Summary
More than two thousand C. elegans genes are targeted for RNA silencing by the mutator complex, a specialized siRNA amplification module which is nucleated by the Q/N-rich protein MUT-16. The mutator complex localizes to Mutator foci adjacent to P granules at the nuclear periphery in germ cells [1]. Here, we show that the DEAD box RNA helicase smut-1 functions redundantly in the mutator pathway with its paralog mut-14 during RNA interference. Mutations in both smut-1 and mut-14 also cause widespread loss of endogenous siRNAs. The targets of mut-14 and smut-1 largely overlap with the targets of other mutator class genes, however, the mut-14 smut-1 double mutant and the mut-16 mutant display the most dramatic depletion of siRNAs, suggesting that they act at a similarly early step in siRNA formation, mut-14 and smut-1 are predominantly expressed in the germline and, unlike other mutator class genes, are specifically required for RNAi targeting germline genes. A catalytically inactive, dominant negative missense mutant of MUT-14 is RNAi-defective in vivo, however, mutator complexes containing the mutant protein retain the ability to synthesize siRNAs in vitro. The results point to a role for mut-14 and smut-1 in initiating siRNA amplification in germ cell Mutator foci, possibly through the recruitment or retention of target mRNAs.
Results and Discussion
mut-14 and smut-1 have Overlapping Roles in Germline RNAi
22G siR-1 is one of a cluster of secondary 22-nt 5′G-containing siRNAs (22G-RNAs) produced from the long non-coding RNA linc-22 [2]. 22G siR-1 formation requires each of the six mutator class genes except the DEAD box RNA helicase mut-14 [1]. Consistent with their roles in 22G siR-1 formation, an siR-1 sensor transgene [3] is desilenced in each mutator mutant except mut-14(pk738) (Figure 1A). Each mutant assayed is presumed null, containing early stop codons or large deletions, except mut-14(pk738), which encodes a protein bearing an amino acid substitution in the conserved catalytic core of the DEAD motif. To generate a null allele of mut-14, mut-14(mg464), hereafter referred to simply as mut-14, we deleted the mut-14 coding sequence [4]. Animals containing the mut-14(pk738) point mutation were deficient in their ability to inactivate germline mRNAs by RNAi but competent to inactivate somatic mRNAs by RNAi (Figure 1B) [1]. In contrast, animals containing the mut-14 deletion were competent for both germline and somatic gene inactivations by RNAi, similar to wild type (Figure 1B).
Figure 1. mut-14 and smut-1 have redundant roles in RNAi.
(A) Diagram of the 22G siR-1 sensor ubl-1∷GFP-siR-1-sensor and images of GFP fluorescence from transgenic wild type and mutant larval stage L4 animals. (B) Assay for germline and somatic RNA interference defects. (C) Images of L4 stage animals containing either the ubl-1∷GFP control transgene, which lacks a 22G siR-1 target site, or the ubl- 1∷GFP-siR- 1-sensor transgene. (D) mCherry expression from smut-1 and mut-14 promoter fusions in L4 animals. Animals are outlined in white and gonads are outlined in magenta. The vector control lacks mCherry sequence and is shown as a control for autofluorescence. See also Figure S1.
Y38A10A.6, hereafter referred to as Synthetic MUTator-1 (smut-1), is one of two closely related paralogs of mut-14, although unlike MUT-14, smut-1 contains a serine instead of an alanine within its DEAD motif (DESD) (Figures S1A-S1B). Similar to the mut-14 deletion and to wild type, smut-1(tm1301), a strain containing a large deletion in smut-1, was susceptible to both germline and somatic RNAi. In contrast, a strain carrying deletions in both mut-14 and smut-1 was defective for germline RNAi but normal for somatic RNAi, similar to mut-14(pk738) (Figure 1B). ZC317.1, the other closely related paralog of mut-14 (Figure S1A), is predicted by RNA-seq [5] to contain an early stop codon that truncates the C-terminal helicase domain (Figures S1B-S1C). We did not observe RNAi defects in a ZC317.1 deletion mutant, nor did we observe somatic RNAi defects in animals containing mutations in all three related helicases (Figure S1D).
GFP expression from the siR-1 sensor was strongly elevated in both the mut-14(pk738) smut-1 and mut-14 smut-1 double mutants but not in mut-14 or smut-1 single deletion mutants (Figure 1C; Figures S1E-S1F). 22G siR-1 levels were moderately reduced in smut-1 (p = 0.026) and to a greater degree in the mut-14 smut-1 double mutant (p < 0.001), but not in the mut-14 single mutant (Figure S1G). The levels of each of two ERGO-1 class 26G-RNAs, which act upstream of the production of certain 22G-RNAs, were also significantly reduced in the mut-14 smut-1 double mutant (p < 0.05), but not in either single mutant (Figure S1G). Although 22G siR-1 is somatic, its formation is initiated by an ERGO-1 class 26G-RNA during oogenesis and/or embryogenesis [3], thus it is possible that mut-14 and smut-1 are indirectly involved in 22G siR-1 formation in the soma via their role in 26G-RNA formation in the germline.
Consistent with a requirement for mut-14 and smut-1 specifically in germline RNAi, mut-14 and smut-1 promoters drive expression of mCherry predominantly in germ cells (Figures 1D). mCherry expression from the smut-1 promoter, but not the mut-14 promoter, was also relatively strong in developing embryos (Figure S1H).
Widespread Loss of Endogenous siRNAs in mut-14 smut-1
We sequenced small RNAs from wild type, and from mut-14 and smut-1 single and double mutants, each of which also contained the siR-1 sensor transgene (Table S1). smut-1 displayed very little change in siRNA levels across each of the six C. elegans chromosomes, relative to wild type, whereas mut-14 displayed widespread but modest loss of siRNAs, which was strongly enhanced in the mut-14 smut-1 double mutant (Figure 2A). siRNAs depleted in mut-14 and mut-14 smut-1 were predominantly 22G-RNAs derived from coding genes, pseudogenes, and transposons (Figure 2B). 2,335 of these features were depleted of siRNAs by >3 fold in mut-14 smut-1 (Figure 2C).
Figure 2. mut-14 smut-1 mutants display widespread loss of endogenous siRNAs.
(A) Log2 ratio of small RNA reads in smut-1, mut-14, and mut-14 smut-1 mutants, relative to control animals, plotted across each chromosome. Each strain also contains the ubl-1 ∷GFP-siR-1-sensor transgene. Control animals contain the siR-1 sensor but are wild type for smut-1 and mut-14. Peaks are colored based on the dominant size class. (B) Proportion of small RNA reads aligning to each genomic feature. (C) The Venn diagram displays genes depleted of siRNAs in smut-1 and mut-14 mutants. Total numbers of targets depleted of siRNAs by >3 fold in each mutant, relative to wild type, are indicated in parentheses. (D) Relative levels of 22G-RNAs in smut-1 and mut-14 mutants (sequencing was done without replicates). (E) Normalized GFP siRNA reads from the ubl-1∷GFP-siR-1-sensor transgene. For simplicity, the 22G siR-1 target site and downstream sequence is not shown. (F) Proportion of small RNA reads aligning to each genomic feature. (G) The Venn diagram displays genes depleted of siRNAs in smut-1 and mut-14(pk738) mutants. Total numbers of targets depleted of siRNAs by >3 fold in each mutant, relative to wild type, are indicated in parentheses. (H) Relative levels of 22G-RNAs in smut-1 and mut-14(pk738) mutants. Mean +/- SD for two biological replicates. (I) MUT-14∷mCherry (red) and catalytically dead MUT-14DKAD∷GFP (green) colocalization at perinuclear Mutator foci. DNA is counterstained with DAPI (blue). See also Figure S1 and Table S1.
To determine which classes of siRNAs are dependent on mut-14 and smut-1 we examined 22G-RNA levels from mRNA targets of the Argonautes WAGO-1, CSR-1, ERGO-1, and ALG-3/4, which represent each of the C. elegans endogenous siRNA pathways [6-10]. ERGO-1 and ALG-3/4 bind 26G-RNAs but trigger formation of 22G-RNAs from target mRNAs [6, 9, 11]. 22G-RNAs derived from WAGO and ERGO-1 targets were strongly depleted in the mut-14 smut-1 double mutant but only modestly or not at all in the single mutants (Figure 2D). In contrast, the levels of 22G-RNAs derived from ALG-3/4 targets were not substantially affected in any of the mut-14 and smut-1 mutants, nor were the levels of primary ALG-3/4 class 26G-RNAs (Figure 2D; Figure S1G). CSR-1 class siRNA levels appeared elevated in the mut-14 smut-1 double mutant, possibly a normalization artifact caused by reduced levels of WAGO and ERGO-1 class siRNAs, as a CSR-1 siRNA that we examined by qRT-PCR was unaffected (Figure 2D; Figure S1G).
The siR-1 sensor is subject to transgene silencing in the germline, independent of 22G siR-1 [3]. siRNAs derived from the siR-1 sensor were depleted in mut-14 and to a greater extent in the mut-14 smut-1 double, but not in the smut-1 single mutant (Figures 2D-2E). We conclude that mut-14 and smut-1 have overlapping roles in WAGO and ERGO-1 pathways, transgene silencing, and exogenous RNAi, although their roles in these pathways may be restricted to the germline and early embryos.
Catalytically Dead mut-14(pk738) is Antagonistic to smut-1
mut-14(pk738) encodes a protein bearing a point mutation that alters an amino acid in the DEAD motif and unlike the mut-14 deletion allele is germline RNAi-defective (Figure 1B). We sequenced small RNAs from wild type, and from mut-14(pk738) and smut-1 single and double mutants (Table S1). The proportion of features depleted of siRNAs was similar between mut-14(pk738) and the mut-14(pk738) smut-1 double mutant (Figure 2F). The vast majority of genes depleted of siRNAs in the mut-14(pk738) smut-1 double mutant were also affected in the mut-14(pk738) single mutant (Figure 2G). WAGO class 22G-RNAs were depleted to similar levels in mut-14(pk738) and the mut-14(pk738) smut-1 double mutant (Figure 2H), both of which resemble the depletion seen in the mut-14 smut-1 double deletion mutant (Figure 2D). In contrast, 22G-RNA levels from ERGO-1 targets were somewhat elevated in mut-14(pk738) but depleted by ∼80% in mut-14(pk738) smut-1, similar to what was observed in the mut-14 smut-1 deletion mutant (Figures 2D and 2H). When fused to mCherry or GFP, wild type MUT-14 and MUT-14 containing the pk738 mutation (MUT-14DKAD) colocalized at the nuclear periphery, indicating that mut-14(pk738) produces a stable protein that localizes to the proper compartment (Figure 2I).
These results suggest that mut-14(pk738) is antagonistic to smut-1 during WAGO class 22G-RNA formation. That mut-14(pk738) is not antagonistic to smut-1 in ERGO-1 class 26G-RNA and ergo-1-dependent 22G-RNA formation may be related to differential expression of smut-1 and mut-14 during oogenesis and embryogenesis, the stages during which 26G-RNAs are produced (Figure S1H) [6, 10].
Comprehensive Analysis of siRNA Defects in Mutator Mutants
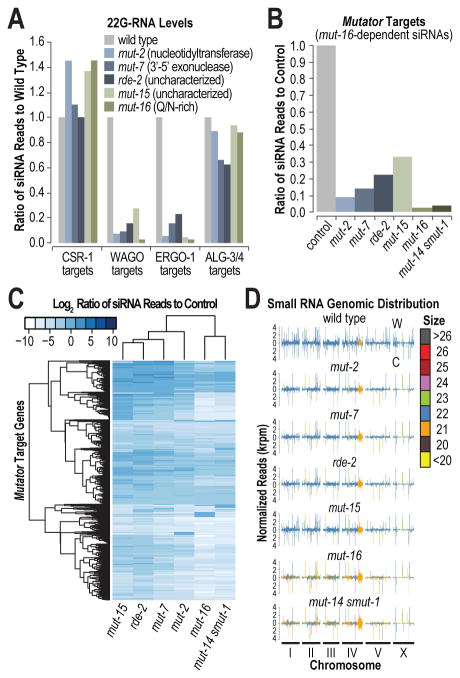
MUT-14 is part of an siRNA amplification module that includes the mutators MUT-2, MUT-7, RDE-2, MUT-15, and MUT-16, and which colocalizes with the RNA-dependent RNA polymerase RRF-1 [1]. To determine if mut-14, smut-1, and the other mutators converge on a common set of targets, we subjected wild type and mutant animals to small RNA sequencing (Table S1). Each of the mutator mutants was depleted of 22G-RNAs derived from WAGO and ERGO-1 targets, but not substantially from CSR-1 or ALG-3/4 targets (Figure 3A). Although the genes depleted of siRNAs in each of the mutator mutants largely overlapped, mut-16 displayed the greatest loss of siRNAs (Figure 3A; Figure S2A). Thus, we defined mutator targets as the ∼2,300 genes depleted of siRNAs by >3 fold in mut-16 (Table S2). Of the six mutator mutants, mut-16 and the mut-14 smut-1 double mutant showed the strongest depletion of siRNAs from mutator targets (Figure 3B; Table S2). When clustered by depletion in mutator target siRNAs, mut-16 and the mut-14 smut-1 double mutant assembled more closely with one another than with any of the other mutator mutants (Figure 3C).
Figure 3. mut-14 smut-1 and mut-16 mutants have similar siRNA defects.
(A) Relative levels of 22G-RNAs in wild type, mut-2, mut-7, rde-2, mut-15, and mut-16 mutants. (B) Relative levels of 22G-RNAs derived from mutator target mRNAs in each of the mutator mutants, relative to wild type or a similar control. (C) The heat map displays the log2 ratio of siRNA reads in each mutant, relative to wild type or a similar control, for each of the mutator target genes. (D) Normalized small RNA reads (thousand reads per million total library reads) in wild type and each of the mutator mutants plotted across each chromosome. Peaks are colored based on the dominant size class. siRNAs are predominantly 22-nt in wild type animals. W, Watson strand; C, Crick strand. See also Figure S2 and Tables S1-S2.
There was a genome-wide enrichment, particularly from mutator target genes, for 21-nt small RNA species in mut-16 and mut-14 smut-1 mutants, but not in any of the other mutator mutants (Figure 3D; Figures S2B-S2D). We examined residual siRNAs in each mutat or mutant to determine if they contained modifications that were absent in wild type animals that might to point to a specific role in siRNA maturation. We observed an ∼3 fold increase in the proportions of siRNAs containing nontemplated 3′ uracil (U) additions in mut-16 and mut-14 smut-1 but not in any of the other mutator mutants (Figure S2E). The proportion of siRNAs containing 3′ nontemplated Us was elevated -8.5 fold in the mut-14(pk738) smut-1 double mutant and nearly every siRNA-yielding gene had elevated levels of nontemplated Us, including CSR-1 targets (Figures S2E-S2F). The reason for this is unclear, however, the size distribution and elevated levels of nontemplated Us observed in residual siRNAs in mut-16 and mut-14 smut-1 are features more common to CSR-1 class siRNAs than to WAGO class (Figure S2G) [3, 8, 12]. It is possible that some mutator targets also produce CSR-1 class siRNAs or that in the absence of the mutator pathway some mutator targets are misrouted into the CSR-1 pathway.
We did not find evidence for siRNA precursors in any of our libraries, although our analysis was limited to sequences <30-nt long, which may indicate that the mutators function to facilitate RNA-dependent RNA polymerase activity on target mRNAs and are not involved in processing siRNAs into their 22-nt mature form. mut-16, but not mut-14 or smut-1, is required for formation and proper localization of the mutator complex (Figure S2H) [1], indicating that mut-16 acts upstream of other mutators during siRNA biogenesis. Because mut-16 and the mut-14 smut-1 double mutant have the most dramatic affects on siRNA levels and show similar anomalies in residual siRNAs, we propose that they act at a similarly early step in siRNA formation. Although mut-14 and smut-1 are not required for mutator protein localization, they could be required to recruit mRNAs into Mutator foci to initiate siRNA amplification.
Compartmentalization of the Mutator Pathway is Restricted to the Germline
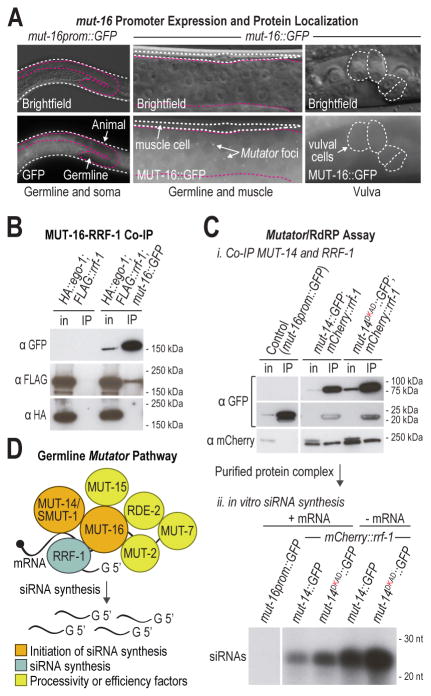
Each of the mutators except mut-14 and smut-1 are required for both somatic and germline RNAi [1]. It is possible that another gene fulfills the function in somatic cells that mut-14 and smut-1 serve in the germline. Alternatively, mut-14 and smut-1 could have a role in RNAi that is specifically required in germ cells. What distinguishes germline RNAi from somatic RNAi? In germ cells, each of the mutator proteins localize to perinuclear compartments, called Mutator foci, adjacent to P granules [1]. To determine if the mutators are also compartmentalized in the soma, we examined GFP expression in animals containing either the mut-16 promoter (mut-16prom∷GFP) or the mut-16 promoter and coding sequence fused to GFP (mut-16∷GFP). mut-16 is required for mutator complex formation in both the soma and the germline and for its localization to the nuclear periphery in germ cells [1]. Free GFP expressed from the mut-16 promoter was present throughout the germline and soma, in contrast to mut-14 and smut-1 which are predominantly expressed in the germline (Figures 1D and 4A). The MUT-16∷GFP translational fusion protein formed distinct perinuclear foci in the germline but appeared diffuse throughout the cytoplasm in somatic cells (Figure 4A; Figure S3A). MUT-7∷GFP also appeared diffuse in somatic cells, indicating that the mutators are not compartmentalized in the soma, consistent with our previous observations (Figure S3B) [1]. Thus, there are at least two features that distinguish germline RNAi from somatic RNAi: 1) the requirement for either mut-14 or smut-1 and 2) compartmentalization of the mutator complex. Therefore, it is possible that the role of mut-14 and smut-1 is related to compartmentalization of the RNAi pathway.
Figure 4. Mutator complex containing catalytically dead MUT-14 is competent for siRNA formation in vitro.
(A) Expression and localization of GFP derived from a mut-16 transcriptional fusion (mut-16prom∷GFP) and a mi/M6 translational fusion (mut-16∷GFP) in adult animals. Mutator foci and muscle and vulval cells are indicated. (B) Western blot analysis of MUT-16∷GFP, FLAG∷RRF-1, and HA∷EGO-1 from cell lysates (in) and anti-GFP co-immunoprecipitates (IP). (C) In vitro assay for siRNA synthesis. Western blot analysis of free GFP (mut-16prom∷GFP) and GFP and mCherry fusion proteins (mut-14∷GFP; mCherry∷rrf-1; mut-14 smut-1 and mut-14DKAD∷GFP; mCherry∷rrf-1; mut-14) from cell lysates (in) and anti-GFP co-immunoprecipitates (IP) (top panel). A faint background band is observed in each of the input samples after probing with mCherry antibody. In vitro synthesized siRNAs following co-immunoprecipitation of mutator complexes containing wild type MUT-14 (mut-14∷GFP; mCherry∷rrf-1; mut-14 smut-1) or catalytically dead MUT-14 (mut-14DKAD∷GFP; mCherry∷rrf-1; mut-14) (bottom panel). Immunoprecipitated protein complexes were incubated with a mixture of radiolabeled and non-labeled nucleotides and in the presence or absence of an in vitro transcribed mRNA (B0250.8). (D) Schematic depicting siRNA formation by the mutator complex in the germline. See also Figure S3 and Tables S3-S4.
MUT-14 Catalytic Activity is Dispensable for siRNA Formation in vitro
We performed a yeast two-hybrid screen to test mutator protein interactions with other validated and predicted small RNA factors, including SMUT-1, which was identified in a screen for gene inactivations that desilence an RNAi sensor (Table S3) [13, 14]. As predicted by its role in nucleating the mutator complex and its prion-like Q/N-rich region, MUT-16 was the most promiscuous factor, binding to 12 of the 58 proteins assayed. MUT-16-interactors included the mutators SMUT-1 and RDE-2, the Argonautes WAGO-1 and ERGO-1, the RNA-dependent RNA polymerase EGO-1, and several chromatin factors, including MES-4 and GFL-1 (Figure S3C; Table S4). These results are consistent with the requirement for mut-16 in siRNA formation from WAGO and ERGO-1 targets and point to a direct interaction between MUT-16 and SMUT-1. EGO-1 may not associate with MUT-16 in vivo in wild type animals, as we did not observe EGO-1 at Mutator foci [1]. Furthermore, in MUT-16∷GFP immunoprecipitates we detected FLAG∷RRF-1 but not HA∷EGO-1 (Figure 4B). ego-1 functions redundantly with rrf-1 [7] suggesting that in the absence of rrf-1, EGO-1 might bind to MUT-16. In co-immunoprecipitation assays of mCherry∷EGO-1 and MUT-16∷GFP we detected only a very weak interaction between EGO-1 and MUT-16 in rrf-1 mutants (Figure S3D). EGO-1 also functions in the CSR-1 pathway in P granules and therefore may have a stronger affinity for factors involved in CSR-1 class siRNA formation than for the mutators [7, 8].
Because RRF-1 co-immunoprecipitates with MUT-16 and is therefore associated with the mutator complex, we reasoned that we could pull down any factor in the complex and perform siRNA synthesis in vitro. This would allow us to test whether or not complexes containing wild type or catalytically dead MUT-14 are competent for siRNA synthesis in an in vitro context in which the pathway is not compartmentalized. We immunoprecipitated wild type MUT-14 (MUT-14∷GFP) and catalytically dead MUT-14 (MUT-14DKAD∷GFP) and incubated the purified complexes with a mixture of radiolabeled and non-labeled ribonucleotides. Both MUT-14∷GFP and MUT-14DKAD∷GFP co-immunoprecipitated mCherry∷RRF-1 (Figure 4C). Complexes containing either wild type or catalytically dead MUT-14 immunoprecipitated from mut-14 or the mut-14 smut-1 double mutant were proficient for siRNA synthesis (Figure 4C; Figure S3E). In contrast, a control immunoprecipitate containing free GFP (mut-16prom∷GFP), failed to produce siRNAs, as did FLAG∷RRF-1 complex immunoprecipitated from mut-16 mutants in which the Mutator complex does not form (Figure 4C; Figure S3E) [1]. Addition of an in vitro transcribed B0250.8 mRNA, an endogenous target of mut-14 and smut-1 (Table S2), was not necessary and was actually inhibitory to siRNA formation, indicating that the complex co-immunoprecipitated RNA (Figure 4C). It is unclear if the complex contained RNA in vivo or if it bound RNA during incubation of the cell lysate with GFP antibody.
Our results demonstrate that mut-14 and smut-1 are not required for RNAi in the soma or for siRNA formation in vitro, two distinct contexts in which the mutator complex is not compartmentalized. Nor are they required for localization of other mutator proteins. Yet the data points to an essential role for mut-14 and smut-1 at an early step in siRNA formation in the germline, in which the mutator complex is compartmentalized. DEAD box helicases have numerous roles in RNA processing, such as localized unwinding of RNA duplexes and as RNA clamps [15]. The DEAD box helicases UAP56 and Vasa, which are closely related to MUT-14 and SMUT-1 (blastp p values ≤ 5 × 10-19, 24% identity), function in the Drosophila germline on opposite sides of nuclear pores to transport RNA from the sites of transcription in the nucleus to perinuclear piRNA processing compartments called nuage [16]. It is possible that MUT-14 and SMUT-1 are involved in transporting mRNAs from P granules, sites of mRNA surveillance, into mutator foci nucleated by MUT-16 to initiate siRNA amplification. The more modest reduction in siRNA levels in the other mutators suggests that they serve accessory roles in siRNA biogenesis (Figure 4D).
Experimental Procedures
mut-14(mg464) and ZC317.1 (mgDf465) deletion alleles were generated using MosDEL [4]. Transgenes were generated using Life Technologies Multisite Gateway Technology and introduced into C. elegans using MosSCI (Table S5) [17]. C. elegans were cultured at 20°C [18]. Immunofluorescence assays were done as described [1]. For yeast two-hybrid assays, candidate gene sequences were cloned into bait and prey vectors using Gateway Technology and then transformed into Saccharomyces cerevisiae GAL4 and GAL80 deletion strains Y8800 (prey, MAT a) and Y8930 (bait, MATα). To assess RNAi defects in mutator mutants, animals were fed E. coli expressing dsRNA with sequence homology to germline or somatic genes. Taqman qRT-PCR assays were done as described (Table S5) [10]. Small RNA sequencing was done as described [3]. Sequences were aligned to the C. elegans reference genome WS204 using CASHX v. 2.0 [19]. Co-immunoprecipitation and Western blot assays were done as described [1]. RdRP assays were done as described [20].
Supplementary Material
Highlights.
mut-14 and smut-1 are required for germline RNAi and endogenous siRNA formation.
MUT-14 and SMUT-1 likely function at the initiation step of siRNA amplification.
MUT-14 containing a point mutation in the DEAD motif is dominant to SMUT-1.
siRNA synthesis in vivo but not in vitro requires catalytically active MUT-14.
Acknowledgments
Thanks to Ulandt Kim and Yanqun Wang for Illumina sequencing. Strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440), and Shohei Mitani of the Japanese National Bioresources Project. Mos strains were generated by the Ewbank and Segalat labs as part of the NEMAGENETAG project funded by the European Community and distributed by M. Carre-Pierrat at the UMS 3421, supported by the CNRS. This work was supported by the NIH (GM44619 to GR), Colorado State University (TAM), the Massachusetts General Hospital Executive Committee of Research (CMP and TAM), the Damon Runyon Cancer Research Foundation (CMP and TAM), and an ERC Starting Grant (202819) from the Ideas Program of the European Union Seventh Framework Program (RFK).
Footnotes
Supplemental Information: Supplemental Information, including supplemental experimental procedures, three supplemental figures, and five supplemental tables, can be found online.
Accession Numbers: All small RNA sequencing data is available from NCBI GEO under the accession number GSE54320.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Phillips CM, Montgomery TA, Breen PC, Ruvkun G. MUT-16 promotes formation of perinuclear Mutator foci required for RNA silencing in the C. elegans germline. Genes Dev. 2012;26:1433–1444. doi: 10.1101/gad.193904.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nam JW, Bartel DP. Long noncoding RNAs in C. elegans. Genome Res. 2012;22:2529–2540. doi: 10.1101/gr.140475.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery TA, Rim YS, Zhang C, Dowen RH, Phillips CM, Fischer SEJ, Ruvkun G. PIWI Associated siRNAs and piRNAs Specifically Require the Caenorhabditis elegans HEN1 Ortholog henn-1. PLoS Genet. 2012;8:e1002616. doi: 10.1371/journal.pgen.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frøkjaer-Jensen C, Davis MW, Hollopeter G, Taylor J, Harris TW, Nix P, Lofgren R, Prestgard-Duke M, Bastiani M, Moerman DG, et al. Targeted gene deletions in C. elegans using transposon excision. Nat Methods. 2010;7:451–453. doi: 10.1038/nmeth.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerstein MB, Lu ZJ, Van Nostrand EL, Cheng C, Arshinoff BI, Liu T, Yip KY, Robilotto R, Rechtsteiner A, Ikegami K, et al. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science. 2010;330:1775–1787. doi: 10.1126/science.1196914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasale JJ, Gu W, Thivierge C, Batista PJ, Claycomb JM, Youngman EM, Duchaine TF, Mello CC, Conte D. Sequential rounds of RNA-dependent RNA transcription drive endogenous small-RNA biogenesis in the ERGO-1/Argonaute pathway. Proc Natl Acad Sci USA. 2010;107:3582–3587. doi: 10.1073/pnas.0911908107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu W, Shirayama M, Conte D, Vasale J, Batista PJ, Claycomb JM, Moresco JJ, Youngman EM, Keys J, Stoltz MJ, et al. Distinct argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline. Mol Cell. 2009;36:231–244. doi: 10.1016/j.molcel.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claycomb JM, Batista PJ, Pang KM, Gu W, Vasale JJ, van Wolfswinkel JC, Chaves DA, Shirayama M, Mitani S, Ketting RF, et al. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell. 2009;139:123–134. doi: 10.1016/j.cell.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conine CC, Batista PJ, Gu W, Claycomb JM, Chaves DA, Shirayama M, Mello CC. Argonautes ALG-3 and ALG-4 are required for spermatogenesis-specific 26G-RNAs and thermotolerant sperm in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2010;107:3588–3593. doi: 10.1073/pnas.0911685107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han T, Manoharan AP, Harkins TT, Bouffard P, Fitzpatrick C, Chu DS, Thierry-Mieg D, Thierry-Mieg J, Kim JK. 26G endo-siRNAs regulate spermatogenic and zygotic gene expression in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2009;106:18674–18679. doi: 10.1073/pnas.0906378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gent JI, Lamm AT, Pavelec DM, Maniar JM, Parameswaran P, Tao L, Kennedy S, Fire AZ. Distinct phases of siRNA synthesis in an endogenous RNAi pathway in C. elegans soma. Mol Cell. 2010;37:679–689. doi: 10.1016/j.molcel.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Wolfswinkel JC, Claycomb JM, Batista PJ, Mello CC, Berezikov E, Ketting RF. CDE-1 affects chromosome segregation through uridylation of CSR-1-bound siRNAs. Cell. 2009;139:135–148. doi: 10.1016/j.cell.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Kim JK, Gabel HW, Kamath RS, Tewari M, Pasquinelli AE, Rual JF, Kennedy S, Dybbs M, Bertin N, Kaplan JM, et al. Functional genomic analysis of RNA interference in C. elegans. Science. 2005;308:1164–1167. doi: 10.1126/science.1109267. [DOI] [PubMed] [Google Scholar]
- 14.Robert VJ, Sijen T, van Wolfswinkel J, Plasterk RHA. Chromatin and RNAi factors protect the C. elegans germline against repetitive sequences. Genes Dev. 2005;19:782–787. doi: 10.1101/gad.332305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Under P, Jankowsky E. From unwinding to clamping - the DEAD box RNA helicase family. Nat Rev Mol Cell Biol. 2011;12:505–516. doi: 10.1038/nrm3154. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Wang J, Xu J, Zhang Z, Koppetsch BS, Schultz N, Vreven T, Meignin C, Davis I, Zamore PD, et al. UAP56 couples piRNA clusters to the perinuclear transposon silencing machinery. Cell. 2012;151:871–884. doi: 10.1016/j.cell.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frøkjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahlgren N, Sullivan CM, Kasschau KD, Chapman EJ, Cumbie JS, Montgomery TA, Gilbert SD, Dasenko M, Backman TWH, Givan SA, et al. Computational and analytical framework for small RNA profiling by high-throughput sequencing. RNA. 2009;15:992–1002. doi: 10.1261/rna.1473809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang G, Reinhart BJ, Bartel DP, Zamore PD. A biochemical framework for RNA silencing in plants. Genes Dev. 2003;17:49–63. doi: 10.1101/gad.1048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.