Abstract
The immune responses to viruses provide a means to quickly alert the host to the presence of an invader, activating a range of intrinsic and adaptive antiviral mechanisms. Several research groups have made advances in understanding the innate immune response to HIV-1, although their findings differ. Some investigators find that the virus slips under the radar of the pattern recognition receptors that sense viruses by co-opting host factors that restrict accessibility of the viral nucleic acids, while others find that the virus is sensed and activates a type-I interferon response. This article reviews the recent findings and discusses the similarities and differences.
Introduction
The innate immune response is an ancient means by which an organism responds quickly to an invading bacterial or viral pathogen by recognizing conserved, widely expressed structures termed pathogen-associated molecular patterns (PAMPs) that are common to microbes and not present as normal cellular constituents (O'Neill, 2013; Paludan and Bowie, 2013). The response is fast because it does not require clonal expansion of B- or T-cell clones bearing rare antigen-specific receptors and difficult to escape because it is based on PAMPs that include proteins, lipids, lipoprotein glycan molecules, and nucleic acids that cannot easily be altered genetically. Nucleic acids, including single-stranded DNA, double-stranded DNA, and double-stranded RNA, are compelling as PAMPs as these are essential components of the pathogen that cannot be readily altered. The sensors, termed pattern recognition receptors (PRRs), are a large family of proteins that are expressed on the cell surface, in endosomes, or in the cytoplasm and includes the toll-like receptors, the RIG-I-like receptors, cyclic GMP-AMP synthase (cGAS), interferon-γ-inducible protein (IFI16), and DNA-dependent activators of interferon (IFN) regulatory factors (DAI) (Goubau et al., 2013; Gurtler and Bowie, 2013; Paludan and Bowie, 2013). Monocyte-derived macrophages (MDM) and dendritic cells (MDDC) are myeloid cell-types rich in PRRs that, as phagocytic scavengers, act as “canaries in a coal mine.” They continually sample the environment and send out distress signals in the form of type-I IFN, chemokines, and inflammatory cytokines to warn bystander cells of an incursion by invading microbes and to activate various intrinsic restriction factors.
MDM and MDDC are targets of HIV-1 and thus, it is reasonable to suspect that the virus is subject to being sensed. Viral components that could potentially be sensed include incoming virion structural molecules (proteins and the single-stranded viral RNA genome), reverse transcription intermediates (single-stranded DNA, RNA/DNA hybrids, and double-stranded DNA), and the viral proteins that are synthesized in an infected cell, as they assemble to form a new virion. To date, each of these components has been implicated in sensing. Nevertheless, HIV-1 is a fairly successful pathogen and has presumably evolved mechanisms to escape the innate immune response, either by avoiding detection in the first place or by resisting the antiviral defense mechanisms activated by the innate immune response.
The question of innate immune system sensing of HIV-1 has, over the past few years and particularly in the past few months, become a hot topic. The picture that has emerged is fascinating and complex and is not yet entirely figured out. The models proposed do not entirely agree with one another and the discrepancies between them imply that they cannot all be correct. In this review, I focus on three recent publications, each of which presents a different perspective on how HIV is sensed and presents different models to explain the mechanism. For the sake of simplicity (or at least minimizing confusion), this review focuses on preintegration sensing and does not address the previously reported postintegration sensing mechanism (Manel et al., 2010); nor does it consider findings recently reported on the sensing of HIV-1 by resting CD4 T cells that results in rapid cell death by pyroptosis (Doitsh et al., 2014; Monroe et al., 2014).
Model I (Chen Lab). HIV-1 Can Be Sensed, but Sensing Is Prevented by SAMHD1 (Gao et al., 2013a)
In the course of HIV replication, the cell is infected by a large number of virions, resulting in the production of multiple reverse transcription complexes in each cell. Gao et al. (2013a) find that when the human myeloid cell line, THP-1, is infected with HIV-1, the cells respond by activating a type-I IFN response as detected by the dimerization of the IRF3 signaling protein, phosphorylation of STAT1, and 100-fold induction of IFNβ mRNA. The response was blocked by a reverse transcription inhibitor but not by an integrase inhibitor, pointing to the viral DNA as the targeted PAMP. siRNA knockdown of cGAS and STING in THP-1 blocked the response. cGAS is a cytoplasmic sensor for double-stranded DNA that catalyzes the synthesis of the unusual cyclic dinucleotide, cyclic GMP-AMP (cGAMP) that activates STING, which in turn activates TBK and NFkB (Ishikawa et al., 2009; Abe et al., 2013; Diner et al., 2013; Gao et al., 2013b; Li et al., 2013; Sun et al., 2013; Wu et al., 2013). The article only briefly looks at primary cells, but the results show that infected MDM and MDDC produce cGAMP and activate IRF3. The response required the addition of the lentiviral accessory protein Vpx to the cells as a means of overcoming the SAMHD1-mediated block to reverse transcription (Hrecka et al., 2011; Laguette et al., 2011). The investigators also report that the murine leukemia virus is sensed by cGAS, but in this case, sensing only occurs when the cytoplasmic exonuclease TREX1 is knocked down (Gao et al., 2013a). TREX1 has been proposed to degrade reverse transcription by-products, preventing their sensing (Yan et al., 2010). The authors support a model in which HIV-1 can be sensed and induces a potent innate immune response, but that in primary MDDC and MDM, where SAMHD1 blocks reverse transcription, sensing is prevented.
Model II (Manel Lab). HIV-1 Is not Sensed, but Alterations That Increase Capsid Affinity for Cyclophilin Result in Sensing (Lahaye et al., 2013)
Lahaye et al. (2013) find that HIV-1 is not sensed by MDDC and removal of SAMHD1 with Vpx does not help. Upon entry of HIV-1, the capsid associates with the cellular proline isomerase cyclophilin (CypA). Blocking the interaction either by knockout of CypA or by mutations in the CypA binding loop of capsid blocks replication at reverse transcription. However, introducing a mutation in the CypA binding loop of capsid (termed HIVac for affinity capsid) that results in an increased affinity for CypA, causes the virus to be sensed (Lahaye et al., 2013). HIV-2, unlike HIV-1, was sensed without the need to make any mutations in capsid. Mutation of the HIV-2 capsid to increase its affinity for CypA caused the virus to be sensed even better. HIV-2 caused MDDCs to mature and to produce type-I IFN as well as the chemokine IP-10. HIV2ac was sensed, but failed to be imported into the nucleus (as surmised by its failure to produce 2-LTR circles, a hallmark of nuclear virus), suggesting that sensing occurred in the cytoplasm. shRNA knockdown showed that the sensing required cGAS. The authors support a complex model in which sensing is determined by exactly how much CypA is bound by the capsid. CypA is said to be required to open up the capsid to allow for reverse transcription. Excessive CypA binding opens the capsid too much, leaving the viral DNA accessible to cGAS. Thus, evolution has precisely engineered HIV-1 to bind just the right amount of CypA to uncoat the virus but not to be sensed, allowing the virus to slip in just under the wire.
Model III (Towers Lab). HIV-1 Is Not Sensed Because of Capsid Binding to CypA and CPSF6 (Rasaiyaah et al., 2013)
In addition to binding CypA upon infection, capsid binds to CPSF6, a protein that plays a role in poly-adenylation of mRNA in the cell (Lee et al., 2010). CPSF6 binds the capsid to chaperone the virus to the nuclear pore for transit into the nucleus through association with the importin protein TNPO3. Mutations in the capsid have been identified that block these two interactions: N74D blocks CPSF6 binding and P90A [the same residue mutated by Lahaye et al. (2013)] blocks the interaction with CypA. Mutation of either of these residues prevented replication of the virus in MDM. Rasaiyaah et al. (2013) report that native HIV-1 is not sensed in MDM, but that introduction of either mutation results in a virus that is sensed as detected by an increase in the production of type-I IFN and activation of IRF3 and NFkβ. The block to infection was relieved by treating the cells with the anti-IFN receptor antibody, demonstrating that the block to infection was mediated by type-I IFN binding to its receptor. CPSF6 knockdown also resulted in sensing. The N74D mutant-infected cells produced cGAMP, implicating cGAS as the sensor. In addition, treatment of MDM with cyclosporin-like drugs that prevent the interaction of capsid with CypA also allow the virus to be sensed. The investigators propose that interaction of the virus with CPSF6 and CypA evolved as a means to prevent the cytoplasmic DNA sensors from accessing the viral reverse transcription intermediates. They support a model in which CPSF6 and CypA suppress reverse transcription until the virus traffics to the nuclear pore, preventing its detection in the cytoplasm. A potential problem with the model is that mutations that prevent CypA and CPSF6 binding are generally thought to prevent reverse transcription, making it difficult for these virus to be sensed. The model requires at least a small amount of viral DNA synthesis in the mutated viruses.
Perspectives
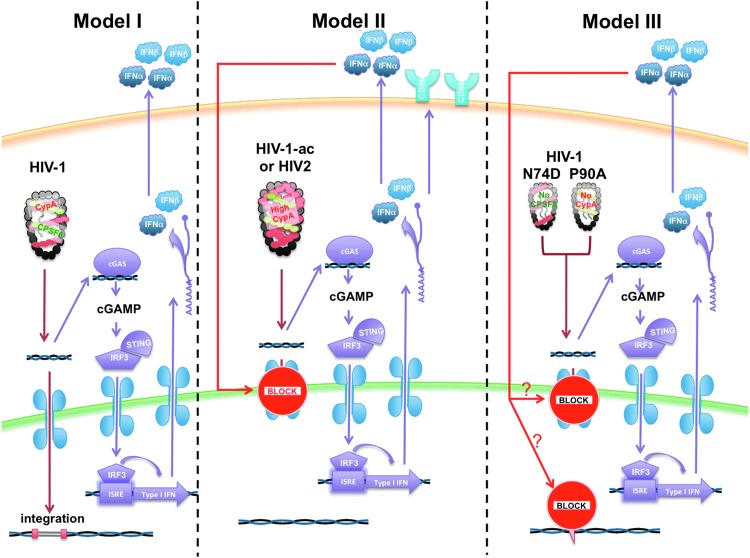
Each of the models presents a somewhat different view of HIV sensing (Figure 1). All three groups agree that the major sensor for HIV-1 in myeloid cells is cGAS and that it is viral DNA reverse transcription intermediates that are sensed. Other findings differ and are difficult to reconcile. The Chen laboratory finds that in the absence of SAMHD1, HIV-1 is sensed. In contrast, the Manel and Towers laboratories find that HIV-1 can be sensed only when mutations that alter CypA or CPSF6 binding to the capsid are introduced. The Manel and Towers laboratories also differ with respect to the effect of CypA on sensing. The Manel laboratory finds that increased CypA binding by HIV-1 capsid promotes sensing, while the Towers laboratory finds that decreased CypA binding to the capsid is required.
FIG. 1.
Models for sensing of HIV-1 in myeloid cells. In Model I (Chen laboratory), native HIV-1 is sensed in myeloid cells upon depletion of SAMHD1 (not shown). In Model II (Manel laboratory), HIV-1 is not sensed, even after removal of SAMHD1. Capsid mutations that increase the affinity for CypA allow for sensing. In Model III (Towers laboratory), native HIV-1 is not sensed because the capsid is protected by CypA and CPSF6. Mutations that prevent binding of the host factors result in sensing. In each model, the viral replication intermediates are sensed by cGAS, resulting in signaling through STING, resulting in type-I IFN production.
A question raised by these findings is that of how activation of an innate immune response can block infection in a single round of virus replication. If the trigger that activates the response is a reverse transcription intermediate, the cell has to respond very rapidly, otherwise all is lost. cGAS has to detect the DNA, activate signaling through STING, turn on transcription of IFN genes, generate new IFN proteins, and then signal through the IFN receptor, all before reverse transcription and integration is completed, a process that takes only a few hours. Such a mechanism is likely to be more useful as a means of alerting the neighboring cells to the presence of the virus rather than protecting the target cell from infection.
Whether native HIV-1 is sensed or not, this area of investigation is important. First, it provides a fascinating insight into the strategies that have evolved by which the virus escapes the innate response. Second, it may provide new ideas for how to increase the innate immune response to the virus, for example, through the use of a drug that affects capsid interactions with CypA or by the development of vaccine immunogens similar to HIVac that provoke a stronger innate immune response, which in turn amplify the adaptive immune response to the vaccine. A clearer picture will emerge as these models are further tested and as additional groups report their findings.
Acknowledgments
The author thanks Nicolin Bloch for providing Figure 1 and Megan Schultz and Cynthia Chen for critical reading of the manuscript. The author is supported by grants from the NIH (AI058864, AI067059).
Disclosure Statement
No competing financial interests exist.
References
- Abe T., Harashima A., Xia T., Konno H., Konno K., Morales A., et al. (2013). STING recognition of cytoplasmic DNA instigates cellular defense. Mol Cell 50,5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner E.J., Burdette D.L., Wilson S.C., Monroe K.M., Kellenberger C.A., Hyodo M., et al. (2013). The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep 3,1355–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsh G., Galloway N.L., Geng X., Yang Z., Monroe K.M., Zepeda O., et al. (2014). Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505,509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Wu J., Wu Y.T., Du F., Aroh C., Yan N., et al. (2013a). Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341,903–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P., Ascano M., Wu Y., Barchet W., Gaffney B.L., Zillinger T., et al. (2013b). Cyclic [G(2',5')pA(3',5')p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153,1094–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubau D., Deddouche S., and Reis E.S.C. (2013). Cytosolic sensing of viruses. Immunity 38,855–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtler C., and Bowie A.G. (2013). Innate immune detection of microbial nucleic acids. Trends Microbiol 21,413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrecka K., Hao C., Gierszewska M., Swanson S.K., Kesik-Brodacka M., Srivastava S., et al. (2011). Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474,658–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Ma Z., and Barber G.N. (2009). STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461,788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguette N., Sobhian B., Casartelli N., Ringeard M., Chable-Bessia C., Segeral E., et al. (2011). SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474,654–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahaye X., Satoh T., Gentili M., Cerboni S., Conrad C., Hurbain I., et al. (2013). The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity 39,1132–1142 [DOI] [PubMed] [Google Scholar]
- Lee K., Ambrose Z., Martin T.D., Oztop I., Mulky A., Julias J.G., et al. (2010). Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe 7,221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.D., Wu J., Gao D., Wang H., Sun L., and Chen Z.J. (2013). Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341,1390–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N., Hogstad B., Wang Y., Levy D.E., Unutmaz D., and Littman D.R. (2010). A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467,214–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe K.M., Yang Z., Johnson J.R., Geng X., Doitsh G., Krogan N.J., et al. (2014). IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343,428–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA. (2013). Immunology. Sensing the dark side of DNA. Science 339,763–764 [DOI] [PubMed] [Google Scholar]
- Paludan S.R., and Bowie A.G. (2013). Immune sensing of DNA. Immunity 38,870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasaiyaah J., Tan C.P., Fletcher A.J., Price A.J., Blondeau C., Hilditch L., et al. (2013). HIV-1 evades innate immune recognition through specific cofactor recruitment. Nature 503,402–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wu J., Du F., Chen X., and Chen Z.J. (2013). Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339,786–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Sun L., Chen X., Du F., Shi H., Chen C., et al. (2013). Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339,826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N., Regalado-Magdos A.D., Stiggelbout B., Lee-Kirsch M.A., and Lieberman J. (2010). The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol 11,1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]