Abstract
To assess sex disparities in AIDS clinical and laboratory outcomes in the highly active antiretroviral therapy (HAART) era we conducted a systematic review of the published literature on mortality, disease progression, and laboratory outcomes among persons living with HIV and starting HAART. We performed systematic PubMed and targeted bibliographic searches of observational studies published between January, 1998, and November, 2013, that included persons starting HAART and reported analyses of mortality, progression to AIDS, or virologic or immunologic treatment outcomes by sex. Risk ratios (relative risks, odd ratios, and hazard ratios) and 95% confidence intervals were obtained. Sixty-five articles were included in this review. Thirty-nine studies were from North America and Europe and 26 were from Latin America, Asia, and Africa. Forty-four studies (68%) showed no statistically significant difference in risk of mortality, progression to AIDS, or virologic or immunologic treatment outcomes by sex. Decreased risk of death among females compared to males was observed in 24 of the 25 articles that included mortality analyses [pooled risk ratio 0.72 (95% confidence interval=0.69–0.75)], and decreased risk of death or AIDS was observed in 9 of the 13 articles that examined the composite outcome [pooled risk ratio=0.91 (0.84–0.98)]. There was no significant effect of sex on the risk of progression to AIDS [pooled risk ratio=1.15 (0.99–1.31)]. In this systematic review, females starting HAART appeared to have improved survival compared to males. However, this benefit was not associated with decreased progression to either AIDS or to differences in virologic or immunologic treatment outcomes.
Introduction
Since early in the HIV-1 epidemic, sex disparities in HIV-1 transmission, infection, and disease outcome have been examined and have often shown contradictory results. HIV-infected females tend to be younger, have lower HIV-1 RNA, and have higher CD4+ lymphocyte counts at the time of HIV-1 infection or entry into care than males.1–6 However, despite these favorable baseline characteristics, females do not consistently have lower rates of HIV-1 disease progression or death. Studies from the era before highly active antiretroviral therapy (HAART) showed mixed results: while some studies found increased rates of death among females compared with males, others found no difference in death or disease progression by sex.7–11
In the HAART era, HIV-1 disease progression and treatment outcomes by sex have also been inconsistent. A review article by Nicastri et al. in 2007 examined sex differences in HIV-1 outcomes among individuals receiving HAART.12 They reviewed 41 cohort studies from 2002 to 2005 that included data on the effect of sex on timing of HAART initiation, adverse events following treatment initiation, and clinical and laboratory outcomes. That review highlighted the heterogeneous, but largely nonsignificant, differences by sex. Of note, that review included only six studies form outside North America or Europe. In developing countries, females represent a larger proportion of persons living with HIV/AIDS and have had increased access to HAART in recent years. Additionally, the epidemic in North America and Europe has shifted over time with females representing a larger proportion of persons living with HIV/AIDS.13
This systemic review was performed to assess sex disparities in AIDS clinical and laboratory outcomes among persons starting HAART in both developed and developing countries.
Materials and Methods
Studies included in this systematic review were observational cohort studies of HIV-infected adults starting HAART that analyzed outcomes of death, progression to AIDS, virologic outcomes (viral response), or immunologic outcomes (CD4+ lymphocyte count response). A PubMed search of all articles published from January, 1998, though November, 2013, included MeSH terms of “HIV-1” or “Acquired Immunodeficiency Syndrome,” “prognosis” or “mortality” or “disease progression, ” and “gender” or “sex” or “male” or “female. ” Articles were limited to human studies written in English. Following the PubMed search, titles were reviewed and selected for abstract review, and from abstract review, selected for manuscript review-based meeting inclusion criteria and the absence of exclusion criteria. A targeted bibliographic review was also performed on selected articles to search for additional studies meeting inclusion criteria. All studies with risk ratio assessments (hazard ratios, odds ratios, relative risk ratios) for sex and clinical or laboratory outcomes that noted a statistical analysis by way of 95% confidence interval were included. Studies were excluded if they included only one sex group (since conclusions regarding disparities could not be obtained), if they did not include or report specific outcome data of sex analyses (i.e., risk ratios and corresponding confidence intervals), or if they included cohort participants who were not started on HAART. Randomized trials were excluded since clinical trial participants may not accurately reflect general epidemiologic patterns of all individuals infected with HIV-1.
Studies meeting inclusion criteria were reviewed. Particular attention was made to the studies' cohort location (North America and Europe or Latin America, Africa, and Asia), size of the cohort, and percentage of females in the cohort. Given the increased risk of mortality among HIV-infected individuals with anemia and the increased rates of anemia among females, inclusion of hemoglobin in multivariable analyses was also noted.14–16 For consistency, “males” and “females” were used in the reporting of results to indicate the biological definition of sex rather than the psychosocial, gender terminology of “men” and “women.”
Statistical analysis
To compare percentages of females included in studies by cohort geographic region and over time (year of publication), we used the Wilcoxon rank-sum test and univariable regression models, respectively. We compared risk assessments (hazard ratios, relative risk ratios, odds ratios) graphically with forest plot displays. Weighted-pooled risk ratios were calculated using meta-analysis statistics. For consistency, results from studies were assessed as the risk ratio of females compared to males, including confidence intervals. All statistical analyses and graphic displays were performed by Stata 12.1 (Stata Corporation, College Station, TX).
Results
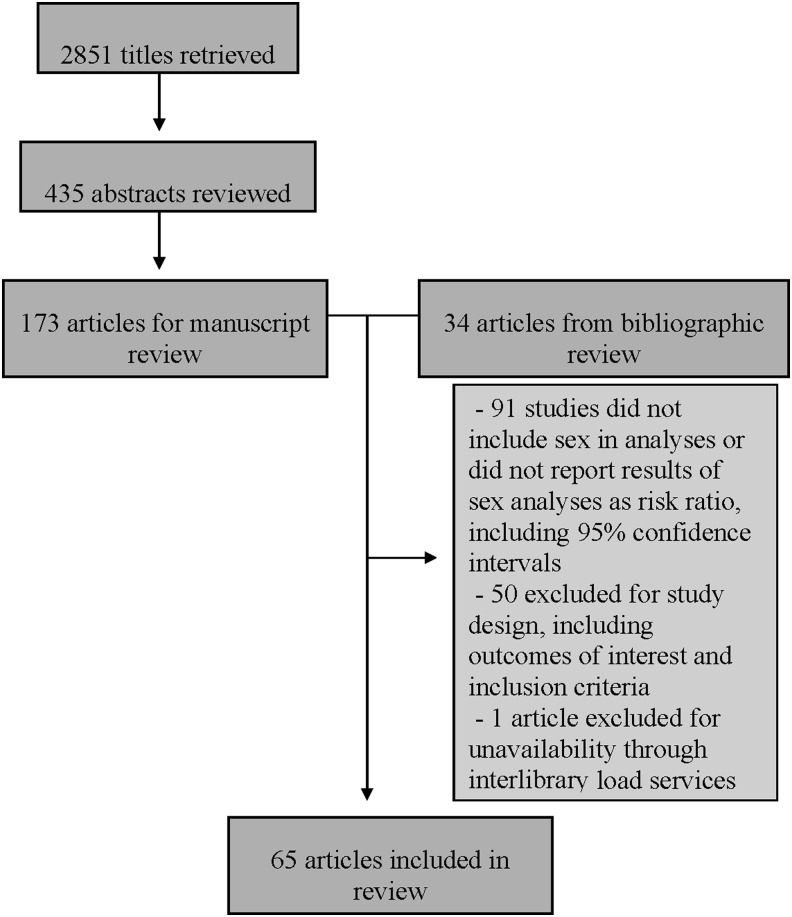
There were 2,851 articles identified in the PubMed search (Fig. 1). After reviewing titles for inclusion criteria, 435 were selected for abstract review. From those abstracts, 173 were selected for manuscript review. An additional 34 articles were identified from bibliographic review, yielding a total of 207 manuscripts reviewed. From those 207 articles, 141 met at least one exclusion criterion and were therefore excluded. One study was unavailable through interlibrary loan services and could not be retrieved.
FIG. 1.
Search results. PubMed Search Query: (HIV[mh] OR Acquired Immunodeficiency Syndrome[mh]) AND (Prognosis[mh] OR Mortality[mh] OR Disease Progression[mh]) AND (gender[tw] OR sex[mh] OR sex[tw] OR (male[mh] AND female[mh])) AND English[la] AND adult[mh] AND human[mh] AND 1998:2013[dp].
Of the 65 articles included in the review, 13 included sex as their primary variable of interest in the analyses. Studies generally included similar baseline variables of sex, age, HIV-1 transmission risk factor, and often CD4+ lymphocyte count and HIV-1 RNA in multivariable analyses. Eleven studies adjusted for hemoglobin in multivariable analyses. Fifty-one unique epidemiologic cohorts were included, 32 of which were multisite cohorts. Forty studies were from cohorts in North America (Canada and the United States) or Europe. There were 17 studies from African cohorts, five from Asian cohorts, one Latin American cohort, one from the ART-LINC cohort (a multicenter cohort from Africa, Asia, and Latin America), and one from the HIV Netherlands, Australia, and Thailand Collaboration cohort. Cohorts from North America and Europe had smaller percentages of females compared to those from Latin America, Africa, and Asia (median percentage of females 22.6 vs. 54.3%, p<0.001). The percentage of females included in study cohorts did not statistically change over time among studies from North America and Europe (0.97% per year of publication, p=0.07). Forty-one studies examined the clinical outcomes of progression to AIDS and/or death; 28 examined virologic and/or immunologic outcomes. None of the studies included transgender or transsexual individuals.
Clinical outcomes: mortality and disease progression
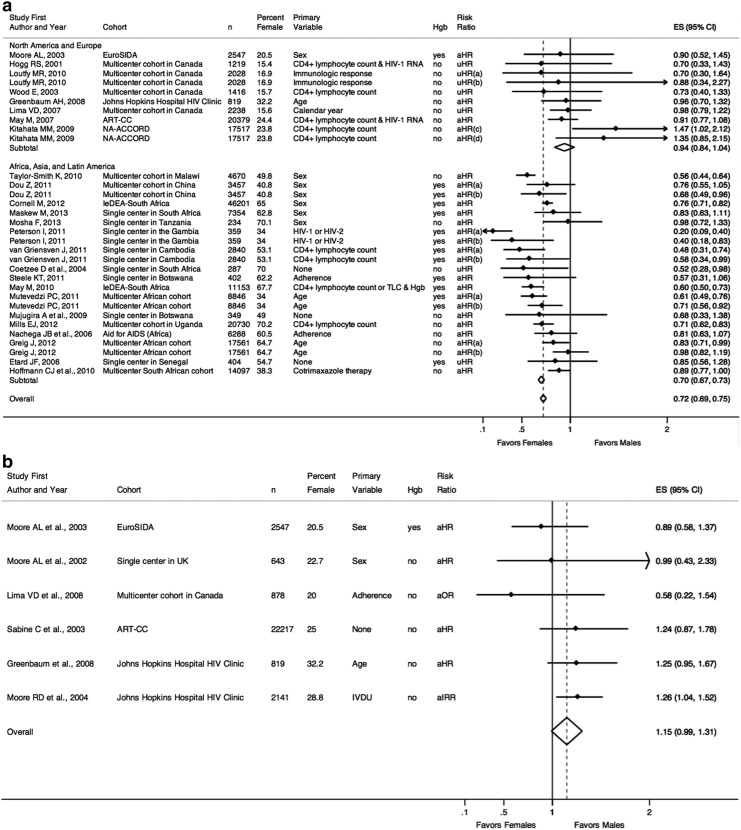
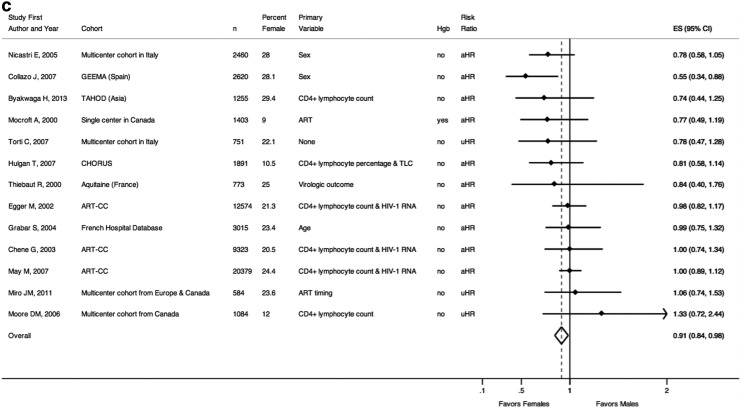
Figure 2a–c includes studies that examined the outcomes of death and/or AIDS by sex.5,17–56 Figure 2a displays the studies that examined risk of death (stratified by global region), Fig. 2b includes those that examined risk of AIDS, and Fig. 2c includes those that examined risk of the composite outcome of AIDS or death.
FIG. 2.
(a) Risk of death. (a) “Early” mortality following HAART initiation, as defined by the study. (b) “Late” mortality following HAART initiation, as defined by the study. (c) Mortality among patients with CD4+ lymphocyte count 351–500 cells/μl at HAART initiation. (d) Mortality among patients with CD4+ lymphocyte count >500 cells/μl at HAART initiation. n, number of individuals in the study cohort; Hgb, hemoglobin (as a marker for anemia), indicated as whether or not included in multivariable analyses; uHR, univariable hazard ratio; aHR, adjusted hazard ratio from multivariable analyses; ES, effect size of risk ratio, reported as comparing females to males; 95% CI, 95% confidence interval; Favors Females, a risk ratio less than one indicates a decreased risk of death for females compared to males; Favors Males, a risk ratio greater than one indicates an increased risk of death for females compared to males. (b) Risk of AIDS. n, number of individuals in the study cohort; Hgb, hemoglobin (as a marker for anemia), indicated as whether or not included in multivariable analyses; aIRR, adjusted incidence rate ratio from multivariable analyses; aHR, adjusted hazard ratio from multivariable analyses; aOR, adjusted odds ratio from multivariable analyses; ES, effect size of risk ratio, reported as comparing females to males; 95% CI, 95% confidence interval; Favors Females, a risk ratio less than one indicates a decreased risk of AIDS for females compared to males; Favors Males, a risk ratio greater than one indicates an increased risk of AIDS for females compared to males. (c) Risk of AIDS or death. n, number of individuals in the study cohort; Hgb, hemoglobin (as a marker for anemia), indicated as whether or not included in multivariable analyses; TLC, total lymphocyte count; uHR, univariable hazard ratio; aHR, adjusted hazard ratio from multivariable analyses; ES, effect size of risk ratio, reported as comparing females to males; 95% CI, 95% confidence interval; Favors Females, a risk ratio less than one indicates a decreased risk of AIDS or death for females compared to males; Favors Males, a risk ratio greater than one indicates an increased risk of AIDS or death for females compared to males.
Figure 2a reports risk of death estimates from studies stratified by region. The overall pooled risk ratio for risk of death from the 25 studies demonstrated a statistically significant decreased risk of death for females compared to males [pooled risk ratio=0.72 (95% confidence interval=0.69–0.75)]. This association was particularly driven by studies from developing countries [pooled risk ratio=0.70 (0.69–0.75)], which included results of “early” and “late” mortality following HAART initiation. Of the studies from North America and Europe, only two point estimates for adjusted hazards ratios (aHR) indicated increased risk of death for females. A large study of patients from the NA-ACCORD cohort examined outcomes of early (CD4+ lymphocyte count 351–500 cells/μl or CD4+ lymphocyte count >500 cells/μl) versus deferred (waiting for CD4+ lymphocyte count to fall below either threshold) initiation of HAART.26 Among individuals who started HAART with CD4+ lymphocyte count 351–500 cells/μl, females had a 47% increased risk of death [aHR=1.47 (1.02–2.12)], though sex was no longer statistically significant when hepatitis C virus infection and injection drug use history were included in multivariable models (numeric data not provided). Among those with CD4+ lymphocyte count >500 cells/μl at HAART initiation, females again had an increased risk of death, though not statistically significant [aHR=1.35 (0.85–1.32)]. Thus, the only study that found a statistically significant increased risk of mortality for females found these results to no longer be significant when analyses accounted for additional baseline variables.
Figure 2b lists the studies that examined progression to AIDS. All six studies included cohorts from developed regions. The pooled risk ratio for risk of AIDS demonstrated a slightly increased risk of AIDS for females but was not statistically significant [pooled risk ratio=1.15 (0.99–1.31)]. Two studies specifically looked at sex as the primary variable, both of which yielded an aHR of less than one (indicating a decreased risk of AIDS progression for females), though neither was statistically significant.5,28 Of the studies whose point estimates were greater than one, one study from the Johns Hopkins Hospital clinic cohort that examined HIV-1 outcomes and substance abuse found a statistically significantly increased risk of AIDS among females compared to males.30
Lastly, Fig. 2c lists the results from those studies that used a composite outcome of AIDS or death. Assessed together, risk of AIDS or death was slightly decreased for females compared to males [pooled risk ratio=0.91 (0.84–0.98)]. Of these 13 studies, only one was from a developing region.55 Nine of the studies had risk ratios less than one (indicating decreased risk of AIDS or death for females). Only two had risk ratios indicating increased risk for females. One multicenter study from Spain demonstrated a 45% decreased risk of AIDS or death for females [aHR=0.55 (0.34–0.88)].31 In this study, females tended to be older at baseline and had a higher CD4+ lymphocyte count and lower HIV-1 RNA. One study from British Columbia, Canada, demonstrated an increased risk of AIDS or death for females compared to males starting HAART; however, this risk was not statistically significant and was assessed only in univariable analysis [univariable HR=1.33 (0.72–2.44)].
Laboratory outcomes: virologic and immunologic response to HAART
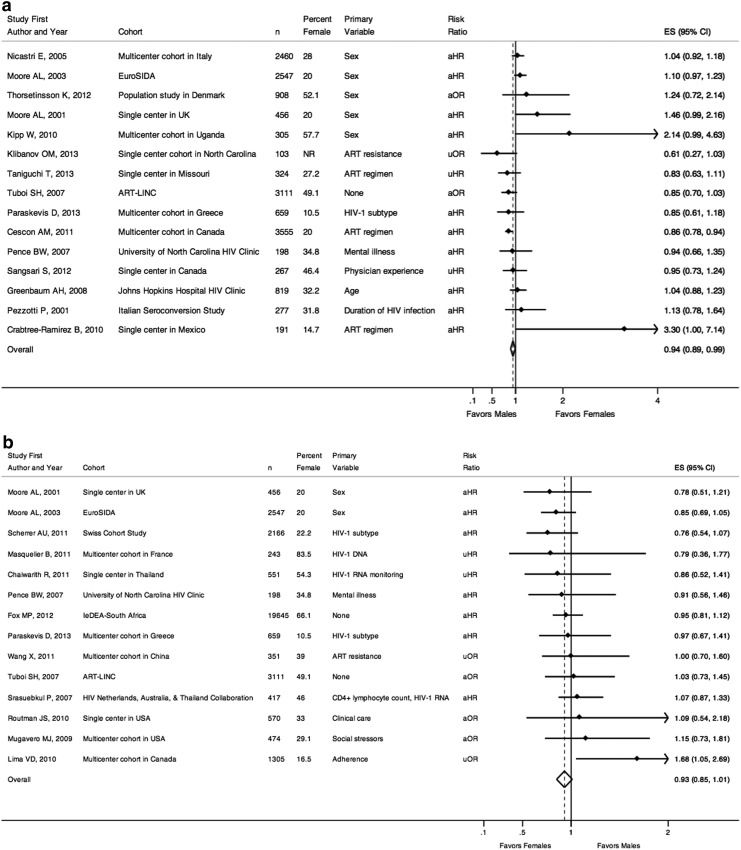
Figure 3a and b display study results of virologic outcomes.5,24,33,57–77 Fifteen studies examined likelihood of virologic suppression, of which three were from developing regions. Together, there was a slightly decreased likelihood of viral suppression for females compared to males [pooled risk ratio=0.94 (0.89–0.99)]. Of the seven studies with point estimates less than one (suggesting decreased rates of viral suppression for females) one multicenter study from Canada reported a small but statistically significant effect of sex [aHR for females=0.86 (0.78–0.94)].70 Five of the studies specified sex as the primary variable of interest and all had point estimates greater than one, reflecting increased likelihood of suppression for females, though none met statistical significance.5,33,61,62,66 Of the eight studies that demonstrated point estimates greater than one, only a small study of patients starting HAART in Mexico demonstrated a borderline significant effect in which females had a 3-fold increased likelihood of achieving viral suppression in univariable analyses [univariable HR 3.30 (1.00–7.14)].63
FIG. 3.
(a) Risk of virologic suppression. n, number of individuals in the study cohort; uHR, univariable odds ratio; uOR, univariable odds ratio; aHR, adjusted hazard ratio from multivariable analyses; aOR, adjusted odds ratio from multivariable analyses; ES, effect size of risk ratio, reported as comparing females to males; 95% CI, 95% confidence interval; Favors Males, a risk ratio less than one indicates a decreased likelihood of virologic suppression for females compared to males; Favors Females, a risk ratio greater than one indicates an increased likelihood of virologic suppression for females compared to males; (b) Risk of virologic failure. n, number of individuals in the study cohort; uHR, univariable odds ratio; uOR, univariable odds ratio; aHR, adjusted hazard ratio from multivariable analyses; aOR, adjusted odds ratio from multivariable analyses; ES, effect size of risk ratio, reported as comparing females to males; 95% CI, 95% confidence interval; Favors Females, a risk ratio less than one indicates a decreased risk of virologic failure for females compared to males; Favors Males, a risk ratio greater than one indicates an increased risk of virologic failure for females compared to males.
Figure 3b examines those studies that looked at risk of virologic failure (either viral rebound following suppression or failure to suppress) and sex. Overall, there was no statistically significant difference in risk of virologic failure for males and females [pooled risk ratio=0.93 (0.85–1.01)]. Of the nine studies, five studies were from developing countries.57,59,74–76 Two studies from Europe by Moore et al. included sex as the primary variable of interest, both of which showed decreased rates of virologic failure for females compared to males in multivariable models; however, neither result was statistically significant.5,61
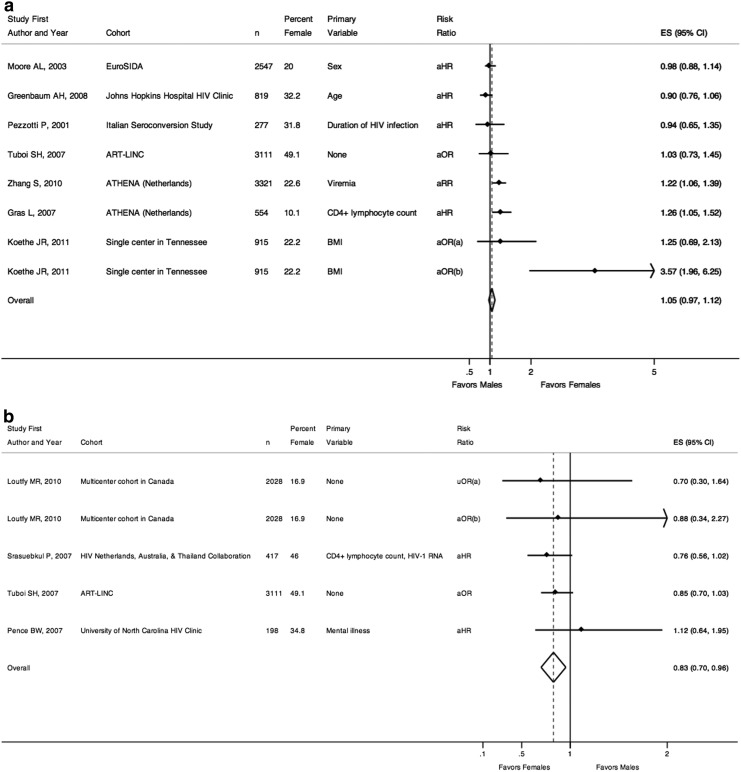
Lastly, Fig. 4a and b examine immunologic results and sex.5,24,42,57–60,78–81 Figure 4a reports studies that looked at likelihood of immunologic success (appropriate or robust CD4+ lymphocyte count recovery after starting HAART) and sex, and together, showed no significant difference [pooled risk ratio=1.05 (0.97–1.12)]. Seven studies were included, one of which was from developing regions.57 Only one study included sex as the primary variable of interest and showed no difference in immunologic responses by sex [aHR=0.98 (0.88–1.14)].5 Two studies of a national cohort from the Netherlands demonstrated a statistically significant increased rate of immunologic success for females compared to males.78,80 Additionally, a single-center study from the United States found that among patients with a CD4+ lymphocyte count of <200 cells/μl at HAART initiation, females were over 3-fold more likely than males to have a count >350 cells/μl after 12 months in multivariable analyses.81
FIG. 4.
(a) Risk of immunologic response. (a) Odds of achieving CD4+ lymphocyte count >200 cells/μl after 12 months for patients with CD4+ lymphocyte count <200 cells/μl at HAART initiation. (b) Odds of achieving CD4+ lymphocyte count >350 cells/μl after 12 months for patients with CD4+ lymphocyte count <200 cells/μl at HAART initiation. n, number of individuals in the study cohort; aHR, adjusted hazard ratio from multivariable analyses; aOR, adjusted odds ratio from multivariable analyses; aRR, adjusted relative risk from multivariable analyses; ES, effect size of risk ratio, reported as comparing females to males; 95% CI, 95% confidence interval; Favors Males, a risk ratio less than one indicates a decreased likelihood of immunologic response for females compared to males; Favors Females, a risk ratio greater than one indicates an increased likelihood of immunologic response for females compared to males. (b) Risk of immunologic failure. (a) Odds of CD4+ lymphocyte count <200 cells/μl after 12 months of HAART. (b) Odds of CD4+ lymphocyte count <200 cells/μl after 24 months of HAART. n, number of individuals in the study cohort; uHR, univariable hazard ratio; aHR, adjusted hazard ratio from multivariable analyses; aOR, adjusted odds ratio from multivariable analyses; ES, effect size of risk ratio, reported as comparing females to males; 95% CI, 95% confidence interval; Favors Females, a risk ratio less than one indicates a decreased risk of immunologic failure for females compared to males; Favors Males, a risk ratio greater than one indicates an increased risk of immunologic failure for females compared to males.
Figure 4b includes four studies that examined rates of immunologic failure (inappropriate or lack of CD4+ lymphocyte count recovery following HAART initiation) and sex. Together, these studies suggest a decreased risk of immunologic failure for females compared to males [pooled risk ratio=0.83 (0.70–0.96)]. Two of the studies were from developing countries and both suggested decreased rates of immunologic failure among females compared to males, though neither was statistically significant individually. A Thai study found that 12 weeks after starting HAART, females were 24% less likely to have a CD4+ lymphocyte count of less than 200 cells/μl compared to males [aHR=0.76 (0.56–1.02)].59 Similarly, a large study using the ART-LINC data found that among patients starting HAART, females were 15% less likely [aHR=0.85 (0.70–1.03)] to have a discordant outcome of viral suppression but a CD4+ lymphocyte count increase of 50 cells/μl or less after 6 months of therapy.57
Discussion
This systematic review assessed published studies to answer the question of whether sex differences in HIV-1 clinical and laboratory outcomes exist among persons starting antiretroviral therapy in the HAART era. A broad, systematic search of the literature identified studies that focused on sex as well as studies that included sex in multivariable analyses. The studies came from both developed and developing countries. Together, these studies suggest that around the globe, females with HIV-1 infection have slightly improved survival outcomes compared to males. However, studies showed no clear sex disparity in HIV-1 disease progression or in treatment effects of viral suppression and immunologic recovery.
While most studies individually yielded statistically nonsignificant results, collectively, there was suggestion of improved survival of females with HIV-1 compared to males. Improved female survival was observed from studies from both resource-rich and resource-limited countries with all but one study reporting risk ratios favoring females. Additionally, studies that adjusted for anemia all had point estimates of mortality less than one, demonstrating decreased risk of death in females compared to males when controlling for anemia.5,17,44–46,48–52 The difference in mortality was seen without parallel evidence in the outcomes related to HIV-1 disease outcomes—namely progression to AIDS or virologic or immunologic outcomes—a pattern that has been seen in a meta-analysis of sex and HIV outcomes among participants in clinical trials.2 Interpretation of these results is limited without knowledge of cause of death (AIDS-related or non-AIDS-related). In developed countries, death from non-AIDS-defining events (NADEs) has increasingly replaced AIDS-related causes of mortality.82 Studies from HIV-1 cohorts from developing countries, as well, have suggested the importance of non-AIDS mortality. A recent study from a large South African cohort found a decreased risk of death for females starting HAART that was parallel to sex disparities seen in the background population, not just among the HIV-1 infected.45 Attention to possible differences in rates of non-AIDS mortality is needed to better understand the observed mortality difference in this review.
The results for disease progression to AIDS and immunologic and virologic treatment effects did not show a clear sex disparity, though the results were limited by the small number of studies included. There was a significant, small, decreased risk of immunologic failure for females compared to males, but this was drawn from only four studies.
This study has a number of limitations. While the search was designed to capture a broad catchment of studies, it was limited by only including those studies that identified “sex” or “gender” or “male” or “female” words as MeSH terms. This was done to not miss studies that included sex as the primary predictor variable, but in turn, meant that many studies that examined HIV-1 outcomes and included sex in their analyses but did not identify sex by MeSH terms were not included in this study. Additionally, this study only queried PubMed as a search engine. The literature on HIV-1 outcomes is vast and it is likely that some studies were not included. However, the retrieval of nearly 3,000 articles (the majority of which did not focus on sex primarily) speaks to the large sample drawn. Similarly, many studies were excluded for not reporting risk ratios point estimates for sex or for simply stating that sex was not predictive of the outcome of interest. Without a point estimate, we were unable to assess if the nonsignificant result they reported was in favor of females or males and thus these studies were excluded. It is impossible to know if the majority of those studies yielded unreported point estimates contradictory to the studies reported in this review. However, that the majority of the studies in this review, too, had statistically nonsignificant results argues that the sample included may be representative of others' results. Lastly, this study is limited by its lack of formal meta-analysis. Given that studies from developed countries, in particular, are limited by small percentages of females included in cohorts and decreased power, a meta-analysis would allow for more definitive statements regarding HIV-1 outcomes and sex. However, a meta-analysis could not be pursued given the heterogeneity of studies and their primary statistical aims, as well as the risk of oversampling from cohorts used repeatedly in multiple studies.
With the above limitations acknowledged, this systematic review addressed several questions regarding sex disparities in HIV-1 outcomes among persons starting antiretroviral therapy. Females starting HAART in developed and developing countries had improved survival compared to males; however, this benefit was not seen in risk of progression to AIDS or in virologic or immunologic outcomes of HIV-1 therapy. Questions regarding cause of death and sex require further investigation, with particular attention to noninfectious morbidity and mortality.
Acknowledgments
Supported by the National Institutes of Health: T32 AI007474 (J.L.C.), NIAID K23AI080227 (V.V.M.), and K24 AI65298 (T.R.S.). Vanderbilt University has received research grants from Pfizer, Bristol Myers Squibb, and Virco to perform HIV observational studies.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Meditz AL, MaWhinney S, Allshouse A, et al. : Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis 2011;203:442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soon GG, Min M, Struble KA, et al. : Meta-analysis of gender differences in efficacy outcomes for HIV-positive subjects in randomized controlled clinical trials of antiretroviral therapy (2000–2008). AIDS Patient Care STDs 2012;26:444–453 [DOI] [PubMed] [Google Scholar]
- 3.Ballesteros-Zebadua P, Villarreal C, Cocho G, Huerta L, and Estrada JL: Differences in HIV-1 viral loads between male and female antiretroviral-untreated Mexican patients. Arch Med Res 2013;44:296–301 [DOI] [PubMed] [Google Scholar]
- 4.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, and Quinn TC: Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001;344:720–725 [DOI] [PubMed] [Google Scholar]
- 5.Moore AL, Kirk O, Johnson AM, et al. : Virologic, immunologic, and clinical response to highly active antiretroviral therapy: The gender issue revisited. J Acquir Immune Defic Syndr 2003;32:452–461 [DOI] [PubMed] [Google Scholar]
- 6.Braga P, Cardoso MR, and Segurado AC: Gender differences in survival in an HIV/AIDS cohort from Sao Paulo, Brazil. AIDS Patient Care STDs 2007;21:321–328 [DOI] [PubMed] [Google Scholar]
- 7.Chaisson RE, Keruly JC, and Moore RD: Race, sex, drug use, and progression of human immunodeficiency virus disease. N Engl J Med 1995;333:751–756 [DOI] [PubMed] [Google Scholar]
- 8.Rothenberg R, Woelfel M, Stoneburner R, Milberg J, Parker R, and Truman B: Survival with the acquired immunodeficiency syndrome. Experience with 5833 cases in New York City. New Engl J Med 1987;317:1297–1302 [DOI] [PubMed] [Google Scholar]
- 9.Melnick SL, Sherer R, Louis TA, et al. : Survival and disease progression according to gender of patients with HIV infection. The Terry Beirn Community Programs for Clinical Research on AIDS. JAMA 1994;272:1915–1921 [PubMed] [Google Scholar]
- 10.Farzadegan H, Hoover DR, Astemborski J, et al. : Sex differences in HIV-1 viral load and progression to AIDS. Lancet 1998;352:1510–1514 [DOI] [PubMed] [Google Scholar]
- 11.Prins M, Robertson JR, Brettle RP, et al. : Do gender differences in CD4 cell counts matter? AIDS (London, England) 1999;13:2361–2364 [DOI] [PubMed] [Google Scholar]
- 12.Nicastri E, Leone S, Angeletti C, et al. : Sex issues in HIV-1-infected persons during highly active antiretroviral therapy: A systematic review. J Antimicrob Chemother 2007;60:724–732 [DOI] [PubMed] [Google Scholar]
- 13.May MT, Sterne JA, Costagliola D, et al. : HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: A collaborative analysis. Lancet 2006;368:451–458 [DOI] [PubMed] [Google Scholar]
- 14.Moore RD, Keruly JC, and Chaisson RE: Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol 1998;19:29–33 [DOI] [PubMed] [Google Scholar]
- 15.Shah S, Smith CJ, Lampe F, et al. : Haemoglobin and albumin as markers of HIV disease progression in the highly active antiretroviral therapy era: Relationships with gender. HIV Med 2007;8:38–45 [DOI] [PubMed] [Google Scholar]
- 16.Melekhin VV, Shepherd BE, Stinnette SE, Rebeiro PF, Turner MM, and Sterling TR: Hemoglobin may contribute to sex differences in mortality among HIV-infected persons in care. PloS One 2012;7:e44999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etard JF, Ndiaye I, Thierry-Mieg M, et al. : Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: A 7-year cohort study. AIDS (London, England) 2006;20:1181–1189 [DOI] [PubMed] [Google Scholar]
- 18.Coetzee D, Hildebrand K, Boulle A, et al. : Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS (London, England) 2004;18:887–895 [DOI] [PubMed] [Google Scholar]
- 19.Mujugira A, Wester CW, Kim S, Bussmann H, and Gaolathe T: Patients with advanced HIV type 1 infection initiating antiretroviral therapy in Botswana: Treatment response and mortality. AIDS Res Hum Retroviruses 2009;25:127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogg RS, Yip B, Chan KJ, et al. : Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA 2001;286:2568–2577 [DOI] [PubMed] [Google Scholar]
- 21.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, and Montaner JS: Is there a baseline CD4 cell count that precludes a survival response to modern antiretroviral therapy? AIDS (London, England) 2003;17:711–720 [DOI] [PubMed] [Google Scholar]
- 22.Nachega JB, Hislop M, Dowdy DW, et al. : Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr 2006;43:78–84 [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann CJ, Fielding KL, Charalambous S, et al. : Reducing mortality with cotrimoxazole preventive therapy at initiation of antiretroviral therapy in South Africa. AIDS (London, England) 2010;24:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenbaum AH, Wilson LE, Keruly JC, Moore RD, and Gebo KA: Effect of age and HAART regimen on clinical response in an urban cohort of HIV-infected individuals. AIDS (London, England) 2008;22:2331–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lima VD, Hogg RS, Harrigan PR, et al. : Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS (London, England) 2007;21:685–692 [DOI] [PubMed] [Google Scholar]
- 26.Kitahata MM, Gange SJ, Abraham AG, et al. : Effect of early versus deferred antiretroviral therapy for HIV on survival. New Engl J Med 2009;360:1815–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima VD, Harrigan R, Murray M, et al. : Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS (London, England) 2008;22:2371–2380 [DOI] [PubMed] [Google Scholar]
- 28.Moore AL, Sabin CA, Johnson MA, and Phillips AN: Gender and clinical outcomes after starting highly active antiretroviral treatment: A cohort study. J Acquir Immune Defic Syndr 2002;29:197–202 [DOI] [PubMed] [Google Scholar]
- 29.Sabine C: AIDS events among individuals initiating HAART: Do some patients experience a greater benefit from HAART than others? AIDS (London, England) 2005;19:1995–2000 [DOI] [PubMed] [Google Scholar]
- 30.Moore RD, Keruly JC, and Chaisson RE: Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J Acquir Immune Defic Syndr 2004;35:46–51 [DOI] [PubMed] [Google Scholar]
- 31.Collazos J, Asensi V, and Carton JA: Sex differences in the clinical, immunological and virological parameters of HIV-infected patients treated with HAART. AIDS (London, England) 2007;21:835–843 [DOI] [PubMed] [Google Scholar]
- 32.Mocroft A, Gill MJ, Davidson W, and Phillips AN: Are there gender differences in starting protease inhibitors, HAART, and disease progression despite equal access to care? J Acquir Immune Defic Syndr 2000;24:475–482 [DOI] [PubMed] [Google Scholar]
- 33.Nicastri E, Angeletti C, Palmisano L, et al. : Gender differences in clinical progression of HIV-1-infected individuals during long-term highly active antiretroviral therapy. AIDS (London, England) 2005;19:577–583 [DOI] [PubMed] [Google Scholar]
- 34.Torti C, Lapadula G, Maggiolo F, et al. : Predictors of AIDS-defining events among advanced naive patients after HAART. HIV Clin Trials 2007;8:112–120 [DOI] [PubMed] [Google Scholar]
- 35.Hulgan T, Shepherd BE, Raffanti SP, et al. : Absolute count and percentage of CD4+lymphocytes are independent predictors of disease progression in HIV-infected persons initiating highly active antiretroviral therapy. J Infect Dis 2007;195:425–431 [DOI] [PubMed] [Google Scholar]
- 36.Thiebaut R, Morlat P, Jacqmin-Gadda H, et al. : Clinical progression of HIV-1 infection according to the viral response during the first year of antiretroviral treatment. Groupe d'Epidemiologie du SIDA en Aquitaine (GECSA). AIDS (London, England) 2000;14:971–978 [DOI] [PubMed] [Google Scholar]
- 37.Egger M, May M, Chene G, et al. : Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet 2002;360:119–129 [DOI] [PubMed] [Google Scholar]
- 38.Grabar S, Kousignian I, Sobel A, et al. : Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS (London, England) 2004;18:2029–2038 [DOI] [PubMed] [Google Scholar]
- 39.Chene G, Sterne JA, May M, et al. : Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: Analysis of prospective studies. Lancet 2003;362:679–686 [DOI] [PubMed] [Google Scholar]
- 40.Moore DM, Hogg RS, Chan K, Tyndall M, Yip B, and Montaner JS: Disease progression in patients with virological suppression in response to HAART is associated with the degree of immunological response. AIDS (London, England) 2006;20:371–377 [DOI] [PubMed] [Google Scholar]
- 41.May M, Sterne JA, Sabin C, et al. : Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: Collaborative analysis of prospective studies. AIDS (London, England) 2007;21:1185–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loutfy MR, Genebat M, Moore D, et al. : A CD4+ cell count <200 cells per cubic millimeter at 2 years after initiation of combination antiretroviral therapy is associated with increased mortality in HIV-infected individuals with viral suppression. J Acquir Immune Defic Syndr 2010;55:451–459 [DOI] [PubMed] [Google Scholar]
- 43.Taylor-Smith K, Tweya H, Harries A, Schoutene E, and Jahn A: Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J 2010;22:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dou Z, Xu J, Jiao JH, et al. : Gender difference in 2-year mortality and immunological response to ART in an HIV-infected Chinese population, 2006–2008. PloS One 2011;6:e22707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cornell M, Schomaker M, Garone DB, et al. : Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: A multicentre cohort study. PLoS Med 2012;9:e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maskew M, Brennan AT, Westreich D, McNamara L, MacPhail AP, and Fox MP: Gender differences in mortality and CD4 count response among virally suppressed HIV-positive patients. J Womens Health (Larchmt) 2013;22:113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosha F, Muchunguzi V, Matee M, et al. : Gender differences in HIV disease progression and treatment outcomes among HIV patients one year after starting antiretroviral treatment (ART) in Dar es Salaam, Tanzania. BMC Pub Health 2013;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson I, Togun O, de Silva T, et al. : Mortality and immunovirological outcomes on antiretroviral therapy in HIV-1 and HIV-2-infected individuals in the Gambia. AIDS (London, England) 2011;25:2167–2175 [DOI] [PubMed] [Google Scholar]
- 49.van Griensven J. and Thai S: Predictors of immune recovery and the association with late mortality while on antiretroviral treatment in Cambodia. Transact R Soc Trop Med Hyg 2011;105:694–703 [DOI] [PubMed] [Google Scholar]
- 50.Steele KT, Steenhoff AP, Newcomb CW, et al. : Early mortality and AIDS progression despite high initial antiretroviral therapy adherence and virologic suppression in Botswana. PloS One 2011;6:e20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.May M, Boulle A, Phiri S, et al. : Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: A collaborative analysis of scale-up programmes. Lancet 2010;376:449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mutevedzi PC, Lessells RJ, Rodger AJ, and Newell ML: Association of age with mortality and virological and immunological response to antiretroviral therapy in rural South African adults. PloS One 2011;6:e21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mills EJ, Bakanda C, Birungi J, Yaya S, and Ford N: The prognostic value of baseline CD4(+) cell count beyond 6 months of antiretroviral therapy in HIV-positive patients in a resource-limited setting. AIDS (London, England) 2012;26:1425–1429 [DOI] [PubMed] [Google Scholar]
- 54.Greig J, Casas EC, O'Brien DP, Mills EJ, and Ford N: Association between older age and adverse outcomes on antiretroviral therapy: A cohort analysis of programme data from nine countries. AIDS (London, England) 2012;26(Suppl 1):S31–S37 [DOI] [PubMed] [Google Scholar]
- 55.Byakwaga H, Petoumenos K, Ananworanich J, et al. : Predictors of clinical progression in HIV-1-infected adults initiating combination antiretroviral therapy with advanced disease in the Asia-Pacific region: Results from the TREAT Asia HIV observational database. J Int Assoc Provid AIDS Care 2013;12:270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miro JM, Manzardo C, Mussini C, et al. : Survival outcomes and effect of early vs.deferred cART among HIV-infected patients diagnosed at the time of an AIDS-defining event: A cohort analysis. PloS One 2011;6:e26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuboi SH, Brinkhof MW, Egger M, et al. : Discordant responses to potent antiretroviral treatment in previously naive HIV-1-infected adults initiating treatment in resource-constrained countries: The antiretroviral therapy in low-income countries (ART-LINC) collaboration. J Acquir Immune Defic Syndr 2007;45:52–59 [DOI] [PubMed] [Google Scholar]
- 58.Pence BW, Miller WC, Gaynes BN, and Eron JJ, Jr: Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2007;44:159–166 [DOI] [PubMed] [Google Scholar]
- 59.Srasuebkul P, Ungsedhapand C, Ruxrungtham K, et al. : Predictive factors for immunological and virological endpoints in Thai patients receiving combination antiretroviral treatment. HIV Med 2007;8:46–54 [DOI] [PubMed] [Google Scholar]
- 60.Pezzotti P, Pappagallo M, Phillips AN, et al. : Response to highly active antiretroviral therapy according to duration of HIV infection. J Acquir Immune Defic Syndr 2001;26:473–479 [DOI] [PubMed] [Google Scholar]
- 61.Moore AL, Mocroft A, Madge S, et al. : Gender differences in virologic response to treatment in an HIV-positive population: A cohort study. J Acquir Immune Defic Syndr 2001;26:159–163 [DOI] [PubMed] [Google Scholar]
- 62.Kipp W, Alibhai A, Saunders LD, et al. : Gender differences in antiretroviral treatment outcomes of HIV patients in rural Uganda. AIDS Care 2010;22:271–278 [DOI] [PubMed] [Google Scholar]
- 63.Crabtree-Ramirez B, Villasis-Keever A, Galindo-Fraga A, del Rio C, and Sierra-Madero J: Effectiveness of highly active antiretroviral therapy (HAART) among HIV-infected patients in Mexico. AIDS Res Hum Retroviruses 2010;26:373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Routman JS, Willig JH, Westfall AO, et al. : Comparative efficacy versus effectiveness of initial antiretroviral therapy in clinical trials versus routine care. Clin Infect Dis 2010;50:574–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mugavero MJ, Raper JL, Reif S, et al. : Overload: Impact of incident stressful events on antiretroviral medication adherence and virologic failure in a longitudinal, multisite human immunodeficiency virus cohort study. Psychosom Med 2009;71:920–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thorsteinsson K, Ladelund S, Jensen-Fangel S, et al. : Impact of gender on response to highly active antiretroviral therapy in HIV-1 infected patients: A nationwide population-based cohort study. BMC Infect Dis 2012;12:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klibanov OM, Dolder CR, Assefa F, and Ekwonu TJ: Baseline antiretroviral resistance and clinical outcomes in an urban HIV clinic. AIDS Patient Care STDs 2013;27:205–207 [DOI] [PubMed] [Google Scholar]
- 68.Taniguchi T, Grubb JR, Nurutdinova D, et al. : Efavirenz outperforms boosted atazanavir among treatment-naive HIV-1-infected persons in routine clinical care. J Int Assoc Provid AIDS Care 2013;12:138–141 [DOI] [PubMed] [Google Scholar]
- 69.Paraskevis D, Touloumi G, Bakoyannis G, et al. : Effect of HIV type 1 subtype on virological and immunological response to combination antiretroviral therapy: Evidence for a more rapid viral suppression for subtype A than subtype B-infected Greek individuals. AIDS Res Hum Retroviruses 2013;29:461–469 [DOI] [PubMed] [Google Scholar]
- 70.Cescon AM, Cooper C, Chan K, et al. : Factors associated with virological suppression among HIV-positive individuals on highly active antiretroviral therapy in a multi-site Canadian cohort. HIV Med 2011;12:352–360 [DOI] [PubMed] [Google Scholar]
- 71.Sangsari S, Milloy MJ, Ibrahim A, et al. : Physician experience and rates of plasma HIV-1 RNA suppression among illicit drug users: An observational study. BMC Infect Dis 2012;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scherrer AU, Ledergerber B, von Wyl V, et al. : Improved virological outcome in white patients infected with HIV-1 non-B subtypes compared to subtype B. Clin Infect Dis 2011;53:1143–1152 [DOI] [PubMed] [Google Scholar]
- 73.Masquelier B, Taieb A, Reigadas S, et al. : Cellular HIV-1 DNA quantification and short-term and long-term response to antiretroviral therapy. J Antimicrob Chemother 2011;66:1582–1589 [DOI] [PubMed] [Google Scholar]
- 74.Chaiwarith R, Praparattanapan J, Nuntachit N, Kotarathitithum W, Sirisanthana T, and Supparatpinyo K: Impact of the frequency of plasma HIV-1 RNA monitoring on the outcome of antiretroviral therapy. Curr HIV Res 2011;9:82–87 [DOI] [PubMed] [Google Scholar]
- 75.Fox MP, Cutsem GV, Giddy J, et al. : Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquir Immune Defic Syndr 2012;60:428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X, Yang L, Li H, et al. : Factors associated with HIV virologic failure among patients on HAART for one year at three sentinel surveillance sites in China. Curr HIV Res 2011;9:103–111 [DOI] [PubMed] [Google Scholar]
- 77.Lima VD, Bangsberg DR, Harrigan PR, et al. : Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acquir Immune Defic Syndr 2010;55:460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gras L, Kesselring AM, Griffin JT, et al. : CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr 2007;45:183–192 [DOI] [PubMed] [Google Scholar]
- 79.Florence E, Lundgren J, Dreezen C, et al. : Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med 2003;4:255–262 [DOI] [PubMed] [Google Scholar]
- 80.Zhang S, van Sighem A, Gras L, et al. : Clinical significance of transient HIV type-1 viraemia and treatment interruptions during suppressive antiretroviral treatment. Antivir Ther 2010;15:555–562 [DOI] [PubMed] [Google Scholar]
- 81.Koethe JR, Jenkins CA, Shepherd BE, Stinnette SE, and Sterling TR: An optimal body mass index range associated with improved immune reconstitution among HIV-infected adults initiating antiretroviral therapy. Clin Infect Dis 2011;53:952–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: Collaborative analysis of 13 HIV cohort studies. Clin Infect Dis 2010;50:1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]