Abstract
The cytokine TGF-β plays an integral role in regulating immune responses. TGF-β has pleiotropic effects on adaptive immunity, especially in the regulation of effector and regulatory CD4+ T cell responses. Many immune and nonimmune cells can produce TGF-β, but it is always produced as an inactive complex that must be activated to exert functional effects. Thus, activation of latent TGF-β provides a crucial layer of regulation that controls TGF-β function. In this review, we highlight some of the important functional roles for TGF-β in immunity, focusing on its context-specific roles in either dampening or promoting T cell responses. We also describe how activation of TGF-β controls its function in the immune system, with a focus on the key roles for members of the integrin family in this process.
Keywords: TGF-β, integrins, T cells, Th17 cells, Tregs, dendritic cells
PROCESSING, SECRETION, AND STRUCTURE OF TGF-β
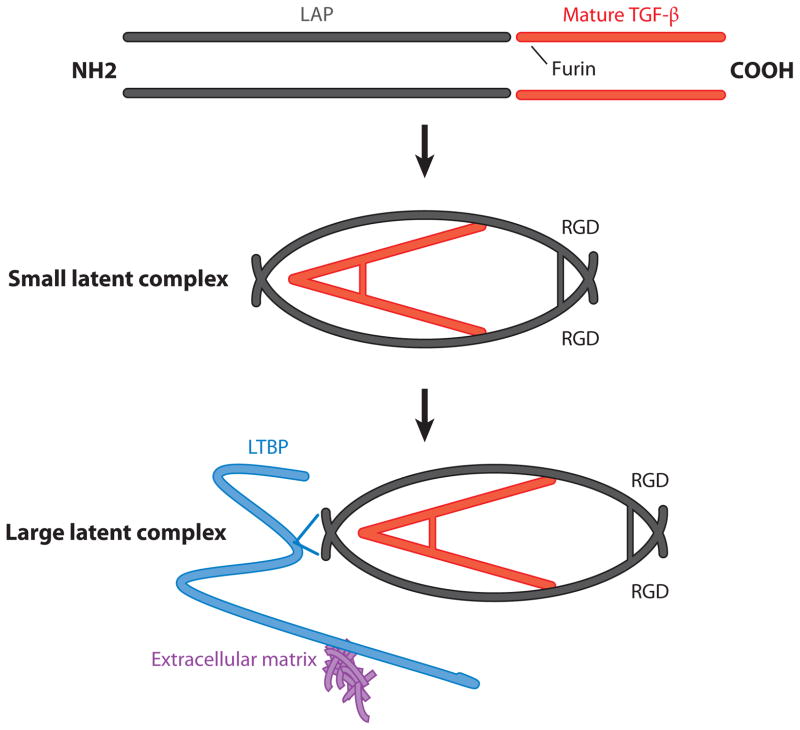
The TGF-β family in mammals includes three members, TGF-β1, 2, and 3. Each is synthesized as a precursor that includes a signal peptide to direct TGF-βs to the endoplasmic reticulum, a large N-terminal portion called the latency-associated peptide (LAP), and a short C-terminal fragment, which is the mature cytokine (1, 2). After the signal peptide is removed, the remaining polypeptide translocates to the lumen of the endoplasmic reticulum, where it is assembled into a dimer with three interchain disulfide bonds and cleaved by the endoprotease furin. Furin cleavage can also occur in the extracellular space after secretion of unprocessed TGF-β. Following furin-mediated cleavage, the disulfide-linked homodimer of the C-terminal fragment remains noncovalently associated with the disulfide-linked homodimer of the N-terminal LAP to form the small latent complex (Figure 1). Furin-mediated cleavage is required for TGF-β activity, and loss of furin from specific cell types (for example, T cells) in mice results in phenotypes very similar to those seen in mice lacking TGF-β production from the same cells (3).
Figure 1.
Synthesis and processing of TGF-β isoforms into small and large latent complexes. TGF-β genes encode an N-terminal latency-associated peptide (LAP) and a C-terminal mature cytokine that form dimeric structures. The LAP and the mature TGF-β cytokine are cleaved from each other by the enzyme furin but remain noncovalently associated. The LAP region folds around the mature cytokine, blocking access of TGF-β to its receptor, and is termed the small latent complex. In the LAP of TGF-β1 and 3 (but not TGF-β2) there is an arginine-glycine-aspartic acid (RGD) site that facilitates binding to integrins. In some cells, the small latent complex can associate with latent TGF-β binding protein (LTBP) via interactions with LAP, forming the large latent complex. LTBP can facilitate binding of the large latent complex to proteins of the extracellular matrix.
In most, but not all, cells the two copies of the N-terminal LAP are themselves chemically cross-linked by disulfide bonds with two cysteine residues in another small family of proteins called latent TGF-β binding proteins (LTBPs) (Figure 1). LTBP1, 3, and, to a lesser degree, 4 can all be associated in this fashion with the small latent complex to form a structure called the large latent complex. LTBP1 and 3 can form disulfides with all three TGF-β isoforms, but LTBP4 appears to associate only with TGF-β1. After secretion, LTBPs interact with other components of the extracellular matrix (especially fibrillin family members) and can be covalently cross-linked to some matrix proteins (for example, fibronectin) through the action of a family of extracellular enzymes called tissue transglutaminases.
The recently solved crystal structure of the small latent complex shows that the active cytokine is encircled by the larger LAP in a wreath-like structure, effectively covering all the contact sites that must interact with TGF-β receptors to induce cellular responses (4). Importantly, each monomer of the LAP is composed of a series of relatively rigid α helices and β sheets, but the active cytokine is held in place by multiple contacts with a single flexible loop that has been termed the latency lasso and effectively holds the active cytokine in a straitjacket. As long as this lasso maintains close contacts with the active cytokine, TGF-β can be stored in the extracellular space with little or no evidence of active TGF-β signaling or cellular responses.
The three isoforms of TGF-β are highly homologous. The active cytokine regions of the TGF-β isoforms show 71–79% amino acid sequence identity, with the LAP regions showing slightly less homology (36–51% sequence identity). The functions of the different isoforms appear to be similar in vitro, but the diversity of phenotypes of knockout mice for each isoform suggests distinct functions for the isoforms in vivo (5). The predominate isoform expressed in the immune system is TGF-β1 (6; see also http://www.immgen.org), and mice lacking this isoform either die embryonically or, if surviving to birth, develop multiorgan inflammation and die by 3–4 weeks of age (7, 8). Thus, this initial work highlighted a crucial role for TGF-β in dampening self-harmful inflammatory responses. However, as described later in this review, TGF-β also has important functions in promoting inflammatory responses.
TGF-β SIGNALING
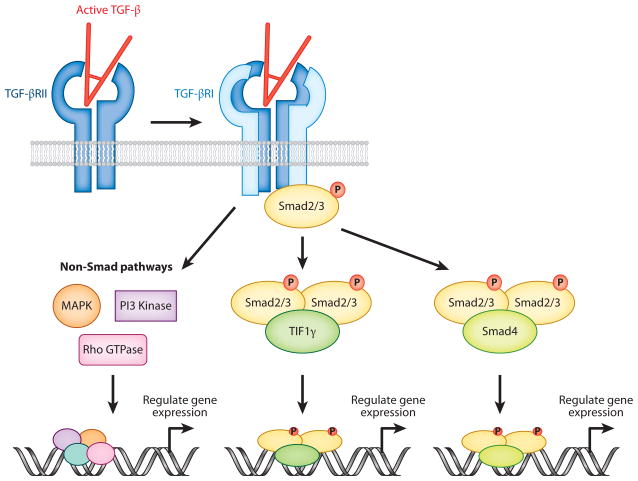
TGF-β triggers signaling in cells via binding to the TGF-β receptor complex. This complex is a tetrameric structure, composed of two type I TGF-β receptors (TGF-βRI) and two type II TGF-β receptors (TGF-βRII). Initially, TGF-β binds to a homodimer of TGF-βRII, which complexes with a homodimer of TGF-βRI (9). Both receptors are serine/threonine kinases, and upon engagement with TGF-β, TGF-βRII phosphorylates the cytoplasmic domain of TGF-βRI (Figure 2). For optimal signaling by TGF-β2, an additional receptor, β-glycan (also called TGF-βRIII), is required and enhances initial binding of TGF-β2 to TGF-βRII.
Figure 2.
Signaling pathways triggered by TGF-β. Active TGF-β binds to a dimer of TGF-βRII, which associates with a dimer of TGF-βRI to form a tetrameric receptor complex. TGF-βRII then phosphorylates the cytoplasmic domain of TGF-βRI, which activates the kinase activity of TGF-βRI. TGF-βRI can then associate with and phosphorylate receptor Smads (Smad2 or 3), which subsequently form a complex with either Smad4 or TIF1γ. The activated Smad complexes then act to modulate gene expression by binding to Smad-responsive elements in genes. TGF-β receptor activation can also trigger non-Smad-mediated signaling events (e.g., via MAPK, Rho GTPases, or PI3 kinase) to regulate gene expression.
Ligation of the TGF-β receptor complex triggers recruitment of the intracellular receptor Smad (R-Smad) proteins Smad2 and Smad3 to the cytoplasmic domain of activated TGF-βRI, which directly phosphorylates Smad2/3. Once phosphorylated, Smad2/3 forms a trimeric structure with Smad4, which translocates to the nucleus to activate or repress gene expression via binding to Smad-responsive regulatory regions (Figure 2). Alternatively, Smad2/3 can form a complex with TIF1γ to regulate gene expression (10). However, it is now appreciated that, at least in some cell types, alternative Smad activation can facilitate TGF-β signaling. Thus, Smad1 and 5, classically involved in signaling by bone morphogenic proteins, can be phosphorylated upon binding of TGF-β to its receptors (11). Indeed, recent evidence has suggested that, during differentiation of Th17 cells, different isoforms of TGF-β may activate different Smad signaling pathways (12). Hence, it appears that alternate Smad signaling pathways may be initiated by TGF-β in a context-dependent manner. Given the potential differences in functional activity of Smad2 versus Smad3 in the immune system (see below), further work is required to determine the biological relevance of differential Smad activation in regulation of immunity.
In addition to classic Smad-mediated signaling events, TGF-β can trigger numerous Smad-independent signaling pathways. For example, signaling via MAP kinases, PI3 kinase, and Rho GTPases can all be triggered by TGF-β receptor engagement (Figure 2) (13). Much remains to be learned about how and when Smad-dependent and -independent signaling pathways are triggered by TGF-β in immune cells and the relative contributions of these pathways to the pleiotropic effects of TGF-β on immune responses.
Once TGF-β binds to the receptor complex, several mechanisms can modulate the intensity and duration of downstream signals. One important pathway is upregulation of Smad7, an inhibitory Smad (I-Smad) that is induced by TGF-β and binds to TGF-βRI directly to inhibit Smad2/3 binding (14, 15). Smad7 also promotes TGF-βRI inactivation [via recruitment of the phosphatase PP1 (16)] and degradation [via recruitment of the ubiquitin ligase Smurf2 (17)]. Other important inhibitory pathways include the dephosphorylation of R-Smads, which inhibits Smad signaling, and hyperphosphorylation of R-Smads, which targets them for ubiquitination and degradation (18). Thus, TGF-β signaling concomitantly triggers pathways that prevent overly exuberant responses.
FUNCTION OF TGF-β IN THE IMMUNE SYSTEM
As stated above, mice lacking TGF-β1 die of multiorgan inflammation early in life (7, 8). TGF-β1-deficient animals crossed with mice lacking MHC class II molecules (19) or β2-microglobulin (20) were substantially protected from inflammation, indicating that the adaptive immune response is a fundamentally important target for TGF-β. TGF-β can be made by, and act upon, many types of immune cells (6). In this review we focus on the important role of TGF-β in regulating adaptive immunity, focusing on its crucial role in modulating T cell and dendritic cell (DC) function.
Regulation of T Cells by TGF-β Receptor Signaling
To address the importance of T cells as a major direct target for TGF-β in vivo, Gorelik & Flavell (21) generated mice expressing a dominant-negative TGF-βRII specifically in T cells using the CD4 promoter. These mice developed enhanced T cell activation and an age-related wasting disorder associated with multiorgan inflammation and development of autoantibodies (21). This phenotype was similar to that seen in TGF-β1−/− mice, but not nearly as severe and with a delayed onset. In another study where a dominant-negative TGF-βRII was driven in T cells via the CD2 promoter, mice developed expansion of memory CD8+ T cells, but they did not show any overt inflammatory phenotype (22). These studies left open the possibility that TGF-β acting on non–T cells was important in preventing T cell–mediated inflammatory disease, but they were confounded by the likelihood that expression of the dominant-negative TGF-βRII did not completely inhibit TGF-β signaling in T cells. Subsequently, T cell–specific deletion of either TGF-βRII (23, 24) or TGF-βRI (25) via expression of Cre recombinase under the control of the CD4 promoter region (CD4-Cre) resulted in rapid lethal inflammatory disease similar to that seen in TGF-β1−/− mice, definitively identifying a central role for TGF-β acting directly on T cells.
However, a recent study has questioned the crucial requirement for TGF-β in postnatal homeostatic regulation of T cells. Zhang & Bevan (26) produced mice in which a conditional TGF-βRII was deleted via expression of dLck-Cre, a T cell–specific Cre that is switched on later in T cell development. Indeed, TGF-βRIIflox/flox × dLck-Cre mice show delayed TGF-βRII deletion, with considerable TGF-βRII expression seen on T cells in the first 3 weeks of life, in contrast to the complete absence of TGF-βRII expression on T cells in TGF-βRIIflox/flox × CD4-Cre mice (26). Strikingly, in contrast to TGF-βRIIflox/flox × CD4-Cre mice that succumbed to inflammatory disease at 3–5 weeks of age, TGF-βRIIflox/flox × dLck-Cre mice survived into adulthood, showing low levels of T cell activation and minimal pathology (26). T cells from these mice showed enhanced proliferation in a lymphopenic environment, suggesting that in mature animals TGF-β signals to T cells may be principally important for dampening T cell expansion in response to exogenous stimuli (26).
Role of Smad-Mediated TGF-β Signaling in Regulation of T Cells
Analyses of mice lacking expression of Smad proteins in T cells support a crucial role for T cell–intrinsic TGF-β signaling in prevention of inflammation. Mice lacking Smad2 specifically in T cells and globally deficient in Smad3 develop lethal autoimmunity early in life akin to that of TGF-β1−/− mice, suggesting a critical role for Smad-mediated signaling in regulating T cell function (27, 28). Mice either lacking Smad2 in T cells or globally lacking Smad3 had relatively normal T cell homeostasis, suggesting functional redundancy of Smad2 and 3 in T cells (27, 28). Mice lacking Smad4 specifically in T cells displayed dysregulation of T cell homeostasis and developed intestinal inflammation and colorectal cancer (29). However, these mice did not develop rapid lethal onset of inflammation akin to that in TGF-β1−/− mice or Smad2/3 knockout mice (29), suggesting that Smad2/3 can mediate TGF-β signaling in T cells in a Smad4-independent manner.
As it does in other cells, the I-Smad Smad7 negatively regulates T cell responses to TGF-β. Overexpression of Smad7 in T cells enhanced Th1 and Th2 cytokine production in mouse models of allergic asthma, although T cell homeostasis was unaffected in unchallenged mice (30). Similarly, mice overexpressing Smad7 in T cells developed worse disease during experimental autoimmune encephalomyelitis (EAE), and mice lacking Smad7 in T cells were protected from EAE (31). In humans, isolated T cells from patients with active multiple sclerosis showed enhanced expression of Smad7 compared to T cells from patients in remission (31). T cells from patients with chronic inflammatory bowel disease also showed heightened Smad7 expression, and inhibiting Smad7 reduced proinflammatory cytokine expression in these cells (32), at least in part by enabling suppression by regulatory T cells (Tregs) (33). Thus, the balance between R-Smad and I-Smad expression and function appears to be key in the regulation of T cell responsiveness to TGF-β during health and disease.
TGF-β Signaling in Regulation of T Cell Proliferation, Survival, and Homing
Early studies showed that TGF-β can be a potent inhibitor of T cell proliferation (34). Several mechanisms drive TGF-β-mediated inhibition of T cell proliferation, including suppression of IL-2 production, downregulation of c-myc, and upregulation of cyclin-dependent kinase inhibitors (6). However, inhibition of proliferation is abrogated in activated T cells, with costimulation via CD28 important in blocking TGF-β-mediated inhibition of proliferation (35).
In some contexts, TGF-β also plays an important role in promoting cell death to limit T cell expansion after activation. For example, during bacterial infection with Listeria monocytogenes, reduced TGF-β signaling in T cells resulted in enhanced clonal expansion of CD8+ T cells (36). This enhanced T cell expansion was due to diminished apoptosis in proliferating short-lived effector cells, with TGF-β normally acting to reduce expression of the antiapoptotic protein Bcl2 in such cells to induce cell death (36). Similar findings were seen in a model of chronic viral infection, in which the upregulation of the proapoptotic protein Bim by TGF-β resulted in enhanced CD8+ T cell apoptosis (37).
However, in other contexts TGF-β has been shown to promote survival of activated T cells. Thus, TGF-β in conjunction with IL-2 can inhibit activation-induced cell death during T cell expansion by blocking FasL-mediated apoptosis (38). In addition, TGF-β has been shown to promote survival of naive CD4+ T cells in vivo. In mice whose T cells lack TGF-βRII expression, early T cell development is normal, but the numbers of naive CD4+ T cells in the periphery are dramatically reduced, and this is associated with enhanced T cell death (6). Additionally, TGF-β can promote homeostasis of CD4+ T cells by promoting expression of IL-7Rα (via downregulation of the negative regulator Gfi-1), with this pathway most important in T cells with low-affinity T cell receptors (TCRs) (39). TGF-β can also promote migration and/or retention of T cells in specific peripheral tissues. For example TGF-β induces expression of the G protein–coupled receptor GPR15 in Tregs, which is required for homing of these cells to the large intestine (40). Thus, in different contexts TGF-β can either inhibit or enhance T cell proliferation, survival, and subsequent accumulation at specific tissue sites.
REGULATION OF EFFECTOR AND REGULATORY T CELL DIFFERENTIATION AND FUNCTION BY TGF-β
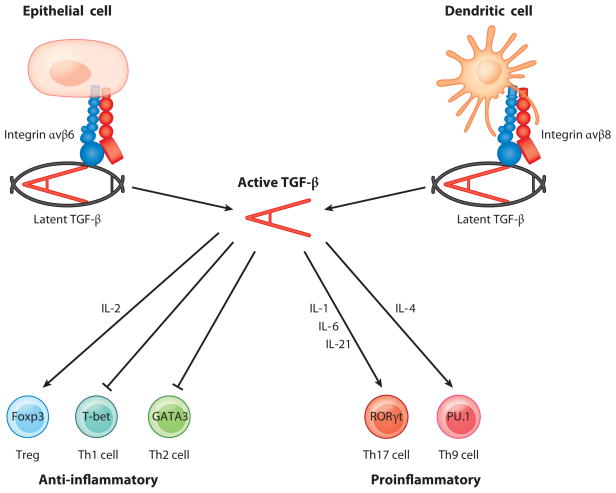
TGF-β plays fundamental roles in regulating differentiation of T cells into effector and regulatory subsets (Figure 3). The effects of TGF-β on T cell differentiation are highly context specific: TGF-β can drive either pro- or anti-inflammatory responses, depending on the amount of TGF-β presented to the cells and on intracellular and local extracellular factors present at the time of exposure to TGF-β.
Figure 3.
Regulation of multiple CD4+ T cell phenotypes by TGF-β. The latent complex of TGF-β must be activated to function, and recent data highlight a key role for integrin αvβ6 expressed by epithelial cells and integrin αvβ8 expressed by dendritic cells in activating TGF-β. Once activated, TGF-β performs important functions in controlling both anti-inflammatory and proinflammatory T cell responses, with the presence of other cytokines dictating the functional outcome of TGF-β signaling in T cells.
Th1 Cells
TGF-β is a potent suppressor of Th1 effector T cell differentiation. Differentiation of Th1 cells is driven by IL-12 produced by myeloid cells, which drives T cells to produce large amounts of IFN-γ and to express the transcription factor T-bet (41). IFN-γ contributes to a positive feedback loop to drive further Th1 differentiation, and innate sources of IFN-γ have the same effect (42). TGF-β potently inhibits Th1 cell differentiation by inhibiting production of T-bet, and ectopic expression of T-bet in Th1 cells renders them resistant to TGF-β inhibition (43). TGF-β downregulates expression of IL-12Rβ2 on T cells, thus reducing their responsiveness to IL-12 (44). TGF-β can also act indirectly to inhibit Th1 cell differentiation by inhibiting IFN-γ production by natural killer cells (45).
The importance of TGF-β in limiting Th1 cell differentiation during homeostasis in vivo is strikingly demonstrated in mice lacking TGF-βRII on T cells. As previously described, these mice develop massive T cell activation and a wasting disorder early in life, and this phenotype is characterized by a specific upregulation of Th1 cell differentiation (23, 24). TGF-β-mediated inhibition of Th1 cells appears to require Smad2/3-mediated signaling, as mice globally lacking Smad3 and lacking Smad2 in T cells also develop Th1-mediated autoimmune disease (27, 28).
However, it has been reported that, under certain in vitro conditions in the presence of IFN-γ, TGF-β can enhance Th1 cell differentiation (46). Additionally, recent data suggest that TGF-β in combination with IFN-γ and IL-4 can mediate induction of a specific subset of CD103+ Th1 cells (47). However, the importance of TGF-β-mediated enhancement of Th1 cell differentiation remains unclear, especially given that loss of TGF-β signaling and function in vivo is associated with enhanced Th1 cell differentiation.
Th2 Cells
TGF-β has also been shown to potently downregulate differentiation of Th2 cells. Inhibition of Th2 differentiation is due to modulation of the transcription factor GATA3, a key transcription factor in Th2 cell induction (48, 49). TGF-β downregulates expression of GATA3, and ectopic expression of GATA3 prevents TGF-β-mediated inhibition of Th2 differentiation. TGF-β also inhibits GATA3 function indirectly by inducing the transcription factor Sox4, which binds directly to GATA3 and inhibits its transcriptional activity (50). Additionally, Sox4 inhibits production of the Th2 cytokine IL-5 by binding to the IL-5 promoter and preventing GATA3-mediated induction of gene expression (50).
Regulation of Th2 differentiation by TGF-β also appears to be important in vivo. Although loss of TGF-β signaling in T cells in mice primarily drives T cells toward a Th1 phenotype, concomitant loss of the Th1-associated transcription factor T-bet in T cells causes rapid multiorgan inflammation associated with enhanced Th2 cell differentiation (23). Conversely, retroviral-mediated overexpression of TGF-β by T cells prevents Th2 cell differentiation in vivo and protects mice from allergic inflammation and airway hyperresponsiveness in mouse models or allergic airway disease (51).
Foxp3+ Tregs
TGF-β also contributes to suppression of T cell responses by regulating Foxp3+ Tregs. Tregs are a subset of CD4+ T cells that are crucial in suppression of self-harmful T cell responses (52). Thus, mice with a nonfunctional mutation in Foxp3 (53) or with a targeted knockout of Foxp3 in T cells (54) die of multiorgan inflammation very early in life, akin to mice lacking TGF-β1. Also, humans carrying an inactivating mutation in the FOXP3 gene develop immunodysregulation, polyendocrinopathy, and enteropathy X-linked (IPEX) syndrome, a severe autoimmune disease generally resulting in death by age 2 (55). There are two major subtypes of Tregs: natural Tregs (nTregs), which are produced in the thymus early in life, and induced Tregs (iTregs), which are generated in the periphery from naive CD4+ T cells (56). TGF-β appears to play important but distinct roles in the regulation of both nTreg and iTreg populations.
TGF-β and nTreg development
TGF-β was initially thought to be dispensable for nTreg production in the thymus, as mice lacking expression of TGF-β1 showed similar numbers of thymic Tregs (57). Also, in mice lacking TGF-βRII expression by T cells, nTreg numbers were similar to (24) or increased (23) compared with those in control mice. However, subsequent work showed that in mice lacking TGF-β signaling in T cells, nTregs were almost completely absent for the first 5 days after birth (25). Thereafter, an IL-2-dependent expansion of nTregs occurred, explaining the similar/higher nTreg numbers observed in previous studies of mice lacking TGF-βRII in T cells (25). Subsequent work showed that TGF-β signaling in T cells protects nTregs from apoptosis during thymic development by suppression of proapoptotic proteins and upregulation of the antiapoptotic protein Bcl2 (58).
TGF-β and iTreg development
TGF-β plays a more clear-cut role in promoting iTreg development. In combination with IL-2, TGF-β promotes the conversion of naive CD4+ T cells to iTregs by upregulating expression of Foxp3 (59–61). Both Smad2 and Smad3 contribute to Foxp3 induction by distinct mechanisms. In the setting of TCR engagement, Smad3 interacts with an enhancer region of the Foxp3 gene called CNS1 (62, 63). A recent report suggests that, in vivo, Smad3 binding to the CNS1 enhancer region is required for normal Foxp3 Treg numbers in the mouse gut, but not in other organs (64). Smad3 also modulates Foxp3 expression by forming an enhanceosome complex along with NFATc2 and CREB at the Foxp3 promoter (65). TGF-β-induced expression of Foxp3 is partially reduced in Smad3 knockout T cells (28, 66, 67), suggesting an important functional role for Smad3 in promoting iTreg induction.
Smad2 does not bind directly to the CNS1 region (62), but it does appear to play a role in the TGF-β-mediated iTreg induction, given that T cells lacking Smad2 have a reduced ability to upregulate Foxp3 expression (68, 69). Loss of both Smad2 and Smad3 resulted in complete ablation of Foxp3 upregulation by TGF-β (28), supporting a cooperative interaction between Smad2 and 3 in the induction of iTregs. In addition to Smad-mediated effects, TGF-β can indirectly promote Foxp3 induction by inhibiting factors that normally suppress Foxp3, such as the transcriptional repressor Gfi-1 (70).
Expression of Foxp3 induced in vitro by TGF-β is unstable in iTregs because of incomplete demethylation of the so-called Treg-specific demethylated region (TSDR) present upstream of the Foxp3 gene (71, 72). However, Tregs induced in vivo appear to express Foxp3 stably and display a demethylated TSDR region (72). Thus, further studies are required to determine the mechanisms regulating the stability of Foxp3 induction by TGF-β in different immunological contexts.
TGF-β-mediated induction of Foxp3 is enhanced by the vitamin A metabolite retinoic acid (RA) (73), which can be secreted by DCs and macrophages to promote iTreg induction in the intestine (74, 75), lung (76–78), and skin (79). Ligated RA receptor complexes bind to regulatory elements in the Foxp3 promoter and enhancer regions and promote binding of phosphorylated Smad3 to the CNS1 enhancer region of the Foxp3 gene (80). RA also facilitates iTreg induction indirectly by inhibiting proinflammatory cytokine production by effector/memory T cells and dampening the responsiveness of T cells to proinflammatory cytokines (which normally block iTreg induction) (81, 82). Finally, RA can enhance TGF-β-mediated Foxp3 expression by promoting histone acetylation at the Foxp3 promoter (83).
Roles of TGF-β in Treg maintenance and function
Mice lacking TGF-β1 (57) or TGF-βRII on T cells (23, 24) display marked reductions in Foxp3+ Treg numbers in the periphery, suggesting a role for TGF-β in maintenance of these cells. However, repopulation of mice lacking TGF-βRII on T cells with wild-type Tregs did not prevent multiorgan inflammation (23, 24). One mechanistic explanation for these results is that Foxp3+ Treg-mediated suppression of effector T cells requires TGF-β responsiveness in those cells. Indeed, responsiveness of T cells to TGF-β is required for Treg-mediated suppression in T cell–dependent models of colitis (84).
In studies in which peripheral Treg numbers are diminished in the absence of TGF-β production/signaling, it is not possible to directly address the relative contribution of nTregs versus iTregs to the phenotypes observed. To assess the role of iTregs in controlling immune responses directly, researchers generated mice lacking the CNS1 enhancer of the Foxp3 gene that is required for TGF-β-mediated upregulation of Foxp3 expression and iTreg induction (85). These mice did not develop the severe, early-onset multiorgan inflammation seen in mice lacking all Tregs, indicating that nTregs are sufficient to prevent this phenotype. However, in older mice lacking iTregs, Th2-mediated pathology developed in the lung and intestine, with altered bacterial species detected in the gut (85). Similarly, Haribhai et al. (86) found that selective depletion of iTregs, using a diphtheria toxin depletion model, resulted in T cell activation and pathology in the liver, intestine, and lung, indicating that iTregs are important in controlling immune responses in some peripheral tissues. These findings fit with data suggesting that mucosal surfaces such as the intestine are a major site of iTreg induction driven by the commensal flora. However, a recent report that profiled the TCR specificity of Tregs in the intestine concluded that most intestinal Tregs were nTregs, with TCR specificity regulated by the microflora (87). Thus, further work is required to definitively apportion the contributions of iTregs and nTregs at mucosal surfaces.
Recent work has suggested that an additional CD4+ Foxp3− Treg population has an important suppressive function and that this subset is characterized by cell surface expression of the LAP region of TGF-β. Investigators have proposed that these cells, termed Th3-type Tregs, are important in the suppressive effects of anti-CD3 antibody in several autoimmune diseases (88). TGF-β has also been shown to induce expression of CD73 on activated T cells independently of Foxp3 expression, which can induce adenosine to suppress T cell proliferation (89). Additionally, in conjunction with IL-27, TGF-β can drive induction of Tr1 cells, which are a Foxp3−-suppressive CD4+ T cell subpopulation characterized by high expression of IL-10 (90, 91). Thus, the potential role of TGF-β in regulating Foxp3− Treg subsets requires further exploration.
Th17 Cells
As noted above, TGF-β plays several important suppressive roles in T cell biology. However, research in the past decade has highlighted a fundamental role for TGF-β in enhancing proinflammatory T cell responses. TGF-β is critical in the induction of Th17 cells—CD4+ T cells that are characterized by the production of IL-17 and the expression of the transcription factor RORγt. Th17 cells play crucial roles in immunity to certain extracellular bacteria and fungi and in the development of autoimmunity (92). Induction of Th17 cells by TGF-β requires proinflammatory cytokines such as IL-6, IL-1β, and IL-21, and recent studies suggest that IL-6 and IL-1 may play distinct roles in driving the Th17 differentiation in specific anatomical locations (93, 94). IL-23 is also important in the induction of Th17 cells, with IL-6 shown to induce the expression of both the IL-23 receptor and IL-21 in T cells, thereby enhancing Th17 differentiation (95).
Although TGF-β was first identified as a key inducer of Th17 cell differentiation in mice, initial studies suggested that TGF-β was not required for Th17 induction in humans (96, 97). However, follow-up studies have shown that the role for TGF-β in Th17 cell differentiation is conserved between mice and humans (98, 99), although in some contexts Th17 cell differentiation does appear to occur in the absence of TGF-β (100).
Despite this potential TGF-β-independent Th17 cell induction, the functional importance of TGF-β in Th17 cell differentiation has been shown in several in vivo mouse models. Thus, mice lacking TGF-βRII on T cells have reduced numbers of Th17 cells and are protected from development of EAE, and local injection of TGF-β-blocking antibody also protects against EAE (101). Overexpression of active TGF-β by T cells results in exacerbated CNS inflammation and more severe EAE (102). A recent report suggests that in addition to TGF-β1, production of the alternative TGF-β isoform TGF-β3 by Th17 cells is crucial for induction of their effector function (12), suggesting that isoform-specific effects may be important in Th17 differentiation.
Although TGF-β can drive Th17 differentiation in vitro, exposure to high concentrations of TGF-β can generate Th17 cells that do not drive inflammation in the EAE model because of upregulation of IL-10 (103). The addition of IL-23 during Th17 cell differentiation suppressed expression of IL-10 and rescued pathogenicity in the EAE model (103). However, whether similar regulation would be relevant in the context of the much smaller amounts of TGF-β likely to be activated in vivo remains to be determined.
The signaling mechanisms underlying TGF-β’s role in differentiation of Th17 cells are controversial. In particular, it is not clear what role, if any, Smad signaling plays in TGF-β-mediated Th17 differentiation. Smad3 does not appear to be required (67, 68), and other studies have shown that, in T cells lacking expression of Smad2 and Smad3 (28) or Smad 4 (104), induction of RORγt in T cells in response to TGF-β and IL-6 is unaltered. Additionally, TGF-β has been shown to suppress expression of the transcription factor Eomesodermin (Eomes) during Th17 cell induction, which alleviates Eomes-mediated suppression of RORγt in a Smad2/3-independent manner (105).
However, one study suggested that Smad2 can directly bind to and synergize with RORγt (69), another suggested that Smad2 induced expression of the IL-6 receptor on T cells (106), and each effect was purported to be important for Th17 cell differentiation. Also, Smad signaling may promote Th17 differentiation indirectly by dampening production of cytokines (for example IL-2) that inhibit Th17 induction (28). Thus, further work is required to clarify the role of Smad signaling in Th17 cell induction.
The Role of TGF-β in the Induction of iTregs Versus Th17 Cells
An important consideration is how TGF-β can induce such functionally diverse cell populations in different immunological contexts (e.g., iTregs in the presence of IL-2 and Th17 cells in the presence of IL-6, IL-1β, and/or IL-21). A study by Zhou et al. (107) suggested that, upon initial sensing of TGF-β, CD4+ T cells upregulate both Foxp3 and RORγt and that the concentration of TGF-β present is crucial in determining whether a cell commits to becoming an iTreg or a Th17 cell. Thus, low concentrations of TGF-β block expression of the IL-23 receptor and favor Foxp3 expression, whereas high concentrations of TGF-β in conjunction with IL-6 and IL-21 result in upregulation of the IL-23 receptor and commitment to become RORγt+ Th17 cells (107). The relative importance of differential TGF-β concentrations and different cytokine milieus in modulating the induction of iTregs versus Th17 cells in vivo remains to be determined.
Mechanistically, Foxp3 and RORγt can directly interact with each other, and Foxp3 binding to RORγt inhibits RORγt function by preventing its interaction with the IL-17 gene promoter (104, 107, 108). The presence of IL-6, IL-21, and IL-23 blocks this Foxp3-mediated inhibition of RORγt function, driving differentiation of Th17 cells (104, 107, 108). Antagonism of Foxp3 by IL-6 also appears to be important in nTregs, as coculture of these cells with IL-6 causes downregulation of Foxp3 expression and upregulation of IL-17 production (104). Together these data show the potential plasticity between the Treg and Th17 lineages, highlighting the importance of understanding how therapeutic manipulation of one subset may have unexpected effects on the other.
Th9 Cells
TGF-β induces another subset of proinflammatory CD4+ T cells, termed Th9 cells, which secrete IL-9, and can drive inflammation in various disease settings (109). Induction of Th9 cells requires TGF-β in conjunction with IL-4 (110, 111), with TGF-β inducing the key transcription factor PU.1 (112, 113). The addition of IL-4 to in vitro cultures blocks TGF-β-mediated Treg induction in favor of IL-9 production (110). TGF-β can also skew Th2 cells toward a Th9 phenotype (111). However, recent evidence using an IL-9 reporter mouse suggests that, in vivo, the majority of IL-9 is produced by innate lymphocyte cells termed ILC2s (114). Thus, the physiological role of TGF-β-mediated IL-9 production in T cells warrants further investigation.
REGULATION OF CD8+ T CELLS BY TGF-β
In addition to regulation of CD4+ T cell subsets, TGF-β plays an important role in controlling CD8+ T cells. Thus, TGF-β can potently inhibit CD8+ T cell proliferation in vitro, although in contrast to its action in CD4+ T cells, inhibition appears to be independent of Smad3 signaling (115). As mentioned above, dampening of TGF-β signaling in T cells (via expression of a dominant-negative TGF-βRII under the control of the CD2 promoter) resulted in expansion of CD8+ T cells (22), and expression of the construct by the CD4 promoter led to enhanced CD8+ T cell activation (21). However, complete loss of TGF-β signaling in T cells results in reduced thymic development of CD8+ T cells (23), with recent work showing that TGF-β induction of IL-7Rα expression is important in CD8+ T cell development (39). Interestingly, a recent report suggests that reduced TGF-β signaling (via expression of dominant-negative TGF-βRII) results in lymphoproliferation of memory CD8+ T cells but that complete blockade of TGF-β signaling (via genetic deletion of TGF-βRII) does not result in similar lymphoproliferation (116). Thus, reduction of TGF-β signaling appears to play distinct functional roles compared with complete blockade of TGF-β function in regulation of CD8+ T cells.
TGF-β has also been shown to potently suppress the effector functions of cytotoxic T cells through multiple mechanisms, including inhibition of perforin and IFN-γ expression, and mice expressing dominant-negative TGF-βRII in T cells show enhanced cytotoxic T lymphocyte (CTL) function in tumor models (6). Thus, TGF-β appears to control the homeostatic expansion and activation of CD8+ T cells in vivo.
TGF-β signaling in CD8+ T cells is important in the induction of cell death during bacterial infection, preventing overproliferation by promoting apoptosis in short-lived effector cells (36, 37). TGF-β also induces a specialized intraepithelial subset of CD8+ T cells in the intestine that is characterized by expression of CD8αα and is important in mucosal immune regulation (117). Recent data also suggest that CD4+ T cells can be reprogrammed to upregulate CD8+ T cell–associated genes, via downregulation of the transcription factor ThPOK and upregulation of Runx3. This process is driven by TGF-β and results in a unique CD4+ CTL-like effector cell (118, 119). Thus, as for CD4+ T cells, the regulation of CD8+ T cells by TGF-β is highly context dependent, so that TGF-β can either inhibit or enhance effector function.
Recently, investigators have found populations of CD8+ T cells that can suppress immune responses and are controlled by TGF-β (120). Several populations of potential CD8+ suppressor cells have been identified. For example, CD8+ T cells expressing LAP on their cell surface (mostly Foxp3−) can suppress EAE via TGF-β-dependent and IFN-γ-dependent mechanisms (121). A recent study identified a population of CD8+ Foxp3− T cells from the intestine that can suppress CD4+ T cell proliferation, demonstrating that these cells from Crohn’s patients have reduced suppressive ability (122). However, the investigators suggested that TGF-β inhibited the suppressive capacity of these CD8+ suppressor cells.
In addition to CD8+Foxp3− suppressor cells, a small population of CD8+Foxp3+ Tregs have been identified in the thymus, and these cells can be induced from CD8+Foxp3− T cells by TGF-β (123). Although initial reports suggested that these cells lacked suppressive function (123), subsequent studies have shown that these CD8+Foxp3+ T cells are induced by TGF-β during graft-versus-host disease and are functionally suppressive (124, 125). Thus, in addition to its effect on classical CD4+ iTregs, TGF-β can also drive the differentiation of an important CD8+Foxp3+ Treg population.
REGULATION OF DENDRITIC CELLS BY TGF-β
TGF-β signaling can also regulate the adaptive immune response by effects on DCs. Although expression of dominant-negative TGF-βRII in DCs did not affect their homeostasis (45), ablation of TGF-βRII in DCs (using CD11c-Cre) caused spontaneous multiorgan inflammation and death by 15 weeks of age (126). DCs lacking TGF-βRII express normal levels of MHC class II and costimulatory molecules but produce more IFN-γ, which reduces these DCs’ ability to induce Foxp3+ Tregs (126).
TGF-β is also important in regulating Langerhans cells, a specialized subset of DCs present in the skin. Early descriptions of mice lacking expression of TGF-β1 reported a complete lack of Langerhans cells in these mice (127), and in vitro studies suggested that TGF-β could inhibit the maturation and antigen-presenting ability of Langerhans cells (128). Deletion of TGF-βRII specifically in Langerhans cells (via Langerin-Cre) resulted in the absence of Langerhans cells, and deletion of TGF-β1 from these cells had the same effect (129). TGF-β-mediated differentiation of Langerhans cells appears to involve Axl, a receptor tyrosine kinase that is induced by TGF-β during Langerhans cell development (130). Langerhans cells isolated from patients with atopic dermatitis display reduced TGF-β signaling and TGF-β receptor expression, suggesting potential functional importance of TGF-β signaling in Langerhans cells in this disease (131).
IMPORTANT SOURCES OF TGF-β FOR REGULATION OF ADAPTIVE IMMUNITY
Studies have clearly shown that T cells themselves are an important source of TGF-β for regulating adaptive immune responses. Conditional ablation of the TGF-β1 gene in T cells results in lethal multiorgan inflammation in mice associated with increased T cell activation and enhanced differentiation of T cells into both Th1 and Th2 cells (132). Surprisingly, in contrast to mice lacking TGF-β1 production by all cells and mice lacking TGF-βRII specifically on T cells, Foxp3+ Treg numbers were not reduced in mice lacking TGF-β1 in T cells. In fact, Treg numbers were elevated in the mesenteric lymph nodes of these mice, indicating that Foxp3 induction/maintenance does not require TGF-β production by T cells (132). In mice specifically lacking TGF-β1 expression in Tregs (via expression of Foxp3-Cre), numbers of Foxp3+ Tregs in lymph nodes were again higher than in control mice, suggesting an important role for autocrine TGF-β signaling in limiting Foxp3+ Treg cell numbers but not in their induction or maintenance (133).
Mice lacking TGF-β1 specifically in Foxp3+ Tregs are healthy and do not develop inflammation (133), indicating a redundant role for Treg-derived TGF-β in Treg-mediated immune homeostasis. Whether or not TGF-β produced by Tregs is crucial to their ability to suppress inflammation is less clear. Although one study demonstrated that TGF-β produced by Tregs was critical for suppression of disease in a T cell transfer colitis model (132), another study showed that TGF-β1−/− Tregs were fully capable of suppressing colitis (84). Thus, further work is required to clarify the importance of Treg-derived TGF-β in suppression of T cell–mediated inflammation.
Mice lacking TGF-β1 production by both activated CD4+ T cells and Foxp3+ Tregs (using OX40-Cre) develop multiorgan inflammation and T cell activation akin to that of mice lacking TGF-β1 production in all T cells (133). Together, these data indicate a crucial role for TGF-β production by activated T cells. Furthermore, Th17 cell differentiation was almost completely absent in these mice but was normal in mice lacking TGF-β1 in Foxp3+ cells, indicating that autocrine TGF-β production is key to the induction of Th17 cells. Accordingly, TGF-β1flox/flox × OX40-Cre mice are almost completely protected from EAE (133).
Although ablation of T cell–derived TGF-β1 clearly impairs T cell homeostasis, the inflammatory phenotype observed is mild compared to that of mice either completely lacking TGF-β1 or lacking TGF-β responsiveness in T cells. This indicates that other non–T cell sources of TGF-β are important in signaling to T cells. Given that TGF-β is produced as an inactive complex by a wide variety of cells, TGF-β function may be better controlled at the level of its activation rather than at the level of latent TGF-β production. Below, we describe in detail mechanisms known to activate TGF-β and the known functional relevance of such mechanisms in regulation of the immune system.
MECHANISMS OF TGF-β ACTIVATION
Because active TGF-β is maintained in a latent state by noncovalent association with the LAP, it is relatively easy to activate TGF-β in vitro through a variety of mechanisms that lead to protein denaturation. For example, heat, extremes of pH, and ionizing radiation all activate TGF-β in vitro. Heat is commonly used in the laboratory to activate all available latent TGF-β as a way to calculate the fraction that is activated in biological samples. However, no convincing data implicate changes in temperature or pH as a way to activate TGF-β in vivo. Radiation treatment induces tissue fibrosis in a variety of organs, and radiation of mammary tissue has been suggested to cause fibrosis, at least in part, by activating TGF-β (134).
Binding to Thrombospondin 1
The secreted extracellular matrix protein thrombospondin 1 contains a short exposed linear peptide sequence (KRFK) that binds to a specific sequence in the LAP of TGF-β1 (LSKL) and interferes with its noncovalent association with the active cytokine (135, 136). In vitro, addition of either intact thrombospondin 1 or the relevant peptide to latent TGF-β has been shown to activate TGF-β (135, 136). Mice homozygous for an inactivating mutation in thrombospondin 1 manifest some, but not all, phenotypes of TGF-β1-deficient mice, including inflammation of multiple organs and epithelial hyperplasia at multiple sites (137).
Enzymatic Activation
Latent TGF-β can be activated by a variety of glycosidases, including N-glycanase, sialidase, and neuraminidase (138). Incubation of the latent complex with influenza virus induced activation that was dependent on neuraminidase expressed on the surface of viral particles (139). However, these studies have involved in vitro activation of TGF-β, so the in vivo significance of activation by deglycosylation is unclear.
A more extensive literature has implicated two families of proteases in TGF-β activation—serine proteases, especially plasmin and cathepsin D, and metalloproteases, including MMP9 and MMP14. The crystal structure of the small latent complex identified accessible proteolytic cleavage sites and suggested how proteases could lead to release of free active TGF-β from the LAP latency lasso (4). However, as with glycosidases, most of the data implicating proteases in activation are based on in vitro observations with purified proteins or cultured cells. The phenotypes of mice lacking specific candidate proteases (i.e., plasminogen or its activators or metalloproteases) are not suggestive of effects of loss of TGF-β function (140). Such results could be explained by functional redundancy or by the dominance of other roles for these proteases independent of TGF-β activation. At least one metalloprotease (MMP14) can cooperate with the integrin αvβ8 in TGF-β activation (141), and mice lacking the integrin β8 subunit have multiple phenotypes consistent with loss of TGF-β activity (see below) (142, 143).
Regulation by Other TGF-β-Binding Proteins
As noted above, there are four members of the LTBP family, LTBP1, 2, 3, and 4, and three of these, LTBP1, 3, and 4, can form disulfide bonds with TGF-β LAPs prior to secretion in so-called large latent complexes. LTBPs are structurally closely related to another family of proteins called fibrillins. There are three mammalian isoforms, fibrillin 1, 2, and 3, all of which form components of elastin microfibrils. All LTBPs and fibrillins contain several domains called TGF-β-binding (TB) domains (144), although only a small minority of these TB domains actually form disulfide bonds with TGF-β LAPs (145). LTBPs and fibrillins also contain a large but variable number of epidermal growth factor–like domains that bind to extracellular calcium and contribute to the relatively rigid structure of these proteins. LTBP1 and 2 and all three fibrillins contain arginine-glycine-aspartic acid (RGD) sequences, but only the RGD sequence in fibrillin 1 has been shown to be present in a surface-exposed loop and to interact with RGD-binding integrins (146). LTBPs bind directly to fibrillins, which is an important step in anchoring latent TGF-β to elastin-containing fibers in the extracellular matrix (144).
Mutations in the fibrillin 1 gene have been shown to cause Marfan syndrome, a human disease characterized by joint laxity, lens dislocation, skeletal deformities, pulmonary emphysema, and aortic aneurysms (147). Similarly, mice with a truncation mutation in fibrillin 1 develop pulmonary emphysema and aortic aneurysms (148). Both manifestations are associated with excess TGF-β signaling, and both can be inhibited by blocking antibodies to TGF-β. These results strongly suggest that intact fibrillin 1 is normally an inhibitor of TGF-β activation, perhaps as a consequence of sequestering latent TGF-β in elastin fibers. These findings also suggest that release of latent TGF-β from the extracellular matrix could in some circumstances facilitate its activation.
It is clear that the relationship between TGF-β tethering through LTBPs and fibrillins is complex because defects in these tethers can either enhance or inhibit TGF-β activation. For example, mice homozygous for a null mutation in LTBP3 develop abnormalities in bone development and severe developmental emphysema that appear to be due to a deficiency in TGF-β signaling (149, 150). However, the functional importance of LTBP-fibrillin interactions in regulating TGF-β function in the immune system is at present completely unclear.
Regulation of TGF-β Activation by Glycoprotein A Repetitions Predominant Protein
Recent evidence demonstrates that TGF-β can be tethered to the surface of T cells through association with glycoprotein A repetitions predominant protein (GARP) (151–153). GARP is a transmembrane protein that, like LTBPs, is covalently linked to TGF-β1 LAP through disulfide bond formation prior to TGF-β1 secretion (154). Unlike LTBPs, which are widely expressed, GARP expression appears to be tightly restricted to Foxp3-expressing Tregs, and latent TGF-β expression on activated Tregs is dependent on expression of GARP (155). Recent work suggests that when GARP and LTBP1 are coexpressed, GARP outcompetes LTBP1 for association with LAP. Association of TGF-β1 with GARP can facilitate activation of the latent complex by other mechanisms, for example via the integrin αvβ6 and, to a lesser extent, integrin αvβ8 (154).
ACTIVATION OF TGF-β BY INTEGRINS
The possibility that members of the integrin family could activate latent TGF-β was first suggested by the phenotype of mice lacking the integrin β6 subunit of the integrin αvβ6 (156, 157). Expression of this integrin is restricted to epithelial cells and is highly induced on epithelial cells in several organs by tissue injury and inflammation (158, 159). Integrin β6 knockout mice develop exaggerated inflammatory responses in the lungs and skin in response to usually trivial insults, but despite exaggerated inflammation they are protected from tissue fibrosis at multiple sites (157). Both phenotypic features are consistent with a failure to optimally activate TGF-β. Cells expressing integrin αvβ6 can activate both TGF-β1 and TGF-β3 by binding to a common integrin recognition motif, the linear tripeptide RGD that is present in an exposed loop in the LAPs of TGF-β1 and 3 (157, 160).
The integrin αvβ8 binds to the same RGD site and can also activate TGF-β1 and 3 (141). Mice globally lacking the integrin β8 subunit generally die during embryonic development or immediately after birth as a consequence of intracerebral hemorrhage caused by defective vascular development in the central nervous system (143). This effect appears to be due to impaired TGF-β signaling as a consequence of impaired presentation of active TGF-β by neuroepithelial cells, which normally express the integrin αvβ8, to endothelial cells, which do not (142, 161). Mice lacking integrin αvβ8 also develop cleft palate, a prominent finding in mice deficient in TGF-β3 (142, 143).
Work from Munger’s laboratory strongly suggests that these two integrins, αvβ6 and αvβ8, are essential for all the developmental and homeostatic roles of TGF-β1 and 3. Knock-in of a point mutation in the TGF-β1 gene, which changed the RGD sequence to RGE and eliminated integrin binding but did not affect TGF-β1 secretion or any other known mechanism of TGF-β1 activation, completely recapitulated all known effects of the knockout of TGF-β1, including massive multiorgan inflammation in young mice (162). These data suggest a critical role for integrin-mediated TGF-β1 activation in suppressing self-harmful immune responses. The Munger group also generated mice completely lacking expression of the integrin β8 subunit that survived developmental defects by crossing integrin β8 knockout mice into an outbred line (CD1). Treatment of these mice, beginning before or immediately after birth, with a blocking antibody against the integrin αvβ6, or crossing these mice to mice lacking integrin αvβ6 recapitulated essentially all the findings in mice lacking TGF-β1 and 3 (163), including severe multiorgan inflammation (a central feature of TGF-β1 knockout mice) and a high prevalence of cleft palate [a central feature in TGF-β3 knockout mice (164)]. Furthermore, mice lacking integrin-mediated activation of TGF-β1 (via an RGD to RGE knock-in mutation) and completely lacking TGF-β3 expression developed intracerebral hemorrhage, a prominent feature of integrin β8 subunit knockout mice (143) not seen in single knockouts of TGF-β1 or 3. Taken together, these data indicate that integrins αvβ6 and αvβ8 are crucial activators of TGF-β for maintenance of immune homeostasis and for controlling TGF-β function during development.
In addition to integrins αvβ6 and αvβ8, there are four other integrins that recognize the RGD site in the LAP region of TGF-β1: α 8β1 (165), αvβ1 (166), αvβ3 (167, 168), and αvβ5 (166, 169). Integrins αvβ3 and αvβ5 can both activate TGF-β in vitro when they are expressed on contractile fibroblasts or airway smooth muscle cells (168–171). Recent evidence suggests that one or more αv-containing integrins on myofibroblasts, other than αvβ6 and αvβ8, contribute to tissue fibrosis in multiple solid organs (172) and that TGF-β activation by αvβ5 contributes to proliferation of airway smooth muscle following allergen sensitization and challenge in vivo (171). These data suggest that multiple RGD-binding integrins may play important roles in activating TGF-β that contribute to tissue pathology in adult mammals. However, no roles for activation of TGF-β by integrins other than αvβ6 and αvβ8 have yet been demonstrated to be relevant to immune responses.
Mechanisms of TGF-β Activation by Integrins
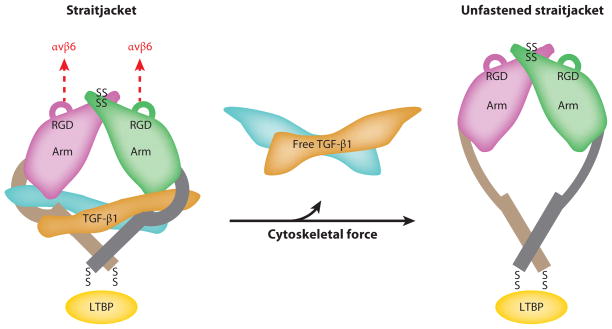
Integrin αvβ6 appears to activate TGF-β1 and 3 by binding to the RGD sequence in the respective LAPs and exerting deforming physical force on the tethered latent complex. Although expression of this integrin is restricted to epithelial cells, which are not generally considered to be highly contractile cells, considerable evidence supports an important role for actin-myosin contraction and mechanical deformation of the latent complex in integrin αvβ6–mediated TGF-β activation. This process clearly depends on interactions between the β6 cytoplasmic domain and the actin cytoskeleton, because deletions in the cytoplasmic domain that impair cytoskeletal engagement or inhibition of actin polymerization (by cytochalasin D) completely prevent TGF-β activation (157). This process is also blocked by pharmacologic inhibition of nonmuscle myosin (with blebbistatin) and by plating αvβ6-expressing epithelial cells on flexible substrates that interfere with the transmission of contractile force to the tethered latent complex (173). Deletion of LTBP1, required for tethering the latent complex to the extracellular matrix, also completely inhibits αvβ6-mediated TGF-β activation, and this effect can be rescued by a short fusion protein composed of a region of LTBP1 containing the cysteine residues that form disulfide bonds with LAP and the domain that directly binds fibronectin (174). These data underscore the fact that LTBP1 supports αvβ6-mediated TGF-β activation entirely through mechanically tethering the latent complex. Finally, the solved crystal structure of the small latent complex identified a single unstructured region of TGF-β LAP (the latency lasso) that forms the critical contacts covering the sites on active TGF-β that are required for binding to TGF-β receptors (4). The solved structure also demonstrated that the site for tethering LAP and the exposed RGD-containing loop that binds αvβ6 are located on opposite poles of the latent complex, and it fully explained how linear force applied across the tethered complex would preferentially unfold the latency lasso and thus release the active cytokine (Figure 4). Although there is clear evidence for integrin αvβ6–mediated TGF-β activation in regulation of the immune system (163), expression of this integrin is restricted to epithelial cells. Thus, modulation of the immune system by this mechanism must be via epithelial cell–immune cell cross talk.
Figure 4.
Latent TGF-β can be activated by integrins via generation of physical force. Physical force generated by contraction of the actin cytoskeleton of cells expressing certain TGF-β-activating integrins (e.g., αvβ6) unfolds the unstructured region (straitjacket) of the latency-associated peptide that forms the major contact points with the active TGF-β dimer, thereby releasing active TGF-β and freeing it to interact with TGF-β receptors. Integrins αvβ3 and αvβ5 are proposed to activate latent TGF-β via a similar mechanism (not shown). Adapted from Reference 4 and reprinted with permission from Nature Publishing Group and Prof. Timothy Springer (Harvard University).
In vitro studies have shown that more professional contractile cells, for example myofibroblasts, also activate latent TGF-β through mechanical deformation of the tethered latent complex. Wipff and coworkers (170) showed that this effect could be induced in the absence of intact cells, using cytoskeletal preparations from myofibroblasts, and that TGF-β activation clearly required transmission of contractile force, because this effect was also prevented when fibroblasts were plated on flexible substrates. As noted above, myofibroblasts do not express αvβ6, and studies with blocking antibodies suggested that integrins αvβ3 and αvβ5 both contributed to this effect.
However, the role of mechanical deformation in TGF-β activation by integrin αvβ8 is less clear. The β8 cytoplasmic domain is completely divergent from other integrin β subunits and does not contain sequences known to interact with linker proteins that connect integrins to the actin cytoskeleton (141). In contrast to αvβ6-mediated TGF-β activation, activation by integrin αvβ8 is retained even after the entire β8 cytoplasmic domain is deleted. Furthermore, in at least some cultured cell lines, αvβ8-mediated TGF-β activation appears to require proteolytic cleavage of TGF-β LAP by the transmembrane metalloproteinase MMP14 (141). However, it is not yet certain whether MMP-mediated cleavage is universally required for αvβ8-mediated TGF-β activation, and additional proteins that associate with αvβ8 could facilitate transmission of mechanical force across the integrin-bound latent complex.
Activation of TGF-β2
As mentioned earlier, there are three mammalian isoforms of TGF-β, TGF-β1, 2, and 3. All developmental effects and regulation of immune homeostasis by TGF-β1 and 3 appear to depend on activation by the integrins αvβ6 and αvβ8, which bind to exposed RGD sequences in TGF-β1 and 3 LAP. However, TGF-β2 LAP does not contain an RGD sequence and does not appear to bind to these integrins. Homology modeling based on the solved crystal structure of the small latent complex of TGF-β1 suggests that TGF-β2 could also be activated by mechanical deformation (4), but whether such a mechanism is physiologically relevant to TGF-β2 activation and whether TGF-β2 can be activated by other integrins remain to be determined.
FUNCTIONAL SIGNIFICANCE OF INTEGRIN-MEDIATED TGF-β ACTIVATION IN IMMUNITY
The most convincing evidence for the importance of distinct mechanisms of TGF-β activation in immunity comes from studies of mice defective in integrin αvβ6, αvβ8, or both. As noted above, mice expressing latent TGF-β1 with a point mutation that prevents integrin binding manifest all the features of TGF-β1 knockout mice, including profound multiorgan inflammation (162). Mice deficient in the integrin β8 subunit that are treated starting at birth with a blocking monoclonal antibody to the integrin αvβ6 have the same phenotype, with similar severity of multiorgan inflammation (163), suggesting, as noted above, that activation of TGF-β responsible for these features is likely entirely explained by effects of integrins αvβ6 and αvβ8.
However, the temporal pattern of phenotypic effects of combined inhibition of integrins αvβ6 and αvβ8 is striking. If β8-deficient mice are treated with αvβ6-blocking antibody beginning at 3 days after birth, the inflammatory phenotype is much milder. Thus, whereas β8 knockout mice treated with αvβ6-blocking antibody before or at birth nearly all die from severe multiorgan inflammation before age 25 days, all the mice treated beginning at 3 days of age survived well beyond 25 days and only manifested the mild lung inflammation seen in αvβ6-deficient mice (163). Given that this time window corresponds to the period of rapid T cell development in the thymus, these results suggest combined roles in TGF-β activation for integrins αvβ6 and αvβ8 during thymic development. However, the nature of these effects and of the relevant αvβ6- and αvβ8-expressing cells remains to be determined.
Role of Integrin αvβ8–Mediated TGF-β in Regulating Intestinal Homeostasis
Integrin αvβ8 is more widely expressed than αvβ6, but in the absence of effective antibodies for immunostaining, the precise distribution of integrin αvβ8 expression remains to be determined. Inbred mice globally lacking the integrin β8 subunit usually die during embryonic development from intracerebral hemorrhage (143), so they have not been useful for studies of the role of integrin αvβ8–mediated TGF-β activation in immune responses of adult animals. Mice deficient in integrin αvβ8 in hematopoietic cells, generated by crossing mice expressing a floxed allele of integrin β8 with mice expressing Cre recombinase under the control of the Vav-1 promoter, live well into adulthood, but by as early as 1 month of age they have an increase in spontaneously activated CD4+ and CD8+ T cells and profound increases in circulating levels of IgE, IgA, and IgG1 (175). By 6–10 months of age these mice develop severe colonic inflammation, and they subsequently develop circulating autoantibodies. All these phenotypic features are essentially identical to those seen in mice with hypomorphic TGF-β signaling in T cells that resulted from overexpression of a dominant-negative TGF-βRII receptor in these cells (21), and a similar phenotype was observed in mice lacking all αv integrins from hematopoietic cells (176).
In the absence of effective antibodies recognizing murine αvβ8, relative expression in hematopoietic cells was assessed by quantitative PCR. Expression of β8 appeared to be largely restricted to CD4+ T cells and DCs, with little detectible β8 mRNA in B cells, neutrophils, monocytes, or CD8+ T cells (175; A.C. Melton & D. Sheppard, unpublished data). Additional conditional knockout lines were thus generated using CD11c-Cre to induce deletion in DCs or CD4-Cre to induce deletion in T cells. Mice lacking integrin β8 in DCs completely mimicked the phenotype of mice lacking β8 in all hematopoietic cells, whereas mice lacking β8 only in T cells had no spontaneous phenotype (175). T cell activation, increased immunoglobulin production, and colonic inflammation thus all appear to be due to a defect in activation of TGF-β by integrin αvβ8 on DCs and its subsequent presentation to T cells. The function of integrin αvβ8 expressed by CD4+ T cells remains to be determined.
Recent studies identify potential explanations for this phenotype. Mice lacking integrin αvβ8 on DCs have a significant reduction in the number of Tregs in the colonic mucosa (175). Work has shown that the integrin β8 subunit is preferentially expressed by intestinal CD103+ DCs (177, 178). CD103+ DCs in the gut are a subset of DCs proposed to contribute to intestinal tolerance by preferentially inducing naive T cells to express the transcription factor Foxp3 and become iTregs (74, 75). CD103+ DCs from the intestine appear specialized to activate TGF-β, a function dependent on high expression of integrin αvβ8 (177, 178). This enhanced integrin αvβ8–mediated TGF-β activation is crucial to induction of iTregs, as CD103+ intestinal DCs lacking integrin β8 are no longer able to induce iTregs in vitro, and lack of β8 expression on DCs inhibits the preferential induction of iTregs in the intestine in vivo (178).
Additionally, mice lacking integrin αvβ8 in DCs have a marked reduction in the numbers of Th17 cells in the colonic mucosa (179). Cytokines released by these cells (for example, IL-22) play a major role in maintaining the barrier function of intestinal epithelial cells, so loss of these cells could also contribute to the disruption in intestinal homeostasis in mice lacking the integrin αvβ8 on DCs.
Role of Integrin αvβ8–Mediated TGF-β Activation in Induction of Th17 Cells
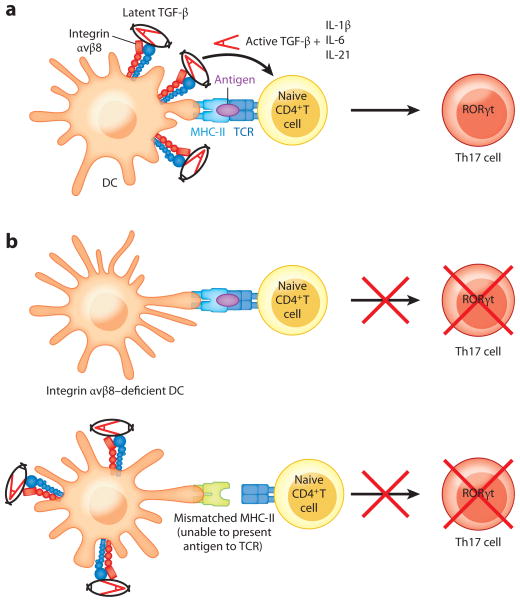
Mice lacking integrin αvβ8 on DCs are relatively healthy for several months, which allowed examination of the role of DC-αvβ8-mediated TGF-β activation in models of immune-mediated disease. Despite a baseline increase in T cell activation, mice lacking integrin αvβ8 on DCs were dramatically protected from EAE (179), with a similar phenotype observed in mice lacking all αv integrins in myeloid cells (180). This protection was associated with a nearly complete absence of Th17 cells in the spinal cords and draining lymph nodes of mice lacking integrin αvβ8 on DCs. In vitro Th17 induction assays demonstrated that wild-type DCs loaded with a peptide derived from myelin could induce differentiation of naive TCR transgenic T cells into Th17 cells in the absence of exogenous TGF-β but that this effect was markedly reduced when integrin αvβ8–deficient DCs were used in the assay (179). This difference could be rescued by the addition of a low concentration of active TGF-β or the addition of wild-type DCs, but not by the addition of DCs with a mismatched MHC class II molecule and thus incapable of presenting the myelin peptide to the T cells (179). These results strongly suggest that TGF-β, activated by integrin αvβ8 and simultaneously presented with antigen, is required for induction of antigen-specific Th17 cells (Figure 5).
Figure 5.
Th17 cell induction via dendritic cell (DC)–expressed, integrin αvβ8–mediated TGF-β activation requires MHC-II–antigen–TCR interaction. (a) Activation of TGF-β by integrin αvβ8 expressed by DCs, in the presence of proinflammatory cytokines, can induce Th17 cell differentiation when the DC presents antigen via MHC-II to naive T cells. (b) In the absence of integrin αvβ8 expression, DCs are unable to induce Th17 cell differentiation. Additionally, DCs that express integrin αvβ8 but express a mismatched haplotype of MHC-II that cannot present antigen to T cells cannot induce Th17 cells.
Role of Integrin αvβ8–Mediated TGF-β Activation in Allergic Asthma
IL-17 has been suggested to play a role in allergic asthma and has been shown to be increased in the blood of patients with severe asthma and to correlate with asthma severity (181). Mice lacking integrin αvβ8 on DCs are also protected from airway hyperresponsiveness in two different models of allergic asthma, induced by sensitization and challenge with either ovalbumin or house dust mite (182). Although IL-17 induces release of neutrophil-recruiting chemokines from epithelial cells and is associated with neutrophilic inflammation in multiple disease models, there are no differences in the numbers of neutrophils recruited into the airways of allergen-challenged control mice or of mice lacking integrin αvβ8 on DCs. As in the colonic epithelium and the spinal cords of mice undergoing EAE, the major cellular abnormality in the lungs of allergen-challenged mice lacking integrin αvβ8 on DCs was a profound reduction in Th17 cells (182). In the lungs, Th17 cells are not the largest population of cells capable of making IL-17 as there are large numbers of IL-17-producing innate γδT cells. The number of IL-17-producing γδT cells also increases with allergen challenge, but this effect was not different between control mice and mice lacking integrin αvβ8 on DCs.
In these models, IL-17 released by Th17 cells appears to act directly on airway smooth muscle to increase its contractility. Overnight incubation of murine tracheas with IL-17 increased force generation in response to bronchoconstrictor agonists (182). This effect appeared to be due to activation of NF-κB, which increased transcription and protein synthesis of the small GTPase RhoA and its effector kinase, Rho kinase, leading to inhibition of myosin phosphatase, increased phosphorylation of myosin, and enhanced actin-myosin contraction. Importantly, all these biochemical effects were readily apparent in smooth muscle dissected from control mice sensitized and challenged with allergen in vivo but were markedly blunted in muscle dissected from sensitized and challenged mice lacking integrin αvβ8 on DCs (182). Tracheal rings dissected from control mice generated more contractile force in vitro than rings from mice lacking αvβ8 on DCs, but this difference was abolished by adding back IL-17. These results raise the possibility that targeting integrin αvβ8–mediated TGF-β activation might be an effective intervention in allergic asthma.
Immune Effects of Integrin αvβ8–Mediated TGF-β Activation on Fibroblasts
Integrin αvβ8 is also expressed on a number of nonimmune cells, including fibroblasts. Deletion of integrin αvβ8 from fibroblasts in adult mice, using tamoxifen-inducible collagen 1α2-ERcre, protected mice from airway inflammation induced by the introduction of adenovirus that expresses IL-1 in the airways and also inhibited airway inflammation and mucus metaplasia in response to allergen sensitization and challenge (183). This protection appeared to be explained by reduction in production of the chemokines CCL2 and CCL20 and impaired recruitment of DCs into the lungs. These findings suggest that integrin αvβ8–mediated TGF-β activation can have very different effects on immune responses when integrin αvβ8 is expressed on different cell types. These findings also provide an additional rationale for considering inhibition of integrin αvβ8–mediated TGF-β activation as a potential therapeutic intervention in allergic asthma.
Unique Roles of the Integrin αvβ6 in Immunity
As described in detail above, mice lacking expression of TGF-β1 develop T cell–mediated inflammation early in life, and mice deficient in both integrins αvβ6 and αvβ8 have profound abnormalities in adaptive immunity. However, work on the TGF-β-activating integrin αvβ6, an integrin expressed specifically on epithelial cells, has shown that the principal immune defects involve cells of the innate immune system.
Mice globally lacking the integrin β6 subunit are born at the expected Mendelian frequency with no apparent developmental defects in B cells or T cells. In the first few weeks of life, they develop spatially restricted baldness that is due to infiltration of the dermis with macrophages (156). This appears to be an exaggerated response to low-grade trauma because the bald areas correspond to locations where mothers pick up their offspring with their teeth. These areas heal and look normal after pups are weaned from their mothers (156). Integrin β6 knockout mice also develop low-grade inflammation in their lungs, characterized by accumulations of T cells and B cells around blood vessels and conducting airways. These mice also have increased numbers of alveolar macrophages and a dramatic change in alveolar macrophage morphology, with accumulation of large macrophages with foamy cytoplasm suggestive of an activated phenotype (156). Lung inflammation also appears to be an exaggerated response to low-grade injury because the phenotype is much more severe when mice are maintained in nonventilated cages and therefore exposed to ammonia released from urine-soaked bedding (X. Huang & D. Sheppard, unpublished observations).
Alveolar macrophages in integrin subunit β6 knockout mice have a marked increase in production of metalloproteases, most prominently MMP12 (184). mRNA encoding MMP12 is increased up to 200-fold in macrophages from these mice, and increased MMP12 eventually leads to the development of destructive emphysema (185). The role of MMP12 and loss of TGF-β signaling in this phenotype was demonstrated by complete prevention of emphysema in integrin β6 knockout mice by combined loss of MMP12 and by rescue of the increase in MMP12 expression by transgenic expression of active TGF-β by pulmonary epithelial cells. Thus, integrin αvβ6–mediated TGF-β activation appears crucial in regulating alveolar macrophage–mediated regulation of immune responses. However, how epithelial-expressed integrin αvβ6 regulates adaptive immune responses to modulate immunity requires further study.
Role of Integrin αvβ6 in Regulating Intraepithelial Mast Cell Protease Expression
Intraepithelial mast cells play an important role in expulsion of parasites from the gastrointestinal tract. TGF-β contributes to the accumulation or differentiation of a specific population of intraepithelial intestinal mast cells that express the murine mast cell protease mMCP1 and are greatly expanded after infection of mice with the parasite Nippostrongylus brasiliensis (186). β6 knockout mice have a profound defect in accumulation of intraepithelial mMCP1-expressing intestinal mast cells in response to Nippostrongylus infection (186).
TGF-β has been suggested as an important modulator of the airway remodeling seen in mice chronically challenged with allergens in models of allergic asthma. β6 knockout mice sensitized and then repeatedly challenged with intra-airway ovalbumin were protected from the induction of airway hyperresponsiveness (187). However, contrary to expectations, these mice had the same airway remodeling seen in wild-type mice.
Because expression of integrin αvβ6 is restricted to epithelial cells, the epithelial microenvironment was sampled with a specially designed murine airway “brush.” There were few informative differences between control mice and integrin β6 knockout mice in epithelial cell genes, but there were profound differences in a series of genes encoding proteins restricted to mast cells (187). Even in the absence of allergen challenge, the epithelial microenvironment in β6 knockout mice manifested large increases in the mast cell proteases mMCP4, 5, and 6 and chymotrypsin. After chronic allergen challenge, mMCP1 was dramatically increased in the airway microenvironment of control mice, but this response was essentially absent in the airways of integrin β6 knockout mice (187). In vitro differentiation of mast cells in the presence or absence of active TGF-β1 demonstrated that TGF-β1 signaling is absolutely required for induction of mMCP1 expression but completely suppresses expression of mMCP4 and 6. Examination of whole mounts of tracheas from wild-type and β6 knockout mice demonstrated a large increase in the numbers of mast cells within the airways of integrin β6 knockout mice even in the absence of allergen challenge (187).
In vitro studies examining contractility of mouse tracheal rings appeared to explain the protection from airway hyperresponsiveness seen in the integrin β6 knockout mice. Sugimoto et al. (187) found that mMCP1, which was not expressed in mast cells in integrin β6 knockout mice, increased tracheal ring contractility through indirect effects on the airway epithelium (187). In contrast, mMCP4, which was increased in the airway epithelium of integrin β6 knockout mice, acted directly on airway smooth muscle to suppress the enhanced contractility induced by the Th2 cytokine IL-13, a well-characterized mediator of enhanced airway smooth muscle contractility in this model. Thus, integrin αvβ6 appears to be a key activator of TGF-β for regulation of mast cells during allergic responses in the airway.
CONCLUSIONS AND FUTURE PERSPECTIVES
We have discussed some of the pleiotropic effects of TGF-β on immunity, with a focus on the effects of TGF-β on T cells. Depending on the presence of other secreted factors and cell surface coreceptors, TGF-β can suppress adaptive immune responses (through induction and stabilization and function of Tregs and by directly suppressing Th1 cells, Th2 cells, and CD8+ T cells) or can enhance adaptive responses (through induction of Th17 cells, Th9 cells, and CD4+ CTL-like effector cells). It is thus clear that global inhibition of TGF-β or TGF-β signaling will be an imprecise strategy for therapeutically modulating adaptive immunity. A more detailed understanding of regulation of cofactors that drive these divergent effects and the relative importance of specific pathways downstream of TGF-β receptor ligation will thus be required to optimally translate what we have learned about TGF-β signaling in immunity into effective therapies.
One important regulator of TGF-β function is its extracellular activation. In this review we have focused on activation by a subset of integrins. Two integrins, αvβ6 and αvβ8, appear to jointly regulate much of the TGF-β1 activation relevant to adaptive immunity, given that combined inhibition of these integrins largely mimics the immune dysregulation seen in mice with complete loss of TGF-β signaling in T cells. Because a loss of one of these integrins by itself results in much less severe phenotypes, targeting integrin αvβ6 or αvβ8 could provide more precision for therapeutic intervention. However, much remains to be learned about the relative contributions of each of these integrins on specific cell types and how their expression and function are regulated during development of the immune system and in the setting of immune-mediated diseases.
Acknowledgments
We apologize to those whose work we did not cite because of space restrictions. Work in the laboratory of M.A.T. is funded by grants from the Biotechnology and Biological Sciences Research Council and Medical Research Council. Work in the laboratory of D.S. is funded by grants HL108794, HL102292, HL53947, and AI077439 from the National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT
M.A.T. receives research funding from the Manchester Collaborative Centre for Inflammation Research, which is jointly funded by the University of Manchester, Astra Zeneca, and GlaxoSmithKline. D.S. receives research funding from Pfizer to develop therapeutic antibodies targeting integrin αvβ8 and from a sponsored research agreement with BiogenIdec to develop therapeutic antibodies targeting integrin αvβ6.
Contributor Information
Mark A. Travis, Email: mark.travis-2@manchester.ac.uk.
Dean Sheppard, Email: dean.sheppard@ucsf.edu.
LITERATURE CITED
- 1.Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, et al. TGF-β latency: biological significance and mechanisms of activation. Stem Cells. 1997;15:190–97. doi: 10.1002/stem.150190. [DOI] [PubMed] [Google Scholar]
- 2.Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-β: structural features and mechanisms of activation. Kidney Int. 1997;51:1376–82. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 3.Pesu M, Watford WT, Wei L, Xu L, Fuss I, et al. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455:246–50. doi: 10.1038/nature07210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi M, Zhu J, Wang R, Chen X, Mi L, et al. Latent TGF-β structure and activation. Nature. 2011;474:343–49. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci. 2003;116:217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 6.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni AB, Huh C-G, Becker D, Geiser A, Lyght M, et al. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–74. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–99. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol. 2009;19:385–94. doi: 10.1016/j.tcb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Shi YG, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 11.Daly AC, Randall RA, Hill CS. Transforming growth factor β-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol. 2008;28:6889–902. doi: 10.1128/MCB.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–99. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu YB, Gudey SK, Landstrom M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347:11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi H, Abdollah S, Qiu YB, Cai JX, Xu YY, et al. TheMAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell. 1997;89:1165–73. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 15.Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, et al. Identification of Smad7, a TGF β-inducible antagonist of TGF-β signalling. Nature. 1997;389:631–35. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 16.Shi W, Sun C, He B, Xiong W, Shi X, et al. GADD34-PP1c recruited by Smad7 dephosphorylates TGFβ type 1 receptor. J Cell Biol. 2004;164:291–300. doi: 10.1083/jcb.200307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu HT, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Mol Cell. 2000;6:1365–75. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 18.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letterio JJ, Geiser AG, Kulkarni AB, Dang H, Kong L, et al. Autoimmunity associated with TGF-beta1-deficiency in mice is dependent on MHC class II antigen expression. J Clin Investig. 1996;98:2109–19. doi: 10.1172/JCI119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi S, Yoshida K, Ward JM, Letterio JJ, Longenecker G, et al. β2-microglobulin-deficient background ameliorates lethal phenotype of the TGF-β1 null mouse. J Immunol. 1999;163:4013–19. [PubMed] [Google Scholar]
- 21.Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 22.Lucas PJ, Kim S-J, Melby SJ, Gress RE. Disruption of T cell homeostasis in mice expressing a T cell–specific dominant negative transforming growth factor β II receptor. J Exp Med. 2000;191:1187–96. doi: 10.1084/jem.191.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455– 71. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity. 2006;25:441–54. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–40. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 26.Zhang N, Bevan MJ. TGF-β signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nat Immunol. 2012;13:667–73. doi: 10.1038/ni.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu A-D, Wang Y, Lin L, Zhang SS, Wan YY. Requirements of transcription factor Smad-dependent and -independent TGF-β signaling to control discrete T-cell functions. Proc Natl Acad Sci USA. 2012;109:905–10. doi: 10.1073/pnas.1108352109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takimoto T, Wakabayashi Y, Sekiya T, Inoue N, Morita R, et al. Smad2 and Smad3 are redundantly essential for the TGF-β-mediated regulation of regulatory T plasticity and Th1 development. J Immunol. 2010;185:842–55. doi: 10.4049/jimmunol.0904100. [DOI] [PubMed] [Google Scholar]
- 29.Kim BG, Li CL, Qiao WH, Mamura M, Kasperczak B, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–19. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 30.Nakao A, Miike S, Hatano M, Okumura K, Tokuhisa T, et al. Blockade of transforming growth factor β/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med. 2000;192:151–58. doi: 10.1084/jem.192.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleiter I, Song J, Lukas D, Hasan M, Neumann B, et al. Smad7 in T cells drives T helper 1 responses in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain. 2010;133:1067–81. doi: 10.1093/brain/awq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J Clin Investig. 2001;108:601–9. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fantini MC, Rio A, Fina D, Caruso R, Sarra M, et al. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology. 2009;136:1308–16. doi: 10.1053/j.gastro.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 34.Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, et al. Production of transforming growth factor β by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–50. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sung JL, Lin JT, Gorham JD. CD28 co-stimulation regulates the effect of transforming growth factor-β1 on the proliferation of naive CD4+ T cells. Int Immunopharmacol. 2003;3:233–45. doi: 10.1016/S1567-5769(02)00276-X. [DOI] [PubMed] [Google Scholar]
- 36.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-β and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–44. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-intrinsic transforming growth factor-β signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31:145–57. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerwenka A, Kovar H, Majdic O, Holter W. Fas- and activation-induced apoptosis are reduced in human T cells preactivated in the presence of TGF-β. J Immunol. 1996;156:459–64. [PubMed] [Google Scholar]
- 39.Ouyang W, Oh SA, Ma Q, Bivona MR, Zhu J, Li MO. TGF-β cytokine signaling promotesCD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor α expression. Immunity. 2013;39:335–46. doi: 10.1016/j.immuni.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SV, Xiang WV, Kwak C, Yang Y, Lin XW, et al. GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science. 2013;340:1456–59. doi: 10.1126/science.1237013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Garra A, Gabrysova L, Spits H. Quantitative events determine the differentiation and function of helper T cells. Nat Immunol. 2011;12:288–94. doi: 10.1038/ni.2003. [DOI] [PubMed] [Google Scholar]
- 42.Schoenborn JR, Wilson CB. Regulation of interferon-γ during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 43.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor β-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorham JD, Guler ML, Fenoglio D, Gubler U, Murphy KM. Low dose TGF-β attenuates IL-12 responsiveness in murine Th cells. J Immunol. 1998;161:1664–70. [PubMed] [Google Scholar]
- 45.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat Immunol. 2005;6:600–7. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 46.Smeltz RB, Chen J, Shevach EM. Transforming growth factor-β1 enhances the interferon-γ-dependent, interleukin-12-independent pathway of T helper 1 cell differentiation. Immunology. 2005;114:484– 92. doi: 10.1111/j.1365-2567.2005.02115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tofukuji S, Kuwahara M, Suzuki J, Ohara O, Nakayama T, Yamashita M. Identification of a new pathway for Th1 cell development induced by cooperative stimulation with IL-4 and TGF-β. J Immunol. 2012;188:4846–57. doi: 10.4049/jimmunol.1103799. [DOI] [PubMed] [Google Scholar]
- 48.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-β inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165:4773–77. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 49.Heath VL, Murphy EE, Crain C, Tomlinson MG, O’Garra A. TGF-β1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol. 2000;30:2639–49. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 50.Kuwahara M, Yamashita M, Shinoda K, Tofukuji S, Onodera A, et al. The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-β and suppresses TH2 differentiation. Nat Immunol. 2012;13:778–86. doi: 10.1038/ni.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hansen G, McIntire JJ, Yeung VP, Berry G, Thorbecke GJ, et al. CD4+ T helper cells engineered to produce latent TGF-β1 reverse allergen-induced airway hyperreactivity and inflammation. J Clin Investig. 2000;105:61–70. doi: 10.1172/JCI7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 54.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor FoxP3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 55.van der Vliet HJJ, Nieuwenhuis EE. IPEX as a result of mutations in FOXP3. Clin Dev Immunol. 2007;2007:89017. doi: 10.1155/2007/89017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–58. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 57.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–67. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–53. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen WJ, Jin WW, Hardegen N, Lei KJ, Li L, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting edge: IL-2 is essential for TGF-β-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–26. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 61.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-β to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–27. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 62.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 63.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–12. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med. 2012;209:1529–35. doi: 10.1084/jem.20112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu L, Kitani A, Strober W. Molecular mechanisms regulating TGF-β-induced Foxp3 expression. Mucosal Immunol. 2010;3:230–38. doi: 10.1038/mi.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jana S, Jailwala P, Haribhai D, Waukau J, Glisic S, et al. The role of NF-κB and Smad3 in TGF-β-mediated Foxp3 expression. Eur J Immunol. 2009;39:2571–83. doi: 10.1002/eji.200939201. [DOI] [PubMed] [Google Scholar]
- 67.Martinez GJ, Zhang Z, Chung Y, Reynolds JM, Lin X, et al. Smad3 differentially regulates the induction of regulatory and inflammatory T cell differentiation. J Biol Chem. 2009;284:35283–86. doi: 10.1074/jbc.C109.078238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu L, Wang J, Zhang F, Chai Y, Brand D, et al. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez GJ, Zhang Z, Reynolds JM, Tanaka S, Chung Y, et al. Smad2 positively regulates the generation of Th17 cells. J Biol Chem. 2010;285:29039–43. doi: 10.1074/jbc.C110.155820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu J, Davidson TS, Wei G, Jankovic D, Cui K, et al. Down-regulation of Gfi-1 expression by TGF-β is important for differentiation of Th17 and CD103+ inducible regulatory T cells. J Exp Med. 2009;206:329–41. doi: 10.1084/jem.20081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Floess S, Freyer J, Siewert C, Baron U, Olek S, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:169–78. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–63. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 73.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun C-M, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β–and retinoic acid–dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun C-M, Hall JA, Blank RB, Bouladoux N, Oukka M, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coleman MM, Ruane D, Moran B, Dunne PJ, Keane J, Mills KHG. Alveolar macrophages contribute to respiratory tolerance by inducing FoxP3 expression in naive T cells. Am J Respir Cell Mol Biol. 2013;48:773–80. doi: 10.1165/rcmb.2012-0263OC. [DOI] [PubMed] [Google Scholar]
- 77.Khare A, Krishnamoorthy N, Oriss TB, Fei M, Ray P, Ray A. Cutting edge: Inhaled antigen upregulates retinaldehyde dehydrogenase in lung CD103+ but not plasmacytoid dendritic cells to induce Foxp3 de novo in CD4+ T cells and promote airway tolerance. J Immunol. 2013;191:25–29. doi: 10.4049/jimmunol.1300193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210:775–88. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, et al. Skin-draining lymph nodes contain dermis-derived CD103− dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood. 2010;115:1958–68. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 80.Xu L, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity. 2010;33:313–25. doi: 10.1016/j.immuni.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hill JA, Hall JA, Sun C-M, Cai Q, Ghyselinck N, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi cells. Immunity. 2008;29:758–70. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, et al. STAT6 inhibits TGF-β1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J Biol Chem. 2008;283:14955–62. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–33. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 84.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, et al. T cells that cannot respond to TGF-β escape control by CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:737–46. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–99. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–22. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–62. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pires da Cunha A, Weiner HL. Induction of immunological tolerance by oral anti-CD3. Clin Dev Immunol. 2012;2012:425021. doi: 10.1155/2012/425021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Regateiro FS, Howie D, Nolan KF, Agorogiannis EI, Greaves DR, et al. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-β. Eur J Immunol. 2011;41:2955–65. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- 90.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–41. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 91.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, et al. A dominant function for interleukin 27 in generating interleukin 10–producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–89. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 92.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 93.Hu W, Troutman TD, Edukulla R, Pasare C. Priming microenvironments dictate cytokine requirements for T helper 17 cell lineage commitment. Immunity. 2011;35:1010–22. doi: 10.1016/j.immuni.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shaw MH, Kamada N, Kim Y-G, Nunez G. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209:251–58. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 96.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17–producing human T helper cells. Nat Immunol. 2007;8:942–49. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 97.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, et al. Development, cytokine profile and function of human interleukin 17–producing helper T cells. Nat Immunol. 2007;8:950–57. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 98.Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol. 2008;9:641–49. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, et al. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating humanTH-17 responses. Nat Immunol. 2008;9:650–57. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 100.Ghoreschi K, Laurence A, Yang X-P, Tato CM, McGeachy MJ, et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–71. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–56. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 102.Bettelli E, Carrier YJ, Gao WD, Korn T, Strom TB, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–38. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 103.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–97. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 104.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ichiyama K, Sekiya T, Inoue N, Tamiya T, Kashiwagi I, et al. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-β is mediated by suppression of Eomesodermin. Immunity. 2011;34:741–54. doi: 10.1016/j.immuni.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 106.Malhotra N, Robertson E, Kang J. SMAD2 is essential for TGFβ-mediated Th17 cell generation. J Biol Chem. 2010;285:29044–48. doi: 10.1074/jbc.C110.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou L, Lopes JE, Chong MMW, Ivanov II, Min R, et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, et al. Foxp3 inhibits RORγt-mediated IL-17A mRNA transcription through direct interaction with RORγt. J Biol Chem. 2008;283:17003–8. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 109.Jabeen R, Kaplan MH. The symphony of the ninth: the development and function of Th9 cells. Curr Opin Immunol. 2012;24:303–7. doi: 10.1016/j.coi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nat Immunol. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, et al. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9–producing subset. Nat Immunol. 2008;9:1341–46. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 112.Chang H-C, Sehra S, Goswami R, Yao W, Yu Q, et al. The transcription factor PU. 1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–34. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goswami R, Jabeen R, Yagi R, Duy P, Zhu J, et al. STAT6-dependent regulation of Th9 development. J Immunol. 2012;188:968–75. doi: 10.4049/jimmunol.1102840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilhelm C, Hirota K, Stieglitz B, van Snick J, Tolaini M, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–77. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McKarns SC, Schwartz RH. Distinct effects of TGF-β1 on CD4+ and CD8+ T cell survival, division, and IL-2 production: a role for T cell intrinsic Smad3. J Immunol. 2005;174:2071–83. doi: 10.4049/jimmunol.174.4.2071. [DOI] [PubMed] [Google Scholar]
- 116.Ishigame H, Mosaheb MM, Sanjabi S, Flavell RA. Truncated form of TGF-β RII, but not its absence, induces memory CD8+ T cell expansion and lymphoproliferative disorder in mice. J Immunol. 2013;190:6340–50. doi: 10.4049/jimmunol.1300397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Konkel JE, Maruyama T, Carpenter AC, Xiong Y, Zamarron BF, et al. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat Immunol. 2011;12:312–19. doi: 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mucida D, Husain MM, Muroi S, van Wijk F, Shinnakasu R, et al. Transcriptional reprogramming of mature CD4+ helper T cells generates distinct MHC class II–restricted cytotoxic T lymphocytes. Nat Immunol. 2013;14:281–89. doi: 10.1038/ni.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat Immunol. 2013;14:271–80. doi: 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dinesh RK, Skaggs BJ, La Cava A, Hahn BH, Singh RP. CD8+ Tregs in lupus, autoimmunity, and beyond. Autoimmun Rev. 2010;9:560–68. doi: 10.1016/j.autrev.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen M-L, Yan B-S, Kozoriz D, Weiner HL. Novel CD8+ Treg suppress EAE by TGF-β- and IFN-γ-dependent mechanisms. Eur J Immunol. 2009;39:3423–35. doi: 10.1002/eji.200939441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rabinowitz KM, Wang Y, Chen EY, Hovhannisyan Z, Chiang D, et al. Transforming growth factor β signaling controls activities of human intestinal CD8+ T suppressor cells. Gastroenterology. 2013;144:601–12. doi: 10.1053/j.gastro.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mayer CT, Floess S, Baru AM, Lahl K, Huehn J, Sparwasser T. CD8+Foxp3+ T cells share developmental and phenotypic features with classical CD4+Foxp3+ regulatory T cells but lack potent suppressive activity. Eur J Immunol. 2011;41:716–25. doi: 10.1002/eji.201040913. [DOI] [PubMed] [Google Scholar]
- 124.Robb RJ, Lineburg KE, Kuns RD, Wilson YA, Raffelt NC, et al. Identification and expansion of highly suppressive CD8+FoxP3+ regulatory T cells after experimental allogeneic bone marrow transplantation. Blood. 2012;119:5898–908. doi: 10.1182/blood-2011-12-396119. [DOI] [PubMed] [Google Scholar]
- 125.Sawamukai N, Satake A, Schmidt AM, Lamborn IT, Ojha P, et al. Cell-autonomous role of TGFβ and IL-2 receptors in CD4+ and CD8+ inducible regulatory T-cell generation during GVHD. Blood. 2012;119:5575–83. doi: 10.1182/blood-2011-07-367987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramalingam R, Larmonier CB, Thurston RD, Midura-Kiela MT, Zheng SG, et al. Dendritic cell– specific disruption of TGF-β receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J Immunol. 2012;189:3878–93. doi: 10.4049/jimmunol.1201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor β1 in Langerhans cell biology: The skin of transforming growth factor β1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–22. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Strobl H, Knapp W. TGF-β1 regulation of dendritic cells. Microbes Infect. 1999;1:1283–90. doi: 10.1016/s1286-4579(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 129.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGFβ1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–52. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bauer T, Zagorska A, Jurkin J, Yasmin N, Koeffel R, et al. Identification of Axl as a downstream effector of TGF-β1 during Langerhans cell differentiation and epidermal homeostasis. J Exp Med. 2012;209:2033–47. doi: 10.1084/jem.20120493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peng W-M, Maintz L, Allam J-P, Novak N. Attenuated TGF-β1 responsiveness of dendritic cells and their precursors in atopic dermatitis. Eur J Immunol. 2013;43:1374–82. doi: 10.1002/eji.201242955. [DOI] [PubMed] [Google Scholar]
- 132.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–91. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 133.Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. Autocrine transforming growth factor-β1 promotes in vivo Th17 cell differentiation. Immunity. 2011;34:396–408. doi: 10.1016/j.immuni.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-β activation in irradiated murine mammary gland. J Clin Investig. 1994;93:892–99. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ribeiro SMF, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-β. J Biol Chem. 1999;274:13586–93. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- 136.Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, et al. Regulation of transforming growth factor-β activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270:7304–10. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- 137.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, et al. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93:1159–70. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 138.Miyazono K, Heldin CH. Role for carbohydrate structures in TGF-β-1 latency. Nature. 1989;338:158– 60. doi: 10.1038/338158a0. [DOI] [PubMed] [Google Scholar]
- 139.Schultz-Cherry S, Hinshaw VS. Influenza virus neuraminidase activates latent transforming growth factor β. J Virol. 1996;70:8624–29. doi: 10.1128/jvi.70.12.8624-8629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Worthington JJ, Klementowicz JE, Travis MA. TGFβ: a sleeping giant awoken by integrins. Trends Biochem Sci. 2011;36:47–54. doi: 10.1016/j.tibs.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 141.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, et al. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP–dependent activation of TGF-β1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires β8 integrin expression in the neuroepithelium. J Neurosci. 2005;25:9940–48. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. β8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Todorovic V, Rifkin DB. LTBPs, more than just an escort service. J Cell Biochem. 2012;113:410–18. doi: 10.1002/jcb.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saharinen J, Keski-Oja J. Specific sequence motif of 8-Cys repeats of TGF-β binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-β. Mol Biol Cell. 2000;11:2691–704. doi: 10.1091/mbc.11.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jovanovic J, Iqbal S, Jensen S, Mardon H, Handford P. Fibrillin-integrin interactions in health and disease. Biochem Soc Trans. 2008;36:257–62. doi: 10.1042/BST0360257. [DOI] [PubMed] [Google Scholar]
- 147.Nijbroek G, Sood S, McIntosh I, Francomano CA, Bull E, et al. Fifteen novel FBN1 mutations causing Marfan syndrome detected by heteroduplex analysis of genomic amplicons. Am J Hum Genet. 1995;57:8–21. [PMC free article] [PubMed] [Google Scholar]
- 148.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, et al. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–11. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 149.Colarossi C, Chen Y, Obata H, Jurukovski V, Fontana L, et al. Lung alveolar septation defects in Ltbp-3-null mice. Am J Pathol. 2005;167:419–28. doi: 10.1016/S0002-9440(10)62986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dabovic B, Chen Y, Colarossi C, Zambuto L, Obata H, Rifkin DB. Bone defects in latent TGF-β binding protein (Ltbp)-3 null mice: a role for Ltbp in TGF-β presentation. J Endocrinol. 2002;175:129–41. doi: 10.1677/joe.0.1750129. [DOI] [PubMed] [Google Scholar]
- 151.Oida T, Weiner HL. TGF-β induces surface LAP expression on murine CD4Tcells independent of Foxp3 induction. PLoS ONE. 2010;5:e15523. doi: 10.1371/journal.pone.0015523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci USA. 2009;106:13439–44. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-β on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci USA. 2009;106:13445–50. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang R, Zhu J, Dong X, Shi M, Lu C, Springer TA. GARP regulates the bioavailability and activation of TGFβ. Mol Biol Cell. 2012;23:1129–39. doi: 10.1091/mbc.E11-12-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Edwards JP, Fujii H, Zhou AX, Creemers J, Unutmaz D, Shevach EM. Regulation of the expression of GARP/latent TGF-β1 complexes on mouse T cells and their role in regulatory T cell and Th17 differentiation. J Immunol. 2013;190:5506–15. doi: 10.4049/jimmunol.1300199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, et al. Inactivation of the integrin β6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–28. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, et al. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 158.Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrinβ-6 messenger RNA in primate epithelial tissues. J Histochem Cytochem. 1993;41:1521–27. doi: 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- 159.Breuss JM, Gallo J, Delisser HM, Klimanskaya IV, Folkesson HG, et al. Expression of the β-6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodelling. J Cell Sci. 1995;108:2241–51. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 160.Annes JP, Rifkin DB, Munger JS. The integrin αVβ6 binds and activates latent TGFβ3. FEBS Lett. 2002;511:65–68. doi: 10.1016/s0014-5793(01)03280-x. [DOI] [PubMed] [Google Scholar]
- 161.Cambier S, Gline S, Mu D, Collins R, Araya J, et al. Integrin αvβ8-mediated activation of transforming growth factor-β by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–94. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, et al. Absence of integrin-mediated TGFβ1 activation in vivo recapitulates the phenotype of TGFβ1-null mice. J Cell Biol. 2007;176:787–93. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, et al. Mice that lack activity of αvβ6- and αvβ8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci. 2009;122:227–32. doi: 10.1242/jcs.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, et al. Abnormal lung development and cleft palate in mice lacking TGF–β3 indicates defects of epithelial–mesenchymal interaction. Nat Genet. 1995;11:415–21. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 165.Lu M, Munger JS, Steadele M, Busald C, Tellier M, Schnapp LM. Integrin α8β1 mediates adhesion to LAP-TGFβ1. J Cell Sci. 2002;115:4641–48. doi: 10.1242/jcs.00145. [DOI] [PubMed] [Google Scholar]
- 166.Munger JS, Harpel JG, Giancotti FG, Rifkin DB. Interactions between growth factors and integrins: Latent forms of transforming growth factor-β are ligands for the integrin αvβ1. Mol Biol Cell. 1998;9:2627–38. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ludbrook SB, Barry ST, Delves CJ, Horgan CM. The integrin αvβ3 is a receptor for the latency-associated peptides of transforming growth factors β1 and β3. Biochem J. 2003;369:311–18. doi: 10.1042/BJ20020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J Immunol. 2005;175:7708–18. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- 169.Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. Increased expression of integrin αvβ5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol. 2006;168:499–510. doi: 10.2353/ajpath.2006.041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol. 2007;179:1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tatler AL, John AE, Jolly L, Habgood A, Porte J, et al. Integrin αvβ5-mediated TGF-β activation by airway smooth muscle cells in asthma. J Immunol. 2011;187:6094–107. doi: 10.4049/jimmunol.1003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Henderson N, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–24. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Giacomini MM, Travis MA, Kudo M, Sheppard D. Epithelial cells utilize cortical actin/myosin to activate latent TGF-β through integrin αvβ6-dependent physical force. Exp Cell Res. 2012;318:716–22. doi: 10.1016/j.yexcr.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin αVβ6-mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. J Cell Biol. 2004;165:723–34. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, et al. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–65. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, et al. Ulcerative colitis and autoimmunity induced by loss of myeloid αv integrins. Proc Natl Acad Sci USA. 2007;104:15823–28. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Paidassi H, Acharya M, Zhang A, Mukhopadhyay S, Kwon M, et al. Preferential expression of integrin αvβ8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology. 2011;141:1813–20. doi: 10.1053/j.gastro.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Worthington JJ, Czajkowska BI, Melton AC, Travis MA. Intestinal dendritic cells specialize to activate transforming growth factor-β and induce Foxp3+ regulatory T cells via integrin αvβ8. Gastroenterology. 2011;141:1802–12. doi: 10.1053/j.gastro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. Expression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Investig. 2010;120:4436–44. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Acharya M, Mukhopadhyay S, Paidassi H, Jamil T, Chow C, et al. αv integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Investig. 2010;120:4445–52. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010;104:1131–37. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 182.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, et al. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18:547–54. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J Clin Investig. 2011;121:2863–75. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, et al. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci USA. 2000;97:1778–83. doi: 10.1073/pnas.97.4.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, et al. Loss of integrin αvβ6-mediated TGF-β activation causes Mmp12-dependent emphysema. Nature. 2003;422:169–73. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 186.Knight PA, Wright SH, Brown JK, Huang X, Sheppard D, Miller HR. Enteric expression of the integrin αvβ6 is essential for nematode-induced mucosal mast cell hyperplasia and expression of the granule chymase, mouse mast cell protease-1. Am J Pathol. 2002;161:771–79. doi: 10.1016/s0002-9440(10)64236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, et al. The αvβ6 integrin modulates airway hyperresponsiveness in mice by regulating intraepithelial mast cells. J Clin Investig. 2012;122:748–58. doi: 10.1172/JCI58815. [DOI] [PMC free article] [PubMed] [Google Scholar]