Abstract
We developed a new high-dose combination of infusional gemcitabine with busulfan/melphalan for lymphoid tumors. Gemcitabine dose was escalated by extending infusions at a fixed rate of 10 mg/m2/min in sequential cohorts, in daily, 3-dose or 2-dose schedules. Each dose immediately preceded busulfan (adjusted targeting AUC 4,000 μM.min−1/day × 4 days) or melphalan (60 mg/m2/day × 2 days). We enrolled 133 patients (80 Hodgkin’s lymphoma (HL), 46 non-Hodgkin’s lymphoma (NHL), 7 myeloma), median 3 prior regimens; primary refractory disease in 63% HL/45% NHL and PET-positive tumors at transplant in 50% patients. Two patients died from early posttransplant infections. The major toxicity was mucositis. The daily and 3-dose schedules caused substantial cutaneous toxicity. In contrast, the 2-dose schedule was better tolerated, which allowed us to extend the infusions from 15 to 270 minutes. Pretransplant values of C-reactive protein, b-type natriuretic peptide, ferritin or haptoglobin did not correlate with toxicity. Overall response and complete response rates were 87%/62% (HL), 100%/69% (B-LCL), 66%/66% (T-NHL), and 71%/57% (myeloma). At median follow-up of 24 months (3–63), the event-free/overall survival rates are 54%/72% (HL), 60%/89% (B-LCL), 70%/70% (T-NHL) and 43%/43% (myeloma). In conclusion, gemcitabine/busulfan/melphalan is a feasible regimen with substantial activity against a range of lymphoid malignancies. This regimen merits further evaluation in phase II and III trials.
Keywords: Gemcitabine, high-dose chemotherapy, autologous transplantation, lymphoma, myeloma, Hodgkin, phase 1
INTRODUCTION
High-dose chemotherapy (HDC) with autologous stem cell transplantation (ASCT), using regimens such as BEAM (carmustine/etoposide/cytarabine/melphalan), is standard treatment of chemosensitive relapsed Hodgkin’s lymphoma (HL) and diffuse large B-cell non-Hodgkin’s lymphoma (DLCL). [1,2] However, relapse remains a major problem, particularly in patients with primary refractory disease or high-risk features at relapse. [3,4] Populations with a first remission shorter than 12 months, exposed to multiple pretransplant salvage regimens or with active tumor at HDC present a 2-year year event-free survival (EFS) of less than 30% and are in clear need for more active high-dose regimens. [3–7]
Alkylating agents, such as busulfan, constitute the backbone of HDC regimens based on their steep concentration-response effect. Precise and predictable systemic exposure is an important factor of their therapeutic window. The intravenous formulation of busulfan avoids problems inherent to its oral administration, such as large interdose variability in absorption or hepatic first-pass effect, and results in markedly reduced toxicity. [8,9] In a prior study by our group of an intravenous combination of busulfan and melphalan (Bu/Mel), prospective targeted adjustment of individual busulfan doses based upon pharmacokinetic exposure resulted in absence of venoocclusive disease, [10] with superior tolerability compared to earlier versions of this two-drug combination. [11] This regimen had an antitumor effect in lymphomas at least comparable to that of BEAM.
Alkylating agent activity is dependent on the extent of DNA damage and repair. Thus, combination of alkylating agents and drugs known to inhibit DNA damage repair would be predicted to produce additive or synergistic effects. Gemcitabine is a nucleoside analogue that has been shown to inhibit DNA damage repair caused by prior exposure to alkylating agents. [12] Similar to cytarabine and fludarabine, gemcitabine undergoes complex intracellular activating metabolism, with sequential phosphorylation by deoxycytidine kinase (dCK) to its active metabolite difluorodeoxycytidine triphosphate (dFdCTP), which is incorporated into DNA and is considered to be largely responsible for the cytotoxic effect. [13] Gemcitabine presents two distinct mechanistic advantages over cytarabine: it is locked into DNA (“masked chain termination effect”) becoming less susceptible to removal by 3′-5′-proofreading exonucleases, and it inhibits ribonucleotide reductase, which decreases the pool of normal deoxynucleotide triphosphates competing with dFdCTP for incorporation into DNA. [12] Preclinical experiments indicate that the antitumor effect of gemcitabine is highly dependent on both its dose and duration of exposure. [14–16] Gemcitabine causes limited extramedullary toxicity at standard doses and shows clinical antitumor activity against HL [17,18] and NHL. [19,20] Therefore, it presents a promising profile for combination with alkylators in HDC studies for lymphomas.
While gemcitabine presents greater affinity than fludarabine and cytarabine for dCK, this enzyme becomes saturated when gemcitabine plasma levels (Css) exceed 20 to 30 μmol/L. [21] In contrast, infusing gemcitabine at a fixed dose rate (FDR) of 10 mg/m2/min seems to minimize saturation of dCK and optimize dFdCTP formation. [22,23] Randomized trials comparing FDR infusions of gemcitabine to shorter 30-minute infusions at comparable doses have shown substantial pharmacokinetic advantages for the FDR schedule, with several fold greater accumulation of dFdCTP and increased tumor responses albeit with increased hematological toxicities. [24,25] Autologous hematopoietic support circumvents myelotoxicity of HDC, which allows for substantial prolongation of gemcitabine infusions. [26] We hypothesized that infusional gemcitabine can be safely combined at high doses with busulfan/melphalan with ASCT. We report here the results of a dose- and schedule-finding study of gemcitabine, busulfan and melphalan (Gem/Bu/Mel) in patients with refractory or poor-risk relapsed lymphoid malignancies.
PATIENTS AND METHODS
Preclinical Studies
Experiments were done to determine the cytotoxicity of busulfan, melphalan and gemcitabine, individually and in varying combinations. The human lymphoma cell line J45.01 was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and grown in RPMI 1640 medium (Mediatech, Manassas, VA) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Inc., Lawrenceville, GA) and 100 U/ml penicillin and 100 μg/mlstreptomycin at 37°C in a humidified atmosphere of 5% CO2. Busulfan (Sigma-Aldrich, St. Louis, MO) and melphalan (Sigma-Aldrich) were dissolved in dimethyl sulfoxide (DMSO) immediately prior to cellular drug exposures. The final concentration of DMSO in all experiments did not exceed 0.08% by volume. Gemcitabine (Eli Lilly, Indianapolis, IN) was dissolved in phosphate-buffered saline (PBS) and stored at 4°C. Cell suspensions (8 ml of 0.5 × 106 cells/ml) were grown in T25 flask in the presence of busulfan, melphalan or gemcitabine at their IC10 (15 μg/mL, 0.6 μM and 0.03 μM, respectively) or solvent alone and continuously incubated at 37°C for 48 hr. Cell aliquots were then analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. [27] The remaining cells were collected by centrifugation, washed with PBS and lysed with cell lysis buffer (Cell Signaling Technology, Danvers, MA) as recommended by the manufacturer. Total protein concentrations in the cell lysates were determined using a BCA Protein Assay kit (Thermo Fisher Scientific, Rockford, IL). Western blot analysis was done by separating protein extracts on polyacrylamide-SDS gels and blotting onto nitrocellulose membranes (Bio-Rad, Hercules, CA), which were probed with antibodies against PARP1 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), γ-H2AX (Millipore, Bedford, MA) and β-actin (Sigma-Aldrich). Immunoblot analyses by chemiluminescence were done using the Immobilon Western Chemiluminescent HRP Substrate (Millipore). All cytotoxicity data are the average ± standard deviation of at least 4 independent experiments and all Western blots were done at least 2 times.
Patient Population
The clinical study protocol was approved by the Clinical Research Committee and the Institutional Review Board of MD Anderson Cancer Center, where all patients were treated. All patients provided written informed consent to participate in the study.
Eligibility included patients ages 18–65 with HL or NHL and primary refractory tumors (defined by less than CR to first-line treatment or relapse within 3 months), or refractory/poor-risk relapsed disease, including first CR shorter than 12 months, more than one relapse or progression, or active tumor at the time of transplant. Patients with myeloma were eligible if no prior response to first-line therapy including lenalidomide, bortezomib or thalidomide, or relapsed after a prior autologous stem-cell transplant. Additional eligibility criteria included adequate renal function (serum creatinine clearance ≥50 ml/min and/or serum creatinine ≤1.8 mg/dL), hepatic function (SGOT and/or SGPT ≤3 × upper limit of normal; serum bilirubin and alkaline phosphatase ≤2 × upper limit of normal), pulmonary function (FEV1, FVC and DLCO ≥50%) and cardiac function (left ventricular ejection fraction ≥40% without uncontrolled arrhythmias or symptomatic cardiac disease), a Zubrod performance status 0–1, no evidence of uncontrolled infection, no prior whole brain irradiation, and no radiation therapy in the month prior to enrollment. Patients with active hepatitis B were excluded. Patients with chronic hepatitis C or positive hepatitis C serology were excluded if they had evidence of either cirrhosis or stage 3–4 liver fibrosis. Patients with HL and NHL and a prior autologous transplantation were not eligible. Patients were not eligible for enrollment until their non-hematologic toxicity from previous therapies had downgraded to at least grade 1.
Restaging studies were obtained within 30 days prior to enrollment, and subsequently at 1 month, 3 months, 6 months after SCT, and every 6 months thereafter as feasible. Responses were assessed, before any planned post-HDC treatment, at 1 month for HL and NHL patients and at 3 months for myeloma patients. Staging studies for patients with HL and NHL included CT and PET scans, which were interpreted using mediastinal blood pool activity as the reference background, [28] and bone marrow biopsy when applicable. Myeloma patients had serum and urine electrophoresis, quantitative immunoglobulin and free light chain studies, bone marrow biopsy and bone survey. C-reactive protein (CRP), b-type natriuretic peptide (BNP), ferritin, haptoglobin and troponin in serum were measured at the time of admission.
High-Dose Chemotherapy
The treatment schema is shown in Table 1. Patients received an intravenous test dose of busulfan of 32 mg/m2 over 45 minutes on day −10. The conditioning regimen started on day −8. Three schedules of gemcitabine were tested: daily × 6 (days −8 to −5, and −3 to −2), three doses (days −8, −6 and −3), and two doses (days −8 and −3). Each dose of gemcitabine was administered as a loading bolus of 75 mg/m2, targeting a steady state concentration of 15 μmol/L, followed by a continuous infusion per cohort, and immediately followed by the corresponding dose of busulfan or melphalan. Busulfan was infused daily over 3 hours from days −8 to −5 targeting an average daily AUC of 4,000 μM.min−1, with the first two therapeutic doses calculated from the pharmacokinetic parameters derived from the prior test dose. If necessary, a dose adjustment for the third and fourth doses was made following the analysis of the first therapeutic dose, targeting an aggregate AUC of 16,000 μM.min−1. The sampling process and analytical methodology have been previously described. [10] In cases where pharmacokinetic dosing of busulfan was not feasible, patients received a fixed daily dose of 105 mg/m2. After a day of rest on day −4 melphalan was administered at 60 mg/m2 daily over 30 minutes on days −3 and −2.
Table 1.
Treatment schedule
| Day | −10 | −9 | −8 | −7 | −6 | −5 | −4 | −3 | −2 | −1 | 0 | +1 | +8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gemcitabine | Daily | X | X | X | X | X | X | |||||||
| 3-dose | X | X | X | |||||||||||
| 2-dose | X | X | ||||||||||||
| Busulfan | x (test) | X | X | X | X | |||||||||
| Melphalan | X | X | ||||||||||||
| PBPC | X | |||||||||||||
| Rituximab (CD20+ tumors) | X | X | ||||||||||||
Supportive Care
Acetaminophen, azoles and metronidazole were avoided from day −10 to −1. Phenytoin 600 mg oral daily was started the evening before the test dose of busulfan until 24 hours after the last dose of busulfan. Hydration with 0.9% saline was administered at 125 mL/hour from admission until day −1. Supportive oral care was uniform using palifermin (60 mcg/kg/d IV, days −13 to −11 and 0 to +2), oral cryotherapy throughout each melphalan infusion and oral and throat rinses with caphosol (30 mL four times a day) and glutamine (15 g four times a day). The infusion of PBPC was on day 0. G-CSF was administered at a dose of 5 mcg/kg/day subcutaneously beginning on day +5 until neutrophil recovery. Institutional transplant guidelines for antiemetics, antibacterial, antifungal and antiviral prophylaxis, and red blood and platelet transfusions were followed.
Trial Design
The primary endpoints were to determine the optimal schedule and maximum tolerated dose (MTD) of gemcitabine when added to busulfan and melphalan and to define the dose-limiting toxicity. Secondary endpoints were to describe the toxicity profile of the new regimen and to derive preliminary estimates of EFS and overall survival (OS) produced by this regimen.
In this schedule- and dose-finding study gemcitabine was combined with fixed doses of busulfan and melphalan. Dose levels were determined by prolonging the infusion of gemcitabine by prespecified time intervals maintaining its infusion rate (Table 3). Patients were assigned a dose level using the continual reassessment method [29] based on the toxicity data available at the time of their enrollment targeting a dose-limiting toxicity (DLT) probability closest to 0.15. Toxicities were scored according to the National Cancer Institute Common Toxicity Criteria, version 3.0. [30] DLT was defined as grade 3 mucositis or skin toxicity lasting for more than 3 days before downgrading, or as any grade 4 or 5 nonhematologic toxicity. After the daily and 3-dose schedules were found to be excessively toxic at their starting levels, dose escalation proceeded on the 2-dose schedule with 6 initially planned dose levels (levels 1–6). After level 6 was established as safe, the protocol was amended to escalate above this level. After the MTD was identified at level 9, this level was expanded to a total of 50 patients to fully characterize its side effect profile. The method of Thall et al. was employed to perform the interim monitoring. [31]
Table 3.
Toxicities per dose level (2-dose schedule)
| Level | N | Gem infusion (min) | Gem dose (mg/m2) | Mucositis | Skin | DLT | ||
|---|---|---|---|---|---|---|---|---|
| G2 | G3 | G2 | G3 | |||||
| 1 | 3 | 15 | 225 | 33% | 0 | 0 | 0 | 0% |
| 2 | 3 | 40 | 475 | 0 | 0 | 0 | 0 | 0% |
| 3 | 3 | 60 | 675 | 33% | 0 | 0 | 0 | 0% |
| 4 | 6 | 90 | 975 | 80% | 0 | 0 | 0 | 0% |
| 5 | 14 | 120 | 1,275 | 59% | 23% | 12% | 0 | 6% |
| 6 | 22 | 180 | 1,875 | 71% | 6% | 18% | 0 | 2% |
| 7 | 14 | 210 | 2,175 | 60% | 27% | 33% | 0 | 7% |
| 8 | 14 | 240 | 2,475 | 64% | 28% | 21% | 7% | 7% |
| 9 (MTD) | 50 | 270 | 2,775 | 54% | 28% | 18% | 3% | 14% |
Statistical Analyses
Results of the cytotoxicity assay were compared using Student’s t test. For categorical variables, Fisher’s exact test and its generalizations were used to assess association with grade 3 toxicities. All p-values are two-sided. No adjustment of p-values for multiple comparisons was performed.
Overall response rate (RR) and complete response (CR) rates were calculated among patients with measurable disease at the time of HDC following the usual criteria. [32,33] The EFS was defined as the time from transplant to either relapse or death, whichever occurred first, or to last contact. Overall survival (OS) was defined as the time from transplant to death or last contact. Kaplan-Meier survival curves were used to estimate unadjusted time-to-event distribution (EFS and OS). [34] All calculations used R v2.12.1 and OpenBUGS v3.1.2 rev 668.
RESULTS
Preclinical Experiments
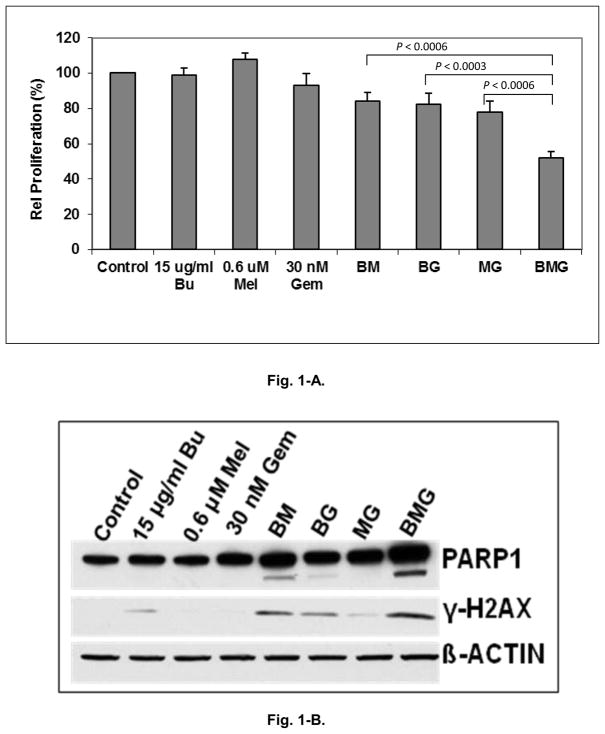
Exposure of J45.01 cells individually to busulfan, melphalan or gemcitabine did not show significant effects on cell proliferation (Fig. 1-A). Two-drug combinations (busulfan/melphalan, gemcitabine/busulfan or gemcitabine/melphalan) resulted in mean inhibition of proliferation of 16%, 18% and 22%, respectively. Exposure of this refractory cell line to Gem/Bu/Mel resulted in an average 48% inhibition of proliferation, significantly greater than any of the two-drug combinations (P values <0.001). To determine a possible mechanism of the cytotoxicity of Gem/Bu/Mel, immunoblot staining was performed. Exposure to Gem/Bu/Mel produced increased cleavage of PARP1 and phosphorylation of histone 2AX (Fig. 1-B), indicative of activation of apoptosis and increased DNA damage response, respectively.
Figure 1.
Cytotoxicity of Gem/Bu/Mel on chemotherapy-resistant J45.01 cells. Fig. 1-A. Cell proliferation. Fig. 1-B. Immunoblot staining. Bu or B: busulfan; Mel or M: melphalan; Gem or G: gemcitabine
Patient Enrollment
One hundred and thirty-three patients were enrolled between January 2007 and August 2011 (Table 2). Their median age was 41 (18–65). Eighty patients had HL, 46 NHL (35 B lymphomas and 11 T lymphomas) and 7 had myeloma. No patients received a prior autologous SCT but were heavily pretreated with a median 3 prior regimens (range 2 to 9). Thirty-five HL patients and 6 NHL patients had previously received gemcitabine. Patients had extensive tumor involvement (median 3 organs). In the HL subgroup, extranodal disease, B symptoms, a bulky nodal mass (>5 cm) were present in 50%, 14% and 36% of patients, respectively, and 43% of patients had more than one prior relapse. In the NHL subgroup, an International Prognostic Index at the time of relapse of 0–1, 2–3 or >3 was present in 17%, 62% and 20% of patients, respectively, and 50% of patients had more than one prior relapse. Half of all patients with HL and NHL had PET-positive tumors at the time of HDC. Almost 30% of all enrolled patients (23 HL, 11 NHL and 5 myeloma patients) had progressive disease at the time of admission.
Table 2.
Patient Characteristics (N=133)
| Age: median (range) | 41 (18–65) |
| Gender: Male / female | 77 / 56 |
|
| |
| Hodgkin’s lymphoma (N=80) | |
| Primary refractory | 53 |
| Relapsed | 27 |
| Remission ≤1 yr | 72 (90%) |
| Extranodal disease at relapse/PD | 40 (50%) |
| B symptoms at relapse/PD | 11 (14%) |
| No. clinical risk factors (*) | |
| 1 | 20 (25%) |
| 2 | 43 (56%) |
| 3 | 17 (21%) |
| No. prior relapses/PD: | |
| 1 | 46 (57%) |
| >1 | 34 (43%) |
| Bulky tumor at relapse/PD | 29 (36%) |
| Prior treatment | |
| No. prior chemotherapy regimens: median (range) | 3 (2–6) |
| Prior radiotherapy | 22 |
| Disease status at HDC | |
| Clinical response: CR / PR / PD | 39 / 18 / 23 |
| PET: Positive / Negative | 41 / 39 |
|
| |
| NHL (N=46) | |
| B-LCL | 30 |
| Primary DLCL | 18 |
| Transformed | 4 |
| Primary CNS LCL | 4 |
| Primary mediastinal LCL | 4 |
| Burkitt’s lymphoma | 3 |
| Follicular lymphoma | 2 |
| T-NHL | 11 |
| Anaplastic large cell | 6 |
| Peripheral TCL NOS | 2 |
| NK/T | 2 |
| Angioimmunoblastic | 1 |
| Primary refractory (B-LCL / FL / BL / T-NHL) | 21 |
| Relapsed (B-LCL / FL / BL / T-NHL) | 25 |
| Time to relapse | |
| ≤12 months | 19 |
| >12 months | 6 |
| No. prior relapses/PD: | |
| 1 | 23 |
| >1 | 23 |
| Secondary IPI (**) | |
| 0–1 | 7 (17%) |
| 2–3 | 25 (62%) |
| >3 | 8 (20%) |
| Elevated LDH at relapse/PD | 13 |
| Prior treatment | |
| No. prior chemotherapy regimens: median (range) | 3 (2–9) |
| Prior radiotherapy | 10 |
| Disease status at HDC | |
| Clinical status: CR / PR / PD | 21 / 14 / 11 |
| PET status : Positive / Negative | 23 / 23 |
|
| |
| Myeloma (N=7) | |
| Primary refractory | 4 |
| Relapsed and refractory | 3 |
| No. prior relapses relapses/PD: | |
| 1 | 3 |
| >1 | 4 |
| Stage III | 7 |
| Poor-prognosis cytogenetic abnormalities (CGA) (**) | 3 |
| Prior treatment | |
| No. prior chemotherapy regimens: median (range) | 3 (2–5) |
| Prior radiotherapy | 3 |
| Disease status at HDC | |
| Clinical status: CR / PR / PD | 0 / 2 / 5 |
Risk factors: B symptoms at relapse, extranodal disease (both at relapse), or remission duration <1 year.
Poor-prognosis CGA: 17p deletion, t(14;16), t(4;14) and 13 deletion.
Hematologic Recovery
The source of stem cells was peripheral blood for all patients. The median times to neutrophil and platelet engraftment were 9 days (range, 8–12) and 11 days (range, 8–31), respectively.
Regimen-Related Toxicities
The daily and 3-dose schedules of gemcitabine resulted in excessive cutaneous and mucosal toxicity at their starting levels. One of two patients on the daily schedule and both patients on the 3-dose schedule developed G4 bullous dermatitis. Toxicity was markedly reduced in the 2-dose schedule, which allowed escalation of gemcitabine infusion from level 1 (15-minute infusion, daily dose and total dose of 225 and 450 mg/m2, respectively) to level 9 (4.5-hour infusion, daily dose of 2,775 mg/m2, total dose 5,550 mg/m2), which was established as the MTD (Table 3). After the MTD was identified at level 9 with mucositis being the DLT, this level was expanded to a total of 50 patients to fully characterize its toxicity profile. No regimen-related deaths or G4 toxicities were seen on the 2-dose schedule, which was associated with the following side effect profile:
Mucositis
G2-3 mucositis was common at dose level 4 and above. It started at median day +4 (range, day +1 to +7) and lasted at its maximal severity for a median of 2 (range, 1–8) days. Sixty-five percent of patients required a narcotic patient-controlled analgesia pump for a median of 6 (range, 3–17) days.
Skin effects
An early G1-2 erythematous rash was common and resolved with a few days of over-the-counter sunburn creams (e.g., Noxzema ®) or topical steroids. Two patients who developed a G3 rash received a short course of intravenous methylprednisolone at 1 mg/kg/d.
Hepatic effects
An early and self-limited elevation of transaminases was common (75% patients) across all dose levels, starting on median d-1 (range, d-6 to 0), peaking at a median value of 124 (range, 41–1120) IU/L and resolving within 1 week. A transient bilirubin elevation was seen in 11% patients in the first week post-transplant (median peak of 2.4, 1.6–4.4) mg/dL. There were no cases of venoocclusive disease.
Pulmonary effects
There were two cases of steroid-responsive G2 pneumonitis, in levels 6 and 9, respectively, both in patients with previous mantle field radiotherapy. Four additional patients had asymptomatic infiltrates on d+30 restaging CT scans with negative microbiological studies on bronchioalveolar lavage. They promptly resolved spontaneously and were attributed to G1 pneumonitis. There were no significant DLCO changes in the study population from before (median 75% of predicted, 53–113%) to one month post-transplant (median 77%, 38–113%) (P=0.75).
Other toxicities
Diarrhea was mild with only 8 G2 cases. There was one case of G2 renal toxicity. No neurological or cardiac toxicities were observed.
The median values on admission of CRP, BNP, ferritin and haptoglobin were 7 (range, 0.35–189) mg/L, 79 (7–612) pg/mL, 330 (9–15,063) ng/mL and 82 (9–446) mg/dL, respectively. All patients had a troponin value of 0 on admission. There were no differences between patients who experienced G3 toxicity in the 2-day schedule and those who did not with respect to values of C-reactive protein (P=0.9), B- atrial natriuretic peptide (P=0.4), ferritin (P=0.2) or haptoglobin (P=0.3). Likewise, none of the following variables predicted occurrence of G3 toxicity in the 2-day schedule: dose level below the MTD, number of prior lines of therapy, number of organs involved, or diagnosis. There appeared to be a higher incidence of G3 toxicities among patients younger than 41 years old not reaching statistical significance (31% vs 17%, P=0.06).
Infections
Two patients experienced fatal infections: one with Candida albicans sepsis in the setting of severe grade 4 mucositis (daily schedule) and one with Pseudomonas aeruginosa pneumonia (2-dose schedule). The latter infection had been previously diagnosed and treated before transplant but reoccurred fatally during myelosuppression. The following additional infections resolved with antimicrobials: E coli bacteremia (N=2), K pneumoniae bacteremia (N=1), S epidermidis bacteremia (N=2), C difficile-positive diarrhea (N=2), RSV pneumonia (N=1), and E faecalis urinary tract infection (N=1).
Busulfan Pharmacokinetic Studies
Busulfan PK parameters were calculated from blood samples of 121 patients (91% of the study file). These patients had their first and second therapeutic doses determined based on the PK analyses after the test dose, and their third and fourth doses subsequently adjusted based on the PK analyses after the first dose. In seven percent of patients, dose 1 AUC was more than 20% higher (N=3) or more than 20% lower (N=4) than the value predicted after the test dose. For the remaining 93% of patients the interdose variation of calculated clearance estimates between the test dose and the first therapeutic dose was less than 20%. The overall mean of the variation of the calculated test-to-therapeutic clearance was 8.3% (95% confidence interval (CI), −11% to 28%). The mean (% coefficient of variation) population clearance, volume of distribution and plasma half life for once-daily dosing were 99 mL/min/m2 (16.7%), 25 L/m2 (14%) and 2.9 hours (16.8%) from the first therapeutic dose. These data did not differ significantly from those previously estimated with Bu/Mel, [10] where the mean clearance of busulfan after its first therapeutic dose was 97.9 mL mL/min/m2 (15.7%) (P=0.6), which indicates an absence of PK interaction between gemcitabine and busulfan. The mean and median daily AUCs from the first therapeutic dose in the present study were 3,726 and 3,666 μMol × min (95% CI, 2,825 to 4,625), respectively.
Tumor Responses
Tumor responses in HL and NHL patients were assessed at 1 month post-HDC. Among 41 patients with HL and measurable disease the CR and RR were 62% and 88%, respectively. There were no significant differences per previous gemcitabine exposure. The response rate was 100%, 88%, 80% and 90%, respectively, among patients with a prior CR, prior PR, no previous response, and no prior gemcitabine. Likewise, CR rates did not vary significantly among those subgroups (50%, 75%, 60% and 67%, respectively).
All 17 patients with B-LCL with measurable disease responded, with 15 CRs. Both patients with FL had a CR. Three patients with primary refractory Burkitt’s lymphoma had a CR. Two of three patients with T-NHL and measurable tumors (angioimmunoblastic and peripheral T-cell lymphoma, respectively) had a CR; a third one (anaplastic large-cell lymphoma) experienced short-lived tumor shrinkage that did not meet response criteria. Of note, all six NHL patients with prior gemcitabine treatment, none of whom had previously responded to this drug, had a CR following Gem/Bu/Mel.
Finally, all 7 patients with myeloma had measurable disease: five of them responded, four of them with a CR, at 3 months.
Post-HDC Treatment
Twenty-eight patients (21 HL, 7 NHL) who had bulky (>5 cm) PET-positive lesions received radiotherapy to their PET-positive sites starting at 1–2 months following transplant at 30.6–41.4 Gy. Post-transplant radiotherapy was well tolerated from hematological and non hematological standpoints. Irradiated sites included mediastinum, axillary/supraclavicular lymph nodes, thoracic spine, mesentery, retroperitoneum and nasopharynx. Two patients with refractory DLCL received a planned matched unrelated donor nonmyeloablative transplant around 3 months after Gem/Bu/Mel. One of them died from complications of the allogeneic procedure, whereas the other one tolerated it without difficulty.
Three patients with myeloma received maintenance lenalidomide.
Patient Outcomes
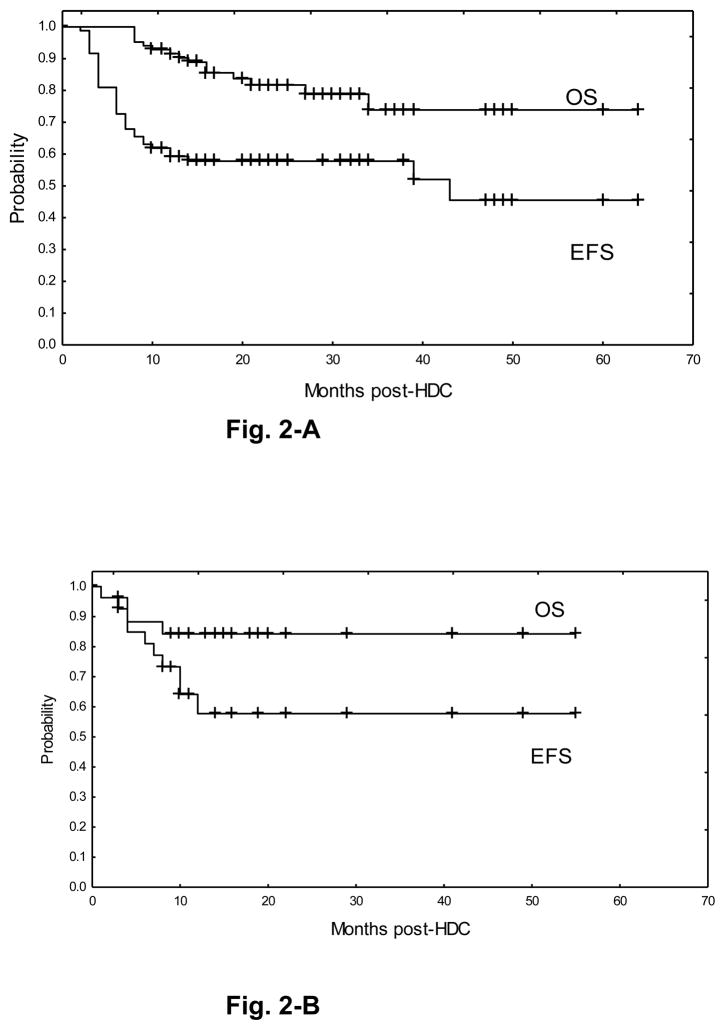
Median follow-up of the whole study population is 24 months (range, 3–63). The EFS and OS rates of the HL subgroup are 54% and 72%, respectively, with median EFS of 43 months and median OS not reached (Figure 2-A). Forty-three patients are alive in CR at 6–60 months after HDC, including 16 of 21 patients treated at the MTD. Thirty-seven patients experienced tumor relapse after HDC and 20 of these died from relapse.
Figure 2.
Event-free survival (EFS) and overall survival (OS) of the main histologic subsets. Fig. 2-A: Hodgkin’s lymphoma. Fig. 2-B: B-large cell lymphoma.
Eighteen of 30 patients with B-LCL (60%) are alive in CR, including 11 of 18 DLCL, 3 of 4 transformed LCL, 2 of 4 primary mediastinal LCL and 2 of 4 primary CNS LCL (Figure 2-B). Twelve of 24 patients with B-LCL treated at the MTD are in CR. Seven of 10 patients with T-NHL are alive in CR (4 of 6 ALCL, 2 of 2 PTCL, 1 of 1 NK/T) (Figure 2-C). One of the 2 patients with follicular lymphoma is alive in CR at 15 months after HDC; the other one is alive with relapsed disease. All three patients with Burkitt’s lymphoma experienced rapid tumor relapse and died shortly after HDC. Lastly, three of the seven patients with myeloma are alive and free of progression at 46–48 months (1 CR, 2 stable residual disease) and four patients died from relapse.
DISCUSSION
Our study shows that high doses of infusional gemcitabine can be safely added to busulfan/melphalan with ASCT. The optimal schedule includes two doses of gemcitabine, each one preceding the first doses of busulfan and melphalan, respectively, which caused a manageable side-effect profile of stomatitis and skin rash. Busulfan pharmacokinetics were similar to those previously estimated with Bu/Mel, [10] which indicates no pharmacokinetic interaction between gemcitabine and busulfan. The resulting regimen presented high antitumor activity in the population of patients with refractory or poor prognosis relapsed lymphomas enrolled.
In lymphomas, as in other tumor types, two major factors determine the clinical activity of gemcitabine and other nucleoside analogs: substrate specificity for their activating nucleoside kinases and the expression of these enzymes in tumor tissues. The content of dCK, the rate-limiting enzyme in the activation of pyrimidine analogs, is several fold higher in lymphocytes than in epithelial cells, and in most tumor tissues compared to their normal counterparts. [35,36] The affinity of dCK for gemcitabine is higher than for fludarabine, cytarabine or cladribine, which may account for its broader spectrum of activity. [12] However, this enzyme becomes saturated at high intracellular levels of gemcitabine. [21] Furthermore, when present at high intracellular levels parental gemcitabine acts as a substrate inhibitor of dCK. [37] These observations appear to be the basis for the decline in the ability of the cells to accumulate dFdCTP at gemcitabine concentrations above 20 μmol/L. As a consequence, merely increasing the dose of gemcitabine in a standard 30-minute infusion will augment its extracellular and intracellular concentrations but will not result in a parallel increase of the active metabolite dFdCTP. While prolonged infusions of gemcitabine, optimizing dFdCTP formation, seem its most pharmacologically rational administration schedule, [23] their clinical applicability is hampered by increased myelotoxicity. [24,25] Phase 1 studies exploring dose escalation of gemcitabine infused at FDR were limited by myelosuppression. [38–40] In contrast, ASCT allowed us in a prior trial to circumvent myelotoxicity and fully dose escalate gemcitabine in multiple 4-hour infusions combined with a high-dose triplet of docetaxel/melphalan/carboplatin. [26] The resulting regimen was feasible and markedly active against refractory solid tumors. We observed a dose-linear pharmacokinetic behavior of parental gemcitabine and prolonged intracellular retention times of dFdCTP, which accounted, at least in part, for the predictable side effects of that regimen. Furthermore, we measured exponential increases of intracellular dFdCTP levels over the course of repeated doses of gemcitabine, consistent with its known mechanisms of metabolic self-potentiation. Those observations prompted us to further explore infusional gemcitabine specifically targeting lymphomas. Building on prior work from our group and others with intravenous busulfan/nucleoside analogue regimens, [41,42] we used busulfan/melphalan as an alkylator platform for combination with gemcitabine. Our preclinical experiments showing greater cytotoxicity with Gem/Bu/Mel provided the basis, albeit their normoxic conditions and other limitations of in vitro studies, for using these three drugs combined.
We designed Gem/Bu/Mel based on the overriding principles of using a prolonged infusion schedule of gemcitabine in a sequence that would facilitate synergism with busulfan and melphalan based on inhibition of DNA damage repair. [12,43] In addition to this well established synergy mechanism, we have recently reported that gemcitabine induces chromatin relaxation, increasing DNA access and cytotoxicity of busulfan in lymphoma cell lines. [44] Confirming our hypothesis, we saw high activity of Gem/Bu/Mel in the population of heavily pretreated and refractory patients with HL, B-NHL and T-NHL enrolled in this trial. At median follow-up of 2 years their EFS rates are 54%, 60% and 70%, respectively. T and B lymphocytes express dCK at high levels, [45] which likely contributes to these results. While longer follow-up is needed, these outcomes are encouraging, as most post-transplant relapses in similarly high-risk populations typically occur early after transplant.
Since we treated a small number of myeloma patients, no conclusions can be drawn about the activity of Gem/Bu/Mel in this tumor. Gemcitabine has potent activity against myeloma cell lines in vitro, including cells resistant to other drugs. [46,47] Unfortunately, gemcitabine has not been adequately tested clinically in this disease. In a small phase 2 trial of weekly 30-minute infusions little activity was observed against refractory tumors. [48] As in other preclinical models, gemcitabine cytotoxicity against myeloma cells correlates with dFdCTP intracellular accumulation. [46] While myeloma plasma cells have been shown to express dCK, [49] gene profile studies indicate lower expression than normal or malignant B/T lymphocytes. [50,51] This strengthens the rationale for studying gemcitabine in prolonged infusions in myeloma.
Toxicities of this regimen depended on the gemcitabine schedule and dose. The daily and 3-dose schedules had substantially more cutaneous toxicity than the 2-dose schedule. Mucositis, was the DLT and its incidence (54% G2 and 28% G3 at the MTD) appeared increased compared to our prior study with Bu/Mel (49% G2, 7% G3). We considered dose level 9 using the 2-dose schedule to be the MTD to take forward into phase II and III studies.
It would be useful to identify predictive markers of severe toxicity. Previous reports have correlated high pretransplant values of the inflammatory biomarkers CRP and ferritin (which is also a maker of iron overload) with G3-4 toxicity in patients receiving allogeneic and autologous stem cell transplants, respectively. [52–55] Likewise, levels of haptoglobin, another acute phase protein, have been inversely associated with hematological toxicity of gemcitabine at standard doses. [56] High pretransplant values of BNP, secreted in response to volume expansion, were associated with hepatic VOD and treatment-related mortality in a study in allogeneic stem cell transplant. [57] We could not identify any serum marker or patient-related characteristic associated with G3 or greater toxicity after Gem/Bu/Mel. It is possible that polymorphic genetic variation of relevant enzymes involved in the metabolism of these drugs might predict toxicity.
In conclusion, the dose of gemcitabine can be substantially escalated at an infusion rate that optimizes its intracellular activation, in combination with busulfan/melphalan with ASCT. This regimen induced high response and CR rates in patients with refractory or poor prognosis Hodgkin’s and NHL with encouraging preliminary outcome results. Further investigation of this regimen is underway in disease-specific studies.
References
- 1.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous Bone Marrow Transplantation as Compared with Salvage Chemotherapy in Relapses of Chemotherapy-Sensitive Non-Hodgkin’s Lymphoma. N Engl J Med. 1005;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 2.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus HM, Loberiza FR, Jr, Zhang MJ, et al. Autotransplants for Hodgkin’s disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR) Bone Marrow Transplant. 2001;27:387–396. doi: 10.1038/sj.bmt.1702796. [DOI] [PubMed] [Google Scholar]
- 4.Vose JM, Zhang M-J, Rowlings PA, et al. Autologous transplantation for diffuse aggressive non-Hodkin’s lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2011;19:406–413. doi: 10.1200/JCO.2001.19.2.406. [DOI] [PubMed] [Google Scholar]
- 5.Guglielmi C, Gomez F, Philip T, et al. Time to relapse has prognostic value in patients with aggressive lymphoma enrolled onto the Parma trial. J Clin Oncol. 1998;16:3264–3269. doi: 10.1200/JCO.1998.16.10.3264. [DOI] [PubMed] [Google Scholar]
- 6.Gisselbrecht C, Glass B, Mounier N, et al. R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-Cell lymphoma (DLBCL) followed by autologous stem cell transplantation: CORAL study. J Clin Oncol. 2009;27:15s. (suppl, abstr 8509) [Google Scholar]
- 7.Caballero MD, Pérez-Simón JA, Iriondo A, et al. High-dose therapy in diffuse large cell lymphoma: results and prognostic factors in 452 patients from the GEL-TAMO Spanish Cooperative Group. Ann Oncol. 2003;14:140–151. doi: 10.1093/annonc/mdg008. [DOI] [PubMed] [Google Scholar]
- 8.Andersson BS, Kashyap A, Gian V, et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: A phase II study. Biol Blood Marrow Transplant. 2002;8:145–154. doi: 10.1053/bbmt.2002.v8.pm11939604. [DOI] [PubMed] [Google Scholar]
- 9.Madden T, de Lima M, Thapar N, et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplant preparative regimens: A comparison with an every 6–hour dosing schedule. Biol Blood Marrow Transplant. 2007;13:56–64. doi: 10.1016/j.bbmt.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Kebriaei P, Madden T, Kazerooni R, et al. Intravenous busulfan plus melphalan is a highly effective, well-tolerated preparative regimen for autologous stem cell transplantation in patients with advanced lymphoid malignancies. Biol Blood Marrow Transplant. 2011;17:412–420. doi: 10.1016/j.bbmt.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carreras E, Rosiñol L, Terol MJ, et al. Veno-occlusive disease of the liver after high-dose cytoreductive therapy with busulfan and melphalan for autologous blood stem cell transplantation in multiple myeloma patients. Biol Blood Marrow Transplant. 2007;13:1448–1454. doi: 10.1016/j.bbmt.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Plunkett W, Huang P, Searcy CE, et al. Gemcitabine: preclinical pharmacology and mechanisms of action. Semin Oncol. 1996;23(5 Suppl 10):3–15. [PubMed] [Google Scholar]
- 13.Plunkett W, Huang P, Xu Y-Z, et al. Gemcitabine: Metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22 (4 suppl 11):3–10. [PubMed] [Google Scholar]
- 14.Hanauske AR, Degen D, Marshall MH, et al. Activity of 2′,2′-difluorodeoxycytidine (Gemcitabine) against human tumor colony forming units. Anti-Cancer Drugs. 1992;3:143–146. doi: 10.1097/00001813-199204000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Csoka K, Liliemark J, Larsson R, et al. Evaluation of the cytotoxic activity of gemcitabine in primary cultures of tumor cells from patients with hematologic or solid tumors. Semin Oncol. 1995;22 (4 Suppl 11):47–53. [PubMed] [Google Scholar]
- 16.Braakhuis BJ, van Haperen VW, Boven E, et al. Schedule-dependent antitumor effect of gemcitabine in in vivo model system. Semin Oncol. 1995;22(4 Suppl 11):42–46. [PubMed] [Google Scholar]
- 17.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071–1079. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- 18.Santoro A, Bredenfeld H, Devizzi L, et al. Gemcitabine in the treatment of refractory Hodgkin’s disease: results of a multicenter phase II study. J Clin Oncol. 2000;18:2615–2619. doi: 10.1200/JCO.2000.18.13.2615. [DOI] [PubMed] [Google Scholar]
- 19.Ng M, Waters J, Cunningham D, et al. Gemcitabine, cisplatin and methylprednisolone (GEM-P) is an effective salvage regimen in patients with relapsed and refractory lymphoma. Br J Cancer. 2005;92:1352–1357. doi: 10.1038/sj.bjc.6602514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crump M, Baetz T, Couban, et al. Gemcitabine, dexamethasone, and cisplatin in patients with recurrent or refractory aggressive histology B-cell non-Hodgkin lymphoma: a Phase II study by the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) Cancer. 2004;101:1835–1842. doi: 10.1002/cncr.20587. [DOI] [PubMed] [Google Scholar]
- 21.Grunewald R, Abbruzzese JL, Tarassoff P, et al. Saturation of 2′,2′-difluorodeoxycytidine 5′-triphosphate accumulation by mononuclear cells during a phase I trial of gemcitabine. Cancer Chemother Pharmacol. 1991;27:258–262. doi: 10.1007/BF00685109. [DOI] [PubMed] [Google Scholar]
- 22.Grunewald R, Kantarjian H, Du M, et al. Gemcitabine in leukemia: A phase I clinical, plasma, and cellular pharmacology study. J Clin Oncol. 1992;10:406–413. doi: 10.1200/JCO.1992.10.3.406. [DOI] [PubMed] [Google Scholar]
- 23.Gandhi V, Plunkett W, Du M, et al. Prolonged infusion of gemcitabine: Clinical and pharmacodynamic studies during a phase I trial in relapsed acute myelogenous leukemia. J Clin Oncol. 2002;20:665–673. doi: 10.1200/JCO.2002.20.3.665. [DOI] [PubMed] [Google Scholar]
- 24.Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 25.Soo RA, Wang LZ, Tham LS, et al. A multicenter randomized phase II study of carboplatin in combination with gemcitabine at standard rate or fixed dose rate infusion in patients with advanced stage non-small-cell lung cancer. Ann Oncol. 2006;17:1128–1133. doi: 10.1093/annonc/mdl084. [DOI] [PubMed] [Google Scholar]
- 26.Nieto Y, Aldaz A, Rifon J, et al. Phase I study of high-dose gemcitabine, administered at fixed dose rate, in combination with docetaxel, melphalan and carboplatin, with autologous hematopoietic progenitor-cell support, in patients with advanced refractory malignancies. Biol Blood Marrow Transplant. 2007;13:1324–1337. doi: 10.1016/j.bbmt.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 29.O’Quigley J, Pepe M, Fisher L. Continual reassessment method: A practical design for phase I clinical trial in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- 30.http://ctep.cancer.gov/protocoldevelopment/electronic.../ctcaev3.pdf
- 31.Thall PF, Simon R, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat Med. 1995;14:357–379. doi: 10.1002/sim.4780140404. [DOI] [PubMed] [Google Scholar]
- 32.Cheson BD, Pfistner B, Juweid ME, et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 33.Rajkumar SV, Harousseau J-L, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statistical Assoc. 1958;53:457–481. [Google Scholar]
- 35.Spasokoukotskaha T, Arner Es, Brösjo O, et al. Expression of deoxycytidine kinase and phosphorylation of 2-chlorodeoxyadenosine in human normal and tumour cells and tissues. Eur J Cancer. 1995;31:202–208. doi: 10.1016/0959-8049(94)00435-8. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda A, Sasaki T. Antitumor activity of sugar-modified cytosine nucleosides. Cancer Sci. 2004;95:105–111. doi: 10.1111/j.1349-7006.2004.tb03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shewach DS, Ensminger WD, Hughes TL. Apparent substrate inhibition of deoxycytidine kinase. Proc Am Assoc Cancer Res. 1994;35:425. (abstract) [Google Scholar]
- 38.Bass AJ, Gockerman JP, Hammett E, et al. Phase I evaluation of prolonged-infusion gemcitabine with irinotecan for relapsed or refractory leukemia or lymphoma. J Clin Oncol. 2002;20:2995–3000. doi: 10.1200/JCO.2002.08.166. [DOI] [PubMed] [Google Scholar]
- 39.Rizzieri DA, Bass AJ, Rosner GL, et al. Phase I evaluation of prolonged-infusion gemcitabine with mitoxantrone for relapsed or refractory acute leukemia. J Clin Oncol. 2002;20:674–679. doi: 10.1200/JCO.2002.20.3.674. [DOI] [PubMed] [Google Scholar]
- 40.Rizzieri DA, Ibom VK, Moore JO, et al. Phase I evaluation of prolonged-infusion gemcitabine with fludarabine for relapsed or refractory acute myelogenous leukemia. Clin Cancer Res. 2003;9:663–668. [PubMed] [Google Scholar]
- 41.Russell JA, Tran HT, Quinlan BN, et al. Once daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–476. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 42.De Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 43.Peters GJ, Ruiz van Haperen VWT, Bergman AM, et al. Preclinical combination therapy with gemcitabine and mechanisms of resistance. Semin Oncol. 1997;23(5 suppl 10):16–24. [PubMed] [Google Scholar]
- 44.Valdez BC, Murray D, Nieto Y, et al. Synergistic cytotoxicity of the DNA alkylating agent busulfan, nucleoside analogs and SAHA in lymphoma cell lines. Leuk Lymphoma. 2012;53:973–981. doi: 10.3109/10428194.2011.634043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toy G, Austin WR, Liao H-I, et al. Requirement for deoxycytidine kinase in T and B lymphocyte development. Proc Natl Acad Sci. 2010;107:5551–5556. doi: 10.1073/pnas.0913900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krett NL, Ayres M, Nabhan C, et al. In vitro assessment of nucleoside analogs in multiple myeloma. Cancer Chemother Pharmacol. 2004;54:113–121. doi: 10.1007/s00280-004-0777-2. [DOI] [PubMed] [Google Scholar]
- 47.Gruber J, Geisen F, Sgonc R, et al. 2′-2′-difluorodeoxycytidine (gemcitabine) induces apoptosis in myeloma cell lines resistant to steroids and 2-chlorodeoxyadenosine (2-CDA) Stem Cells. 1996;14:351–362. doi: 10.1002/stem.140351. [DOI] [PubMed] [Google Scholar]
- 48.Weick JK, Crowley JJ, Hussein MA. The evaluation of gemcitabine in resistant or relapsing multiple myeloma, phase II: a Southwest Oncology Group study. Invest New Drugs. 2002;20:117–121. doi: 10.1023/a:1014493007347. [DOI] [PubMed] [Google Scholar]
- 49.Bieghs L, Caers J, De Bruyne E, et al. The effects of forodesine in murine and human multiple myeloma cells. Adv Hematol. 2010;2010:131895. doi: 10.1155/2010/131895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brune V, Tiacci E, Pfeil I, Döring C, et al. Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. J Exp Med. 2008;205:2251–2268. doi: 10.1084/jem.20080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lenburg ME, Sinha A, Faller DV, et al. Tumor-specific and proliferation-specific gene expression typifies murine transgenic B cell lymphomagenesis. J Biol Chem. 2007;282:4803–4811. doi: 10.1074/jbc.M605870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Artz AS, Wickrema A, Dinner S, et al. Pretreatment C-reactive protein is a predictor for outcomes after reduced-intensity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:1209–1216. doi: 10.1016/j.bbmt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Remberger M, Mattsson J. C-reactive protein levels before reduced-intensity conditioning predict outcome after allogeneic stem cell transplantation. Int J Hematol. 2010;92:161–167. doi: 10.1007/s12185-010-0632-7. [DOI] [PubMed] [Google Scholar]
- 54.Lim ZY, Fiaccadori V, Gandhi S, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2010;34:723–727. doi: 10.1016/j.leukres.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 55.Altes A, Remacha AF, Sarda P, et al. Early clinical impact of iron overload in stem cell transplantation. A prospective study. Ann Hematol. 2007;86:443–447. doi: 10.1007/s00277-007-0266-x. [DOI] [PubMed] [Google Scholar]
- 56.Matsubara J, Ono M, Negishi A, et al. Identification of a predictive biomarker for hematologic toxicities of gemcitabine. J Clin Oncol. 2009;27:2261–2268. doi: 10.1200/JCO.2008.19.9745. [DOI] [PubMed] [Google Scholar]
- 57.Kataoka K, Nannya Y, Iwata H, et al. Plasma brain natriuretic peptide is associated with hepatic veno-occlusive disease and early mortality after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:1631–1637. doi: 10.1038/bmt.2010.26. [DOI] [PubMed] [Google Scholar]