Abstract
Objective: The purpose of this study was to assess whether the diode laser (DL) pulpotomy method is a suitable alternative to formocresol (FC) and ferric sulphate (FS) pulpotomies in human primary teeth. Background data: Pulpotomy is the amputation of infected coronal pulp to maintain radicular pulp vitality and function. Although FC is regarded as the gold standard for pulpotomy in primary teeth, concerns about its safety have been reported. Lasers are an effective nonpharmacological alternative for treating pulp in children. Methods: This study included 120 primary molars in 58 children 5–9 years of age who underwent an identical conventional pulpotomy technique; the molars were allocated to FC, FS, and DL groups. After removal of the coronal tissue, complete hemostasis of the remaining pulp in the DL group was achieved by DL at 1.5 W, 30 Hz, and 50 mJ, with a 10 sec exposure time. For the FC group, diluted FC (1:5 Buckley's formocresol) was used for 5 min., and for the FS group, a 15.5% FS solution was used for 15 sec. Treatments in all groups were completed with stainless steel crowns and monitored clinically and radiographically at 1, 3, 6, 9, and 12 months. Results: The clinical success rates at 12 months were 97%, 95%, and 100%, whereas the radiographic success rates were 87%, 79%, and 75%, for the FC, FS and DL groups, respectively. The differences in the results were not statistically significant according to the χ2 test (p>0.05). Conclusions: DL pulpotomy offers a high clinical success rate, however considering radiographic success rate, it may not replace traditional FC and FS pulpotomies in primary molars.
Introduction
Pulpotomy is a common therapy for cariously exposed pulp in primary molar teeth. With this method, the functional tooth is retained in the oral cavity, without pain and swelling, until it exfoliates.1–4 Over the last 70 years, formocresol (FC) has been a popular material used in the pulpotomy procedure, mainly because it is easy to use and it ensures high clinical success rates.5 However, several studies have shown that FC has hazardous adverse effect, such as mutagenicity and cytotoxicity.6,7 Therefore, a variety of medicaments and nonpharmacologic alternatives have been proposed in the literature to replace FC, such as glutaraldehyde (GH), calcium hydroxide (CH), freeze-dried bone, ferric sulphate (FS), mineral trioxide aggregate (MTA), electrosurgery, and lasers.2–5,8–18 However, it is of note that there has not been any consensus published on the ideal pulpotomy technique.19
FS (15.5%) has been investigated widely and reported in animal and human studies as a hemostatic agent in pulpotomy procedures. On contact with blood, a ferric ion-protein complex is formed, and the membrane of this complex seals the cut vessels mechanically, producing hemostasis, and the agglutinated protein complex forms plugs that occlude the capillary orifices, preventing blood clot formation.20 Based on the available evidence so far, FS and FC produce similar treatment outcomes; however, FS requires much technical sensitivity.21,22 It is worth mentioning that FS has been proposed as a substitute for FC, which some would consider as a new gold standard.1,2,20
Recent advances in laser technology have made lasers more attractive in endodontic applications, where they can be used as an adjunct or alternative method to traditional pulp therapy procedures. Laser treatment has advantages with respect to control of hemorrhage, sterilization, and stimulation effects on the dental pulp cells. Since the effects of ruby laser irradiation on dental pulp tissue were reported,23 other studies using CO2, Nd:YAG, Er:YAG, Er,Cr:YSGG, diode laser (DL), and argon laser (AL) pulpotomies have been published.18,24–26 DLs have been applied widely in oral surgery procedures involving soft tissue. Because these lasers are relatively poorly absorbed by the tooth structure, soft tissue surgery can be performed safely in close proximity to enamel, dentin, and cementum. DLs can be used for ablation, incision, and excision (cutting, vaporization, curettage, coagulation, and hemostasis).26 Furthermore, the laser has the advantages of being portable and compact with a minimum setup time.26 Based on these characteristics and previous studies,18,24–27 the DL could be an alternative for pulpotomy therapy.
The purpose of this in vivo study was to compare the treatment outcome of DL, FC, and FS in vital pulpotomy of carious primary molars.
Materials and Methods
The proposed approach was a randomized cohort study approved by the University of Marmara Health Sciences Ethics Committee (Document number MAR-YC-2008-0131). The participants were 58 children—32 males and 26 females between 5 and 9 years of age—at the University of Marmara, Department of Paediatric Dentistry, in Istanbul. The subjects were categorized as cooperative volunteers (Frankel's class IV) having primary molar teeth requiring vital pulp therapy. Prior to the clinical procedures, informed consent was obtained. All pulpotomies were performed by the same trained and experienced pediatric dentist (B.D). At each follow-up visit, children were examined by the same operator and another pediatric dentist (I.T).
Inclusion criteria
Clinical criteria were:
1. Vital primary molar teeth in children that required pulpotomy treatment for pulpal exposure to caries, and lacked excessive hemorrhage (i.e., controllable within 2 min).
2. Teeth had neither spontaneous pain nor tenderness to percussion.
3. Teeth were deemed to be restorable with stainless steel crowns (SSCs).
Radiographic criteria were:
1. A modified Ekstrand et al.28 criterion was used in the visual examination. The teeth were scored as 3, demineralization involving the middle one third of dentin and 4, demineralization involving the inner one third of dentin.
2. Absence of furcal or periapical radiolucency and widened periodontal ligament spaces.
3. Absence of a pathologic internal or external resorption.
4. Teeth with physiologic root resorption of less than one third of their roots.
Clinical procedures
One-hundred and twenty primary molars were randomly assigned into three groups (n=40), according to the pulpal therapy technique:
Group I–Experimental group: Diode laser (LaserSmile™, Biolase Technology, Inc., Irvine, CA)
Group II–Control Group: Formocresol (Buckley's Formo Cresol®, SultanHealthcare, Hackensack, NJ)
Group III–Control Group: Ferric sulphate (Astringedent™ Ultradent Products Inc., Salt Lake City, UT)
Based on the clinical/radiographic assessment and carious pulp exposure in affected molars, coronal pulpotomies were performed. In each treatment group, coronal pulp amputation was achieved with small and medium slow-speed round burs under local analgesia (Ultracain® DS Ampul - Sanofi Aventis Ltd., Istanbul, Turkey) and rubber dam isolation. The amputation site was cleaned with a sterile spoon excavator, and the initial hemorrhage was controlled using a dry sterile cotton pellet applied with pressure. In cases with excessive and persistent hemorrhage, pulpectomy was performed, and the tooth was eliminated from the study. Complete hemostasis then was achieved as follows, depending on the group assignment.
• In the FC group, the sterile cotton pellets were placed in 19% formaldehyde Buckley's FC solution. They were immediately blotted dry using a sterile cotton roll. The cotton pellet was placed directly over the radicular pulp stumps and left for 5 min for fixation.
• In the FS group, a 15.5% FS solution with a plastic syringe and a cotton-tipped needle were utilized. FS was applied by wiping the cotton tip on the pulp stumps for 15 sec. The pulp cavity was washed with saline to remove any blood clot particles.
• In the DL group, a DL beam at a wavelength of 810 nm was transmitted. The DL fiber tip was kept 1–2 mm from touching the tissue. The pulp at canal orifices was exposed with paramaters of a frequency of 30 Hz and energy of 50 mJ, with a power of 1.5 W for 10 sec with air-cooling operation mode without water.
In each group, a zinc oxide eugenol dressing (ZOE) (Kalzinol®, Dentsply, Konstanz, Germany) was placed directly on the radicular pulpal stumps and sealed by a second layer of glass-ionomer restorative material (Ketac™ Molar, Easy Mix™, 3M ESPE™, Seefeld, Germany). Final crown restorations were completed immediately with SSCs (3M™ Unitek™ Stainless Steel Crowns, Primary, Neuss, Germany).
Follow-up
One-hundred and twenty teeth (57 primary first molars and 63 primary second molars) in 58 children were followed up clinically and radiographically after 1, 3, 6, 9, and 12 months. Two blinded observers evaluated a set of radiographs separately to calculate the interexaminer reliability of radiographic assessments.
Outcome criteria for failure and success
The outcome of success or failure was determined by the following clinical and radiographic criteria: The presence of spontaneous pain, percussion/palpation, abscess, swelling, fistula, or pathologic mobility definitely indicated clinical failure. The presence of periapical radiolucency, widened periodontal ligament space (PDL), pathologic internal/external root resorption, or pathological changes of the alveolar bone in the furcation area were recorded as radiographic failures.
Statistical analysis
The outcome assessment and data analysis were blinded, as the techniques were coded. The intra-and interexaminer agreement of the data for the radiographic assessment were evaluated by Cohen's κ statistics. The χ2 and Fisher's exact tests were performed to compare the results. In the present study, p<0.05 was considered to indicate statistical significance.
Results
The interexaminer reliability of the data for the radiographic assessment was good. The κ coefficient for intra-examiner agreement of data was 0.91.
Clinical results
The clinical success rates related to age, sex, tooth type, and level of physiological root resorption at the 12 month observation time showed no significant differences (Table 1).
Table 1.
Clinical Success Rate at 12 Months According to Age, Gender, Tooth Type, and Level of Physiological Root Resorption
| Clinical success (12 months) | |||
|---|---|---|---|
| Success (n=117) n (%) | Failure (n=3) n (%) | p | |
| Age | |||
| 5 | 8 (100) | 0 (0) | 0.889 |
| 6 | 25 (96.2) | 1 (3.8) | |
| 7 | 51 (98.0) | 1 (2.0) | |
| 8 | 21 (95.4) | 1 (4.3) | |
| 9 | 12 (100) | 0 (7.6) | |
| Gender | |||
| Boys | 68 (97.1) | 2 (2.9) | 0.780 |
| Girls | 49 (98.0) | 1 (2.0) | |
| Physiological root resorption | |||
| Slight | 56 (96.6) | 2 (3.4) | 0.612 |
| Not | 61 (98.3) | 1 (1.6) | |
| Tooth type | |||
| First primary molar | 55 (96.5) | 2 (3.5) | 0.606 |
| Second primary molar | 62 (98.4) | 1 (1.5) | |
The DL group had a clinical success rate of 100% throughout the 12-month follow-up period. The FS group had clinical success rates of 100%, 97%, and 92.5% at 3, 6, and 12 months, respectively. The FC group had a clinical success rate of 100% at 6 months, followed by 97% at 9 and 12 months. (Table 2). However, no significant differences were detected among the clinical success rates in all groups. Moreover, the clinical pathology rates, such as the pathologic mobility, tenderness to percussion, and fistula and extraction rates, were higher in the FS group than in the FC and DL groups.
Table 2.
Clinical Success Rates of Treatment Groups During the 12-Month Follow-Up Period (%)
| Clinical success at follow-up | Diode laser (n=40) n (%) | Formocresol (control) (n=40) n (%) | Ferric sulphate (n=40) n (%) | p |
|---|---|---|---|---|
| Initial | 40 (100) | 40 (100) | 40 (100) | - |
| 1 month | 40 (100) | 40 (100) | 40 (100) | 0.359 |
| 3 month | 40 (100) | 40 (100) | 40 (100) | 0.772 |
| 6 month | 40 (100) | 40 (100) | 39/40 (97.5) | 0.601 |
| 9 month | 40 (100) | 39/40 (97.5) | 39/39 (95) | 0.134 |
| 12 month | 40/40 (100) | 39/40 (97.5) | 38/39 (92.5) | 0.348 |
Radiographic results
When the radiographic success rate in pulpotomies was evaluated in terms of age, sex, and tooth type, the differences in results were not statistically significant (p>0.05). However, according to the results, it can be concluded that the level of root resorption had a significant effect (Table 3).
Table 3.
Radiographic Success Rate at 12 Months According to Age, Gender, Tooth Type, and Level of Physiological Root Resorption
| Radiographic success (12 months) | |||
|---|---|---|---|
| Success (n=97) n (%) | Failure (n=23) n (%) | p | |
| Age | |||
| 5 | 8 (100) | 0 (0) | 0.506 |
| 6 | 22 (84.6) | 4 (15.4) | |
| 7 | 42 (80.7) | 10 (19.2) | |
| 8 | 16 (72.7) | 6 (27.3) | |
| 9 | 9 (75.0) | 3 (25.0) | |
| Gender | |||
| Boys | 59 (84.3) | 11 (15.7) | 0.233 |
| Girls | 38 (76) | 12 (24) | |
| Physiologic root resorption | |||
| Slightly | 42 (72.4) | 16 (27.6) | 0.023* |
| Not | 55 (83.3) | 7 (11.1) | |
| Tooth type | |||
| First primary molar | 43 (75.4) | 14 (24.6) | 0.166 |
| Second primary molar | 54 (85.7) | 9 (14.2) | |
The radiographic success rates in the DL group were 95%, 87%, and 75% at 3, 6, and 12 months, respectively, whereas the success rates in the FS group were 95%, 87%, and 79% at 3, 6, and 12 months, respectively. In the FC group, the success rates for the same observation times were 97%, 92%, and 87%, respectively. The differences among the groups were not statistically significant. Thirty teeth in the DL group were diagnosed as radiographically successful, whereas this number increased to 31 and 35 in the FS and FC groups, respectively (Table 4). Representative radiographs of successful cases are shown in Fig. 1–4. Failed cases detected radiographically alone or in combination with other pathologies are shown in Fig. 5.
Table 4.
Radiographic Success Rates of Treatment Groups During the 12-Month Follow-Up Period (%)
| Radiographic success at follow-up | Diode laser (n=40) n (%) | Formocresol (n=40) n (%) | Ferric sulphate (n=40) n (%) | p |
|---|---|---|---|---|
| Initial | 40 (100) | 40 (100) | 40 (100) | - |
| 1 month | 40 (100) | 40 (100) | 40 (100) | - |
| 3 month | 38/40 (95.0) | 39/40 (97.5) | 38/40 (95) | 0.812 |
| 6 month | 35/40 (87.5) | 37/40 (92.5) | 35/40 (87.5) | 0.708 |
| 9 month | 35/40 (87.5) | 36/40 (90.0) | 33/39 (84.6) | 0.771 |
| 12 month | 30/40 (75) | 35/40 (87.5) | 31/39 (79.5) | 0.358 |
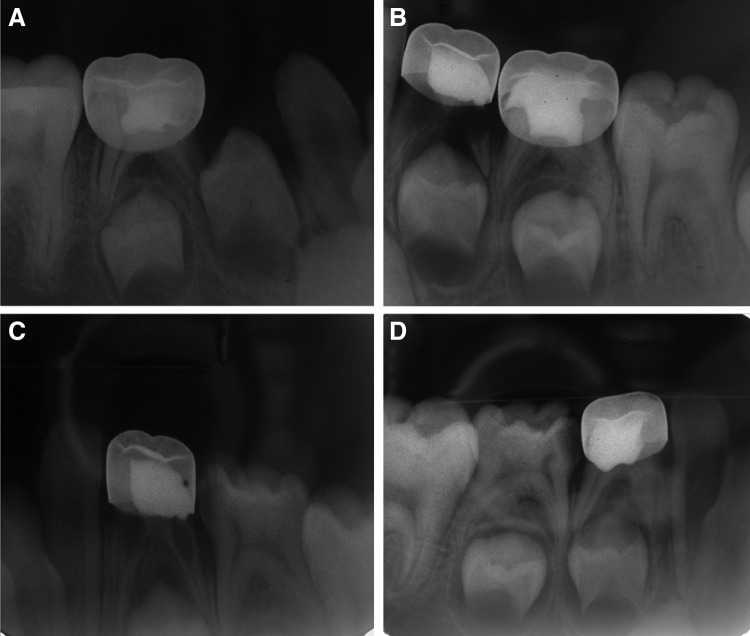
FIG 1.
Radiography of a successful case of formocresol (FC) (maxillary left first primary molar) and diode laser (DL) (maxillary left second primary molar) pulpotomy at 6 months.
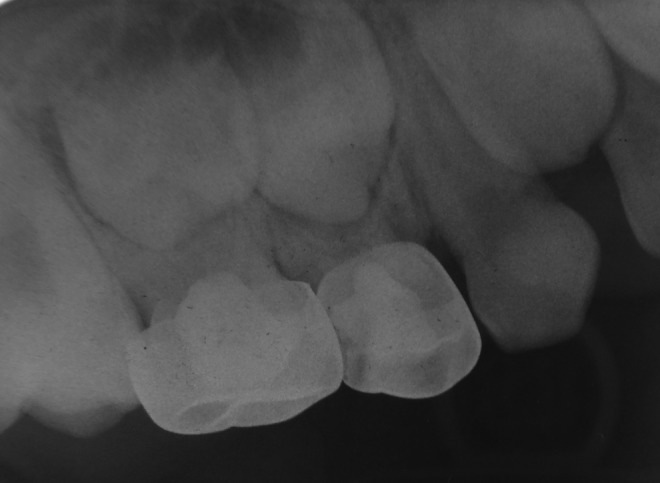
FIG 2.
Radiography of a successful case of formocresol (FC) (maxillary left first primary molar) and diode laser (DL) (maxillary left second primary molar) pulpotomy at 12 months.
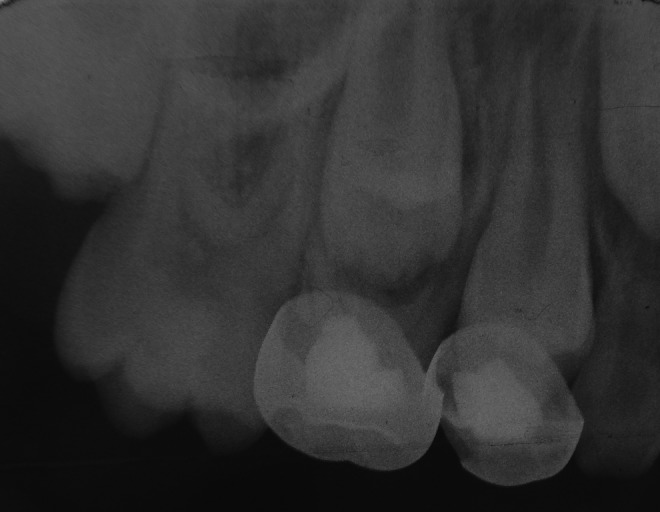
FIG 3.
Radiography of a successful case of ferric sulphate (FS) (mandibular left first primary molar) and diode laser (DL) (mandibular left second primary molar) pulpotomy at 6 months.
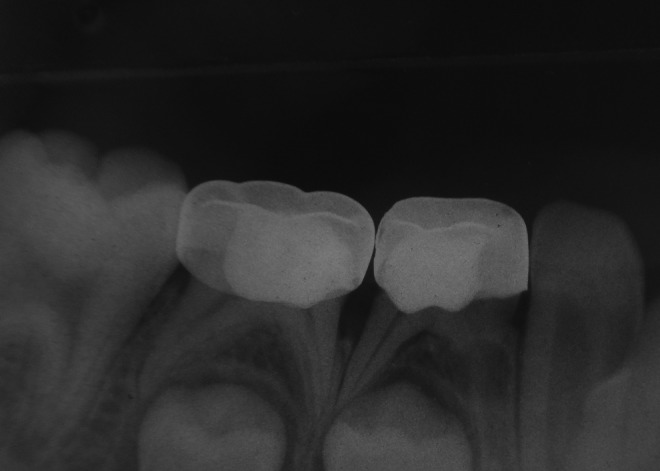
FIG 4.
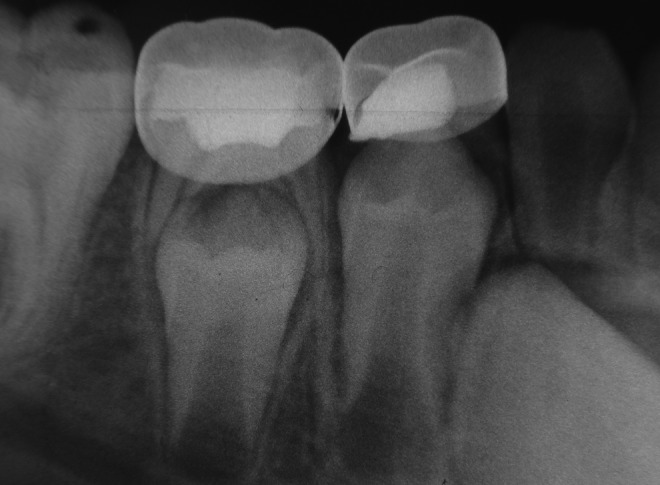
Radiography of a successful case of ferric sulphate (FS) (mandibular left first primary molar) and diode laser (DL) (mandibular left second primary molar) pulpotomy at 12 months.
FIG 5.
Radiography of failed cases at 12 months. (A) Periodontal ligament space (PDL) widening and initial furcal radiolucency in the mandibular left second primary molar in diode laser (DL) pulpotomy. (B) Progressive internal resorption and PDL space disappearing in the mandibular right first primary molar in ferric sulphate (FS) pulpotomy. (C) Internal resorption in the mandibular right first primary molar in FS pulpotomy. (D) Furcal radiolucency and PDL disappearing and in the mandibular left first primary molar in formocresol (FC) pulpotomy.
Note that the most of the radiographic failures in the DL group involved periapical radiolucency and PDL widening, whereas furcal radiolucency and PDL widening rates were the highest in the FC group. For the FS group, however, cases with internal resorption and PDL widening were the most common failures.
Discussion
The objective of pulp therapy in a child patient is the successful treatment of a pulpally involved tooth and retaining the tooth in a healthy condition so that it may fulfil its role as a useful component of a primary and young permanent dentition.1–4
This investigation presented a long-term follow-up study of the clinical and radiographic success rates of pulpotomy utilizing DL, FC, and FS. The FC technique was selected as the control group, as it is still considered the “gold standard” in pediatric dentistry, perhaps because of its ease in use and excellent clinical success.5,15,20 Because FS pulpotomy has achieved results comparable to those obtained by FC pulpotomy,2,12,20 FS was also considered a control group.
The success rate of pulpotomies has been measured traditionally as the percentage of teeth reaching an arbitrary point in time in the absence of clinical or radiographic evidence of disease.2,17,29 Similarly, in the present study, the success rate of the pulpotomy treatments was defined as the absence of any clinical or radiographic pathology at the follow-up appointments.
The direct placement of ZOE over the pulp tissue has been reported as a cause of internal inflammation in FS pulpotomies.29 However, in this study, the ZOE base was chosen to effectively compare the results produced by DL hemostasis with those obtained by the traditional FC and FS hemostasis. The final restorations were completed with the SSCs recommended for pulpotomized primary teeth.30
Therefore, in this study, as a nonpharmacologic technique, DL pulpotomy has been proposed as a reliable alternative to FC and FS pulpotomies. However, human clinical trials that compare laser pulpotomies with existing pulpotomy techniques have shown conflicting results. Any variation in laser application parameters, including the power, frequency, exposure time, and water/air dry-mode, causes different results in pulp tissue. These facts might differentiate the laser-assisted pulpotomy results from each other. The study of Elliot et al.31 evaluated the success rates of CO2 laser pulpotomies on primary canines for 3 months and found that the symptomatic, clinical, or radiographic findings indicated no significant differences between the FC and laser groups. Saltzman et al.4 compared DL with MTA pulpotomy with conventional FC/ZOE pulpotomy. The FC/ZOE pulpotomy ensured a radiographic success rate of 87.5%, whereas this rate was reduced to 70.8% by laser-MTA pulpotomy. However, the radiographic assessment at 15 months revealed no significant difference between the treatment types. Huth et al.2 compared the relative effectiveness of Er.YAG laser, calcium hydroxide, and FS techniques with that of the diluted FC technique in primary molars. The results demonstrated that the total success rate of Er:YAG (78%) was nonsignificantly lower than that achieved by FC (85%) after 2 years. Liu24 compared the effects of Nd:YAG laser pulpotomy with those of FC pulpotomy on human primary teeth for 6–64 months. In the Nd:YAG laser group, the clinical success rate was 97%, and the radiographic success rate was 94%. On the other hand, clinical and radiographic success rates in the control group, 85% and 78%, respectively, were achieved. The author concluded that the success rate of Nd:YAG laser pulpotomy was notably higher than that of FC pulpotomy. In our study, each laser was equipped with a flexible waveguide and fiber with a diameter of 400 mm and was operated with a frequency of 30 Hz and energy of 50 mJ, with a power of 1.5 W for 10 sec with air cooling but without water spray. The lasers were adjusted (standardized) for an effective output power of 1.5 W. This procedure ensured stable and standardized irradiation for each case. The clinical success rate of DL pulpotomy after 12 months was 100%, but the radiographic success rate was 75%. When the radiographic success rates of the FC (87%) and FS groups (79%) were compared with that of the DL group, no significant differences were detected. We concluded that the lower radiographic success rate of our DL group may have been attributable to many factors. Saltzman et al.4 suggested that the heat that is produced by a laser causes an instant and reversible decrease in blood flow for 3–6 min without any hyperemic reaction in the pulp microcirculation. Therefore, laser irradiation resulting in pulp stumps free of hemorrhage could mask truly hyperemic pulp. In addition, the DL itself might have caused medium peripheral thermal damage in the surrounding pulp tissue.
On the other hand, in a comparison to other studies in literature we have reported a detailed analysis of radiographic and clinical results in order to evaluate the long-term success of pulpotomy groups. Therefore, in the present study, periapical radiolucency, widening of the periodontal ligament, and pulp canal calcification were categorized as a radiographic failure, but the teeth presenting one of these pathologies were not treated immediately, and were observed for the follow-up, as they were aysmptomatic and did not show any sign of clinical failure. Therefore, as Smith et al.29 stated, defining osseous changes versus dental changes could be a more realistic approach for developing criteria for the radiographic success of a pulpotomy study. Similarly, in the present study, most of the radiographic failures in the DL group, which were detected at a previous follow-up, did not show any pathologic progression in radiographs at 12-month follow-up. As most of the radiographic failures were not pathologic osseous changes, any clinical symptoms were observed. Therefore, in our opinion, not separating the osseous and dental changes in the radiographic failure criteria may have caused an overly high percentage of radiographic failure outcome.
Our findings suggest that the radiolographic success rates of teeth with physiological root resorption were significantly lower than those of teeth without physiological root resorption. Our result supports the idea of the renewal potential of teeth after injury, with changes from at the beginning of the resorption process until exfoliation.32
In this study, a widened periodontal ligament was the most common radiographic finding in all groups at the 12-month follow-up. In addition to widened periodontal ligament, most of the radiographic failure in the DL group involved periapical radiolucency, whereas furcal radiolucency rate was the highest in the FC group. For the FS group, however, internal resorption and PDL widening were the most common failures. In comparison with previous reports, our results are similar to those recorded by Neamatollahi and Tajik14 and Huth et al.2 and different from those by Saltzman et al.,4 who reported extraction in the laser pulpotomy group for furcal radiolucencies and, pathologic root resorption, and Liu,24 who reported internal resorption in laser pulpotomy group.
Based on the overall evaluation of all outcome parameters determined in this in vivo study, it can be concluded that traditional FC pulpotomy remains the most effective pulp therapy among FC, FS, and DL pulpotomies for primary molars. Also, for a successful pulpotomy procedure, the pulpal status for the FC method is not as crucial as for the DL and FS methods.
Conclusions
DL pulpotomy offers high clinical success rates; however, considering the low radiographic success rate, it may not replace traditional FC and FS pulpotomies in primary molars. However, there is a limited number of in vivo research studies on laser-assisted pulpotomy with the use of different types of lasers in different clinical settings and collected over different follow-up periods. Therefore, additional studies with a larger sample size and longer-term outcomes are required before definitive recommendations can be made.
Acknowledgments
This study was based on work performed by Basak Durmus (Altinok) for the fulfilment of the degree of Doctor of Philosophy, University of Marmara, Istanbul, Turkey. The study was supported by a research grant from The Marmara University Scientific Research Committee (BAPKO) with project numbers SAG-C-DRP-050608-0120 and SAG-D-310510-0182.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fuks A.B. (2002). Current concepts in vital primary pulp therapy. Eur. J. Pediatr. Dent. 3, 115–120 [PubMed] [Google Scholar]
- 2.Huth K.C., Paschos E., Hajek-Al-Khatar N., et al. (2005). Effectiveness of 4 pulpotomy techniques – randomized controlled trial. J. Dent. Res. 84, 1144–1148 [DOI] [PubMed] [Google Scholar]
- 3.Moretti A.B.S., Sakai V.T., Oliveira T.M., et al. (2008). The effectiveness of mineral trioxide aggragate, calcium hydroxide and formocresol for pulpotomies in primary teeth. Int. Endod. J. 41, 547–555 [DOI] [PubMed] [Google Scholar]
- 4.Saltzman B., Sigal M., Clokie C., Rukavina J., Titley K., and Kulkarni G.V. (2005). Assesment of a novel alternative to conventional formocresol-zinc oxide eugenol pulpotomy for the treatment of pulpally involved human primary teeth: diode laser-mineral trioxide aggragate pulpotomy. Int. J. Pediatr. Dent. 15, 437–447 [DOI] [PubMed] [Google Scholar]
- 5.El-Meligy O., Abdalla M., El-Baraway S., El-Tekya M., and Dean J.A. (2001). Histological evaluation of electrosurgery and formocresol pulpotomy techniques in primary teeth in dogs. J. Clin. Pediatr. Dent. 26, 81–85 [DOI] [PubMed] [Google Scholar]
- 6.Zarzar P.A., Rosenbalt A., Takahashi C.S., Takeuchi P.L., and Costa L.A., Jr. (2003). Formocresol mutagenicity following primary tooth pulp therapy: an in vivo study. J. Dent. 31, 479–485 [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer (homepage). (June15, 2004) Available at http://www.iarc.fr/ENG/Press_Release/archieves/pr153a.html (Last accessed November19, 2007)
- 8.Fuks A.B., Bimstein E., Klein H., Guelmann M. (1990). Assesment of a 2% buffered glutaraldehyde solution in pulpotomized primary teeth of schoolchildren. ASDC J. Dent. Child. 57, 371–375 [PubMed] [Google Scholar]
- 9.Shumayrikh N.M., and Adenubi J.O. (1999). Clinical evaluation of glutaraldehyde with calcium hydroxide and gluteraldehyde with zinc oxide eugenol in pulpotomy of primary molars. Endod. Dent. Traumatol. 15, 259–264 [DOI] [PubMed] [Google Scholar]
- 10.Markovic D., Zivojinovic V., and Vucetic M. (2005). Evaluation of three pulpotomy medicaments in primary teeth. Eur. J. Pediatr. Dent. 6, 133–138 [PubMed] [Google Scholar]
- 11.Fadavi S., and Anderson A.W. (1996). A comparison of the pulpal response to freeze-dried bone, calcium hydroxide, and zinc-oxide eugenol in primary teeth in two cynomolgus monkeys. Pediatr. Dent. 18, 52–56 [PubMed] [Google Scholar]
- 12.Ibrijevic H., and Al-Jame Q. (2003). Ferric sulphate and formocresol in pulpotomy of primary molars: long term follow-up study. Eur. J. Pediatr Dent. 4, 28–32 [PubMed] [Google Scholar]
- 13.Shayegan A., Petein M., and Abbeele A.V. (2008). Beta-tricalcium phosphate, white mineral trioxide aggregate, white Portland cement, ferric sulphate and formocresol used as pulpotomy agents in primary pig teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 105, 536–542 [DOI] [PubMed] [Google Scholar]
- 14.Neamatollahi H., and Tajik A. (2006). Comparison of clinical and radiographic success rates of pulpotomy in primary molars using formocresol, ferric sulphate and mineral trioxide aggregate. JDT 3, 6–14 Available at http://www.journals.tums.ac.ir/upload_files/pdf/_/3304.pdf (Last accessed February28, 2013) [Google Scholar]
- 15.Holan G., Eidelman E., and Fuks A.B. (2005). Long-term evaluation of pulpotomy in primary molars using mineral trioxide aggregate and formocresol. Pediatr. Dent. 27, 129–136 [PubMed] [Google Scholar]
- 16.Farsi N., Alamoudi N., Balto K., and Mushayt A. (2005). Success of minerals trioxide aggregate in pulpotomized primary molars. J. Clin. Pediatr. Dent. 29, 307–311 [DOI] [PubMed] [Google Scholar]
- 17.Dean J.A., Mack R.B., Fulkerson B.T., and Sanders B.J. (2002). Comparison of electrosurgical and formocresol pulpotomy procedures in children. Int. J. Pediatr. Dent. 12, 177–182 [DOI] [PubMed] [Google Scholar]
- 18.Olivi G., and Genovese M.D. (2006). Erbium chromium laser in pulp capping treatment. J. Oral Laser Appl. 6, 291–299 [Google Scholar]
- 19.Anthonappa R.P., King N.M., and Martens L.C. (2013). Is there sufficient evidence to support the long-term efficacy of mineral trioxide aggregate (MTA) for endodontic therapy in primary teeth? Int. Endod. J. 46, 198–204 [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan V., Patchett C.L., and Waterhouse P.J. (2006). Is there a life after Buckley's formocresol? Part I-A narrative review of alternative interventions and materials. Int. J. Pediatr. Dent. 16, 117–127 [DOI] [PubMed] [Google Scholar]
- 21.Peng L., Ye L., Guo X., et al. (2007). Evaluation of formocresol versus ferric sulphate primary molar pulpotomy: a systematic review and meta-analysis. Int. Endod J. 40, 751–757 [DOI] [PubMed] [Google Scholar]
- 22.Fuks A.B. (2008). Vital pulp therapy with new materials for primary teeth: new directions and treatment perspectives. Pediatr. Dent. 30, 211–219 [PubMed] [Google Scholar]
- 23.Adrian J.C., Bernier J.L., and Sprague W.E. (1971). Laser and the dental pulp. J. Am. Dent. Assoc. 83, 113–117 [DOI] [PubMed] [Google Scholar]
- 24.Liu J. (2006). Effects of Nd:YAG laser pulpotomy on human primary molars. J. Endod. 32, 404–407 [DOI] [PubMed] [Google Scholar]
- 25.Gonzales C., Zakariasen K.L., Dederich D.N., and Pruhs R.J. (1996). Potential preventive and therapeutic hard tissue applications of CO2 laser, Nd:YAG laser, and argon lasers in dentistry review. ASDC J. Dent. Child. 63, 196–207 [PubMed] [Google Scholar]
- 26.Boj J.R., Poirier C., Hemandez M., Espasa E., and Espanya A. (2011). Review: Laser soft tissue treatments for paediatric dental patients. Eur. Arch. Paediatr. Dent. 12, 100–105 [DOI] [PubMed] [Google Scholar]
- 27.Alam T., Dawasaz A.A., Thukral N., and Jangam D. (2008). Surgical diode laser excision for peripheral cemento-ossifying fibroma: a case report and literature review. J. Oral Laser Appl. 8, 43–49 [Google Scholar]
- 28.Ekstrand K.R. (2004). Improving clinical visual detection—potential for caries clinical trials. J. Dent. Res. 83, Spec No C:C67–71 [DOI] [PubMed] [Google Scholar]
- 29.Smith N.L., Seale N.S., and Nunn M.E. (2000). Ferric sulphate pulpotomy in primary molars: a retrospective study. Pediatr. Dent. 22, 192–199 [PubMed] [Google Scholar]
- 30.Croll T.P., and Killian C.M. (1992). Zinc oxide eugenol pulpotomy and stainless steel crown restoration of a primary molar. Quintessence Int. 23, 383–388 [PubMed] [Google Scholar]
- 31.Elliot R.D., Roberts M.W., Burkes J., and Philips C. (1999). Evaluation of the carbondioxide laser on vital human primary pulp tissue. Pediatr. Dent. 21, 327–331 [PubMed] [Google Scholar]
- 32.Monteiro J., Day P., Duggal M., Morgan C., and Rodd H. (2009). Pulpal status of human primary teeth with physiological root resorption. Int. J. Pediatr. Dent. 19, 16–25 [DOI] [PubMed] [Google Scholar]