Abstract
Introduction: The purpose of this study was to examine whether a Proline (Pro)-to-Alanine (Ala) exchange at codon 12 (Pro12Ala) polymorphism of the peroxisome proliferator-activated receptor-gamma (PPARγ) is associated with susceptibility to nonalcoholic fatty liver disease (NAFLD), rheumatoid arthritis (RA), and psoriatic arthritis (PsA). Methods: A meta-analysis was conducted on the association between the PPARγ Pro12Ala polymorphism and NAFLD, RA, and PsA. Results: Nine studies, including five on NAFLD, two on RA, and two on PsA, were available for the meta-analysis consisting of 8082 cases and 3790 controls. The meta-analysis revealed no association between the Ala allele of the PPARγ Pro12Ala polymorphism and NAFLD (odds ratios [OR]=0.936, 95% confidence interval [CI]=0.672–1.302, p=0.693). However, stratification by ethnicity indicated an association between the Ala allele and NAFLD in East Asians (OR=0.700, 95% CI=0.496–0.987, p=0.042), but not in Europeans (OR=1.128, 95% CI=0.863–1.475, p=0.378). Analysis using the dominant model showed the same Ala allele pattern in East Asians and Europeans (OR=0.688, 95% CI=0.484–0.978, p=0.037; OR=1.051, 95% CI=0.782–1.413, p=0.742), demonstrating a significant association between the Ala allele and NAFLD in East Asians. The meta-analysis revealed no association between the Ala allele and RA in East Asians (OR=0.467, 95% CI=0.188–1.161, p=0.101), and no association was found between the Ala allele and PsA in Europeans (OR=0.869, 95% CI=0.465–1.627, p=0.662). Conclusions: Our meta-analysis demonstrates that the PPARγ Pro12Ala polymorphism is associated with susceptibility to NAFLD in East Asians, but not in European populations.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a common disorder characterized by an increase in hepatic fat content occurring in individuals with minimal or no alcohol consumption, and who have no other cause for liver disease (Brunt, 2010). Rheumatoid arthritis (RA) is a chronic inflammatory disease of predominantly synovial joints that causes significant morbidity and shortens life expectancy (McInnes and Schett, 2011). Psoriatic arthritis (PsA) is an inflammatory arthritis that occurs in 10–40% of patients with psoriasis. Although the etiology of these diseases is not fully understood, interactions between a susceptible genetic background and environmental factors have been suggested (Choi et al., 2006).
Peroxisome proliferator-activated receptor-γ (PPARγ) is a nuclear receptor that regulates proinflammatory genes, insulin sensitivity, and lipid metabolism (Beaven and Tontonoz, 2006). PPARγ increases the expression of lipoprotein lipase, an enzyme that serves to partition fat to adipocytes, limiting fatty acid flux to the liver (Hui et al., 2008). The PPARγ gene, which is located on chromosome 3, has been known to be linked to NAFLD (Gavrilova et al., 2003). The C/G polymorphism (rs1801282), a Pro-to-Ala exchange that leads to the substitution of proline (Pro) with alanine (Ala) at codon 12, was found to be associated with reductions in both DNA binding and transcriptional activity, and as a consequence, the encoded Ala-allele form has a heavily reduced function (Deeb et al., 1998). This Pro12Ala variant has been associated with increased insulin sensitivity and a lower body mass and protection from type 2 diabetes (Knouff and Auwerx, 2004; Tonjes and Stumvoll, 2007).
The PPARγ Pro12Ala polymorphism has been studied in the context of NAFLD, RA, and PsA. However, published results on the genetic associations of the PPARγ Pro12Ala polymorphism are controversial and inconclusive (Butt et al., 2006; El-Sohemy et al., 2006; Terauchi, 2007; Pan et al., 2009; Dongiovanni et al., 2010; Rey et al., 2010; Cao et al., 2012; Bowes et al., 2012; Yang et al., 2012). This may be due to small sample sizes, low statistical power, and/or clinical heterogeneity. To overcome the limitations of individual studies, resolve inconsistencies, and reduce the likelihood that random errors are responsible for false-positive or false-negative associations, we employed a meta-analysis to further characterize the association (Nath et al., 2005; Lee et al., 2005, 2006) and to investigate whether the PPARγ Pro12Ala polymorphism contributes to susceptibility to NAFLD, RA, and PsA.
Materials and Methods
Identification of eligible studies and data extraction
A search of the literature for studies that examined the association between the PPARγ Pro12Ala polymorphism and NAFLD, RA, PsA was conducted. We utilized the MEDLINE and EMBASE citation indices to identify articles published through December 2012 in which the PPARγ Pro12Ala polymorphism was identified in NAFLD patients and controls. In addition, all references mentioned in the identified articles were reviewed to identify studies not indexed by the electronic databases. The following keywords and subject terms were used in the search: “peroxisome proliferator-activated receptor-γ,” “PPARγ,” “fatty liver,” “rheumatoid arthritis,” and “psoriatic arthritis.” Studies were included in the analysis if (1) they were case–control studies, (2) contained genotype data, (3) included sufficient data to calculate odds ratios (ORs), and (4) the study included patients diagnosed with NAFLD, RA, and PsA based on the diagnostic criteria. No language restriction was applied. We excluded the following: (1) studies that included overlapping data; (2) studies in which the number of genotypes could not be ascertained; (3) studies in which family members were studied because their analysis was based on linkage considerations; and (4) studies in which the genotype distribution in controls was not consistent with the Hardy–Weinberg equilibrium (HWE), as deviation from the HWE among controls suggests the possibility of bias during control selection or genotyping errors. Data regarding the methods and results of meta-analysis were extracted from the original studies by two independent reviewers. Discrepancy between the reviewers was resolved by consensus or a third reviewer. The following information was extracted from each identified study: author, year of publication, ethnicity of the study population, demographics, numbers of cases and controls, and the frequencies of the genotypes and alleles of the PPARγ Pro12Ala polymorphism.
Evaluation of publication bias
The chi-square test was used to determine if the observed genotype frequencies in controls conformed to HWE. Funnel plots are often used to detect publication bias, but require a range of studies of varying sizes and subjective judgments. Therefore, we evaluated publication bias using the Egger's linear regression test (Egger et al., 1997). The Egger's linear regression test measures funnel plot asymmetry on a natural logarithmic scale of ORs.
Evaluation of statistical associations
We performed meta-analyses using (1) allelic contrast (Ala/Ala vs. Pro/Pro), (2) dominant (Ala/Ala+Ala/Pro vs. Pro/Pro), and (3) overdominant (Ala/Ala+Pro/Pro vs. Ala/Pro) models. We could not perform a meta-analysis of recessive and additive models because the frequency of the Ala/Ala genotype was very low. Point estimates of risks, ORs, and 95% confidence intervals (CI) were estimated for each study. In addition, within- and between-study variations and heterogeneities were assessed using Cochran's Q-statistic. The Cochran's Q-statistic test assesses the null hypothesis that all studies evaluated the same effect. The effect of heterogeneity was quantified using I2 with a range between 0% and 100%, representing the proportion of between-study variability attributable to heterogeneity rather than to chance (Higgins and Thompson, 2002). I2 values of 25%, 50%, and 75% were nominally assigned as low, moderate, and high estimates, respectively. The fixed-effects model assumes that a genetic factor has a similar effect on disease susceptibility across all studies investigated and that observed variations among studies are caused by chance alone (Egger et al. 1997). The random-effects model assumes that different studies show substantial diversity and assesses both the within-study sampling error and between-study variance (DerSimonian and Laird, 1986). When study groups are homogeneous, the two models are similar. If the study groups lack homogeneity, the random-effects model usually provides wider CIs than the fixed-effects model. The random-effects model is most appropriate in the presence of significant between-study heterogeneity (DerSimonian and Laird, 1986). Statistical manipulations were undertaken using the Comprehensive Meta-Analysis program (Biosta, Englewood, NJ). The power of each study was computed as the probability of detecting an association between the PPARγ Pro12Ala polymorphism and diseases using a significance level of 0.05 and assuming an OR of 1.5 (small effect size). Power analysis was performed using the statistical program G*Power (www.psycho.uni-duesseldorf.de/aap/projects/gpower).
Results
Studies included in the meta-analysis
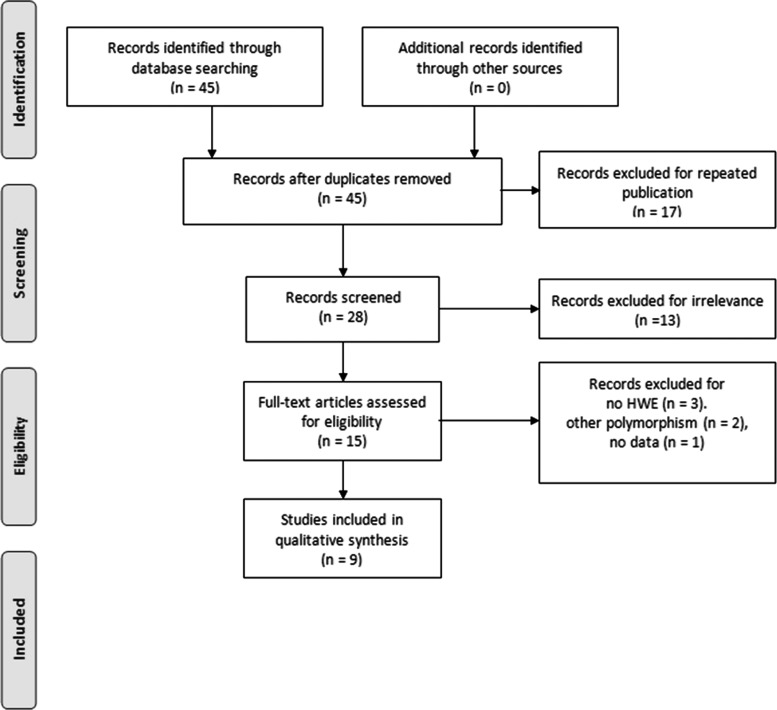
Forty-five studies were identified by electronic and manual searches, 15 of which were selected for full-text review based on title and abstract details (Butt et al., 2006; El-Sohemy et al., 2006; Terauchi, 2007; Hui et al., 2008; Pan et al., 2009; Dongiovanni et al., 2010; Gupta et al., 2010; Rey et al., 2010; Zhou et al., 2010; Cao et al., 2012; Bowes et al., 2012; Gawrieh et al., 2012; Yang et al., 2012; Bhatt et al., 2013; Jalil et al., 2013). Six studies were excluded because one contained no data (Gawrieh et al., 2012), two had other polymorphism data (Hui et al., 2008; Zhou et al., 2010), and three had a genotype distribution in controls that was not in HWE (Gupta et al., 2010; Bhatt et al., 2013; Jalil et al., 2013). Thus, a total of nine studies met our inclusion criteria (Butt et al., 2006; El-Sohemy et al., 2006; Terauchi, 2007; Pan et al., 2009; Dongiovanni et al., 2010; Rey et al., 2010; Cao et al., 2012; Bowes et al., 2012; Yang et al., 2012) (Fig. 1). These studies comprised five on NAFLD, two on RA, and two on PsA. They consisted of four European and five East Asian studies. In total, the studies included 8082 cases and 3790 controls. Selected details of the individual studies are summarized in Table 1. The statistical powers of these five studies ranged from 40.5% to 100%. Four of the studies had a statistical power exceeding 80% (Table 1).
FIG. 1.
Study flowchart.
Table 1.
Details of the Individual Studies Included in the Meta-Analysis
| Numbers | Case | Control | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Country | Ethnicity | Disease | Case | Control | Pro/Pro | Pro/Ala | Ala/Ala | Pro/Pro | Pro/Ala | Ala/Ala | Association pa | Power (%)b |
| Cao, 2012 | China | East Asian | NAFLD | 169 | 699 | 159 | 10 | 0 | 656 | 43 | 0 | 0.910 | 83.7 |
| Yang, 2012 | China | East Asian | NAFLD | 436 | 467 | 406 | 30 | 0 | 409 | 58 | 0 | 0.007 | 85.1 |
| Rey, 2010 | Germany | European | NAFLD | 263 | 363 | 206 | 48 | 9 | 280 | 77 | 6 | 0.878 | 70.6 |
| Dongiovanni, 2010 | Italy | European | NAFLD | 202 | 346 | 166 | 33 | 3 | 295 | 50 | 1 | 0.217 | 64.8 |
| Qing, 2007 | China | East Asian | NAFLD | 155 | 141 | 142 | 13 | 0 | 130 | 11 | 0 | 0.857 | 40.5 |
| Pan, 2009 | China | East Asian | RA | 421 | 207 | 411 | 10 | 0 | 190 | 17 | 0 | 0.002 | 70.7 |
| El-Sohenmy, 2006 | China | East Asian | RA | 473 | 400 | 450 | 23 | 0 | 374 | 25 | 1 | 0.241 | 84.0 |
| Bowes, 2012 | United Kingdom | European | PsA | 5712 | 932 | 4416 | 1216 | 80 | 747 | 174 | 11 | 0.058 | 100 |
| Butt, 2006 | Canada | European | PsA | 251 | 235 | 208 | 41 | 2 | 177 | 51 | 7 | 0.017 | 59.7 |
Allelic association.
Assuming an odds ratio of 1.5 (small effect size) at a level of significance of 0.05.
HWE, Hardy–Weinberg equilibrium; NAFLD, nonalcoholic fatty liver disease; RA, rheumatoid arthritis; PsA, psoriatic arthritis.
Frequencies of the Ala allele of the PPARγ Pro12Ala polymorphism in different ethnic groups
The mean frequency of the Ala allele of the PPARγ Pro12Ala polymorphism was 7.36% among all normal controls, and East Asians had a lower Ala allele prevalence than Europeans (4.1% vs. 10.7%,) (Table 2).
Table 2.
Prevalence of the Ala Allele of the PPARγ Pro12Ala Polymorphism
| Population | No. of studies | Numbers | Ala allele (%) | ||
|---|---|---|---|---|---|
| Case | Control | Case | Control | ||
| European | 4 | 6428 | 1876 | 11.9 | 10.7 |
| East Asian | 5 | 1654 | 1914 | 2.6 | 4.1 |
| Overall | 9 | 8082 | 3790 | 10.1 | 17.4 |
Meta-analysis of the association between the PPARγ Pro12Ala polymorphism and NAFLD
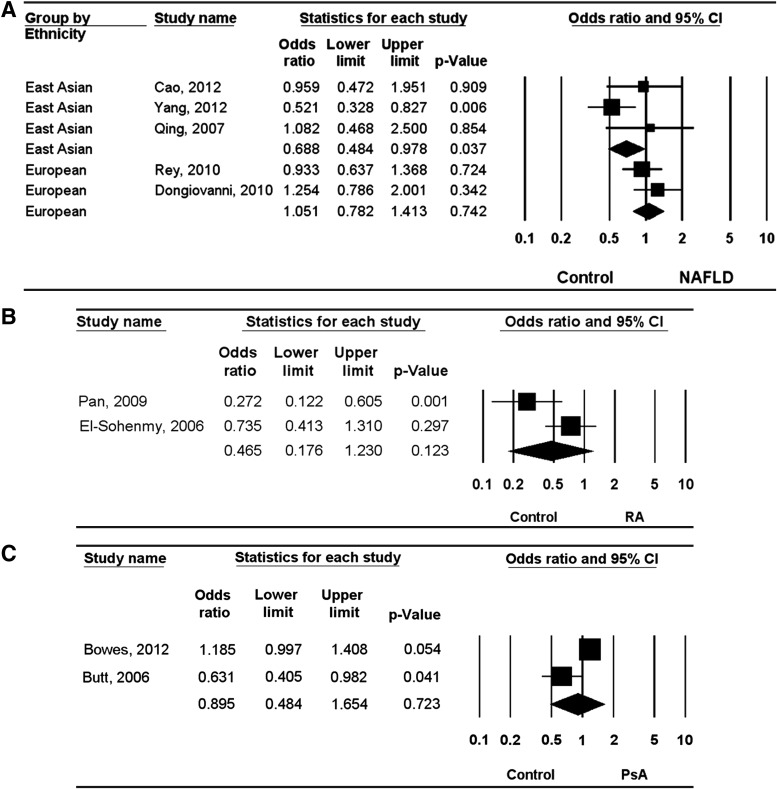
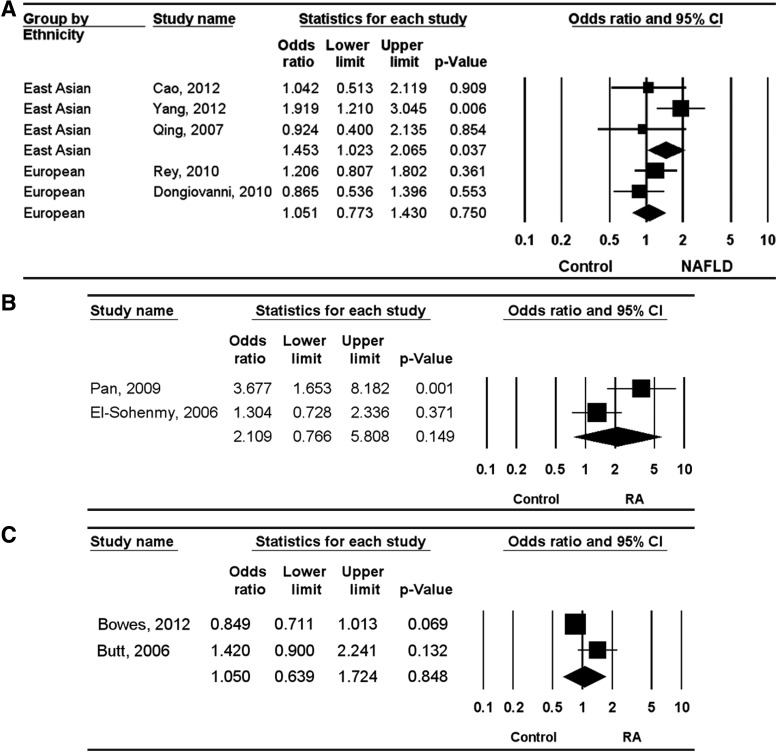
A meta-analysis of all NAFLD patients and of each ethnic group was performed. The meta-analysis revealed no association between the Ala allele and NAFLD (OR=0.936, 95% CI=0.672–1.302, p=0.693) (Table 3). However, stratification by ethnicity indicated a significant association between the Ala allele of the PPARγ Pro12Ala polymorphism and NAFLD in East Asians (OR=0.700, 95% CI=0.496–0.987, p=0.042), but not in Europeans (OR=1.128, 95% CI=0.863–1.475, p=0.378) (Table 3). Analysis using the dominant model showed the same Ala allele pattern in East Asians and Europeans (OR=0.688, 95% CI=0.484–0.978, p=0.037; OR=1.051, 95% CI=0.782–1.413, p=0.742), demonstrating a significant association between the Ala allele and NAFLD in East Asians (Table 3 and Fig. 2). Meta-analysis revealed no association between the Ala/Ala+Pro/Pro genotype of the PPARγ Pro12Ala polymorphism and NAFLD (OR=1.210, 95% CI=0.960–1.525, p=0.107) (Table 3). However, stratification by ethnicity indicated an association between the Ala/Ala+Pro/Pro genotype and NAFLD in East Asians (OR=1.453, 95% CI=1.023–2.065, p=0.037), but not in Europeans (OR=1.051, 95% CI=0.773–1.430, p=0.750) (Table 3 and Fig. 3).
Table 3.
Meta-Analysis of the Association Between the PPARγ Pro12Ala Polymorphism and NAFLD, RA, and PsA
| Test of association | Test of heterogeneity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Polymorphism | Population | Disease | No. of studies | OR | 95% CI | p-value | Model | p-value | I2 |
| Ala vs. Pro allele | Overall | NAFLD | 5 | 0.936 | 0.672–1.302 | 0.693 | R | 0.074 | 53.1 |
| European | NAFLD | 2 | 1.128 | 0.863–1.475 | 0.378 | F | 0.380 | 0 | |
| East Asian | NAFLD | 3 | 0.700 | 0.496–0.987 | 0.042 | F | 0.206 | 36.7 | |
| East Asian | RA | 2 | 0.467 | 0.188–1.161 | 0.101 | R | 0.060 | 71.7 | |
| European | PsA | 2 | 0.869 | 0.465–1.627 | 0.662 | R | 0.004 | 88.1 | |
| Ala/Ala+Ala/Pro vs. Pro/Pro (Dominant) | Overall | NAFLD | 5 | 0.882 | 0.703–1.105 | 0.275 | F | 0.110 | 46.9 |
| European | NAFLD | 2 | 1.051 | 0.782–1.413 | 0.742 | F | 0.337 | 0 | |
| East Asian | NAFLD | 3 | 0.688 | 0.484–0.978 | 0.037 | F | 0.186 | 40.4 | |
| East Asian | RA | 2 | 0.465 | 0.176–1.230 | 0.323 | R | 0.048 | 74.3 | |
| European | PsA | 2 | 0.895 | 0.484–1.654 | 0.723 | R | 0.009 | 85.2 | |
| Ala/Ala+Pro/Pro vs. Ala/Pro (Overdominant) | Overall | NAFLD | 5 | 1.210 | 0.960–1.525 | 0.107 | F | 0.178 | 36.4 |
| European | NAFLD | 2 | 1.051 | 0.773–1.430 | 0.750 | F | 0.297 | 7.96 | |
| East Asian | NAFLD | 3 | 1.453 | 1.023–2.065 | 0.037 | F | 0.186 | 40.4 | |
| East Asian | RA | 2 | 2.109 | 0.766–5.808 | 0.149 | R | 0.040 | 76.2 | |
| European | PsA | 2 | 1.050 | 0.639–1.724 | 0.848 | R | 0.039 | 76.4 | |
OR, odds ratio; CI, confidence interval; F, fixed-effects model; R, random-effects model.
FIG. 2.
Odds ratios (Ors) and 95% confidence intervals (CIs) of individual studies and pooled data for the association between the Pro12Ala polymorphism of peroxisome proliferator-activated receptor-gamma (PPARγ) and nonalcoholic fatty liver disease (NAFLD) (A), rheumatoid arthritis (RA) (B), and psoriatic arthritis (PsA) (C) under dominant model in Europeans and East Asians.
FIG. 3.
ORs and 95% CIs of individual studies and pooled data for the association between the Pro12Ala polymorphism of PPARγ and NAFLD (A), RA (B), and PsA (C) under overdominant model in Europeans and East Asians.
Meta-analysis of the association between the PPARγ Pro12Ala polymorphism and RA, PsA
The meta-analysis revealed no association between the Ala allele and RA in East Asians (OR=0.467, 95% CI=0.188–1.161, p=0.101) (Table 3). Analysis using the dominant and the overdominant models showed the same Ala allele pattern in East Asians (OR=0.465, 95% CI=0.176–1.230, p=0.123; OR=2.109, 95% CI=0.766–5.808, p=0.149) (Table 3 and Figs. 2 and 3). The meta-analysis revealed no association between the Ala allele and PsA in Europeans (OR=0.869, 95% CI=0.465–1.627, p=0.662) (Table 3). Analysis using the dominant and the overdominant models showed no association between the PPARγ Pro12Ala polymorphism and PsA in Europeans (OR=0.895, 95% CI=0.484–1.654, p=0.723; OR=1.050, 95% CI=0.639–1.724, p=0.848) (Table 3 and Figs. 2 and 3).
Heterogeneity and publication bias
Between-study heterogeneity was found during meta-analyses of RA, PsA, and in the meta-analysis of the Ala allele over all groups in NAFLD (Table 3). However, the heterogeneity was resolved by an ethnicity-specific meta-analysis in NAFLD (Table 3). Publication bias causes a disproportionate number of positive studies and poses a problem for meta-analyses. Evidence of publication bias was not found in the meta-analyses of the PPARγ Pro12Ala polymorphism (Egger's regression test p-values>0.1).
Discussion
In this meta-analysis, we combined evidence of the associations between the PPARγ Pro12Ala polymorphism and susceptibility to NAFLD, RA, and PsA. Meta-analysis revealed no association between the Ala allele and NAFLD. However, stratification by ethnicity indicated a significant association between the Ala allele of the PPARγ Pro12Ala polymorphism and NAFLD in East Asians, but not in Europeans. European populations demonstrated a higher frequency of the Ala allele, while East Asians showed a lower frequency of the Ala allele. The differences in the Ala allele among ethnic groups may indicate an ethnic difference in the association between the PPARγ Pro12Ala polymorphism and susceptibility to NAFLD between Europeans and East Asians. However, the meta-analysis revealed no association between the PPARγ Pro12Ala polymorphism and RA and PsA.
PPARγ increases the expression of genes that promote fatty acid storage, whereas it represses genes that induce lipolysis in adipocytes. In patients with NSFLD, hepatic expression of PPARγ is involved in insulin sensitivity, triglyceride clearance, and hepatic steatosis (Lee et al., 2006). An association between positivity for the Ala variant and insulin resistance, type 2 diabetes, and obesity has been reported (Knouff and Auwerx, 2004, Tonjes and Stumvoll, 2007). This association may be explained by the lower activity of the Ala variant in adipose tissue, favoring insulin resistance and, potentially, the flux of FFAs to the liver and NAFLD (Deeb et al., 1998). Our meta-analysis suggested a protective role for the Ala allele of the PPARγ Pro12Ala polymorphism in NAFLD risk. It has been reported that oxidative stress resulting from overproduction of reactive oxidant species (ROS) in over-nutrition conditions contributes to the development and progression of NAFLD (Dimitrova-Shumkovska et al., 2010). Carriers of the PPARγ Ala allele showed increased resistance to oxidative stress (Thamer et al., 2002). The enhanced oxidative stress tolerance of the Ala carriers may imply that the Pro/Pro genotype increases the production of free radicals and ROS (Luo et al., 2008).
The results of this meta-analysis differ from those of a previous meta-analysis on the relationship between the PPARγ Pro12Ala polymorphism and NAFLD risk by Dongiovanni and Valenti (2013). Compared with the Dongiovanni et al. study, the present study included two new studies (Terauchi, 2007; Cao et al., 2012), but excluded two studies as they were not consistent with HWE, and only studies providing genotype data were included (Gupta et al., 2010; Gawrieh et al., 2012). In addition, an ethnicity-specific meta-analysis was conducted for Europeans and East Asians in our meta-analysis. In contrast with the results of the previous study, this meta-analysis revealed an association between the PPARγ Pro12Ala polymorphism and NAFLD in an East Asian population.
The present study has some limitations that should be considered. First, heterogeneity and confounding factors may have distorted the analysis. Second, other PPARγ polymorphisms capable of affecting the PPARγ activity could also be associated with NAFLD. However, the limited amount of data available prevented further meta-analysis. Third, there are varying levels of disease severity, and the NAFLD severity level was unclear. Further research is required to examine whether an association exists between the PPARγ Pro12Ala polymorphism and the activity or clinical features of the disease. Fourth, we included data from European and East Asian patients in our meta-analysis, and thus, our ethnicity-associated results are applicable only to these ethnic groups. Fifth, the number of studies included in this meta-analysis was small, especially in subgroup by ethnicity, and RA and PsA. There were two European studies and three East Asian studies in the subgroup analysis by ethnicity and for each, two studies in RA and PSA. The study numbers may not be sufficient to provide a conclusive result.
In conclusion, this meta-analysis of the PPARγ Pro12Ala polymorphism demonstrated that the PPARγ Pro12Ala polymorphism is associated with susceptibility to NAFLD in East Asians, but not in Europeans. Accordingly, our findings support the notion that the PPARγ Pro12Ala polymorphism plays a role in the pathogenesis of NAFLD. However, meta-analysis did not find an association between the PPARγ Pro12Ala polymorphism and RA, PsA. Since the frequency of the PPARγ Pro12Ala polymorphism is different among various ethnic groups, large-scale studies in populations with different ethnicities are needed to explore the relationships between the polymorphisms of the PPAR gene and the pathogenesis of NAFLD, RA, and PsA.
Acknowledgment
This study was supported in part by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (HI12C1834).
Author Disclosure Statement
The authors have no financial or nonfinancial conflicts of interest to declare.
References
- Beaven SW, Tontonoz P. (2006) Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu Rev Med 57:313–329 [DOI] [PubMed] [Google Scholar]
- Bhatt SP, Nigam P, Misra A, et al. (2013) Association of peroxisome proliferator activated receptor-gamma gene with non-alcoholic fatty liver disease in Asian Indians residing in north India. Gene 512:143–147 [DOI] [PubMed] [Google Scholar]
- Bowes J, Ho P, Flynn E, et al. (2012) Investigation of IL1, VEGF, PPARG and MEFV genes in psoriatic arthritis susceptibility. Ann Rheum Dis 71:313–314 [DOI] [PubMed] [Google Scholar]
- Brunt EM. (2010) Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 7:195–203 [DOI] [PubMed] [Google Scholar]
- Butt C, Gladman D, Rahman P. (2006) PPAR-gamma gene polymorphisms and psoriatic arthritis. J Rheumatol 33:1631–1633 [PubMed] [Google Scholar]
- Cao CY, Li YY, Zhou YJ, et al. (2012) The C-681G polymorphism of the PPAR-gamma gene is associated with susceptibility to non-alcoholic fatty liver disease. Tohoku J Exp Med 227:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Rho YH, Ji JD, et al. (2006) Genome scan meta-analysis of rheumatoid arthritis. Rheumatology (Oxford) 45:166–170 [DOI] [PubMed] [Google Scholar]
- Deeb SS, Fajas L, Nemoto M, et al. (1998) A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet 20:284–287 [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188 [DOI] [PubMed] [Google Scholar]
- Dimitrova-Shumkovska J, Veenman L, Ristoski T, et al. (2010) Chronic high fat, high cholesterol supplementation decreases 18 kDa Translocator Protein binding capacity in association with increased oxidative stress in rat liver and aorta. Food Chem Toxicol 48:910–921 [DOI] [PubMed] [Google Scholar]
- Dongiovanni P, Rametta R, Fracanzani AL, et al. (2010) Lack of association between peroxisome proliferator-activated receptors alpha and gamma2 polymorphisms and progressive liver damage in patients with non-alcoholic fatty liver disease: a case control study. BMC Gastroenterol 10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongiovanni P, Valenti L. (2013) Peroxisome proliferator-activated receptor genetic polymorphisms and nonalcoholic Fatty liver disease: any role in disease susceptibility? PPAR Res 2013:452061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, Phillips AN. (1997) Meta-analysis: principles and procedures. BMJ 315:1533–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sohemy A, Cornelis MC, Park YW, Bae SC. (2006) Catalase and PPARgamma2 genotype and risk of rheumatoid arthritis in Koreans. Rheumatol Int 26:388–392 [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Haluzik M, Matsusue K, et al. (2003) Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 278:34268–34276 [DOI] [PubMed] [Google Scholar]
- Gawrieh S, Marion MC, Komorowski R, et al. (2012) Genetic variation in the peroxisome proliferator activated receptor-gamma gene is associated with histologically advanced NAFLD. Dig Dis Sci 57:952–957 [DOI] [PubMed] [Google Scholar]
- Gupta AC, Chaudhory AK, Sukriti , Pande C, Sakhuja P, et al. (2010) Peroxisome proliferators-activated receptor gamma2 Pro12Ala variant is associated with body mass index in non-alcoholic fatty liver disease patients. Hepatol Int 5:575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558 [DOI] [PubMed] [Google Scholar]
- Hui Y, Yu-Yuan L, Yu-Qiang N, et al. (2008) Effect of peroxisome proliferator-activated receptors-gamma and co-activator-1alpha genetic polymorphisms on plasma adiponectin levels and susceptibility of non-alcoholic fatty liver disease in Chinese people. Liver Int 28:385–392 [DOI] [PubMed] [Google Scholar]
- Jalil SF, Ahmed I, Gauhar Z, et al. (2013) Association of Pro12Ala (rs1801282) variant of PPAR gamma with Rheumatoid Arthritis in a Pakistani population. Rheumatol Int [Epub ahead of print]; DOI: 10.1007/s00296-013-2768-2 [DOI] [PubMed] [Google Scholar]
- Knouff C, Auwerx J. (2004) Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev 25:899–918 [DOI] [PubMed] [Google Scholar]
- Lee YH, Rho YH, Choi SJ, et al. (2006) Association of TNF-alpha -308 G/A polymorphism with responsiveness to TNF-alpha-blockers in rheumatoid arthritis: a meta-analysis. Rheumatol Int 27:157–161 [DOI] [PubMed] [Google Scholar]
- Lee YH, Witte T, Momot T, et al. (2005) The mannose-binding lectin gene polymorphisms and systemic lupus erythematosus: two case-control studies and a meta-analysis. Arthritis Rheum 52:3966–3974 [DOI] [PubMed] [Google Scholar]
- Luo W, Cao J, Li J, He W. (2008) Adipose tissue-specific PPARgamma deficiency increases resistance to oxidative stress. Exp Gerontol 43:154–163 [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–2219 [DOI] [PubMed] [Google Scholar]
- Nath SK, Harley JB, Lee YH. (2005) Polymorphisms of complement receptor 1 and interleukin-10 genes and systemic lupus erythematosus: a meta-analysis. Hum Genet 118:225–234 [DOI] [PubMed] [Google Scholar]
- Pan XF, Song XB, Wang LL, et al. (2009) [Association of the Pro12Ala polymorphism in peroxisome proliferators activated receptor-gamma gene with rheumatoid arthritis in Sichuan Province of China]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 26:87–90 [DOI] [PubMed] [Google Scholar]
- Rey JW, Noetel A, Hardt A, et al. (2010) Pro12Ala polymorphism of the peroxisome proliferator-activated receptor gamma2 in patients with fatty liver diseases. World J Gastroenterol 16:5830–5837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi Y. (2007) [PPARgamma and metabolic syndrome]. Rinsho Byori 55:447–451 [PubMed] [Google Scholar]
- Thamer C, Haap M, Volk A, et al. (2002) Evidence for greater oxidative substrate flexibility in male carriers of the Pro 12 Ala polymorphism in PPARgamma2. Horm Metab Res 34:132–136 [DOI] [PubMed] [Google Scholar]
- Tonjes A, Stumvoll M. (2007) The role of the Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma in diabetes risk. Curr Opin Clin Nutr Metab Care 10:410–414 [DOI] [PubMed] [Google Scholar]
- Yang Z, Wen J, Li Q, et al. (2012) PPARG gene Pro12Ala variant contributes to the development of non-alcoholic fatty liver in middle-aged and older Chinese population. Mol Cell Endocrinol 348:255–259 [DOI] [PubMed] [Google Scholar]
- Zhou YJ, Li YY, Nie YQ, et al. (2010) Influence of polygenetic polymorphisms on the susceptibility to non-alcoholic fatty liver disease of Chinese people. J Gastroenterol Hepatol 25:772–777 [DOI] [PubMed] [Google Scholar]