CLINICAL TRIALS
New treatments and approaches become standards of care when they demonstrate superiority to previously established standards or placebo in phase III trials. Phase II trials are designed to demonstrate antitumor activity and to justify phase III trials that evaluate survival or other measures of clinical benefit. The chance of success is increased if each trial is designed to inform the next; this sequencing is particularly challenging in prostate cancer because of the lack of both measurable disease and a validated PSA- or non–PSA-based surrogate end point that has consistently predicted clinical benefit. Furthermore, given the range of agents and methods available, setting priorities across trials using standard criteria is complicated. PCWG1 recommended that all phase II trials include a clearly defined outcome that would justify proceeding to phase III, but this has not been widely adopted.36,37
PCWG2 emphasizes that specific outcomes on which to base the decision to proceed to phase III will depend on the therapeutic objectives and should increasingly be based on time-to-event outcomes. PCWG2 asserts that the likelihood of favorable results being mirrored in later studies would increase by incorporating several aspects of phase III trials into the phase II setting. Some options include enrolling a contemporary control population using randomized phase II designs to allow for patient heterogeneity, enrolling patients with similar risk profiles for the end point under study, conducting multicenter trials to minimize patient selection and bias, and incorporating end points other than early changes in PSA. PCWG2 also advises prespecifying in the protocol an outcome or outcomes that would justify further development of a compound or approach.
Rational decision making at the phase II to phase III transition point is particularly important given the number of agents currently under development in prostate cancer and the large number of patients already committed to trials. Innovative designs are encouraged, such as including several new potential therapies in randomized phase II trials where the most promising therapies continue to phase III and the least promising are abandoned.
SUMMARY
Since the publication of the original PCWG1 consensus criteria for phase II clinical trials, our knowledge of the biology of prostate cancer has increased substantially, noncytotoxic agents that target potentially important signaling pathways have become available, and there are numerous phase II and III trials that offer the opportunity to assess the relevance of consensus guidelines. The PCWG2 criteria refine existing eligibility criteria and focus on measuring treatment effects that prioritize agents for evaluation in definitive phase III trials to demonstrate clinical benefit. For cytotoxic therapies, many of the changes recommended by PCWG2 update the criteria for reporting post-treatment PSA changes, elucidate how RECIST should be applied to prostate cancer, and clarify how post-treatment bone scan changes should be described. For non-cytotoxic therapies, PCWG2 recommends shifting the focus of designs from response to time-to-event end points, either with attention to defining eligibility by an estimated event frequency or by using a randomized control group. For both types of studies, larger multicenter phase II studies better reflect phase III populations. Phase III trials should be designed to prospectively evaluate new potential biomarkers and new nomograms that may, in turn, inform future studies. PCWG2 recognizes that the optimization of criteria for designing clinical trials in prostate cancer is work in progress and that as the field evolves, the design of clinical trials will change.
Acknowledgments
Supported in part by the Memorial Sloan-Kettering Cancer Center Specialized Program of Research Excellence (SPORE) grant in Prostate Cancer (P50 CA92629), The Department of Defense Prostate Cancer Research Program (PC051382), and the Prostate Cancer Foundation.
The Acknowledgment is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: Howard I. Scher, Susan Halabi, Ian Tannock, Michael Morris, Cora N. Sternberg, Michael A. Carducci, Mario A. Eisenberger, Celestia Higano, Glenn J. Bubley, Robert Dreicer, Daniel Petrylak, Philip Kantoff, Ethan Basch, William D. Figg, Eric J. Small, Alison Martin, Maha Hussain
Provision of study materials or patients: Howard I. Scher
Collection and assembly of data: Howard I. Scher, Mario A. Eisenberger, Celestia Higano, Tomasz M. Beer, Alison Martin
Data analysis and interpretation: Howard I. Scher, Michael Morris, Michael A. Carducci, Mario A. Eisenberger, Glenn J. Bubley, Robert Dreicer, Philip Kantoff, William Kevin Kelly, William D. Figg, Tomasz M. Beer, George Wilding, Alison Martin, Susan Halabi, Maha Hussain
Manuscript writing: Howard I. Scher, Susan Halabi, Ian Tannock, Michael Morris, Cora N. Sternberg, Michael A. Carducci, Mario A. Eisenberger, Celestia Higano, Glenn J. Bubley, Robert Dreicer, Ethan Basch, William Kevin Kelly, William D. Figg, Eric J. Small, Tomasz M. Beer, Alison Martin, Maha Hussain
Final approval of manuscript: Howard I. Scher, Susan Halabi, Ian Tannock, Cora N. Sternberg, Michael A. Carducci, Mario A. Eisenberger, Celestia Higano, Glenn J. Bubley, Robert Dreicer, Daniel Petrylak, Philip Kantoff, Ethan Basch, William Kevin Kelly, William D. Figg, Eric J. Small, Tomasz M. Beer, George Wilding, Alison Martin, Maha Hussain
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Ian Tannock, sanofi-aventis (U), Algeta ASA (U), GPC Biotech (U); Michael A. Carducci, Abbott Laboratories (C), sanofi-aventis (U), Methylgene (C), Cougar Biotech (C); Mario A. Eisenberger, sanofi-aventis (C), GPC (C), Celgene (C); Robert Dreicer, Merck (C), sanofi-aventis (C), Bristol-Myers Squibb (C); Daniel Petrylak, Aventis (C), GPC Biotech (C), Abbott Laboratories (C); Eric J. Small, Cougar Biotechnology (C), Poniard Pharmaceuticals (C); Tomasz M. Beer, Novacea (C) Stock Ownership: Tomasz M. Beer, Novacea Honoraria: Michael A. Carducci, sanofi-aventis, Abbott Laboratories; Mario A. Eisenberger, sanofi-aventis, Ipsen, GPC; Robert Dreicer, Berlex; Daniel Petrylak, Aventis, Celegene, Abbott; William Kevin Kelly, sanofi-aventis, Genetech; Tomasz M. Beer, sanofi-aventis Research Funding: Ian Tannock, sanofi-aventis, Novacea; Mario A. Eisenberger, sanofi-aventis, Clegene, Cytogen; Robert Dreicer, sanofi-aventis, Eli Lilly, Millenium; Daniel Petrylak, Aventis, Celegene, GPC Biotech; William Kevin Kelly, sanofi-aventis, Genetech, Curagen; Eric J. Small, Dendreon, Novartis; Tomasz M. Beer, sanofi-aventis Expert Testimony: None Other Remuneration: None
References
- 1.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: Recommendations from the PSA Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. Erratum: J Clin Oncol 18:2644, 2000; J Clin Oncol 25:1154, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 3.Scher HI, Morris MJ, Kelly WK, et al. Prostate cancer clinical trial end points: “RECIST”ing a step backwards. Clin Cancer Res. 2005;11:5223–5232. doi: 10.1158/1078-0432.CCR-05-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Heller G. Clinical states in prostate cancer: Towards a dynamic model of disease progression. Urology. 2000;55:323–327. doi: 10.1016/s0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 5.Simon SD. Is the randomized clinical trial the gold standard of research? J Androl. 2001;22:938–943. doi: 10.1002/j.1939-4640.2001.tb03433.x. [DOI] [PubMed] [Google Scholar]
- 6.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 7.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 8.US Department of Health and Human Services, Food and Drug Administration. Guidance for industry: Patient-reported outcome measures—Use in medical product development to support labeling claims: Draft guidance released for comment. 2006 Feb; doi: 10.1186/1477-7525-4-79. http://www.fda.gov/CDER/GUIDANCE/5460dft.pdf. [DOI] [PMC free article] [PubMed]
- 9.Armstrong AJ, Garrett-Mayer E, Ou Yang YC, et al. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2007;25:3965–3970. doi: 10.1200/JCO.2007.11.4769. [DOI] [PubMed] [Google Scholar]
- 10.Turk DC, Dworkin RH, Burke LB, et al. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain. 2006;125:208–215. doi: 10.1016/j.pain.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 11.D’Amico AV, Moul J, Carroll PR, et al. Prostate specific antigen doubling time as a surrogate end point for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J Urol. 172:S42–S47. doi: 10.1097/01.ju.0000141845.99899.12. [DOI] [PubMed] [Google Scholar]
- 11a.Arlen PM, Bianco F, Dahut WL, et al. Prostate Specific Antigen Working Group’s guidelines on PSA doubling time. J Urol. doi: 10.1016/j.juro.2008.01.099. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scher HI, Buchanan G, Gerald W, et al. Targeting the androgen receptor: Improving outcomes for castration- resistant prostate cancer. Endocr Relat Cancer. 2004;11:459–476. doi: 10.1677/erc.1.00525. [DOI] [PubMed] [Google Scholar]
- 13.Mohler JL, Gregory CW, Ford OH, III, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 14.Titus MA, Schell MJ, Lih FB, et al. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 14a.Mostaghel EA, Page ST, Lin DW, et al. Intra-prostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 15.Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Zhang ZF, Kelly WK. Hormone and anti-hormone withdrawal therapy: Implications for management of androgen independent prostate cancer. Urology. 1996;47:61–69. doi: 10.1016/s0090-4295(96)80011-2. [DOI] [PubMed] [Google Scholar]
- 18.Beer TM, Garzotto M, Henner WD, et al. Intermittent chemotherapy in metastatic androgen-independent prostate cancer. Br J Cancer. 2003;89:968–970. doi: 10.1038/sj.bjc.6601232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denis LJ, Keuppens F, Smith PH, et al. Maximal androgen blockade: Final analysis of EORTC phase III trial 30853: EORTC Genito-Urinary Tract Cancer Cooperative Group and the EORTC Data Center. Eur Urol. 1998;33:144–151. doi: 10.1159/000019546. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 21.Beekman KW, Fleming MT, Scher HI, et al. Second-line chemotherapy for prostate cancer: Patient characteristics and survival. Clin Prostate Cancer. 2005;4:86–90. doi: 10.3816/cgc.2005.n.015. [DOI] [PubMed] [Google Scholar]
- 22.Smaletz O, Scher HI, Small EJ, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–3982. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 23a.Armstrong AJ, Garrett-Mayer E, Yang YC, et al. A contemporary prognostic nomagram for men with hormone-refractory metastatic prostate cancer: A TAX327 study analysis. Clin Cancer Res. 2007;13:6396–6403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 24.Olbert PJ, Hegele A, Kraeuter P, et al. Clinical significance of a prostate-specific antigen flare phenomenon in patients with hormone-refractory prostate cancer receiving docetaxel. Anticancer Drugs. 2006;17:993–996. doi: 10.1097/01.cad.0000231468.69535.97. [DOI] [PubMed] [Google Scholar]
- 25.Nelius T, Klatte T, de Riese W, et al. Impact of PSA flare-up in patients with hormone-refractory prostate cancer undergoing chemotherapy. Int Urol Nephrol. doi: 10.1007/s11255-007-9221-y. epub ahead of print on June 30, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Carducci MA, Padley RJ, Breul J, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: A randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003;21:679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 27.Fleming MT, Morris MJ, Heller G, et al. Post-therapy changes in PSA as an outcome measure in prostate cancer clinical trials. Nat Clin Pract Oncol. 2006;3:658–667. doi: 10.1038/ncponc0664. [DOI] [PubMed] [Google Scholar]
- 28.Thalmann GN, Sikes RA, Chang S-M, et al. Suramin-induced decrease in prostate-specific antigen expression with no effect on tumor growth in the LNCaP model of human prostate cancer. J Natl Cancer Inst. 1996;88:794–801. doi: 10.1093/jnci/88.12.794. [DOI] [PubMed] [Google Scholar]
- 29.Dixon SC, Knopf KB, Figg WD. The control of prostate-specific antigen expression and gene regulation by pharmacological agents. Pharmacol Rev. 2001;53:73–91. [PubMed] [Google Scholar]
- 30.Beverage JN, Figg WD. The quantification of PSA mRNA copies in pathologically normal pelvic lymph nodes is a viable prognostic indicator. Cancer Biol Ther. 2006;5:1094–1095. doi: 10.4161/cbt.5.9.3287. [DOI] [PubMed] [Google Scholar]
- 31.Seidman AD, Scher HI, Petrylak D, et al. Estramustine and vinblastine: Use of prostate specific antigen as a clinical trial endpoint in hormone-refractory prostatic cancer. J Urol. 1992;147:931–934. doi: 10.1016/s0022-5347(17)37426-8. [DOI] [PubMed] [Google Scholar]
- 32.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–2925. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 33.Yossepowitch O, Bianco FJ, Jr, Eggener SE, et al. The natural history of noncastrate metastatic prostate cancer after radical prostatectomy. Eur Urol. 2007;51:940–948. doi: 10.1016/j.eururo.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levenson RM, Sauerbrunn BJL, Bates HR, et al. Comparative value of bone scintigraphy and radiology in monitoring tumor response in systemically treated prostatic carcinoma. Radiology. 1983;146:513–518. doi: 10.1148/radiology.146.2.6294738. [DOI] [PubMed] [Google Scholar]
- 35.Smith PH, Bono A, Calais da Silva F, et al. Some limitations of the radioisotope bone scan in patients with metastatic prostatic cancer. Cancer. 1990;66:1009–1016. doi: 10.1002/cncr.1990.66.s5.1009. [DOI] [PubMed] [Google Scholar]
- 36.Vickers AJ, Ballen V, Scher HI. Setting the bar in phase II trials: The use of historical data for determining “go/no go” decision for definitive phase III testing. Clin Cancer Res. 2007;13:972–976. doi: 10.1158/1078-0432.CCR-06-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawakami J, Cowan JE, Elkin EP, et al. Androgen-deprivation therapy as primary treatment for localized prostate cancer: Data from Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) Cancer. 2006;106:1708–1714. doi: 10.1002/cncr.21799. [DOI] [PubMed] [Google Scholar]