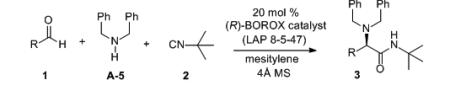
Abstract
The first asymmetric catalyst for the 3-component Ugi reaction was identified as a result of a screen of a large set of different BOROX catalysts. The BOROX catalysts are assembled in-situ from a chiral biaryl ligand, an amine, water, BH3•SMe2 and an alcohol or phenol. The catalyst screen included 13 different ligands, 12 amines and 47 alcohols or phenols. The optimal catalyst (LAP 8-5-47) provides the α-amino amides with excellent asymmetric induction from an aldehyde, 2° amine and an isonitrile. The catalyst is proposed to consist of an ion pair combination of a chiral boroxinate anion and an iminium cation.
Keywords: asymmetric catalysis, Ugi reaction, β-amino amides, VAPOL, BOROX catalyst, catalyst diversity
The multi-component coupling reactions producing α-amino amides from isonitriles have been of interest ever since the first example of this process was discovered by Ugi in 1959.[1] Since that time, the Ugi reaction has been extensively studied and widely used in organic synthesis[2,3] with one of the most salient attractions the diversity associated with the coupling of many components.[4] The four-component Ugi reaction can tolerate variations in the amine component (1° or 2° amines, hydrazines and hydroxyl amines) and in the acid component (carboxylic acids, hydrazoic acid, cyanates, thiocyanates, 2° amine salts, water, H2S, H2Se).[2] The Ugi reaction can also be effected in the absence of the acid component in a three component fashion where the amine component can be either a 1° or 2° amine.[5,6] The Ugi reaction can be catalyzed by both Brønsted and Lewis acids.[7] Pan and List have recently reported for the first time turnover for the three component Ugi reaction with a 1° amine with a non-chiral organocatalyst.[5] Unlike the related Passerini reaction,[8] an asymmetric catalyst has yet to be reported for either the three or four component Ugi reaction.[2d,4c,6,9] Asymmetric catalysts have been reported for closely related Ugi-type reactions involving azomethine imines[10] and the formation of oxazoles from α-isocyanoacetamides.[11]
The Ugi reaction is commonly thought to involve an iminium ion[2a,3,12] and the unsolved problem of an asymmetric catalytic Ugi reaction was an attractive target for the application of the BOROX catalysts that we have developed for asymmetric reactions involving iminium ions in aziridinations,[13] aza-Cope rearrangements[14] and heteroatom Diels-Alder reactions.[15] The BOROX catalyst consists of an ion-pair containing a boroxinate chiral anion with the corresponding cation derived from a protonated substrate.[16] The BOROX catalyst is typically assembled in-situ from the ligand, B(OPh)3 and an imine (or amine) which would produce the catalyst in Scheme 2 with R1 = Ph.[17] We have also shown that the same BOROX catalyst can be directly assembled by a molecule of an imine (or amine) from the ligand, 3 molecules of BH3•SMe2, 3 molecules of water and 2 molecules of phenol.[13d,e,18] This protocol should allow for a facile diversity-oriented generation of an array of BOROX catalysts by incorporation of different ligands and different phenols or alcohols into the boroxinate core during in-situ catalyst assembly (Scheme 2).[19] This essentially instant access to diversity has enabled the identification of the first effective chiral catalyst for the three-component Ugi reaction.
Scheme 2.
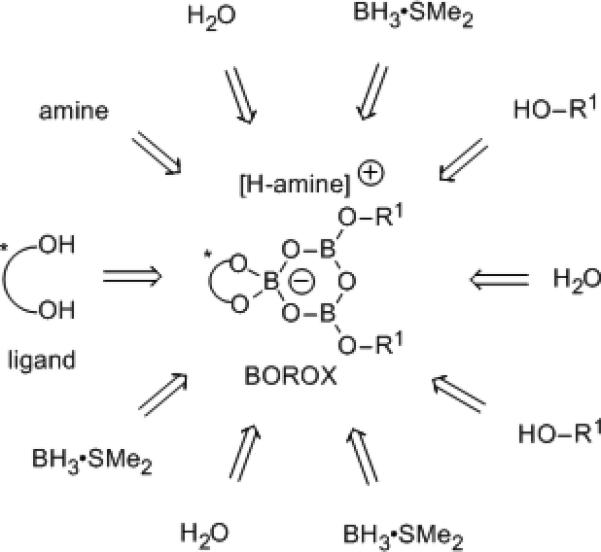
Catalyst Diversity via In-Situ Substrate Induced Assembly
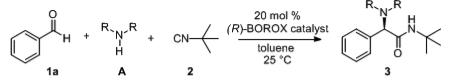
In screening the reactions of benzaldehyde and t-butyl isonitrile with the BOROX catalyst derived from phenol P-11 and the VAPOL ligand L-4, it was found that the primary amine A-6 led only to the formation of imine 4 in quantitative yield (Table 1, entry 6). A number of 2° amines including diethylamine, pyrrolidine and anilines produced no detectable amount of product under these conditions. The reaction with pyrrolidine was examined more closely and it was found that the only identifiable compound present other than starting materials was the aminal 5 (50%, entry 1). Dibenzylamine A-5 was found to give the Ugi-product 3a in 76% yield but, unfortunately, only with an enantioselectivity of 59:41 (entry 5). The bis-pmethoxybenzylamine A-7 gives essentially the same result (entry 7). The catalyst from the VANOL ligand L-1 gave an even lower selectivity and the best catalyst from the BINOL ligands L-10 to L-13 gave an er of 55:45 and even then with reduced yields compared to the VAPOL catalyst (entries 8-12).
Table 1.
Initial Screen with Amines and VANOL, VAPOL and BINOL ligands.a
| |||||||
|---|---|---|---|---|---|---|---|
| entry | ligand | amine | phenol/alcoholb | catalyst# | time (h) | % yield 3c | erd |
| 1 | L-4 | A-1 | P-11 | LAP 4-1-11 | 18 | nde | – |
| 2 | L-4 | A-2 | P-11 | LAP 4-2-11 | 24 | nd | – |
| 3 | L-4 | A-3 | P-11 | LAP 4-3-11 | 24 | nd | – |
| 4 | L-4 | A-4 | P-11 | LAP 4-4-11 | 24 | nd | – |
| 5 | L-4 | A-5 | P-11 | LAP 4-5-11 | 24 | 76 | 41 : 59f |
| 6 | L-4 | A-6 | P-11 | LAP 4-6-11 | 18 | ndg | – |
| 7 | L-4 | A-7 | P-11 | LAP 4-7-11 | 48 | 82 | 57 : 43 |
| 8 | L-1 | A-5 | P-11 | LAP 1-5-11 | 36 | 60 | 47 : 53f |
| 9 | L-10 | A-5 | P-11 | LAP 10-5-11 | 40 | 9h | 44 : 56f |
| 10 | L-11 | A-5 | P-11 | LAP 11-5-11 | 24 | 37 | 55 : 45 |
| 11 | L-12 | A-5 | P-11 | LAP 12-5-11 | 43 | 30 | 55 : 45 |
| 12 | L-13 | A-5 | P-11 | LAP 13-5-11 | 24 | trace | – |
Unless otherwise specified, all reactions were carried out at 0.2 M in 1a (0.25 mmol) in toluene with 2.0 equiv amine and 1.5 equiv of 2 at rt for the indicated time with 20 mol % of the catalyst. The pre-catalyst was prepared by heating 20 mol% of the (R)-ligand, 40 mol% of the phenol or alcohol, 60 mol% H2O, 60 mol% of BH3·SMe2 in toluene at 100 °C for 1 h. After removal of all volatiles, the BOROX catalyst was generated in-situ by the addition of the amine at rt and this was followed by the addition of aldehyde and then the isonitrile.
P-11 is phenol (Table 2).
Isolated yield after chromatography on silica gel. nd = not detected.
Determined by HPLC.
The aminal 5 was formed in 50% yield (1H NMR) and amide 3 could not be detected. Heating this mixture at 80 °C for 18 h resulted in a complex mixture with 3 still not detectable.
Catalyst was generated from (S)-ligand.
The imine 4 was formed in 100% yield as determined by 1H NMR yield with an internal standard. After 89 h, a 100% yield of imine was still observed.
1H NMR yield with internal standard.
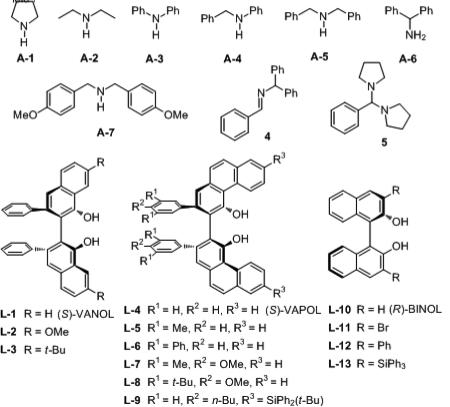
The next two phases of the screening process involved: 1) evaluation of 38 different BOROX catalysts all prepared from the VAPOL ligand and various alcohols and phenols and, 2) the screening of the optimal phenol/alcohol from this study with some newly prepared derivatives of the VANOL and VAPOL ligands. The results from a selected set of 8 of the 38 phenol/alcohols in the VAPOL BOROX catalysts are given in Table 2 (the others are in the Supporting Information). The phenol/alcohol that gives the most selective catalyst with VAPOL is 2,4,6-trimethylphenol P-36 with an enantioselectivity of 70:30 (Table 2, entry 8). There is not a significant effect of the electronic nature of the phenol on the induction (entries 1 vs 5). Essentially the same induction was observed with 3° and 2° alcohols as with phenol P-11 although ethanol stops the reaction (not shown). Both (–) and (+)-menthol give the same induction indicating that auxiliary chiral centers may not be a useful for selectivity modification. A series of BOROX catalysts containing 2,4,6-trimethylphenol P-36 were then generated from a series of newly prepared VANOL and VAPOL ligands. Catalysts prepared from P-36 and the 7,7'-disubstituted VANOL derivatives L-2 and L-3 were hardly any more selective (entries 10 and 11) than those from the parent VANOL ligand which gave essentially racemic material (entry 9)[20] The substituted VAPOL ligands L-5 to L-9, however, were generally significantly more selective than the parent VAPOL ligand. The optimal BOROX catalyst comes from the VAPOL derivative L-8 which gives an er of 85:15 (entry 15). The asymmetric synergism between the ligand and phenol components is revealed by the fact that while the substituted VAPOL ligand L-8 gives just a skosh of improvement in induction over VAPOL with phenol P-11 (entries 17 vs 4), L-8 gives a much greater increase with P-36 than does VAPOL (entries 15 vs 8).
Table 2.
Synergism in the Arrangement of the Substituents in the Boroxinate Core.a
| ||||||
|---|---|---|---|---|---|---|
| entry | ligand | phenol/alcohol | catalyst | time (h) | % yield 3b | erc |
| 1 | L-4 | P-3 | LAP 4-5-3 | 36 | 64 | 57 : 43 |
| 2 | L-4 | P-5 | LAP 4-5-5 | 39 | 86 | 58 : 42 |
| 3 | L-4 | P-6 | LAP 4-5-6 | 24 | 91 | 58 : 42 |
| 4 | L-4 | P-11 | LAP 4-5-11 | 24 | 76 | 59 : 41 |
| 5 | L-4 | P-13 | LAP 4-5-13 | 36 | 75 | 59 : 41 |
| 6 | L-4 | P-26 | LAP 4-5-26 | 39 | 82 | 64 : 36 |
| 7 | L-4 | P-28 | LAP 4-5-28 | 39 | 71 | 60 : 40 |
| 8 | L-4 | P-36 | LAP 4-5-36 | 36 | 72 | 70 : 30 |
| 9 | L-1 | P-36 | LAP 1-5-36 | 39 | 52 | 49 : 51d |
| 10 | L-2 | P-36 | LAP 2-5-36 | 42 | 62 | 54 : 46 |
| 11 | L-3 | P-36 | LAP 3-5-36 | 45 | 62 | 52 : 48 |
| 12 | L-5 | P-36 | LAP 5-5-36 | 39 | 89 | 74 : 26 |
| 13 | L-6 | P-36 | LAP 6-5-36 | 39 | 64 | 35 : 65d |
| 14 | L-7 | P-36 | LAP 7-5-36 | 39 | 93 | 74 : 26 |
| 15 | L-8 | P-36 | LAP 8-5-36 | 39 | 94 | 15 : 85d |
| 16 | L-9 | P-36 | LAP 9-5-36 | 42 | 90 | 71 : 29 |
| 17 | L-8 | P-11 | LAP 8-5-11 | 39 | 74 | 62 : 38e |
| 18 | L-8 | P-26 | LAP 8-5-26 | 39 | 93 | 78 : 22e |
Unless otherwise specified, all reactions were carried out at 0.2 M in 1 (0.25 mmol) in toluene with 2.0 equiv amine and 1.5 equiv of 2 at rt for the indicated time with 20 mol% of the catalyst. The catalyst was made from the (S)-enantiomer of the ligand (≥99% ee) according to the procedure in Table 1.
Isolated yield after chromatography on silica gel.
Determined by HPLC.
Catalyst generated from (R)-ligand.
The ligand for this run was 97% ee.
f The catalyst loading was 10 mol%.
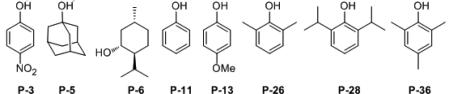
At this point a solvent screen was performed on the optimal catalyst prepared from the substituted VAPOL ligand L-8 and the 2,4,6-trimethyphenol P-36 and the results are given in the supporting information. The induction dropped dramatically with coordinating solvents such as THF and acetonitrile (54:46 and 64:36, respectively) which is consistent with an ion-pair mechanism. The optimal solvent was found to be mesitylene (87:13, Table 3, entry 6) and all further optimizations were performed in this solvent. The stoichiometry of the amine does not seem to have a big impact on the reaction with the yields and inductions falling only slightly in the range of 2.0 to 1.02 equivalents (entries 6-8). With the identification of the VAPOL derivative L-8 as the optimal ligand, a final screen of this ligand and 13 different phenols was performed in mesitylene and some of the results are shown in Table 3 and the rest can be found in the supporting information. The optimal phenol was found to be the 2,6-dimethyl-4-methoxy phenol P-47 and the resultant catalyst (LAP 8-5-47) gave a 90:10 enantiomeric ratio in a total of 91% yield (entry 13). In contrast to the VAPOL derived catalysts which are insensitive to the electronic nature of the phenol (Table 2, entries 1 vs 5), the catalysts derived from the VAPOL derivative L-8 are quite sensitive. The induction slightly increases when the p-methyl group in P-36 is replaced by a methoxyl group (P-47, entries 6 vs 13) and dramatically drops when it is replaced with a nitro group (P-44, entries 6 vs 12). This is consistent with an ion-pair mechanism in which the strength of the electrostatic attraction is important for the asymmetric induction (Scheme 4). The optimized procedure for the amine A-5 (entry 13) was extended to several para-substituted dibenzylamines. All of these dibenzyl amines gave high selectivities (87:13 to 90:10 entries 13-17) with the exception of the p-nitro substituted dibenzylamine A-11 which gave a dramatic drop in the yield and induction (entry 18) and this could be due to destabilization of iminium ion 7 (Scheme 4) Having established the most effective combination of the all of the parts in the boroxinate catalyst, an evaluation of different aryl aldehydes in the Ugi reaction was undertaken with amine A-5 and the results are presented in Table 4. Most of the substrates reacted to give α-amino amides with enantiomeric ratios of 90:10 to 95:5 including aryl aldehydes with both electron-withdrawing and electron-donating groups. The reaction of cyclohexane carboxaldehyde gave racemic product (47%) and this result will require further examination. The reactions in Table 4 were performed in the presence of 4 Å molecular sieves, whereas, most of the previous reactions in this work were without the sieves. The sieves have essentially no effect on the induction but do seem to accelerate the reaction slightly. The rate is slower at 0°C than at 25 °C but the inductions are not substantially different. In many cases the α-amino amide 3 can be crystallized and a few examples are shown where the er can be often enhanced to >99.5 : 0.5. The ligand L-8 can also be recovered from these reactions in high yield (~90%) with no loss in enantiomeric purity (>99.5:0.5). The reaction can be extended to heterocyclic aldehydes as illustrated by the reaction with pyridine carboxaldehydes. 3-Pyrridyl carboxaldehyde is the most reactive, and the 4-pyrridyl isomer reacts slowly (entries 27 & 28) and the 2-pyrridyl isomer is unreactive (not shown).
Table 3.
Screen of Additional Phenol Substituents in the Boroxainte Core with Ligand L-8.a
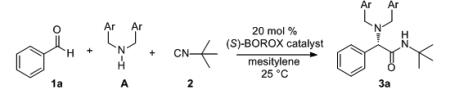
| |||||||
|---|---|---|---|---|---|---|---|
| entry | ligand | amine | phenol/alcohol | catalyst | time (h) | % yield 3ab | erc |
| 1 | L-4 | A-5 | P-11 | LAP 4-5-11 | 38 | 71 | 61 : 39 |
| 2 | L-4 | A-5 | P-36 | LAP 4-5-36 | 42 | 90 | 71 : 29 |
| 3 | L-8 | A-5 | P-11 | LAP 8-5-11 | 41 | 72 | 33 : 67d |
| 4 | L-8 | A-5 | P-26 | LAP 8-5-26 | 39 | 94 | 83 : 17 |
| 5 | L-8 | A-5 | P-28 | LAP 8-5-28 | 39 | 72 | 68 : 32 |
| 6 | L-8 | A-5 | P-36 | LAP 8-5-36 | 39 | 92 | 87 : 13 |
| 7 | L-8 | A-5 | P-36 | LAP 8-5-36 | 39 | 86 | 86 : 14e |
| 8 | L-8 | A-5 | P-36 | LAP 8-5-36 | 39 | 75 | 84 : 16f |
| 9 | L-8 | A-12 | P-36 | LAP 8-12-36 | 39 | 65 | 61 : 39 |
| 10 | L-6 | A-5 | P-36 | LAP 8-5-36 | 41 | 53 | 32 : 68d |
| 11 | L-8 | A-5 | P-39 | LAP 8-5-39 | 38 | 95 | 82 : 18 |
| 12 | L-8 | A-5 | P-44 | LAP 8-5-44 | 38 | 76 | 59 : 41 |
| 13 | L-8 | A-5 | P-47 | LAP 8-5-47 | 38 | 91 | 10 : 90d,g,h |
| 14 | L-8 | A-7 | P-47 | LAP 8-5-47 | 24 | 91 | 12 : 88d |
| 15 | L-8 | A-8 | P-47 | LAP 8-5-47 | 24 | 85 | 10 : 90d |
| 16 | L-8 | A-9 | P-47 | LAP 8-5-47 | 24 | 80 | 12 : 88d |
| 17 | L-8 | A-10 | P-47 | LAP 8-5-47 | 24 | 79 | 13 : 87d |
| 18 | L-8 | A-11 | P-47 | LAP 8-5-47 | 24 | 22 | 27: 73d |
Unless otherwise specified, all reactions were carried out at 0.2 M in 1 (0.25 mmol) in mesitylene with 2.0 equiv amine and 1.5 equiv of 2 at rt for the indicated time with 20 mol% of the catalyst. Entries 14-18 were carried out in the presence of 4Å molecular sieves. The catalyst was prepared according to the procedure in Table 1 with the (S)-ligand unless otherwise specified. Ligand (S)-L-8 was 97% ee and (R)-L-8, (S)-L-4 and (R)-L-6 were >99% ee.
Isolated yield after chromatography on silica gel.
Determined by HPLC.
Catalyst generated from (R)-ligand.
Reaction with 1.2 equiv amine.
Reaction with 1.02 equiv amine.
The er was 89:11 when (S)-L-8 was 97% ee.
The yield was 86% after 24 h.
Scheme 4.
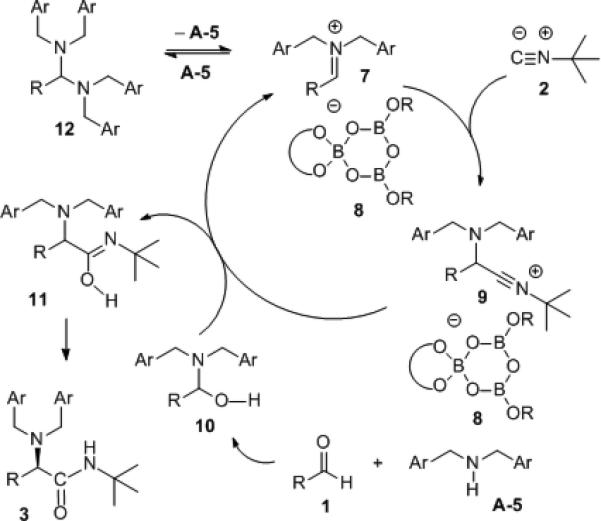
Proposed Mechanism for the 3-Component Ugi Reaction.
Table 4.
Substrate Scope of the Catalytic Asymmetric 3-Component Ugi Reaction.a
| ||||||
|---|---|---|---|---|---|---|
| entry | series | R | time (h) | temp (°C) | % yield 3b | erc |
| 1 | a | C6H5 | 7 | 40 | 87e | 86 : 14 |
| 2d | a | C6H5 | 24 | 25 | 86 (71) | 90 : 10 (>99.5 : 0.5) |
| 3 | a | C6H5 | 66 | 0 | 75 | 92 : 8 |
| 4f | b | 4-NO2C6H4 | 24 | 25 | 83 | 93 : 7 |
| 5f | b | 4-NO2C6H4 | 66 | 0 | 51e | 92 : 8 |
| 6 | c | 4-CF3C6H4 | 24 | 25 | 85 | 91 : 9 |
| 7 | d | 4-BrC6H4 | 24 | 25 | 85 | 93 : 7 |
| 8 | d | 4-BrC6H4 | 48 | 0 | 65g | 95 : 5 |
| 9f | d | 4-BrC6H4 | 48 | 0 | 75h,i | 95 : 5 |
| 10f | e | 3-BrC6H4 | 22 | 25 | 82 | 93 : 7 |
| 11 | e | 3-BrC6H4 | 66 | 0 | 66h | 92 : 8 |
| 12 | f | 3,4-Cl2C6H3 | 24 | 25 | 85 | 94 : 6 |
| 13 | f | 3,4-Cl2C6H3 | 66 | 0 | 54e | 95 : 5 |
| 14 | g | 4-FC6H4 | 24 | 25 | 87e | 91 : 9 |
| 15 | g | 4-FC6H4 | 67 | 0 | 62 | 94 : 6 |
| 16 | h | 4-MeO2CC6H4 | 24 | 25 | 80 | 93 : 7 |
| 17f | h | 4-MeO2CC6H4 | 67 | 0 | 62 | 93 : 7 |
| 18 | i | 4-OAcC6H4 | 24 | 25 | 86 | 85 : 15 |
| 19 | j | 4-AcNHC6H4 | 24 | 25 | 77 (47) | 85 : 15 (96:4) |
| 20f | k | 4-MeC6H4 | 24 | 25 | 84 (47) | 91 : 9 (>99.5 : 0.5) |
| 21 | k | 4-MeC6H4 | 66 | 0 | 80e | 92 : 8 |
| 22 | l | 2-MeC6H4 | 24 | 25 | 76 (56) | 78 : 22 (>99 : 1) |
| 23 | m | 4-t-BuC6H4 | 24 | 25 | 83 | 84 : 16 |
| 24 | n | 4-MeOC6H4 | 40 | 25 | 84j | 88 : 12 |
| 25 | n | 4-MeOC6H4 | 24 | 25 | 70 | 89 : 11 |
| 28 | n | 4-MeOC6H4 | 24 | 0 | 51 | 92 : 8 |
| 27 | o | 3-pyridyl | 25 | 25 | 80 (61) | 90 : 10 (>99 : 1) |
| 28 | p | 4-pyridyl | 70 | 25 | 66 | 89 : 11 |
Unless otherwise specified, all reactions were carried out at 0.2 M in 1 (0.25 mmol) in mesitylene with 2.0 equiv amine and 1.5 equiv of 2 at rt in the presence of 4Å MS for the indicated time with 20 mol% of the catalyst. The catalyst was made from the (R)-enantiomer of the ligand (≥99% ee) according to the procedure in Table 1.
Isolated yield after chromatography on silica gel. Yield in paranthesis is % recovery of the first crop after crystallization
Determined by HPLC. The er in parentheses is of the first crop.
Average of 4 runs.
1H NMR yield with internal standard (Ph3CH).
Average of 2 runs.
Yield was 46% after 24 h.
Reaction at 0.4 M.
Yield was 76% after 66 h. The yield was 60% after 66 h in the absence of 4Å MS.
Run in the absence of 4Å MS.
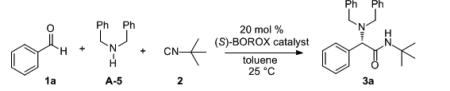
Although t-butylisonitrile was the optimal isonitrile, a number of other isonitriles were effective with inductions ranging from 51:49 to 88:12 under the conditions in Table 4 with benzaldehyde (see supporting information). The catalyst loading can be reduced to 10 mol% with no significant drop in yield or induction if molecular sieves are employed (Scheme 3). The er drops from 90:10 to 87:13 when the loading is reduced from 20 to 10 mol%.[21] Both the 90:10 and the 87:13 mixture can be enhanced to an er of >99.5:0.5 by crystallization with 70-71% recovery for the first crop. The absolute configuration was determined by removal of the benzyl groups to give 6a and the other α-amino amides were assumed to be homo-chiral with 3a.
Scheme 3.
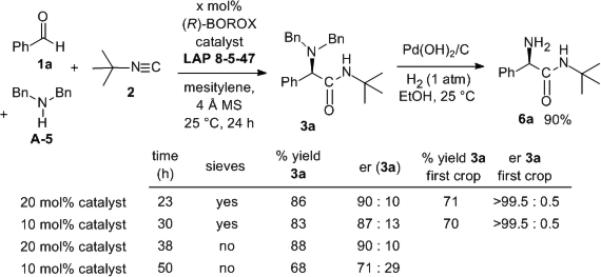
Effect of Molecular Sieves on Catalyst Loading
The involvement of a BOROX catalyst containing a boroxinate core is supported by 1H and 11B NMR studies (see Supporting Information). It is considered likely that the catalyst is the boroxinate anion 8 that exists as an ion pair with the iminium ion 7 and thus that this Ugi reaction is an example of “chiral anion catalysis”.[22] The mechanism can be envisioned to involve the addition of the isonitrile to the iminium cation 7 to give the nitrilium cation 9 which is also ion-paired with the chiral anion catalyst 8. We propose that the next step is hydroxyl exchange between the nitrilium cation 9 and the hemiaminal 10 which would result in the regeneration of the iminium ion 7 and the formation of the product in the form of the tautomer 11. It is also possible that the hemi-aminal 10 is protonated and releases H2O which adds to nitrilium ion 9. Evidence against the presence of free H2O in this reaction comes from the fact that the presence of molecular sieves does not greatly effect the rate of the reaction and the addition of H2O slows down the reaction (see Supporting Information, parts VIII & XVIII). When the reaction is followed by 1H NMR it is observed that there is an initial build-up of the aminal 12 (~15% at 10% completion) and then it slowly disappears and is gone at the end of the reaction. Finally, a four-component version of this reaction with the optimal BOROX catalyst (LAP 8-5-47) was attempted with benzaldehyde and benzoic acid but the Ugi product was racemic (see Supporting Information).
A great diversity of BOROX catalysts can be quickly generated and the optimal combination of the ligand, amine and phenol/alcohol components of the catalyst were sought and found for the three-component asymmetric Ugi reaction. The active catalyst is proposed to involve an ion-pair between a chiral boroxinate anion and an achiral iminium ion.
Supplementary Material
Scheme 1.
Acknowledgments
This work was supported by a grant from the National Institute of General Medical Sciences (GM094478.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a Ugi I, Meyr R, Fetzer U, Steinbrückner Angew. Chem. 1959;71:386. [Google Scholar]; b Ugi I, Steinbrückner C. Angew. Chem. 1960;72:267. [Google Scholar]
- 2.a Dömling A, Ugi I. Angew. Chem. Int. Ed. 2000;39:3169–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. For reviews, see. [DOI] [PubMed] [Google Scholar]; b Ugi I, Werner I, Dömling A. Molecules. 2003;8:53–66. [Google Scholar]; c Zhu J, Bienayme H, editors. Multicomponent Reactions. Wiley-VCH; Weinheim, Germany: 2005. [Google Scholar]; d Dömling A. Chem. Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]; e Wessjohann LA, Rivera DG, Vercillo OE. Chem. Rev. 2009;109:796–814. doi: 10.1021/cr8003407. [DOI] [PubMed] [Google Scholar]; f El Kain L, Grimaud L. Tetrahedron. 2009;65:2153–2171. [Google Scholar]; g Toure BB, Hall DG. Chem. Rev. 2009;109:4439–4486. doi: 10.1021/cr800296p. [DOI] [PubMed] [Google Scholar]; h de Graaff C, Ruijter E, Orru RVA. Chem. Soc. Rev. 2012;41:3969–4009. doi: 10.1039/c2cs15361k. [DOI] [PubMed] [Google Scholar]
- 3.Cheron N, Ramozzi R, El Kaim L, Grimaud L, Fleurat-Lessard P. J. Org. Chem. 2012;77:1361–1366. doi: 10.1021/jo2021554. For citations to the literature on the mechanism, see. [DOI] [PubMed] [Google Scholar]
- 4.a Bienayme H, Hulme C, Oddon G, Schmitt P. Chem. Eur. J. 2000;6:3321–3329. doi: 10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a. For reviews, see. [DOI] [PubMed] [Google Scholar]; b Akritopoulou-Zanze I. Curr. Opin. Chem. Biol. 2008;12:324–331. doi: 10.1016/j.cbpa.2008.02.004. [DOI] [PubMed] [Google Scholar]; c Biggs-Houck JE, Younai A, Shaw JT. Curr Opin. Chem. Biol. 2010;14:371–382. doi: 10.1016/j.cbpa.2010.03.003. [DOI] [PubMed] [Google Scholar]; d Ruijter E, Scheffelaar R, Orru RVA. Angew. Chem. Int. Ed. 2011;50:6234–6246. doi: 10.1002/anie.201006515. [DOI] [PubMed] [Google Scholar]
- 5.Pan SC, List B. Angew. Chem. Int Ed. 2008;47:3622–3625. doi: 10.1002/anie.200800494. This publication includes one example of a 3-component Ugi reaction with a chiral catalyst which proceeds with very low asymmetric induction. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y, Hasui T, Suginome M. Org. Lett. 2007;9:4407–4410. doi: 10.1021/ol701570c. For citations to the literature, see. [DOI] [PubMed] [Google Scholar]
- 7.Okandeji BO, Gordon JR, Sello JK. J. Org. Chem. 2008;73:5595. doi: 10.1021/jo800745a. For citations to the literature, see. [DOI] [PubMed] [Google Scholar]
- 8.a Denmark SE, Fan Y. J. Am. Chem. Soc. 2003;125:7825. doi: 10.1021/ja035410c. [DOI] [PubMed] [Google Scholar]; b Kusebauch U, Beck B, Messer K, Herdtweck E, Dömling A. Org. Lett. 2003;5:4021–4024. doi: 10.1021/ol035010u. [DOI] [PubMed] [Google Scholar]; c Andreana PR, Liu CC, Schreiber SL. Org. Lett. 2004;6:4231–4233. doi: 10.1021/ol0482893. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Denmark SE, Fan Y. J. Org. Chem. 2005;70:9667–9676. doi: 10.1021/jo050549m. [DOI] [PubMed] [Google Scholar]; e Wang S-X, Wang M-X, Wang D-X, Zhu J. Angew. Chem. Int. Ed. 2008;47:388–391. doi: 10.1002/anie.200704315. [DOI] [PubMed] [Google Scholar]
- 9.a Ramon DJ, Yus M. Angew. Chem. Int. Ed. 2005;44:1602–1634. doi: 10.1002/anie.200460548. [DOI] [PubMed] [Google Scholar]; b van Berkel SS, Bögels BGM, Wijdeven MA, Westermann B, Rutjes FPJT. Eur. J. Org. Chem. 2012:3543–3559. [Google Scholar]
- 10.Hashimoto T, Kimura H, Kawamata Y, Maruoka K, Angew K. Chem. Int. Ed. 2012;51:7279–7281. doi: 10.1002/anie.201201905. [DOI] [PubMed] [Google Scholar]
- 11.a Yue T, Wang M-X, Wang D-X, Masson G, Zhu J. Angew. Chem. Int. Ed. 2009;48:6717–6721. doi: 10.1002/anie.200902385. [DOI] [PubMed] [Google Scholar]; b Su Y, Bouma MJ, Alcaraz L, Stocks M, Furber M, Masson G, Xhu J. Chem. Eur. J. 2012;18:12624. doi: 10.1002/chem.201202174. [DOI] [PubMed] [Google Scholar]
- 12. Hemiaminals have also been proposed as intermediates, see ref 3.
- 13.a Gupta AK, Mukherjee M, Wulff WD. Org. Lett. 2011;13:5866–5869. doi: 10.1021/ol202472z. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ren H, Wulff WD. Org. Lett. 2010;12:4908–4911. doi: 10.1021/ol102064b. [DOI] [PubMed] [Google Scholar]; c Mukherjee M, Gupta AK, Lu Z, Zhang Y, Wulff WD. J. Org. Chem. 2010;75:5643–5660. doi: 10.1021/jo101160c. [DOI] [PubMed] [Google Scholar]; d Desai AA, Wulff WD. J. Am. Chem. Soc. 2010;132:13100–13103. doi: 10.1021/ja1038648. [DOI] [PubMed] [Google Scholar]; e Zhang Y, Desai A, Lu Z, Hu G, Ding Z, Wulff WD. Chem. Eur. J. 2008;14:3785–3803. doi: 10.1002/chem.200701558. [DOI] [PubMed] [Google Scholar]
- 14.a Ren R, Wulff WD. J. Am. Chem. Soc. 2011;133:5656–5659. doi: 10.1021/ja1110865. [DOI] [PubMed] [Google Scholar]; b Ren H, Wulff WD. Org. Lett. 2013;15:242–245. doi: 10.1021/ol302769r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman CA, Antilla JC, Chen P, Wulff WD. J. Am. Chem. Soc. 2007;129:7216–7217. doi: 10.1021/ja069019d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu G, Gupta AK, Huang RH, Mukherjee J, Wulff WD. J. Am. Chem. Soc. 2010;132:14669–14675. doi: 10.1021/ja1070224. [DOI] [PubMed] [Google Scholar]
- 17.Gupta AK, Mukherjee M, Hu G, Wulff WD. J. Org. Chem. 2012;77:7932–7944. doi: 10.1021/jo301064u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Li F, Zhao L, He Q, Chen F, Zheng C. Tetrahedron. 2011;67:9199–9203. For catalyst generation in which boric acid is substituted for BH3•SMe2, see. [Google Scholar]
- 19. We have reported one example of a slightly improved catalyst with 2,4,6-trimethylphenol (see reference 14)
- 20.Guan Y, Ding Z, Wulff Chem WD. Eur. J. 2013;19:15565–15571. doi: 10.1002/chem.201302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The yield of 3a with 5 mol% catalyst is 62% after 73 h (er = 74:26) with sieves and 7% yield without sieves.
- 22.Phipps RJ, Hamilton GL, Toste FD. Nature Chemistry. 2012;4:603–614. doi: 10.1038/nchem.1405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


