Abstract
CD4+ count increase has been reported to be different with lopinavir/r (LPV/r) and efavirenz (EFV)-containing regimens. The different effect of these two regimens on other immune function parameters and the relationship with the gain of CD4+ count have not been assessed in a randomized clinical trial. Fifty antiretroviral treatment (cART) naïve HIV-infected individuals were randomized to receive LPV/r or EFV both with tenofovir/emtricitabine for 48 weeks. A substudy of immunological function restoration was performed in 22 patients (LPV/r n=10 and EFV n=12). Activation, thymic function, apoptosis, senescence, exhaustion, Treg cells, interleukin (IL)-7-receptor/IL-7 system, thymic volume, and lymphoid tissue fibrosis were evaluated at baseline and at week 48. Both groups experienced a CD4+ count increase that was higher in the EFV group (ΔCD4+ 88 vs. 315 cells/μl LPV/r vs. EFV, respectively, p<0.001). Despite this difference in CD4+ gain, the change in other immune function parameters was similar in both treatment groups. Most of parameters evaluated tended to normalize after 48 weeks of cART. A significant decrease in levels of activation, senescence, exhaustion, and apoptosis on CD4+ and CD8+ T cells (p<0.001 for all) and a significant increase in markers of thymic function, IL-7 receptor, and in the levels of central memory CD4+ T cells and naive subsets of CD8+ T cells (p<0.001 for all) with respect to baseline values were observed without any difference between groups. These data indicate that the differences in CD4+ gain with different cART regimens are not immunologically meaningful and might explain the similar clinical efficacy of these regimens.
Introduction
Combined antirretroviral treatment (cART) leads to continued viral suppression and immune recovery that reduces HIV-associated morbidity and mortality.1 Two nucleoside retrotranscriptase inhibitors (NRTI) combined with either a boosted protease inhibitor (PI) or with a nonnucleoside retrotranscriptase inhibitor (NNRTI) are the standard of care for initiating therapy.2 Several studies have compared virological efficacy and immune reconstitution of different cART regimens. The ACTG 5142 study described a higher increase of CD4+ T lymphocytes counts from baseline in the PI regimen (lopinavir-ritonavir: LPV/r) compared with the efavirenz (EFV) regimen after 96 weeks of treatment (but not after 48 weeks of cART).3 Similar findings have been reported in other cohort studies.4–6 Conversely, other clinical trials that compared NNRTI and PI-based regimens did not find significant differences in the increases in CD4+ counts in both groups.7–9
It is unclear if the potential differences in recovery of the CD4 T cell count with different cART regimens have any clinical or immunological relevance. First, regarding different clinical outcomes with EFV vs. LPV/r-containing regimens, it has been reported that PI- and NNRTI-based cART regimens are equally effective in protection against Kaposi sarcoma.10 However, some cohort studies showed that PI regimens boosted with ritonavir (RTV) were associated with a 2-fold higher rate of AIDS/death (HR, 2.07) than EFV-containing regimens, although it is possible that confounding factors could be responsible for an overestimate of the true risk associated with boosted PI regimens.11 Second, there are data suggesting that clinically important CD4+ T cell count responses are likely to be better defined in terms of absolute post-cART CD4+ T cell counts rather than change from baseline.12 Finally, the effect of different regimens on parameters involved in immune function and their relationship with different gains of CD4+ have not been assessed in a randomized clinical trial. Moreover, most previous studies addressing the effect of cART on immune restoration have analyzed only a few of the multiple parameters altered in HIV infection.13–18 Therefore, we performed a comprehensive approach of simultaneous measurement of different immunological parameters in CD4+ and CD8+ T cells in a subset of 22 patients out of 50 cART-naive HIV-infected individuals who were randomized to receive LPV/r or EFV both with tenofovir/emtricitabine for 48 weeks. The final objective of the study was to analyze if with a similar level of HIV suppression, different treatment regimens induce different degrees of immunological function restoration.
Materials and Methods
Study population
This is a substudy conducted in a subset of 22 patients out of 50 cART-naive HIV-infected individuals who were randomized to receive LPV/r or EFV both with tenofovir/emtricitabine for 48 weeks at two different Hospitals (Hospital Clinic and Hospital Germans Trias i Pujol) in Barcelona, Spain. Twenty-eight additional patients were recruited in three other hospitals and did not participate in this substudy. There were no differences at baseline between patients included and excluded from the study. Twelve patients received efavirenz (EFV group) and 10 lopinavir/ritonavir (LPV/r group) with a backbone of tenofovir plus emtricitabine. To participate in the study, written informed consent was obtained from all individuals, and the study protocol was evaluated and approved by the Hospitals Ethical Committee.
Viral load measurement
Plasma HIV-RNA was measured using Versant HIV-1 RNA v3.0 (Siemens, Barcelona, Spain), which has a lower limit of detection of 50 copies/ml.
Cell samples
All analyses were done in cryopreserved peripheral blood mononuclear cells (PBMCs). EDTA-anticoagulated blood was obtained by venipuncture; PBMCs were immediately isolated by density gradient centrifugation using Ficoll-Hypaque (Sigma Chemical Co., St. Louis, MO) and frozen in FCS plus 10% DMSO. The viability of thawed PBMCs was always greater than 85%. Analyses were performed in two cell samples from each patient: at baseline and 48 weeks after cART initiation
Immunophenotypic analysis
We used a comprehensive approach of simultaneous measurement of different immunological parameters in CD4+ and CD8+ T cells: differentiation stage (using CD45RA and CD27 markers), activation level (using CD38 and HLADR markers), thymic function (using CD31 marker), apoptosis level (using annexin binding), exhaustion level (using PD-1 and Tim-3 markers), level of senescence (using CD57 marker), interleukin (IL)-7 receptor/IL-7 system (using CD127 marker and measuring the plasma level of IL-7), and T regulatory (Treg) cells level (using CD25, CD127, and FoxP3 markers). All parameters were evaluated using five-color flow cytometry.
PBMCs from each patient were stained with proper antibodies according to eight different antibody panels. A million PBMCs were washed with 2 ml of phosphate-buffered saline (PBS) and stained for surface markers by incubation with the appropriate antibody panel for 30 min at 4°C. For antibody panels one to seven cells were then washed with 2 ml of PBS and resuspended in 250 μl of PBS. For antibody panel 8 (apoptosis) cells were first stained for surface markers as described above, then washed in PBS, resuspended in 250 μl annexin binding buffer, and then incubated with annexin (BD Biosciences, San Diego, CA). For antibody panel 9 (Treg cells), cells were first stained with surface markers as described above, washed with PBS, and resuspended in cytofix/cytoperm solution (BD Biosciences, San Diego, CA) for 20 min at 4°C. The permeabilized cells were washed with 2 ml of Perm/Wash Buffer (BD Biosciences) and stained for intracellular FoxP3-FITC (eBioscience, San Diego, CA) marker at 4°C for 30 min.
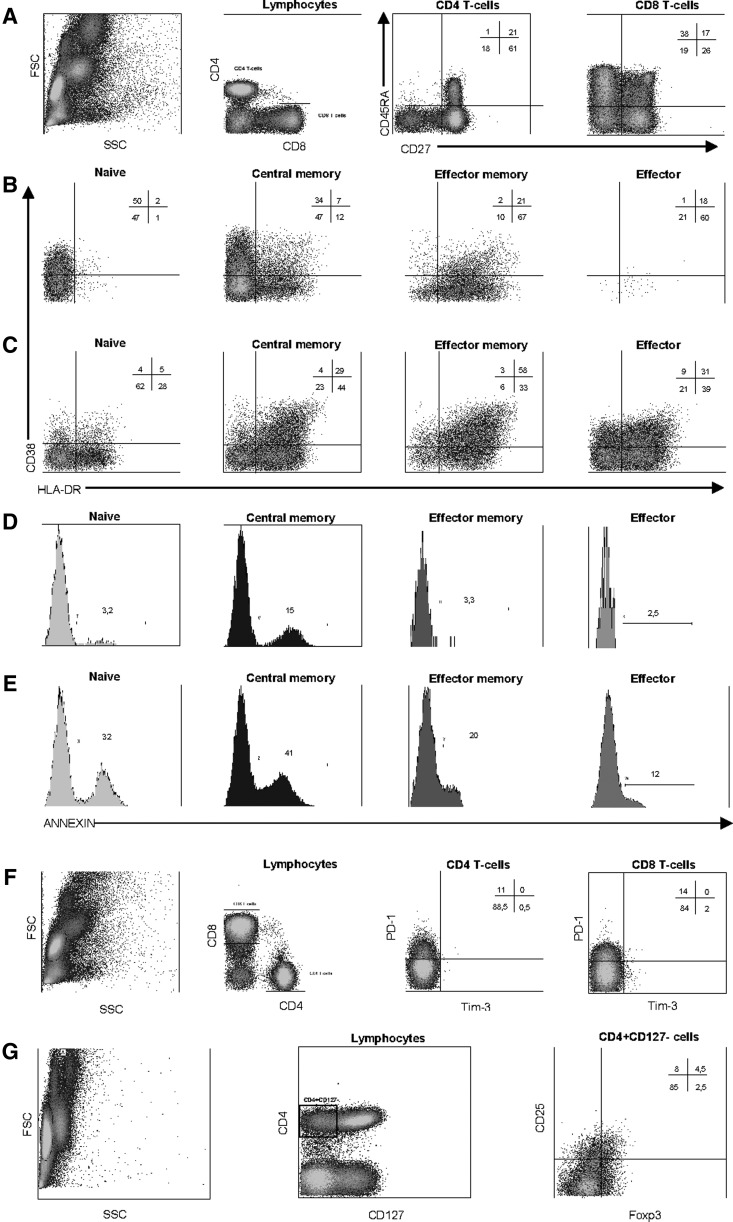
After intracellular marker staining, cells were washed with 2 ml of Perm/Wash buffer and resuspended in 250 μl PBS. Five-color acquisition was performed on Cytomics FC 500 flow cytometer (Beckman Coulter, Fullerton, CA). For each sample, a minimum of 50,000 CD4+ and 50,000 CD8+ events were acquired. Data analysis was performed using CXP software (Beckman Coulter, Fullerton, CA). Gating was done on CD8+bright and CD4+ cells. Based on the expression of CD45RA and CD27 markers, four different differentiation stage subsets of CD4+ and CD8+ cells were defined (see Table 2): naive (CD45RA+CD27+), central memory (CD45RA−CD27+), effector memory (CD45RA−CD27−), and effector (CD45RA+CD27−) cells. Activation, apoptosis, CD31, CD127, and CD57 expression were assessed in total CD4+ and CD8+ and in each of these four differentiation stage subsets. Exhaustion level was assessed in total CD3+ and in total CD3+CD4+ and CD3+CD8+ cells. Treg cells were defined as CD4+CD127−CD25+FoxP3+ cells. Dot plots explaining the flow cytometry gating strategy for defining different T cells subsets can be seen in Fig. 1.
Table 2.
T Cell Subsets Analyzed in the Study, Grouped According to Eight Different Categories
| T cell subset category (antibody panel) | T cell subset |
|---|---|
| Differentiation stage of CD4 and CD8 cells (CD3-FITC/CD27-PE/CD45RA-ECD/CD8-PECy5/CD4-PECy7) | CD4 and CD8 cells (total) |
| Naive cells (CD45RA+CD27+) | |
| Central memory cells (CD45RA−CD27+) | |
| Effector memory cells (CD45RA−CD27−) | |
| Effector cells (CD45RA+CD27−) | |
| Activation of CD4 and CD8 T cells (including naive, central memory, effector memory, and effector cells) (HLADR-FITC/CD27-PE/CD45RA-ECD/CD38-PECy5/CD4-PECy7 or CD8- PECy7) | CD38+DR− |
| CD38+DR+ | |
| CD38−DR+ | |
| Thymic function (CD31) (CD31-FITC/CD27-PE/CD45RA-ECD/CD8-PECy5/CD4-PECy7) | CD31 expression on CD4 and CD8 cells (including naive, central memory, effector memory, and effector cells) |
| Expression of CD127 (CD127-FITC/CD27-PE/CD45RA-ECD/CD8-PECy5/CD4-PECy7) | CD127 expression on CD4 and CD8 cells (including naive, central memory, effector memory, and effector cells) |
| Senescence (CD57) (CD57-FITC/CD27-PE/CD45RA-ECD/CD8-PECy5/CD4-PECy7) | CD57 expression on CD4 and CD8 cells (including naive, central memory, effector memory, and effector cells) |
| Exhaustion (PD-1 and Tim-3) of CD3, CD4, and CD8 T cells (CD4-FITC/Tim-3-PE/CD3-ECD/CD8-PECy5/PD-1-biotin/streptavidin-PECy7) | PD-1+Tim-3− |
| PD-1+Tim-3+ | |
| PD-1−Tim-3+ | |
| Apoptosis (annexin binding) (annexin-FITC/CD27-PE/CD45RA-ECD/CD8-PECy5/CD4-PECy7) | Annexin+ binding on CD4 and CD8 cells (including naive, central memory, effector memory, and effector cells) |
| T regulatory cells (FoxP3-FITC/CD127-PE/CD8-ECD/CD25-PECy5/CD4-PECy7) | CD4+CD25+CD127−FoxP3+ cells |
FIG. 1.
Representative example of flow cytometry data and gating strategy. A representative example for some of the antibody panels used is shown. (A) Expression of CD45RA and CD27 on CD4 and CD8 T cells to define the differentiation stage of these cells. (B, C) Expression of activation markers CD38 and HLA-DR on different subsets of CD4 (B) and CD8 (C) T cells as defined by CD45RA and CD27 expression. (D, E) Annexin binding on different subsets of CD4 (D) and CD8 (E) T cells as defined by CD45RA and CD27 expression. (F) Expression of exhaustion markers PD-1 and Tim-3 on CD4 and CD8 T cells. (G) Gating strategy used to define T regulatory (Treg) CD4 cells as CD127−CD25+FoxP3+. Numbers inside plots or histograms represent the percentage of positive events in each quadrant or region.
Plasma interleukin (IL)-7 levels
IL-7 was measured using a highly sensitive commercial immunoassay (Quantikine HS, R&D Systems, Barcelona, Spain), which has a lower limit of detection of 0.1 pg/ml following the manufacturer's instructions.
Lymphoid tissue
Tonsillar biopsy was performed in eight patients at baseline and 48 weeks after cART initiation. Tonsillar tissue was formalin-fixed and paraffin-embedded for histological study. A trichrome stain, using the Masson method, was used to identify collagen fibers.
Lymphoid tissue collagen deposition was assessed using the Olympus Cell-B Basic Imaging Software (Olympus Corporation, Shinzuku, Tokyo, Japan). From each specimen, the follicular area, defined as the proportion of follicular areas with respect to the total amount of lymphoid tissue, was evaluated by morphometry from low-power fields (×10 objective). To quantify collagen fibers, 10 images from 10 high-power fields (HPFs) (×40 objective) from interfollicular areas were captured on trichrome-stained slides and the percentage of lymphoid tissue occupied by collagen was determined.
Volume and thymic index
Noncontrast chest computerized tomography (CT) was performed in 11 patients at baseline and 48 weeks after cART initiation on a CT scanner (Somatom Sensation 64, Siemens, Erlangen, Germany) with contiguous 5-mm sections. Measurements were always performed by the same radiologist as previously described.19 Thymic tissue in all the slices was carefully delimited on a Leonardo Workstation (Siemens, Erlangen, Germany) obtaining the thymic area in each slice. The thymic areas were multiplied by the slice thickness to obtain the thymic volume in cubic centimeters. Additionally, thymic tissue was scored on a grading scale of 0–5: 0—no soft tissue, with the thymus entirely replaced by fat; 1—minimal soft tissue, barely recognizable; 2—minimal soft tissue, more obvious; 3—moderate soft tissue; 4—moderate soft tissue of greater extent, almost mass like; and 5—mass-like appearance, of concern for hyperplasia or thymoma.20
Statistical analyses
Characteristics of the study population and the different immunological parameters were recorded as median (interquartile range), and comparisons were made using the nonparametric tests Mann–Whitney U-test or Wilcoxon test as appropriate. Correlations between quantitative parameters were explored using the Spearman's rho test. All statistical analyses were performed using SPSS software version 13 (SPSS Inc., Chicago, IL). All p values were two-tailed, and were considered significant when lower than 0.05.
Results
Study population
Twenty-two patients were included in the immunological study. There were no differences in terms of age, sex, baseline CD4+ counts, and HIV-RNA viral load or frequency of hepatitis C virus (HCV) coinfection compared to the population of the main study (data not shown). Of the 22 patients included, 12 received an EFV-containing regimen and 10 an LPV/r-based cART. There were no significant differences between both treatment groups in terms of age, sex, baseline CD4+ counts, baseline HIV-RNA load, and frequency of HCV coinfection (Table 1). All patients reached undetectable HIV plasma viremia and remained so during 48 weeks of therapy. There were no differences in absolute CD4+ count at week 48 (median 530 vs. 641 cells/μl, LPV/r vs. EFV groups, respectively, p=0.25) (Table 1).
Table 1.
Baseline Characteristics of Patients Included in the Study
| Variable | NNRTI-based cART (n=12) | PI-based cART (n=10) | p-value |
|---|---|---|---|
| Age (years)a | 35 (31–45) | 40 (35–44) | 0.4 |
| Sex (M/F) | 12/0 | 10/0 | NA |
| Baseline CD4 counta (median cell/μl) | 317 (228–422) | 407 (269–526) | 0.23 |
| Baseline HIV-RNAa (median log copies/ml) | 4.5 (4.3–5.0) | 4.9 (4.2–5.2) | 0.47 |
| CD4 count at week 48a (median cells/μl) | 641 (474–736) | 530 (446–619) | 0.25 |
| HCV coinfection (%) | 8% (1/12) | 0% (0/10) | 1 |
Median (interquartil range).
NNRTI, nonnucleoside reverse transcriptase inhibitor; CART, combined antiretroviral treatment; PI, protease inhibitor; HCV, hepatitis C virus.
Comparison of immunological parameters at baseline between EFV and LPV/r groups of patients
Different T cell subsets were analyzed grouped in nine different functional categories: T cell differentiation, activation of CD4+ and CD8+ T cells, thymic function (using CD31 as a marker of recent thymic emigrants), expression of IL-7 receptor (CD127), T cell senescence (using CD57 marker), T cell exhaustion (using PD-1 and Tim-3 markers), T cell apoptosis (using annexin binding), and Treg cells (CD4+CD25+FoxP3+CD127−) (Table 2).
Levels of these parameters at baseline were compared between LPV/r and EFV groups of patients. Both groups were comparable for most of the parameters analyzed, with significant differences only for a few of them. Of these parameters, some were significantly increased in the EFV group (level of CD8+ central memory cells, CD31 expression on different subsets of CD8+ cells, and PD-1 expression on CD4+ cells) and some others were significantly increased in the LPV/r group (level of CD8+ effector cells, CD127 expression on effector CD4+ cells, and CD57 expression on CD8+ cells) (data not shown).
Evolution of immunological parameters after cART in the whole population of patients
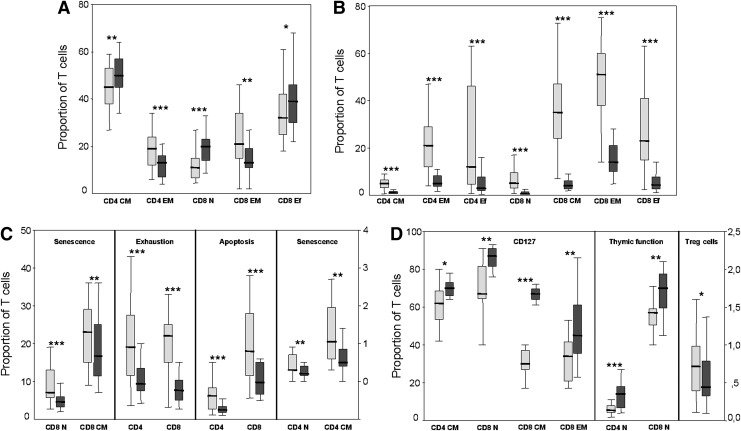
The evolution of all parameters analyzed was measured as the difference between baseline and week 48 of therapy values (delta values). In the whole population of patients most of the parameters significantly changed after cART. In parallel with the significant increase in CD4+ counts after 48 weeks of therapy [from median (IQR) 360 (252–429) to 592 (452–714) cells/μl, p<0.0001] there was a significant increase in the central memory subset of CD4+ T cells and a significant decrease in the effector memory subset of CD4+ T cells, as well as a significant increase in naive and in effector subsets of CD8+ T cells and a significant decrease in the effector memory subset of CD8+ T cells (Fig. 2A).
FIG. 2.
Box-plot graphs showing baseline (light gray boxes) and week 48 (dark gray boxes) levels of different T cell subsets in the whole population of patients. Vertical axes represent the proportion of cells and horizontal axes the different CD4 and CD8 T cells subsets. (A) Differentiation stage (coexpression of CD45RA and CD27) of CD4 and CD8 T cells. (B) Activation (coexpression of CD38 and HLA-DR) of different CD4 and CD8 T cells subsets. (C) Senescence (CD57 expression), exhaustion (PD1 expression), and apoptosis (Annexin binding) of different CD4 and CD8 T cell subsets. In this graph the right vertical axis applies only to senescence of CD4 T cells subsets. (D) CD127 expression, thymic function (CD31 expression), and Treg cells (defined as CD4+CD127−CD25+FoxP3+). In this graph the right vertical axis applies only to Treg cells. Levels of statistical significance for the pairwise comparison (Wilcoxon signed rank test) between baseline and week 48 values are as follows: p<0.05 (*); p<0.01 (**); p<0.001 (***). N, naive; CM, central memory; EM, effector memory; Ef, effector.
Activation levels significantly decreased after cART in the majority of the different CD4+ and CD8+ T cell subsets, and the decrease was especially noticeable for the subsets coexpressing both CD38 and HLA-DR activation markers (Fig. 2B). Interestingly, the extent of decrease of these CD38+HLA-DR+ subsets was much higher in CD8+ than in CD4+ T cell subsets (Fig. 2B), most likely because baseline levels for these subsets were higher in CD8+ than in CD4+ T cells (data not shown). Surprisingly, the levels of effector memory and effector subsets of CD4+ and CD8+ T cells expressing only HLA-DR+ (CD38−HLA-DR+) significantly increased after cART (Fig. 2B).
Other immunological functions (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid) that showed a significant decrease were T cell senescence, T cell exhaustion, and T cell apoptosis. A decrease of senescence after cART was observed in naive and central memory subsets of both CD4+ and CD8+ T cells, with much higher variation in CD8+ subsets, probably due to much higher levels of senescence at baseline in CD8+ subsets than in CD4+ subsets (Fig. 2C). The decrease in T cell exhaustion was observed in both CD4+ and CD8+ T cells (Fig. 2C), as well as in total CD3+ T lymphocytes, and it was restricted to the PD-1 marker with no variation in Tim-3 expression. Overall, Tim-3 expression was only marginal both at baseline and after cART (data not shown). Lastly, the levels of apoptosis also experienced a significant decrease after cART in CD4+ and CD8+ T cells (Fig. 2C) as well as in most subsets of these cells (data not shown).
In contrast to what was observed for activation, senescence, exhaustion, and apoptosis, other immunological functions significantly increased after cART. This was best exemplified by CD127 expression with a significant increase in central memory CD4+ T cells and in all but effector subsets of CD8+ T cells. The highest increase was observed in central memory CD8+ T cells (Fig. 2D). Regarding thymic function, there was a significant increase of CD31 expression on naive CD4+ and CD8+ T cells (Fig. 2D). There was also a small but significant decrease in the level of T regulatory cells in the whole study population. (Fig. 2D).
Comparison of evolution after cART of immunological parameters between EFV and LPV/r groups of patients
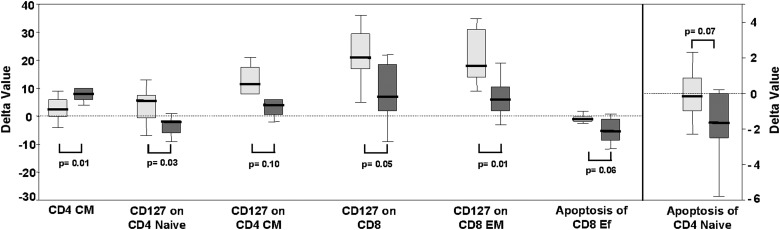
With the hypothesis that with a similar level of HIV suppression, different treatment regimens could induce different degrees of immunological function restoration, we compared the evolution of different immunological parameters between patients treated with LPV/r and EFV-based cART regimens. For this purpose, we compared the variation (delta value, Δ, defined as the difference between baseline value and after 48 weeks of cART value) of each parameter between both groups of patients. Both arms experienced a significant CD4 count increase that was significantly higher in the EFV group [median (IQR) ΔCD4 88 (34–331) vs. 315 (219–359) cells/μl LPV/r vs. EFV, respectively, p<0.001]. Despite the difference in CD4 count increase, there were no significant differences in the delta values between the two groups for the majority of evaluated parameters, suggesting a very similar degree of immunological function restoration. Significant differences between the two groups were observed in only a few parameters. Thus, EFV patients experienced lower increases of central memory CD4+ T cells and lower decreases of apoptosis in effector CD8+ T cells and naive CD4+ T cells than LPV/r patients (Fig. 3). However, EFV patients experienced a higher increase of CD127 expression in different subsets of CD4+ and CD8+ T cells compared to LPV/r patients (p=0.03, p=0.1, p=0.05, p=0.01) (Fig. 3). Regarding plasma levels of IL-7, interestingly we observed a trend to a decrease after cART in the EFV group [10.5 (7.2–15.5) versus 7.4 (4.2–12.6) pg/ml, at baseline and at week 48 respectively, p=0.06] whereas there was no change in the LPV/r group [9.8 (6.3–11.9) versus 10.8 (3.7–13.3) pg/ml, at baseline and at week 48 respectively, p=0.8].
FIG. 3.
Box-plots showing the variation (delta value) in the level of different subsets of T cells after 48 weeks of successful antiretroviral therapy in patients treated with an efavirenz (EFV)-based combined antiretroviral treatment (cART) regimen (light gray boxes) and in patients treated with a lopinavir/r (LPV/r)-containing cART regimen (dark gray boxes). Positive delta values reflect an increase and negative values reflect a decrease in the level of the T cell subset. The y-axis scale on the right applies only to the last subset shown on the x-axis. The dotted line marks the zero delta value. Only T cell subsets with statistically significant differences (p≤0.05) or showing a trend (p≤0.1) between EFV and LPV/r groups of patients are shown. CM, central memory T cells; EM, effector memory T cells; Ef, effector T cells.
Lymphoid tissue collagen deposition
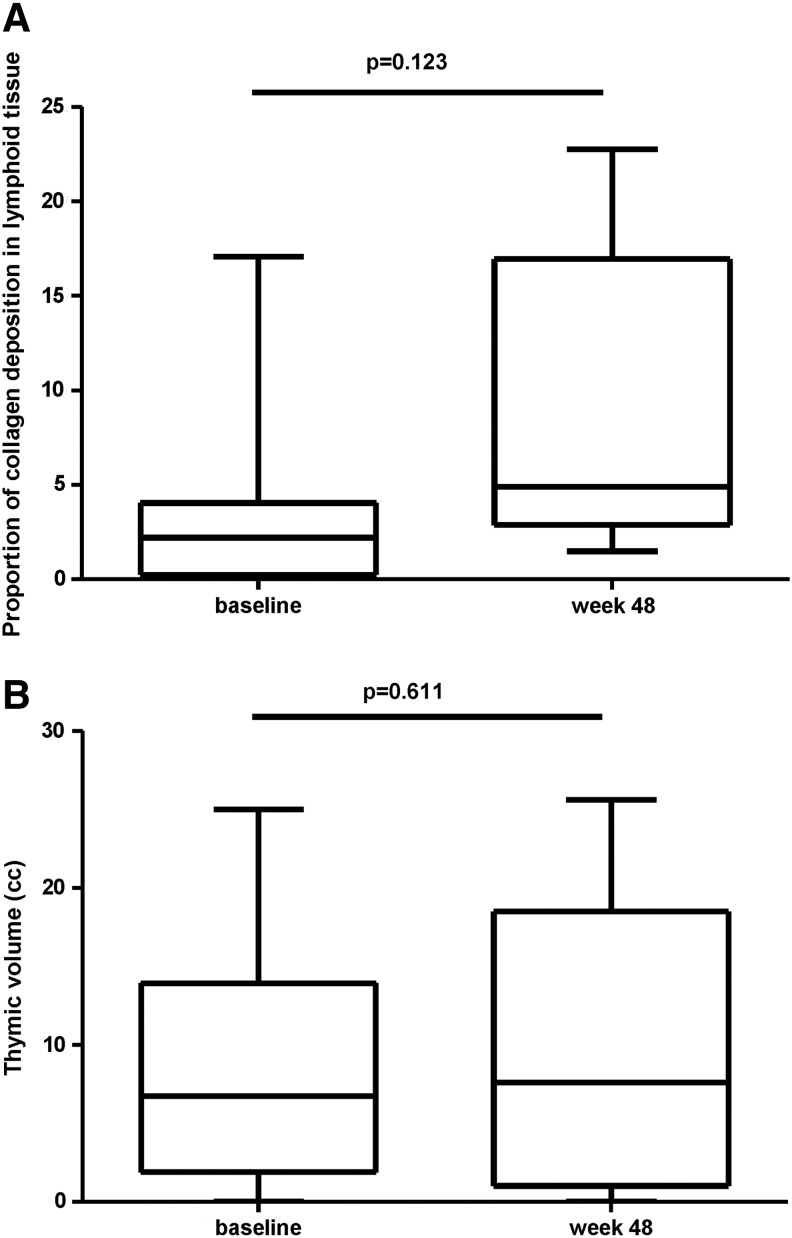
Biopsies of eight patients (three on LPV/r and five on EFV) were suitable for analysis. At baseline patients on LPV/r had a lower percentage of fibrosis than patients on EFV [0.65 (0.5–2.35) vs. 3.76 (0.21–4.05)]. No significant changes were observed after 48 weeks on cART [2.21 (0.24–4.05) and 4.90 (2.89–16.96) (p=0.123) for week 0 and week 48 respectively in all patients] (Fig. 4A).
FIG. 4.
(A) Box-plots showing the variation in the level of collagen deposition as measured as percentage of lymphoid tissue occupied by collagen at baseline before any antiretroviral therapy and after 48 weeks of therapy. Patients on LPV/r and EFV-containing regimens are shown together (n=8). (B) Box-plots showing the variation in the level of thymic volume at baseline before any antiretroviral therapy and after 48 weeks of therapy. Patients on LPV/r and EFV-containing regimens are shown together (n=11).
Volume and thymic index
Eleven patients were analyzed with mediastinal CT scans (five were on LPV/r and six on EFV). The median (IQR) thymic volume did not change significantly from baseline to week 48 after cART initiation [week 0: 6.73 cm3 (1.90–13.92) and week 48: 7.60 cm3 (1.00–18.50) (p=0.611)] (Fig. 4B).
Discussion
This is the first time that an evaluation of the recovery of complete immunohistological function was performed with simultaneous measurement of different immunological parameters in CD4+ and CD8+ T cells, thymic volume, and fibrosis in lymphoid tissue in a randomized clinical trial comparing LPV/r and EFV. Overall, despite a different gain of CD4 count, a similar degree of restoration of immunological function was observed in both treatment groups. We have employed a comprehensive approach of simultaneous measurement of different immunological parameters in CD4+ and CD8+ T cells at baseline and after 48 weeks of cART in a subset of 22 patients out of 50 cART-naive HIV-infected individuals who were randomized to receive LPV/r or EFV both with tenofovir/emtricitabine for 48 weeks. Overall, we have observed a significant immune restoration in the whole cohort. As described by other authors, after 48 weeks of cART, we observed an increase of central memory CD4+ T cells,21–23 thymic recent emigrants,24 and CD127 (IL-7 receptor involved in T cell homeostasis) expression on T cells,25,26 as well as a decrease of effector memory T cells,27 Treg cells,28 and markers of activation, senescence, exhaustion, and apoptosis.13–15,29 However, as reported previously by our group and other authors, cART did not have an effect on lymph node fibrosis after 1 year of treatment.30–33 These data indicate that collagen deposition may limit complete immune reconstitution (although we did not include a control group of healthy individuals to test to what extent immune recovery was complete) and that additional therapeutic strategies are needed to overcome this problem and improve immune recovery.
Some studies3–6 have described a higher increase of CD4+ T lymphocyte counts from baseline in the LPV/r regimen as compared with the EFV regimen. Conversely to what has been shown in these studies,3–6 we found that the CD4+ count increase was significantly lower in the LPV/r group despite the fact that all the patients had an undetectable level of plasma viremia after 48 weeks of cART. The Lake study also showed that patients on LPV/r-containing regimens had a lower CD4+ count increase, although the differences were not significant.7 It is likely that the discrepancy in the results of the Lake study and our clinical trial as compared with the rest of the studies could be due to the small number of patients included. However, it could not be excluded that the higher increase in the expression of CD127 in T cell subsets in the EFV group observed in our clinical trial could also explain this difference, since expression of CD127 has been previously associated with CD4 gains in treated patients.26 Interestingly, only in the EFV group of patients, in whom a higher increase in CD127 expression and in CD4 counts was found, was a decrease of IL-7 plasma levels observed, in agreement with the homeostatic role of this molecule.
In any case, despite this difference in the delta level of increase of the CD4 count between the EFV and LPV/r groups of patients in our study, the degree of restoration of immunological function was similar in both treatment groups. Remarkably, we did not observe a significant difference in the absolute CD4+ count at week 48 between both groups. Our data would support the hypothesis that clinically important CD4+ T cell count responses are likely to be better defined in terms of absolute post-cART CD4+ T cell counts, rather than change from baseline,12 and would explain why most of the studies have not shown meaningful clinical outcome differences between NNRTI and PI regimens.
The main drawback of our study was the small number of patients included in each group. Other authors have also found that restoration of immunological function is similar with different cART strategies.13–18 However, none of them performed a comprehensive analysis of different T cell subsets grouped in eight different functional categories and correlated the differences in total gain of CD4+ T cells with the degree of recovery of other immunological parameters.14–18 Another limitation of our study was that we did not take samples from mucosal tissues (i.e., rectal biopsies). The relevance of gut in the immunopathogenesis of HIV infection and immune recovery with cART is well known. Data about changes in the intestinal mucosal immune system and viral load with different cART regimens deserve further investigation.
In summary, despite the difference in absolute CD4+ increase between LPV/r and EFV-containing regimens, our data indicate that in patients with a similar level of HIV immunosuppression the degree of restoration of immunological function (including immune activation) with cART is similar in both treatment groups. Although this is a study with a low number of patients, these data support the fact that the differences in absolute CD4+ gain with different cART regimens are not immunologically meaningful and explain the similar clinical efficacy of the regimens. Larger clinical trials would be needed to confirm these results.
Supplementary Material
Acknowledgments
This study was partially supported by Grants SAF 2012-39075, EC10-153, TRA-094, PS09/01297, and RD06/0006, as well as by RIS [Red Temática Cooperativa de Grupos de Investigación en Sida del Fondo de Investigación Sanitaria (FIS)] and IDIBAPS (Institut d'Investigacions Biomèdiques August Pi I Sunyer). This study was presented in part at the 20th Conference on Retroviruses and Opportunistic Infections, March 3–6, 2013, Atlanta, GA (Abstract 310).
Author contributions: Berta Torres, Norma I. Rallón, José M. Benito, and Felipe Garcia contributed equally to this study. B.T., E.M., A.C., J.A.A., J.M.G., and F.G. contributed to the design of the clinical study. B.T., M.L., E.M., A.C., J.A.A., L.L., C.L., A.L., E.N., B.C., and F.G. contributed to the implementation of the clinical protocol. N.R., J.M.B., and F.G. contributed to the design of the immunological study. B.T., N.I.R., A.D., L.A., M.S., J.M.B., and F.G. contributed to the immune analysis and interpretation of the immune data. B.T., N.I.R., B.C., J.M.G., J.M.B., and F.G. contributed to the writing of the manuscript. All authors approved the final manuscript.
ClinicalTrials.gov number: NCT00759070.
Author Disclosure Statement
The following authors have received research funding, consultancy fees, or lecture sponsorships, or served on advisory boards: F. García: Abbott, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, and MSD. J.M. Gatell: Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Pfizer, Theratechnologies, and Tibotec. E. Martinez: Abbot, Boehringer-Ingelheim, Bristol-Meyers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec and ViiV Healthcare. E. Negredo: Abbvie, Bristol-Myers Squibb, Gilead Sciences, ViiV, Janssen, and Merck Sharp & Dohme. B. Clotet: BMS, Abbott, Gilead, Janssen, Merck, Siemens, and ViiV.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. : Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338(13):853–860 [DOI] [PubMed] [Google Scholar]
- 2.Thompson MA, Aberg JA, Hoy JF, et al. : Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society–USA panel. JAMA 2012;308(4):387–402 [DOI] [PubMed] [Google Scholar]
- 3.Riddler SA, Haubrich R, DiRienzo AG, et al. : Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med 2008;358(20):2095–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manfredi R, Calza L, and Chiodo F: First-line efavirenz versus lopinavir-ritonavir-based highly active antiretroviral therapy for naive patients. AIDS 2004;18(17):2331–2333 [DOI] [PubMed] [Google Scholar]
- 5.Torti C, Maggiolo F, Patroni A, et al. : Exploratory analysis for the evaluation of lopinavir/ritonavir-versus efavirenz-based HAART regimens in antiretroviral-naive HIV-positive patients: Results from the Italian MASTER Cohort. J Antimicrobe Chemother 2005;56(1):190–195 [DOI] [PubMed] [Google Scholar]
- 6.Barreiro P, Soriano V, Casas E, and Gonzalez-Lahoz J: Different degree of immune recovery using antiretroviral regimens with protease inhibitors or non-nucleosides. AIDS 2002;16(2):245–249 [DOI] [PubMed] [Google Scholar]
- 7.Echeverria P, Negredo E, Carosi G, et al. : Similar antiviral efficacy and tolerability between efavirenz and lopinavir/ritonavir, administered with abacavir/lamivudine (Kivexa), in antiretroviral-naive patients: A 48-week, multicentre, randomized study (Lake Study). Antiviral Res 2010;85(2):403–408 [DOI] [PubMed] [Google Scholar]
- 8.MacArthur RD, Novak RM, Peng G, et al. : A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): A long-term randomised trial. Lancet 2006;368(9553):2125–2135 [DOI] [PubMed] [Google Scholar]
- 9.De Luca A, Cozzi-Lepri A, Antinori A, et al. : Lopinavir/ritonavir or efavirenz plus two nucleoside analogues as first-line antiretroviral therapy: A non-randomized comparison. Antiviral Ther 2006;11(5):609–618 [PubMed] [Google Scholar]
- 10.Portsmouth S, Stebbing J, Gill J, et al. : A comparison of regimens based on non-nucleoside reverse transcriptase inhibitors or protease inhibitors in preventing Kaposi's sarcoma. AIDS 2003;17(11):F17–22 [DOI] [PubMed] [Google Scholar]
- 11.Rates of disease progression according to initial highly active antiretroviral therapy regimen: A collaborative analysis of 12 prospective cohort studies. J Infect Dis 2006;194(5):612–622 [DOI] [PubMed] [Google Scholar]
- 12.Moore DM, Harris R, Lima V, et al.: Effect of baseline CD4 cell counts on the clinical significance of short-term immunologic response to antiretroviral therapy in individuals with virologic suppression. J Acquir Immune Defic Syndr 2009;52(3):357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchetti G, Merlini E, Sinigaglia E, et al. : Immune reconstitution in HIV+ subjects on lopinavir/ritonavir-based HAART according to the severity of pre-therapy CD4+. Curr HIV Res 2012;10(7):597–605 [DOI] [PubMed] [Google Scholar]
- 14.Plana M, Garcia F, Gallart T, et al. : Immunological benefits of antiretroviral therapy in very early stages of asymptomatic chronic HIV-1 infection. AIDS 2000;14(13):1921–1933 [DOI] [PubMed] [Google Scholar]
- 15.Plana M, Ferrer E, Martinez C, et al. : Immune restoration in HIV-positive, antiretroviral-naive patients after 1 year of zidovudine/lamivudine plus nelfinavir or nevirapine. Antiviral Ther 2004;9(2):197–204 [PubMed] [Google Scholar]
- 16.Rizzardini G, Trabattoni D, Capetti A, et al. : An immunological comparison of third companion in advanced drug-naive HIV-infected patients. HIV Clin Trials 2006;7(5):221–228 [DOI] [PubMed] [Google Scholar]
- 17.Gandhi RT, Spritzler J, Chan E, et al.: Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: Results from ACTG 384. J Acquir Immune Defic Syndr 2006;42(4):426–434 [DOI] [PubMed] [Google Scholar]
- 18.Funderburg N, Kalinowska M, Eason J, et al. : Effects of maraviroc and efavirenz on markers of immune activation and inflammation and associations with CD4+ cell rises in HIV-infected patients. PloS One 2010;5(10):e13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franco JM, Rubio A, Martinez-Moya M, et al.: T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood 2002;99(10):3702–3706 [DOI] [PubMed] [Google Scholar]
- 20.McCune JM, Loftus R, Schmidt DK, et al. : High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Invest 1998;101(11):2301–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esser MT, Marchese RD, Kierstead LS, et al. : Memory T cells and vaccines. Vaccine 2003;21(5–6):419–430 [DOI] [PubMed] [Google Scholar]
- 22.Hua W, Jiao Y, Zhang H, et al. : Central memory CD4 cells are an early indicator of immune reconstitution in HIV/AIDS patients with anti-retroviral treatment. Immunol Invest 2012;41(1):1–14 [DOI] [PubMed] [Google Scholar]
- 23.Landay A, da Silva BA, King MS, et al. : Evidence of ongoing immune reconstitution in subjects with sustained viral suppression following 6 years of lopinavir-ritonavir treatment. Clin Infect Dis 2007;44(5):749–754 [DOI] [PubMed] [Google Scholar]
- 24.Vrisekoop N, van Gent R, de Boer AB, et al. : Restoration of the CD4 T cell compartment after long-term highly active antiretroviral therapy without phenotypical signs of accelerated immunological aging. J Immunol 2008;181(2):1573–1581 [DOI] [PubMed] [Google Scholar]
- 25.Dunham RM, Cervasi B, Brenchley JM, et al. : CD127 and CD25 expression defines CD4+ T cell subsets that are differentially depleted during HIV infection. J Immunol 2008;180(8):5582–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benito JM, Lopez M, Lozano S, Gonzalez-Lahoz J, and Soriano V: Down-regulation of interleukin-7 receptor (CD127) in HIV infection is associated with T cell activation and is a main factor influencing restoration of CD4(+) cells after antiretroviral therapy. J Infect Dis 2008;198(10):1466–1473 [DOI] [PubMed] [Google Scholar]
- 27.Kolber MA: CD38+ CD8+ T-cells negatively correlate with CD4 central memory cells in virally suppressed HIV-1-infected individuals. AIDS 2008;22(15):1937–1941 [DOI] [PubMed] [Google Scholar]
- 28.Presicce P, Orsborn K, King E, Pratt J, Fichtenbaum CJ, and Chougnet CA: Frequency of circulating regulatory T cells increases during chronic HIV infection and is largely controlled by highly active antiretroviral therapy. PloS One 2011;6(12):e28118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lange CG, Lederman MM, Madero JS, et al. : Impact of suppression of viral replication by highly active antiretroviral therapy on immune function and phenotype in chronic HIV-1 infection. J Acquir Immune Defic Syndr 2002;30(1):33–40 [DOI] [PubMed] [Google Scholar]
- 30.Diaz A, Alos L, Leon A, et al. : Factors associated with collagen deposition in lymphoid tissue in long-term treated HIV-infected patients. AIDS 2010;24(13):2029–2039 [DOI] [PubMed] [Google Scholar]
- 31.Diaz A, Garcia F, Mozos A, et al. : Lymphoid tissue collagen deposition in HIV-infected patients correlates with the imbalance between matrix metalloproteinases and their inhibitors. J Infect Dis 2011;203(6):810–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estes JD, Haase AT, and Schacker TW: The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Sem Immunol 2008;20(3):181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estes J, Baker JV, Brenchley JM, et al. : Collagen deposition limits immune reconstitution in the gut. J Infect Dis 2008;198(4):456–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.