Abstract
Objective: The purpose of this study was to determine the effectiveness of laser-activated irrigation by photon-induced photoacoustic streaming (PIPS) using Er:YAG laser energy in decontaminating heavily colonized root canal systems in vitro. Materials and methods: Extracted single-rooted human teeth (n=60) were mechanically and chemically prepared, sterilized, inoculated with Enterococcus faecalis for 3 weeks, and randomly assigned to four groups (n=15): Group I (control, no decontamination), Group II (PIPS+6% NaOCl), Group III (PIPS+saline), and Group IV (6% NaOCl). PIPS settings were all preset to 50 μsec pulse, 20 mJ, 15 Hz, for an average power of 0.3 W. After decontamination, the remaining live microbes from all specimens were collected and recovered via plate counting of the colony-forming units (CFUs). Randomized root canal surfaces were examined with scanning electron microscopy and confocal laser microscopy. Mean variance and Dunnett's t test (post-hoc test) comparisons were used to compare mean scores for the three groups with the control group. Results: The CFU analysis showed the following measurements (mean±SE): Group I (control), 336.8±1.8; Group II (PIPS+NaOCl), 0.27±0.21; Group III (PIPS+saline), 225.0±21; and Group IV (NaOCl), 46.9±20.29. Group II had significantly lower CFUs than any other groups (p<0.05). Both imaging analyses confirmed levels of remaining bacteria on examined root surfaces. Conclusions: The use of the PIPS system along with NaOCl showed the most efficient eradication of the bacterial biofilm. It appears that laser-activated irrigation (LAI) utilizing PIPS may enhance the disinfection of the root canal system.
Introduction
Effective cleaning and shaping of the root canal system to maximally eliminate microbes is a prerequisite for successful endodontic treatment.1–3 One important aspect of successful treatment involves the irrigant selected as well as how it is delivered and agitated.4 Various approaches to agitate the irrigant have been tested. Sonic and ultrasonic irrigation techniques appear to be more effective than syringe irrigation alone.4–6 Laser-activated irrigation (LAI) utilizing laser energy has been found to enhance the irrigation efficacy of NaOCl.7,8 This is because the Er:YAG's wavelength is absorbed more effectively by the water molecules within the irrigants, resulting in more aggressive irrigant agitation.9–11
A new LAI system device that has been recently introduced, photon-induced photoacoustic streaming (PIPS), uses a very low power source (subablative) to rapidly pulse laser light energy, which is absorbed by the molecules within the irrigant. This transfer of energy results in a series of rapid and powerful shockwaves, capable of forcefully propelling the irrigant throughout the entire root canal system.12,13 The specially designed Er:YAG laser-based PIPS tip utilizes a tapered tip with 3 mm of polyamide sheathing stripped from its distal end to greatly improve the transfer of light energy into the irrigant. Previous studies indicate that PIPS appears to improve canal wall cleanliness with a greater number of open tubules than when these same irrigants were used without PIPS.13 In comparison with an ultrasonic device, PIPS-activated irrigation was shown to remove more bacteria/film in the root canal space.14 The purpose of this in vitro study was to evaluate the effectiveness of PIPS associated or not with 6% NaOCl in decontaminating root canal systems inoculated with heavily colonized Enterococcus faecalis.
Material and Methods
Sample collection
The procedures utilized in this study conformed to the protocols approved by the Institutional Review Board of the Arizona School of Dentistry and Oral Health (ASDOH) (IRB# 2009-26). Sixty-eight extracted teeth were collected from the clinic in the Department of Oral and Maxillofacial Surgery, and immediately placed in 10% formalin (Fisher Scientific Company LLC, Kalamazoo, MI) fixative solution. Only intact teeth with single canals were selected for this study.
Sample preparation
Each sample tooth was accessed, and patency was established and maintained using a size 15 K-file (Dentsply Maillefer, Tulsa, OK). The coronal third of each canal was enlarged using the crown-down technique, starting with Gates Glidden burs (sizes #4–2). A minimal preparation protocol was followed with the largest file used to working length being a #25/0.08 taper Twisted File (SybronEndo, Orange, CA). During instrumentation, RC Prep (Premium Products, Plymouth Meeting, PA) was used as a lubricant, followed by irrigation with NaOCl (6%) after each instrument use and recapitulation. At the completion of the canal preparation an aliquot of 1 mL 17% ethylenediaminetetraacetic acid (EDTA) was placed into the canal for 1 min to remove the smear layer, which was followed by needle irrigation with 6% NaOCl for 1 min. Teeth were then autoclaved in phosphate-buffered saline (PBS) (PBS, pH 7.2), at 120°C for 20 min. Eight teeth were randomly selected for a plate count technique (described subsequently) test and scanning electron microscopy (SEM) imaging to confirm the patency of dentinal tubules and the complete eradication of the preexisting microbial colonization or biofilm and smear layer.
E. faecalis culture and inoculation
Growth of E. faecalis (ATCC 4083) was maintained by weekly subculturing in trypticase soy agar plates. The agar plates contained BHI agar (Becton, Dickinson, and Co., Sparks, MD), yeast extract (Fisher Biotech, Fair Lawn, NJ), 5 g/mL hemin, 0.3 g/mL vitamin K, and 5% sheep blood (Becton, Dickinson, and Co.). Microorganisms grown on agar plates in a 37°C incubator for 72 h were inoculated into BHI broth and incubated overnight. Cells were scattered by vortexing and repeated passage to ensure a homogeneous population of scattered planktonic bacteria. Cell numbers were then measured by spectrophotometry (Spectronic 20 Genesys, Thermo Electron Scientific Instruments Corporation, Madison, WI) at 600 nm in 1 mL cuvettes (0.1 optical density unit equals ∼108 cells/mL).15
Each tooth sample was transferred to a 2 mL sterile tube. One milliliter of BHI broth containing 108 E. faecalis grown in the exponential phase was delivered, via a syringe with a 30 gauge irrigation needle, into the prepared root canal system. After bacterial inoculation into the canal, the entire tooth specimen was submerged in the BHI broth. All sample tubes were kept in a warm chamber at 37°C for 3 weeks. The medium was changed daily with fresh BHI broth. This process was to establish E. faecalis biofilm. After the incubation period, the medium was aspirated from the tubes. All procedures were conducted under sterile conditions. The outcome key was to count the residual level of colony-forming unit (CFUs).
Cleaning and decontamination of the root canal system
The experiment included 60 teeth that were randomly divided into four independent groups (n=15 per group) (Table 1). Group I was the control group, and did not undergo any irrigation treatment. Group II samples were activated with PIPS utilizing 6% NaOCl as the irrigant, whereas Group III samples were activated with PIPS utilizing saline as the irrigant. Group IV samples were irrigated with 21 mL 6% NaOCl, delivered via a 30 gauge needle syringe for a total of 90 sec, but no PIPS. The 6% NaOCl was chosen for its effectiveness in disinfecting E. faecalis biofilms.16
Table 1.
Treatment Protocol
| Intervention | |||||
|---|---|---|---|---|---|
| 6% NaOCl | Saline | ||||
| Group | Volume (mL) | Time (sec) | Volume (mL) | Time (sec) | Volume/time |
| Group I-control | None | None | None | None | None |
| Group II-(PIPS+NaOCl) | 21 | 90 | 7 | 60 | 28/150 |
| Group III-(PIPS+saline) | None | None | 28 | 150 | 28/150 |
| Group IV-passive NaOCl irrigation | 21 | 90 | 7 | 60 | 28/150 |
PIPS, photon-induced photoacoustic streaming.
Each tooth in all four groups underwent the following procedures: The surface of each tooth sample was wiped with a clean gauze pad soaked with NaOCl, after which the tooth was mounted onto a sterile plastic holder. The apex was sealed with two layers of nail varnish. The PIPS groups (II and III) were exposed to laser irradiation by an Er:YAG laser (Fotona LightWalker DT Ljubljana, Slovenia) with a wavelength of 2940 nm in 30 sec exposure intervals. The laser was set to 50 μsec pulse duration at a 15 Hz pulse rate and 20 mJ of energy, thereby delivering a total of 0.3 W of power. A newly designed PIPS quartz tip was used (600 μm diameter, 9 mm long). The tip was tapered, and had 3 mm of the polyamide sheath stripped back from its end (Fig. 1A). PIPS utilizes a unique tapered and stripped tip that increases the available surface interface for photons of light escaping. Setting for PIPS was established to be below the threshold of dentin ablation (≤20 mJ), thereby avoiding thermal damage as seen with other laser techniques. Also, PIPS utilizes extremely low microsecond pulse durations (50 μsec) generating greater peak powers than longer pulse durations. This creates powerful pressure and shockwaves that travel three dimensionally throughout the fluid-filled root canal systems without the need to place the tip near the morphologically delicate apical third.17 Both the air and water spray feature of the laser unit was set to “off.” The tip was then placed in the coronal pulpal chamber of the access opening only (Fig. 1B), remaining stationary, and was not advanced apically into the root canal during laser activation. The canal system was passively filled with 6% NaOCl via 30 gauge needle syringe. A laser activation using PIPS tip protocol was followed. Thirty seconds on, then 30 sec off, and this cycle was performed three times (i.e., total of 90 sec of activation). The off or “resting” phase in-between laser activation allowed for greater release of the more active forms of NaOCl as described by the literature.7 The amount of NaOCl solution used during each 30 sec exposure measured out to be 7 mL per cycle, hence the total NaOCl irrigation volume used was 21 mL (3×7=21). The canal was then syringe irrigated with sterile saline for 60 sec.
FIG. 1.
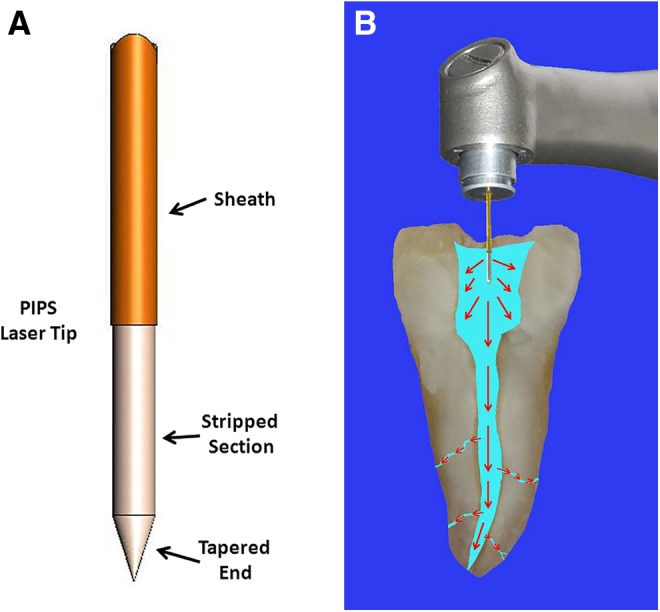
A close-up view of the photon-induced photoacoustic streaming (PIPS) tip and its composition, with stripped sheath that helps to propagate the shockwaves in the root canal system (A). Illustration shows how the PIPS is placed in the coronal aspect of access only, not in the canal, and how it delivers the shock waves (B).
Microbial counts
After root canal decontamination, the root canal was filled with 50 μL sterile BHI broth. Paper points (Course sized, Dentsply Maillefer) were immediately placed into the root canal space to absorb the broth until the canal dried. The paper points were then placed into a microfuge tube containing 500 μL BHI broth, and the tube was vortexed to release the microbes into the broth medium. The vortexed broth was then inoculated onto agar plates containing sheep blood. The formation of bacterial colonies (CFUs) after 24 h was observed, and the CFUs were counted.
SEM
Five teeth were randomly selected from each group. A diamond disc was used to cut a groove along the long axis of the tooth without reaching the root canal system. A chisel was then used to split the tooth open into two pieces. These procedures were performed in a clean area. to avoid contaminating the samples. Both tooth fragments were placed in a sterile 2 mL tube, and one of them was fixed with 3.0% formaldehyde plus 1.5% glutaraldehyde in 0.1M Na cacodylate plus 5 mM Ca2+, 2.5% sucrose, pH 7.4 for SEM. The other one was processed without fixation for confocal laser microscopy (CLM).
The fixed sample was dehydrated with 10% ethanol and sequentially transferred to higher percentages of ethanol until reaching 100% ethanol. The ethanol content was then replaced by hexamethyldisilazane (HMDS) through a graded series of ethanol HMDS mixtures as follows: (1) 25% HMDS in ethanol, (2) 50% HMDS, and (3) 75% HMDS followed by three exchanges in 100% HMDS. The last bath of HMDS was reduced in volume until the liquid just covered the sample. This bath was allowed to evaporate for at least 8 h, leaving the sample completely devoid of any moisture. Subsequently, samples were mounted onto a stub and gold sputter coated for SEM analysis under 15 kW.18 The root canal surface of each specimen was examined randomly under two magnifications, 750×and 1500×, respectively.
CLM
The other halves of the split root samples were used to perform the CLM. The tooth specimens were split into two pieces, using the method described, and placed in 2 mL tubes. Then the root surface of one half was exposed to the reagents of a LIVE/DEAD BacLight Bacterial Viability Kit (Molecular Probes, Inc., Eugene, OR) for 15 min, according to the manufacturer instructions. Negative control tooth specimen only received canal enlargement, using the same method as for experimental tooth samples, and was not infected. The specimen tubes were covered with aluminum foil to prevent the sample from light exposure, and kept in a refrigerator set at 4°. CLM analysis allowed distinguishing viable from nonviable bacteria on root canal walls and in dentin tubules. To detect the presence of green biofilms (living) or red biofilms (dead), we used a Zeiss LSM 510 confocal microscope (Carl Zeiss, Germany) with 40×objective. Green fluorescence was detected using a 30 mW argon 488 nm laser, set at an output of 8% acousto-optic tunable filter (AOTF) and red with 1.5 mW HeNe 543 nm laser, set to output at 50% AOTF. This resulted in 2.4 and 0.75 mW, respectively, of illumination power of the samples.
Data analysis
The ANOVA model was used to compare the CFU means with an overall α ≤0.05. Dunnett's t test (post-hoc test) comparison was used to compare the mean score for the three techniques with the control group.
Results
Effective elimination of E. faecalis biofilm demonstrated by microbial analysis
The recovered bacteria presented in Table 2 shows that Group II had essentially no viable E. faecalis recoverable from the decontaminated canals via the sample collection process. Out of 15 samples, only 2 had recovered colonies (#3, 3 colonies and #15, 1 colony). The traditional NaOCl irrigation group (Group IV) was the next most effective approach to decontaminating the canal, with 10 out of 15 samples having recovered E. faecalis, each ranging between 2 and 296 colonies. The ANOVA procedure suggests that the four different techniques yield significantly different means of recovered live E. faecalis CFU counts, as the F test is highly significant (F=121.514, p value<0.002). The observed power for the analysis is 1.0. The model adequacy measure R2 suggests that 86.7% variability in the recovered live E. faecalis CFU counts can be explained by the one way ANOVA model. All three techniques have significant difference among mean scores from the control group, as suggested by Dunnett's t test (post-hoc test) in Table 3. The maximum difference may be seen in Group II (PIPS+NaOCl).
Table 2.
Recovered Live Enterococcus faecalis After the Treatment
| Recovered live E. faecalis CFU counts | ||||
|---|---|---|---|---|
| Group I (Control) | Group II (PIPS+NaOCl) | Group III (PIPS+saline) | Group IV (NaOCl only) | |
| Mean±SE | 336.8±1.8 | 0.27±0.21 | 225.0±21.2 | 46.9±20.29 |
CFU, colony-forming units.
Table 3.
Dependent Variable: Recovered Live Enterococcus faecalis CFU Counts (Dunnett's t Test)
| Treatment groups (I) | Control group (J) | Mean difference (I-J) | Standard error | Significance | 95% confidence interval upper limit |
|---|---|---|---|---|---|
| PIPS+NaOCl | Control | −336.5333 | 0.76354 | 3.784E-29 | −292.7849 |
| NaOCl Irrigation | Control | −289.8667 | 0.76354 | 1.022E-09 | −246.1182 |
| PIPS+NaCl | Control | −81.8000 | 20.76354 | 0.00175 | −38.0515 |
CFU, colony-forming units; I, J, randomly chosen letters to represent the experimental or control groups.
SEM analysis revealing decontaminated canal walls after PIPS and NaOCl treatment
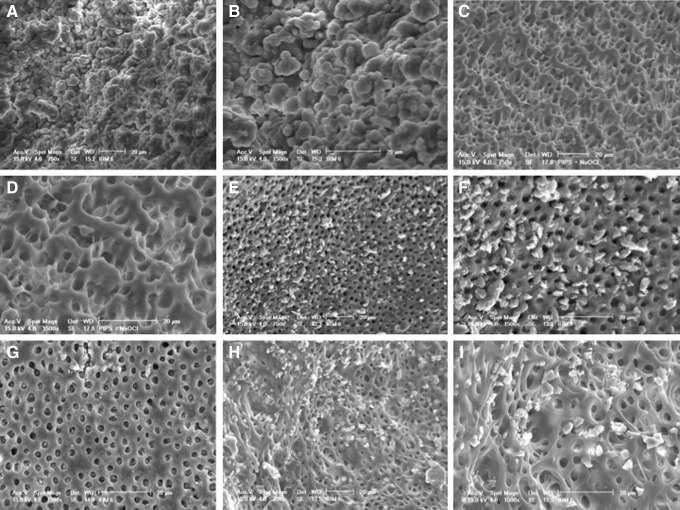
It was shown by other reports that 10 days after inoculation of E. faecalis, a biofilm was formed with microbial penetration into dentinal tubules, and a thick biofilm was established on dentin surface after 2–3 weeks of inoculation.19–21 In our present studies in the control group (Group I), multilayers of bacterial colonization resembling a mature biofilm formation was observed on the root canal wall surface (Fig. 2A, B). In contrast, the PIPS+NaOCl group (Group II) showed a complete depletion of any bacteria or colonies in the samples (Fig. 2C, D). Three weeks of E. faecalis colonization from a saturated loading dose of bacteria were removed by the PIPS+NaOCl cleaning process. In Group III, saline was activated by PIPS, and the effect of PIPS in removing E. faecalis colonies was clearly visible, yet not as significant as when it was accompanied with NaOCl (Fig. 2E, F). For the NaOCl only group (Group IV), there was still significant colonization observed, as shown in Fig. 2G–I. This demonstrates that NaOCl is more effective when laser activated with PIPS.
FIG. 2.
Scanning electron microscope analysis of root canal surface. (A and B) Group I shows E. faecalis colonies attached to the root canal surface. (C and D) Group II (PIPS+ NaOCl) shows a clean root canal surface. (E and F) Group III (PIPS+saline) shows colonies attached to the root canal surface. (G–I) Group IV (irrigation with NaOCl) shows some colonies and the other image shows no colonies.
CLM analysis showing dead bacteria after PIPS and NaOCl treatment
The split-opened tooth samples were examined as shown in Fig. 3A. The negative control tooth sample that was sterile, and not exposed to bacteria, showed no autofluorescence background (Fig. 3B). The control (Group I) showed much green (living) fluorescence (Fig. 3C). Conversely, Group II (PIPS+NaOCl) showed little green and mostly red (dead) fluorescence (Fig. 3D). As anticipated, Group III (PIPS+saline) showed much green fluorescence, indicating less effectiveness when compared with use of NaOCl with PIPS (Fig. 3E). Finally, Group IV (NaOCl and conventional needle irrigation alone) showed red fluorescence limited to the superficial layer only, rather than the deeper penetration seen when NaOCl was activated with PIPS, resulting in the presence of live bacteria in the dentinal tubules (Fig. 3F).
FIG. 3.
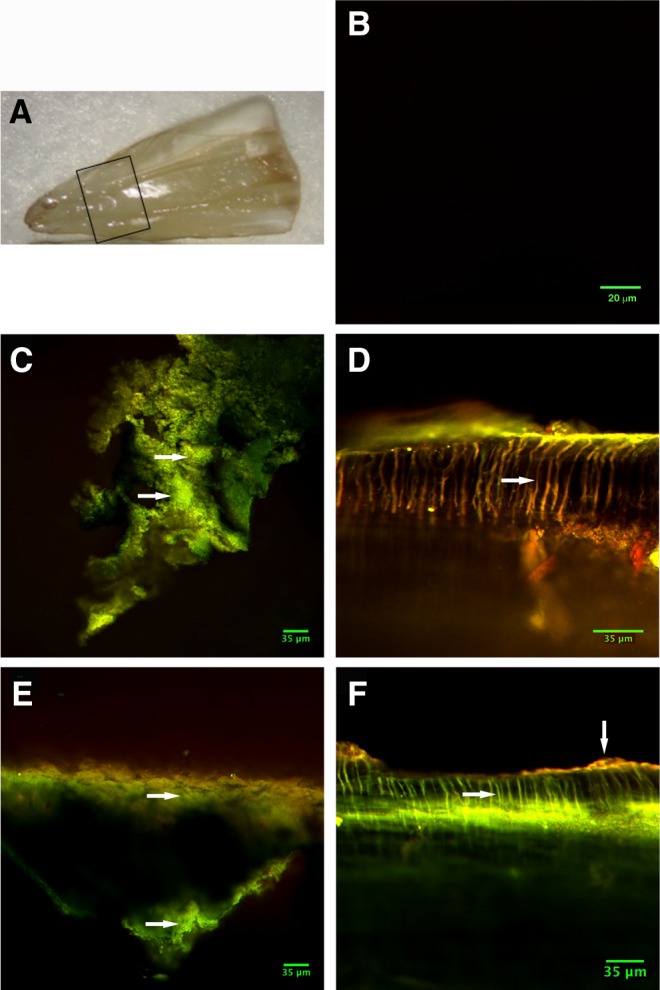
Confocal scanning laser microscopy analysis of live/dead bacteria on root canal surface. (A) Example of a split-opened tooth sample showing the exposed root canal surface. The box at the mid-root area of the root canal system indicates where the imaging analysis was performed. (B) Negative control sterile tooth samples showing no detectable autofluorescence background. (C–F) Experimental samples with live bacterial biofilms are shown in green fluorescence and the dead bacterial biofilm in red fluorescence. (C) Group I, a control sample with no treatment, the green fluorescence (arrows) indicate live bacteria. (D) A representative sample from Group II (PIPS+NaOCl) showing red fluorescence (arrow) in dentinal tubules indicative of dead bacteria. (E) Group III, (PIPS+saline), the green indicates still live bacteria (marked with arrows) with some red dead bacteria. (F) Group IV, NaOCl with no PIPS, shows the red fluorescence (upper vertical arrow) on the superficial layer with green florescence deeper in dentin tubules (lower horizontal arrow) where NaOCl was unable to penetrate without laser activation.
Discussion
This in vitro study model tested laser-activated irrigation using an Er:YAG laser and PIPS technique in conjunction with both NaOCl and saline. Results showed that NaOCl activated by PIPS was the most effective method for removing E. faecalis biofilm in the root canal system when compared with the other irrigation techniques tested. This method both mechanically and chemically debrides and decontaminates the root canal system using Er:YAG laser energy at subablative power levels with a short 50 μsec pulse duration at 15 Hz and 0.3 W of power. The heavy biofilms in the root canal system established by E. faecalis were effectively eliminated when using PIPS in conjunction with NaOCl. This finding could be attributed to the known bactericidal effects of NaOCl enhanced by the photomechanical effect seen when light energy is pulsed in liquid.22 The possible reasons for differences in the efficacy of lasers in endodontic therapy could be the result of the different parameters used in various methods, including the delivery technique, tip design, the time of application within the canal, presence of an aqueous solution that would affect the absorption of the laser beam and power of the laser, and, finally, the density of energy delivered.23
Most of the previous literature cited utilizes the thermal effect of lasers to disinfect the canal. Lasers used in a thermal capacity have inherent disadvantages. Conversely, PIPS utilizes a photoacoustic, subablative technique and does not require heat to create the shockwave.12,24,25 Instead, it is a photoacoustic event and because the energies required are so low, ≤20 mJ, the levels of heat transfer are referred to as “subablative.” There is an negligible increase in temperature in the root canal space up to only 1.5°C.12
PIPS protocol also utilizes alternating 30 sec cycles of activation and 30 sec of “resting” from laser activation. Activation has been shown to be a strong modulator of the reaction rate of NaOCl, whereas during the rest interval, the consumption of available chlorine increases significantly. This effect seems to be more pronounced after irrigant activation by laser.7
One of the major etiologies of endodontic failure is the persistence of a bacterial biofilm following root canal therapy.26 Investigators have examined different methods in order to disinfect the root canal systems. These methods have included various techniques and protocols, including machine-assisted irrigation.27 Lasers have been utilized to eliminate the bacterial biofilms from the root canal system with varying degrees of success. E. faecalis is a well-studied microorganism in the endodontic literature both because of its virulence and because it is the microorganism most often isolated in failed root canal treatments.28 This makes the results of this study noteworthy. Our study shows that following decontamination and mechanical conventional use of NaOCl, the activation of NaOCl with PIPS for 90 sec along with 90 sec of resting, was sufficient to achieve near-zero growth of E. faecalis within the canal system under our experimental settings. This demonstrates that PIPS does have a positive (mechanical) cleaning effect. PIPS is made more effective when used in combination with a known cleaning irrigant such as NaOCl. Most likely, the acoustic streaming and forceful shockwaves created by the tapered and stripped PIPS tip design create a more effective disruption and eradication of biofilm via its photomechanical effects as opposed to the thermal effects of laser energy as described in past literature.25 From the chemical perspective, we assume that the greater ability for disinfection is caused by the photoacoustic effect of PIPS, which actively liberates the antimicrobial hypochlorous acid (HOCl) and hypochlorite ion (OCl-) from NaOCl.7 It should be noted that in the clinical setting, particularly in repeat treatment cases, E. faecalis may live in a starvation phase. They have been found to be more resistant to NaOCl and intracanal medicaments.29,30 Further analysis using the bacteria in the starvation phase is needed to validate the efficiency of PIPS using NaOCl as the irrigant. Another concern is the concentration of NaOCl chosen for the present study. One recent report demonstrated that 6% NaOCl was most effective against 3-week-old E. faecalis biofilm.16 However, the risk of extruding the irrigant out of the apex should be considered. Whether 6% NaOCl is the most optimal concentration for the PIPS disinfection protocol should also be tested.
Our results agree with those of previous studies that have confirmed the efficacy of PIPS in the eradication of bacterial biofilm.14,22 We consider that the very low energy levels (20 mJ) and the high peak power (400 W) produced by the 50 μsec pulse of this Er:YAG laser generate photoacoustic shockwaves that allows streaming of irrigants three dimensionally inside the root canal system without the need to place the tip inside the canals. PIPS can have additional advantages over other systems. Effective canal cleaning resulted even when canal preparation with endodontic instruments was kept to a minimum (in this study #25/08 taper), thus allowing the canal to remain mostly in its natural state. Time savings can result when only minimal instrumentation is required and when all canals can be irrigated at the same time. Even more importantly, minimal instrumentation greatly reduces the chance of iatrogenic events occurring, such as file breakage, ledging, perforation, and root fracture. Because PIPS is a photoacoustic event and not a thermal event, as is the case with most other laser techniques, there is no risk of thermal damage to the tooth structure or periodontium.
Conclusions
Laser-activated irrigation using PIPS protocol and NaOCl significantly enhanced the antimicrobial effect by eliminating bacterial biofilm in vitro. This study suggests that PIPS is a promising adjunctive method to conventional root canal therapy.
Acknowledgments
This work was supported in part by research funds from Boston University School of Dental Medicine Department of Endodontics and Department of Restorative Sciences and Biomaterials; and a grant from the National Institutes of Health R01 DE019156 (G.T.-J.H.). The authors thank Ronald L'Herault (Boston University) for his help with SEM, and Drs. Vickery Trinkaus-Randall (Boston University) and Kristien Zaal (NIAMS, NIH) for their expertise in confocal imaging.
Author Disclosure Statement
Drs. Mohammed Al Shahrani, Christopher V. Hughes, Dan Nathanson, and George T.-J. Huang have no competing financial interests. Dr. DiVito is a partner with Medical Dental Advance Technologies Group (MDATG) and has a financial affiliation as a partner in the MDATG group, which helped support a portion of the present studies (use of the laser machine).
References
- 1.Basmadjian-Charles C.L., Farge P., Bourgeois D.M., and Lebrun T. (2002). Factors influencing the long-term results of endodontic treatment: a review of the literature. Int. Dent. J. 52, 81–86 [DOI] [PubMed] [Google Scholar]
- 2.Siqueira J.F., Jr., and Rocas I.N. (2008). Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 34, 1291–1301 [DOI] [PubMed] [Google Scholar]
- 3.Wong R. (2004). Conventional endodontic failure and retreatment. Dent. Clin. North Am. 48, 265–289 [DOI] [PubMed] [Google Scholar]
- 4.Gu L.S., Kim J.R., Ling J., Choi K.K., Pashley D.H., and Tay F.R. (2009). Review of contemporary irrigant agitation techniques and devices. J. Endod. 35, 791–804 [DOI] [PubMed] [Google Scholar]
- 5.Cameron J.A. (1987). The synergistic relationship between ultrasound and sodium hypochlorite: a scanning electron microscope evaluation. J. Endod. 13, 541–545 [DOI] [PubMed] [Google Scholar]
- 6.Sabins R.A., Johnson J.D., and Hellstein J.W. (2003). A comparison of the cleaning efficacy of short-term sonic and ultrasonic passive irrigation after hand instrumentation in molar root canals. J. Endod. 29, 674–678 [DOI] [PubMed] [Google Scholar]
- 7.Macedo R.G., Wesselink P.R., Zaccheo F., Fanali D., and Van Der Sluis L.W. (2010). Reaction rate of NaOCl in contact with bovine dentine: effect of activation, exposure time, concentration and pH. Int. Endod. J. 43, 1108–1115 [DOI] [PubMed] [Google Scholar]
- 8.Yasuda Y., Kawamorita T., Yamaguchi H., and Saito T. (2010). Bactericidal effect of Nd:YAG and Er:YAG lasers in experimentally infected curved root canals. Photomed. Laser Surg. 28Suppl 2, S75–S78 [DOI] [PubMed] [Google Scholar]
- 9.Blanken J., De Moor R.J., Meire M., and Verdaasdonk R. (2009). Laser induced explosive vapor and cavitation resulting in effective irrigation of the root canal. Part 1: a visualization study. Lasers Surg. Med. 41, 514–519 [DOI] [PubMed] [Google Scholar]
- 10.De Moor R.J., Blanken J., Meire M., and Verdaasdonk R. (2009). Laser induced explosive vapor and cavitation resulting in effective irrigation of the root canal. Part 2: evaluation of the efficacy. Lasers Surg. Med. 41, 520–523 [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto H., Yoshimine Y., and Akamine A. (2011). Visualization of irrigant flow and cavitation induced by Er:YAG laser within a root canal model. J. Endod. 37, 839–843 [DOI] [PubMed] [Google Scholar]
- 12.DiVito E.E., Colonna M.P., and Olivi G. (2011). The photoacoustic efficacy of an Er: YAG laser with radial and stripped tips on root canal dentin walls: an SEM evaluation. J. Laser Dent. 19, 156–161 [Google Scholar]
- 13.DiVito E., Peters O.A., and Olivi G. (2012). Effectiveness of the erbium:YAG laser and new design radial and stripped tips in removing the smear layer after root canal instrumentation. Lasers Med. Sci. 27, 273–280 [DOI] [PubMed] [Google Scholar]
- 14.Peters O.A., Bardsley S., Fong J., Pandher G., and Divito E. (2011). Disinfection of root canals with photon-initiated photoacoustic streaming. J. Endod. 37, 1008–1012 [DOI] [PubMed] [Google Scholar]
- 15.Fimple J.L., Fontana C.R., Foschi F., Ruggiero K., Song X., Pagonis T.C., Tanner A.C., Kent R., Doukas A.G., Stashenko P.P., and Soukos N.S. (2008). Photodynamic treatment of endodontic polymicrobial infection in vitro. J. Endod. 34, 728–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z., Shen Y., and Haapasalo M. (2012). Effectiveness of endodontic disinfecting solutions against young and old Enterococcus faecalis biofilms in dentin canals. J. Endod. 38, 1376–1379 [DOI] [PubMed] [Google Scholar]
- 17.Olivi G. (2013). Laser use in endodontics: evolution from direct laser irradiation to laser-activated irrigation. J. Laser Dent. 21, 58–71 [Google Scholar]
- 18.Braet F., De Zanger R., and Wisse E. (1997). Drying cells for SEM, AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. J. Microsc. 186, 84–87 [DOI] [PubMed] [Google Scholar]
- 19.Saber Sel D., and El-Hady S.A. (2012). Development of an intracanal mature Enterococcus faecalis biofilm and its susceptibility to some antimicrobial intracanal medications; an in vitro study. Eur. J. Dent. 6, 43–50 [PMC free article] [PubMed] [Google Scholar]
- 20.Kishen A., George S., and Kumar R. (2006). Enterococcus faecalis-mediated biomineralized biofilm formation on root canal dentine in vitro. J Biomed. Mater. Res. A 77, 406–415 [DOI] [PubMed] [Google Scholar]
- 21.Case P.D., Bird P.S., Kahler W.A., George R., and Walsh L.J. (2012). Treatment of root canal biofilms of Enterococcus faecalis with ozone gas and passive ultrasound activation. J. Endod. 38, 523–526 [DOI] [PubMed] [Google Scholar]
- 22.De Groot S.D., Verhaagen B., Versluis M., Wu M.K., Wesselink P.R., and Van der Sluis L.W. (2009). Laser-activated irrigation within root canals: cleaning efficacy and flow visualization. Int. Endod. J. 42, 1077–1083 [DOI] [PubMed] [Google Scholar]
- 23.Seet A.N., Zilm P.S., Gully N.J., and Cathro P.R. (2012). Qualitative comparison of sonic or laser energisation of 4% sodium hypochlorite on an Enterococcus faecalis biofilm grown in vitro. Aust. Endod. J. 38, 100–106 [DOI] [PubMed] [Google Scholar]
- 24.Doukas A.G., Zweig A.D., Frisoli J.K., Birngruber R., and Deutsch T.F. (1991). Non-invasive determination of shock wave pressure generated by optical breakdown. Appl. Phys. B 53, 237–245 [Google Scholar]
- 25.Muller P., Guggenheim B., Attin T., Marlinghaus E., and Schmidlin P.R. (2011). Potential of shock waves to remove calculus and biofilm. Clin. Oral Investig. 15, 959–965 [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner J.C. (2004). Microbiologic aspects of endodontic infections. J. Calif. Dent. Assoc. 32, 459–468 [PubMed] [Google Scholar]
- 27.Stuart C.H., Schwartz S.A., Beeson T.J., and Owatz C.B. (2006). Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J. Endod. 32, 93–98 [DOI] [PubMed] [Google Scholar]
- 28.Dumani A., Yoldas O., Yilmaz S., Koksal F., Kayar B., Akcimen B., and Seydaoglu G. (2012). Polymerase chain reaction of enterococcus faecalis and candida albicans in apical periodontitis from Turkish patients. J. Clin. Exp. Dent. 4, e34–e39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Portenier I., Waltimo T., Orstavik D., and Haapasalo M. (2005). The susceptibility of starved, stationary phase, and growing cells of Enterococcus faecalis to endodontic medicaments. J. Endod. 31, 380–386 [DOI] [PubMed] [Google Scholar]
- 30.Aoki A., Sasaki K.M., Watanabe H., and Ishikawa I. (2004). Lasers in nonsurgical periodontal therapy. Periodontol. 200036, 59–97 [DOI] [PubMed] [Google Scholar]