Abstract
It is yet unclear if ethanol-induced motor stimulation in the open field (OF) merely reflects psychomotor stimulating effects of the drug or if this stimulation is driven or modulated by ethanol’s antianxiety properties. In the present study, adolescent rats were administered with different ethanol doses or remained untreated. They were sequentially assessed in the OF, elevated plus maze (EPM), and light-dark box (LDB) and then assessed for ethanol intake. The aims were to assess the relationship between measures of ethanol-induced activity and anxiolysis, analyze ethanol intake as a function of prior ethanol exposure, and associate behavioral responsiveness in these apparatus with ethanol intake during adolescence. The results suggested that the enhanced exploration of the OF observed after 2.5 and 3.25 g/kg ethanol reflected a motor-stimulating effect that appeared to be relatively independent of anxiolysis. The 1.25 g/kg dose induced motor stimulation in the OF and anti-anxiety effects in the EPM, but these effects were relatively independent. The 0.5 g/kg ethanol dose exerted significant anxiolytic effects in the EPM in the absence of stimulating effects in the OF. A multivariate regression analysis indicated that adolescents with a higher frequency of rearing behavior in the OF, higher percentage of open arm entries in the EPM, and lower propensity to enter the central area of the OF exhibited greater ethanol intake. These results indicate that the OF is a valid procedure for the measurement of ethanol-induced stimulation, and provide information towards characterizing subpopulations of adolescents at risk for initiating alcohol drinking.
Keywords: ethanol intake, open field, elevated plus maze, light-dark box, motor stimulation, anxiety
1. Introduction
Ethanol induces appetitive, aversive, and negative (anti-anxiety) effects [1]. The expression of these effects depends on the dosage, time course of intoxication, type of test, and species and strains analyzed. In rats, ethanol’s appetitive effects appear to be associated with low and moderate doses of ethanol (e.g., ≤ 1.5 g/kg) and the early phase of the toxic process when ethanol levels are rising [2]. The aversive effects are more frequently detected at higher doses and later time points [3]. Ethanol’s anxiolytic effects, in turn, are also associated with low and moderate doses and are often assessed by tests (e.g., elevated plus maze [EPM] or light-dark box [LDB]) that take advantage of the rodents’ natural avoidance of open and brightly lit spaces. A greater time spent in these spaces after ethanol is considered an index of anxiolysis.
In a given experiment, however, adequately discriminating which effect of ethanol drives behavior or drug-induced learning is sometimes difficult. An example is conditioned place preference (CPP) studies that employ a conditioned stimulus (CS) that is inherently less preferred [4]. Ciccocioppo and coworkers [5] studied Marchigian Sardinian alcohol-preferring (msP) rats and found that ethanol significantly increased the time spent in a compartment that was non-preferred at the beginning of conditioning. An important caveat of such studies is that it is unclear whether enhanced CS preference after pairings with ethanol reflects the appetitive or anxiolytic properties of the drug. Another example of such ambiguity is studying ethanol’s effects on exploration and distance traveled in the inescapable environment of an open field (OF). Studies that analyze ethanol-induced motor activity using the OF provide similar measures, but the authors’ theoretical interpretations of the results are often different or opposite. Researchers may interpret that changes in the distance traveled in the OF indicate enhanced or reduced anxiety. Karayadian and coworkers [6] considered that decreases in the number of line crossings and rearings in the OF represented behavioral signs of anxiety during ethanol withdrawal. Fukushiro and others [7] assessed the acute and chronic effects of ethanol on OF behavior in mice. They found an enhancement of motor activity following acute ethanol administration, a result that was reported as proof of the ethanol-induced reduction of the aversive effect of the novel environment (i.e., an anxiolytic effect). Yet in other studies ethanol-induced motor stimulation and age-related differences in this variable are thought to reflect sensitivity to the appetitive reinforcing effects of the drug (e.g., [8]). Ethanol-induced motor activation in a novel, inescapable environment has been considered a proxy of ethanol’s appetitive effects [9]. Common neurobiological mechanisms appear to underlie ethanol’s reinforcing and motor-stimulating effects, namely the activation of the mesocorticolimbic dopaminergic pathway [10, 11].
Although OF and EPM tests have become increasingly popular in drug research laboratories and have been used for several decades [12], it is still unknown whether ethanol-induced motor stimulation in the OF represents sensitivity to the appetitive, stimulating effects of the drug or whether it is confounded by the expression of anxiolytic-like behavior.
Infant [13] and adolescent [14] rats have been reported to be significantly more sensitive than adults to the enhancement of OF activity observed after ethanol administration. Adolescents are also less sensitive than adults to the motor-sedative effects of ethanol [14]. These differences have been proposed to reflect greater ethanol-induced appetitive reinforcement in younger individuals, which may underlie their propensity to engage in ethanol self-administration [15]. Moreover, individual differences in ethanol-induced motor activity in an OF have served to identify adolescent rats with a marked predisposition to drink ethanol. Specifically, adolescents that were more sensitive to the stimulant effects of ethanol in the OF drank more ethanol than counterparts that had reduced sensitivity to these stimulant effects in another study [16]. Therefore, elucidating whether ethanol-induced exploration of the OF in adolescents reflects the stimulant effects of the drug or whether it reflects an ethanol-induced reduction of experimental anxiety similar to studies that employ the EPM is important.
The main purpose of the present study was to analyze the relationship between measures of ethanol-induced effects on exploratory behavior in the OF, EPM, and LDB. Adolescent rats were given various doses of ethanol or remained untreated. They were then sequentially assessed in the EPM, OF and LDB. The aim of the present study was to determine whether behavioral scores in one test predicted scores in the other tests. The presence or lack of significant correlations was interpreted as a sign of similitude or difference, respectively, in the psychobiological meaning of the measures provided by the tests.
The animals were then assessed for ethanol intake in a single 24-h test session. The effects of prior ethanol exposure on ethanol intake was examined. Ethanol intoxication can affect subsequent ethanol preference and this effect seems to significantly vary as a function of age of exposure [17]. To our knowledge, the acquisition of ethanol intake preference or aversion after non-reinforced ethanol exposure has not been systematically analyzed in adolescent rats.
Although adolescent drinking is pervasive in most western societies, alcohol-related behaviors exhibit considerable variability. Exposed to similar levels of alcohol availability some youth quickly engage in alcohol drinking, whereas others kept alcohol drinking low and stable [18]. The present study used the natural variability exhibited by adolescent, genetically heterogeneous, rats to identify the predictors of ethanol drinking. Specifically, another aim was to analyze the predictive value of the behavioral measures gathered in each of the tests, upon ethanol intake, through univariate and multivariate statistical analyses (i.e., multiple regression analysis and analysis of variance [ANOVA] as a function of high or low baseline anxiety response). The hypothesis was that anxiety responses and novelty exploration would combine to significantly predict ethanol drinking during adolescence.
2. Materials and Methods
2.1. Experimental design
A 2 (sex: male or female) × 2 (order of testing: animals were first tested in the EPM or OF [EPM-OF or OF-EPM groups]) × 6 (ethanol treatment on postnatal day 28 [PD28]: rats were untreated or treated with 0.0, 0.5, 1.25, 2.5, or 3.25 g/kg ethanol) factorial design was used, with 10–11 animals per group. The untreated animals were removed from their homecage and assessed in the EPM, OF, and LDB but received no ethanol or vehicle intubations.
2.2. Subjects
A total of 246 adolescent male and female Wistar rats, representing 46 litters, were used. The rats were born and reared in the vivarium of the Instituto Ferreyra (INIMEC-CONICET, Córdoba, Argentina). Births were examined daily, and the day of parturition was considered PD0. The pups were kept with their dam in standard maternity cages until weaning on PD21. Weaned animals were housed with five same-sex littermates and given continuous ad libitum access to water and food (ACA Nutricion, Buenos Aires, Argentina) until experimental procedures began on PD28. The colony was kept on a 12 h/12 h light/dark cycle (lights on at 8:00 AM) at an ambient temperature of 22 ± 1°C. The procedures complied with the Guide for the Care and Use of Laboratory Animals [19] and guidelines issued by the Ministry of Animal Care of INIMEC-CONICET. To reduce confounds between litter and treatment effects [20], no more than one male and one female per litter were assigned to each particular cell of the experimental design.
2.3. Drug preparation and administration procedures
Ethanol was administered intragastrically (i.g.) via a 12-cm length of polyethylene-50 tubing (PE-50 Clay Adams, Parsippany, NJ, USA) attached to a 3 ml syringe (Becton Dickinson, Rutherford, NJ, USA) with a 23-gauge needle. Ethanol doses of 0.5, 1.25, 2.5 and 3.25 g/kg resulted from the administration of a volume equivalent to 0.015 ml per gram of body weight of 4.2% 10.5%, 21%, or 27.3% v/v ethanol (Porta Hnos, Córdoba, Argentina) solutions, respectively. An equivalent volume of tap water was administered as vehicle (0.0 g/kg). All of the animals were gently intubated in approximately 5 s, and the solutions were then slowly delivered over 3–4 s into the stomach. The doses and mode of administration were selected based on previous studies [14,16].
2.4. Apparatus
Open field
The apparatus consisted of a gray wooden square box (30 cm length × 30 cm width × 30 cm height) lined with black rubber. Forward locomotion was evaluated by recording the time spent moving around the box in seconds. Locomotion was measured when the animal was in a prone position, moving the four paws simultaneously. Frequency of rearing, wall-climbing, and grooming were also measured. Rearing was measured when the rat stood on its hind legs away from the wall. Wall-climbing and grooming were defined as in [21]. Videotapes were also analyzed with regard to the time spent in the central area of the open field. This central area was virtually defined as a 36 cm2 square that occupied the central square of the arena. The time spent in the central section of an open field is usually considered a measure of anxiety.
Behavior in the OF and across tests was recorded by a video camera positioned on a metal rail that hung from the ceiling. Data was subsequently analyzed by an observer unaware of group assignment.
Elevated plus maze
The EPM was made of black Plexiglas and consisted of two open arms (45 cm × 5 cm) and two closed arms (45 cm length × 5 cm width × 45 cm height) that extended from a common central platform (5 cm × 5 cm) elevated 50 cm above the floor. Each rat was gently placed in the center platform facing an open arm. An entry was considered when the rat crossed into an arm with its four paws at a given time. The following behaviors were recorded: absolute number and percentage of entries into the open arms, number of entries into the closed arms, and total number of arm entries. The first two variables were considered indices of anxiety, and the last two were taken as indices of overall activity. Frequency of grooming, wall-climbing and rearing were also measured and analyzed. Stretched–attend postures towards the open arm were measured but their frequency was minimal across conditions and therefore were not taken into consideration for statistical analysis.
Light-dark box
The LDB was made of Plexiglas and consisted of two compartments of different sizes. One large bright section (25 cm length × 25 cm width × 30 cm height) was illuminated by a 75-W white bulb. Another small dimly lit section (18 cm length × 25 cm width × 30 cm height) was illuminated by a 40-W red bulb. Rodents show an innate aversion to brightly illuminated areas [22]. Both the white and red bulbs were located 45 cm above the box’s floor. The compartments were separated by a 6.5 × 6.5 cm door built into the separating wall. Each rat was initially placed in the center of the bright compartment facing the separating wall. An entry was considered when the rat placed its four paws in one of the two compartments. The total number of transitions between compartments and time spent in the bright side were recorded and considered indices of overall activity and anxiety, respectively. Frequency of grooming, wall-climbing and rearing were also measured.
2.5. Procedures
On PD28, the animals were weighed to the nearest 0.01 g (portable Ohaus L2000; Ohaus, Pine Brook, NJ, USA) and given ethanol (0.5, 1.25, 2.5 or 3.25 g/kg; i.g.) or vehicle (0.0 g/kg, tap water) or simply remained untreated (UT). The latter group of animals was removed from their homecage and underwent behavioral screening with no ethanol or vehicle intubations. Following intubation, the ethanol-treated subjects were returned to a holding cage lined with pine shavings where they remained for 5 min until testing began. Behavioral testing began by gently placing the rat in the central platform of the EPM or central area of the OF. Ten minutes after administration, the animals that were subjected to the EPM were then subjected to the OF, whereas the animals that were first tested in the OF were gently placed on the central platform of the EPM. Half of the animals, which were randomly selected, were tested first in the EPM, and the remaining half were tested first in the OF. The animals remained in the corresponding apparatus for 5 min and were then immediately tested for another 5 min in the LDB test. Each test lasted 5 min (i.e., post-administration intervals of 5–9, 10–14, and 15–19 min). Counterbalanced measurements in the EPM and OF helped reduce the possibility that the order of assessment adversely influenced the results. These procedures have been summarized in Figure 1. Scoring was performed off-line by an observer who was unaware of the experimental conditions using EthoLog software [23]. Each apparatus was cleaned with a sponge that was soaked with water between each test. Illumination during the OF and EPM tests was provided by a fluorescent lamp (75W) positioned on the ceiling approximately 2.5 m from the testing chambers.
Figure 1.
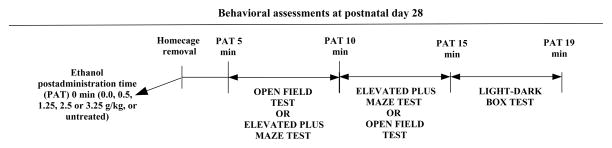
Methods for the analysis of the relationship between ethanol-induced activity and anxiolysis in the open field (OF), elevated plus maze (EPM), and light-dark box (LDB) in adolescent rats. On postnatal day 28, male and female rats were given ethanol (0.5, 1.25, 2.5 or 3.25 g/kg; i.g.) or vehicle (0.0 g/kg, tap water) or simply remained untreated (UT). The latter group of animals was removed from their homecage and underwent behavioral screening with no ethanol or vehicle intubations. Behavioral testing began at post-administration time 5 minutes by gently placing the rat in the central platform of the EPM or central area of the OF. Ten minutes after administration, the animals that were subjected to the EPM were then subjected to the OF, whereas the animals that were first tested in the OF were gently placed on the central platform of the EPM. Half of the animals were tested first in the EPM, and the remaining half were tested first in the OF. The animals remained in the corresponding apparatus for 5 min and were then immediately tested for another 5 min in the LDB. Testing in the LDB began fifteen minutes after administration.
When behavioral testing was completed, each animal was individually housed in cages (22 cm × 20 cm × 30 cm) lined with pine shavings. They were given ad libitum access to food and a graded tube that contained 75% of the water that, according to preliminary studies, they normally drank. The next day (PD29), the animals underwent a 24-h ethanol intake test. They had simultaneous access to three 25-ml graduated glass tubes that were filled with tap water, sucrose (1% v/v), or a compounded solution of sucrose (1% w/v) and ethanol (5% v/v, Porta Hnos, Argentina). Tap water served as vehicle for the sucrose and sucrose-ethanol solutions. The volume consumed from each tube was assessed 24-h after the test began. The selection of a three-bottle choice with sweetened ethanol was based on previous studies that indicated that adding more than two bottles in intake tests significantly increases ethanol intake [24] and that adolescent, uninitiated Wistar rats generally do not consume ethanol concentrations higher than 5%, but they accept ethanol when it is mixed with a mild concentration of sucrose [25]. Moreover, because ethanol mixed with sucrose was provided, the addition of a bottle of 1% sucrose helped determine whether the effects of treatment on ethanol intake were specific for ethanol or for the sweet taste of sucrose.
Sucrose intake and overall fluid intake are expressed as milliliters consumed per 100 g of body weight (ml/100 g), and ethanol intake is expressed as grams of ethanol per kilogram of body weight (g/kg) and as the percentage of ethanol preference over water and sucrose. The percentage of sucrose preference over ethanol and water was also calculated.
2.6. Statistical analysis
Univariate analysis of behavioral responses in the OF, EPM, and LDB and intake scores during three-bottle intake tests
The variables recorded in the OF and EPM were independently analyzed using three-way ANOVAs, with sex (male or female), treatment (0.0, 0.5, 1.25, 2.5 or 3.25 g/kg ethanol or untreated), and order of testing (EPM-OF or OF-EPM) as the between-subjects factors. Similar ANOVAs were used to separately analyze ethanol (g/kg and percent preference) and sucrose (ml/100 g and percent preference) consumption and overall fluid intake (ml/100 g) during the three-bottle choice test. The locus of significant main effects or significant interactions were further analyzed using follow-up ANOVAs and the Fisher post hoc test. In the ANOVAs and post hoc tests, the alpha level was 0.05. The results are expressed as mean ± SEM.
Correlation analyses
Pearson’s r product-moment correlations were calculated for the overall sample of subjects, regardless of ethanol dose, and individually for each treatment group (i.e., groups that received 0.0, 0.5, 1.25, 2.5, or 3.25 g/kg ethanol and untreated animals). Correlations between the following variables were determined: locomotion (distance traveled [s]) in the OF, time spent in the center of the OF (%), total number of open arm entries, total number of closed arm entries, and locomotion (total number of open and closed arm entries) in the EPM, anxiety response (percentage of open arm entries) in the EPM, locomotion (number of transfers between compartments) in the LDB, and anxiety response (time spent in the bright side) of the LDB. Given the large number of correlations performed, the alpha level was adjusted within each dataset for the number of variables under consideration (i.e., 0.05/8). Therefore, only associations with p ≤ 0.00625 were considered significant. The main aim of these analyses was to analyze the overall associations between behaviors in the OF, EPM, and LDB, regardless of ethanol dose, and determine whether greater ethanol-induced locomotion scores in the OF were positively correlated with a greater ethanol-induced percentage of entries into the open arms of the EPM. The latter would support the hypothesis that motor activation in the OF at least partially reflects anxiolytic effects of the drug.
Univariate and multivariate analyses of intake scores in control, ethanol-naive subjects
Preliminary analyses indicated that animals that were untreated or given vehicle on PD28 exhibited similar behavioral responses in the EPM, OF, and LDB. No differences were observed between these conditions with regard to locomotion in the OF, EMP, and LDB (t78 = −0.4, −1.86, and −0.89, respectively; all p < 0.05) or the percent time spent in the open arms of the EPM or time spent in the bright area of the LDB (t78 = −0.03 and −0.60, respectively; all p < 0.05). These conditions were then combined into a single control condition with 80 subjects. Ethanol intake scores in the dataset with the 80 control subjects were further analyzed using univariate and multivariate analyses.
Multiple regression analysis was used to study the relationship between exploratory and anxiety-like behavior and ethanol consumption during the 24-h intake test. The dataset for the multiple regression analysis consisted of 80 subjects originally assigned to the untreated and vehicle-treated groups. The predictive variables included exploratory and anxiety behavior observed in the OF test (i.e., total forward locomotion [s] and percent time spent in the central area of the apparatus-considered a measure of inborn anxiety [26]-, respectively), EPM (total number of arm entries and percentage of open arm entries, respectively), and LDB (time spent in the bright area and number of transfers between compartments, respectively). Rearing in the OF was also included as a predictive variable. Wall-climbing in the OF was discarded because exploration of the correlations between variables indicated a 0.95 association with rearing in the OF. The frequency of grooming and wall-climbing in the EPM and LDB yielded highly skewed distributions because most of the rats exhibited none or very few of these behaviors. No rearing was observed in the LDB or the EPM. The dependent variable was g/kg ethanol consumed in the 24-h ethanol intake test. We used a standard (i.e., “enter” method) multiple regression analysis that simultaneously added all of the independent variables in the model. The regular and adjusted multiple correlation coefficients (R2 and R2a) were calculated. These indices indicate the percentage of variance explained, and the adjusted version decreases with the number of predictive variables introduced into the equation. The standardized regression coefficient (β) was also calculated to provide an estimate of the individual relationships between single predictive variables and ethanol intake.
To assess the level of specificity of the multiple regression model, another multiple regression analysis was conducted with the same predictive variables but using sucrose consumption (ml/100 g in 24 h) as the dependent variable.
Ethanol intake scores in the dataset with the 80 subjects that belonged to the untreated and vehicle-treated groups were further analyzed using ANOVAs as a function of high, medium, and low spontaneous anxiety responses in the EPM and as a function of high, medium, and low anxiety responses in the OF in a separate analysis. Specifically, the animals were classified with low, medium, or high anxiety as a function of the percentage of entries into the open arms of the EPM. The animals that fell into the upper quartile (i.e., 20 animals that exhibited the highest number of entries into the open arms) were classified as the low anxiety (LA) group. The animals that fell into the lower quartile were classified as the high anxiety (HA) group. The animals that fell between the 38th and 62nd percentiles were classified as the medium anxiety (MA) group. Similarly, the animals were classified as low-, medium-, and high-anxiety responders (HA1, MA1, and LA1, respectively) as a function of the percent time spent in the central area of the OF. Ethanol intake (g/kg and percent preference) and sucrose and overall fluid intake (ml/100 g) were then analyzed using separate one-way ANOVAs (comparative factor between groups: level of anxiety response in the EPM or level of anxiety response in the OF) for each of the classification criteria.
3. Results
3.1. Open field scores
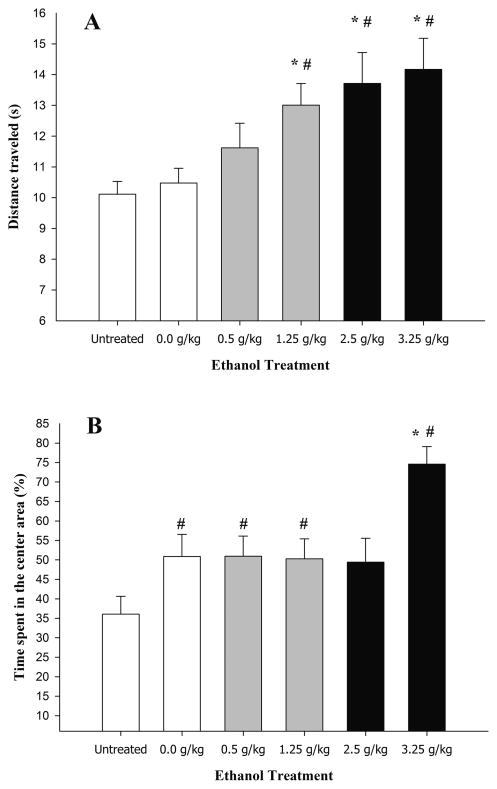
Total forward locomotion (s) in the OF as a function of ethanol treatment is depicted in Figure 2, panel A. Moderate (1.25 g/kg) and high (2.5 and 3.25 g/kg) but not low (0.5 g/kg) doses of ethanol induced significant motor activation in the OF. The ANOVA confirmed these impressions and revealed significant main effects of treatment and order of testing (F5,222 = 5.28 and F1,222 = 16.97, respectively; p < 0.001). The post hoc tests indicated that animals given 3.5, 2.5, or 1.25 g/kg ethanol exhibited significantly higher locomotion scores than untreated and vehicle-treated control animals in the OF. The latter two control groups exhibited similar locomotion scores. Locomotion in animals given 0.5 g/kg ethanol was similar to control subjects. No significant main effects of sex or significant interactions involving sex were observed. Order of testing did not significantly interact with ethanol treatment. The post hoc tests also revealed that locomotion (s) was higher when the OF test occurred prior to the EPM test (13.44 ± 0.50 s) than when OF testing occurred at 10–14 min following ethanol intubation, after termination of the EPM test (10.95 ± 0.38 s).
Figure 2.
Distance traveled in the open field (s) (panel A) and percent time spent in the center area of the open field (panel B) in 28-day-old male and female adolescent rats as a function of ethanol treatment (0.0 [vehicle], 0.5, 1.25, 2.5, and 3.25 g/kg ethanol or untreated [UT]). The rats were tested 5–9 min or 10–14 min post-administration. The data were collapsed across sex (male and female) and order of testing. The ANOVAs indicated that, regardless treatment, locomotion scores and percent time spent in the center were higher when the open field test occurred prior to the elevated plus maze test (EPM) than when testing occurred at 10–14 min following ethanol intubation, after termination of the EPM test. The sex factor did not exert a significant main effect or interact with the remaining factors. The asterisk indicates a significant difference between a given group and the 0.0 g/kg ethanol group. The pound sign indicates a significance difference between a given group and the untreated control group. The vertical bars indicate SEM.
Percent time spent in the center of the OF is depicted in Figure 2 (panel B). The ANOVA revealed significant main effects of treatment and order of testing (F5,222 = 5.89 and F1,222 = 13.81, respectively; p < 0.001). The post hoc tests revealed that animals given 3.25 g/kg ethanol exhibited significantly more time spent in the center of the OF than the remaining groups and that untreated animals spent significantly less time in the central area than animals given 1.25, 0.5 or 0.0 g/kg ethanol. According to the post hoc tests, time spent in the center of the OF (%) was higher when the OF test occurred at post-administration minutes 5–9 (59.86 ± 3.00) than when OF testing occurred at 10–14 min following ethanol intubation, after the EPM test (44.10 ± 3.17 s). No significant main effects of sex or significant interactions involving sex were observed. Order of testing did not significantly interact with ethanol treatment.
3.2. Elevated plus maze scores
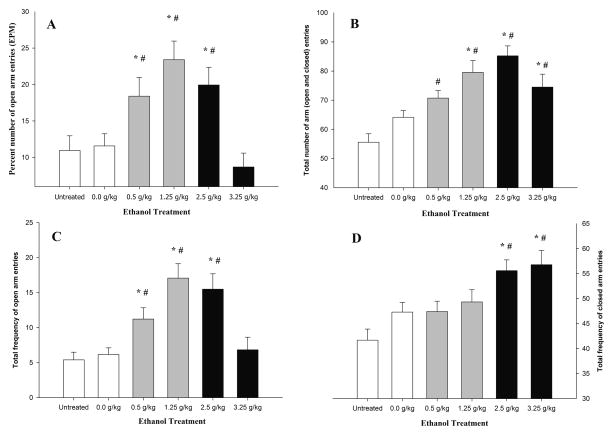
The ANOVA of the percentage of open arm entries revealed significant main effects of treatment and order of testing (F5,222 = 7.11 and F1,222 = 6.78, respectively; p < 0.001). The post hoc tests indicated that animals given 2.5, 1.25, and 0.5 g/kg ethanol exhibited a significantly greater percentage of entries into the open arms of the EPM than vehicle-treated and untreated counterparts. This greater percentage of entries, presumably indicative of an anti-anxiety effect of ethanol, was similar in males and females, and was not significantly affected by order of testing. No significant main effects of sex or significant interactions involving sex were observed. Animals that received 3.25 g/kg ethanol exhibited a similar number of open arm entries as both control conditions. These results are depicted in Figure 3A. The post hoc tests also indicated that the percentage of entries was higher when the animals were tested in the EPM prior to the OF test (17.87 ± 1.35) than when tested at 10–14 min, after termination of the OF test (12.98 ± 1.33).
Figure 3.
Upper panels: Open arm entries (%) (Figure A) in the elevated plus maze (EPM) in 28-day-old male and female adolescent rats as a function of ethanol treatment. Total number of arm entries (Figure B) in the EPM in 28-day-old male and female adolescent rats as a function of ethanol treatment. Lower panels: total frequency of open (Figure C) and closed (Figure D) arm entries in the elevated plus maze in 28-day-old male and female adolescent rats as a function of ethanol treatment. All of the subjects were tested 5–9 min or 10–14 min post-administration. Animals were administered 0.0 [vehicle], 0.5, 1.25, 2.5, or 3.25 g/kg ethanol or were untreated [UT]). The data were collapsed across sex (male and female) and time of assessment (order of testing). The ANOVAs indicated that the total number of arm entries, total number of open arm entries, and total number of closed arm entries were higher when the EPM test occurred prior to the open field test (OF) than when testing occurred at 10–14 min following ethanol intubation, after termination of the OF test. The sex factor did not exert a significant main effect or interact with the remaining variables. The asterisk indicates a significant difference between a given group and the 0.0 g/kg ethanol group. The pound sign indicates a significance difference between a given group and the untreated control group. The vertical bars indicate SEM.
Figure 3B depicts locomotion scores (i.e., total number of arm entries) in the EPM as a function of ethanol treatment. The ANOVA yielded significant main effects of treatment (F5,222 = 10.45, p <0.001) and order of testing (F1,222 = 15.24, p < 0.001). The post hoc tests indicated that animals given 3.25, 2.5, and 1.25 g/kg ethanol exhibited significantly more locomotion than control subjects (either 0.0 g/kg or untreated). Animals given 0.5 g/kg ethanol exhibited significantly greater locomotion (number of arm entries) than untreated animals but were similar to vehicle-treated animals. According to the post hoc tests, the number of entries in the 2.5 g/kg ethanol group was significantly higher than in the remaining groups, with the exception of animals given 1.25 g/kg ethanol, which showed a similar number of entries. The post hoc tests also showed that the overall number of arm entries was higher when testing occurred during the 5–9 min post-administration time period (76.72 ± 2.15) than when testing occurred at 10–14 min, following the OF test (66.21 ± 1.99). Order of testing, however, did not affect the dose-response curve (i.e., order of testing did not significantly interact with ethanol treatment). Sex did not exert significant main effects or interactions.
The total number of entries has been considered a measure of locomotor activity in the EPM, but it conflates locomotion in open (and presumably anxiogenic) and closed sections of the EPM. To better understand the difference between the activating vs. anxiolytic effects of ethanol in the apparatus, the total number of entries was split between open arm entries and closed arm entries, and separate analyses were conducted for each of these variables. Both ANOVAs yielded main effects of treatment (F5,222 = 6.30 and F5,222 = 9.10, respectively; p < 0.001) and order of testing (F1,222 = 10.30 and F1,222 = 4.79, respectively; p < 0.05). As shown in Figure 3C and 3D and confirmed by the post hoc tests, the varying doses of ethanol differentially increased locomotion across open and closed arm entries. Animals that received 0.5 and 1.25 g/kg ethanol exhibited a greater number of entries into the open arms but not closed arms than untreated and vehicle-treated counterparts. Conversely, animals given 3.25 g/kg ethanol exhibited a greater number of entries into the closed arms but not open arms of the EPM than untreated and vehicle-treated counterparts. In the case of subjects given 2.5 g/kg ethanol, the number of arm entries was evenly distributed between both arms; they exhibited a significantly greater number of entries into both arms than both of the control conditions. Open and closed arm entries are depicted in Figure 3C and 3D, respectively. The post hoc tests indicated that locomotion in the EPM was greater in each arm when the test occurred prior to the OF test (open arms: 12.54 ± 1.08; closed arms: 51.59 ± 1.39) compared with locomotion scores observed when the EPM test took place at 10–14 min, after termination of the OF test (open arms: 8.07 ± 0.97; closed arms: 47.68 ± 1.37). Order of testing did not significantly interact with ethanol treatment. No significant main effects of sex or significant interactions involving sex were observed.
3.3. Light-dark box scores
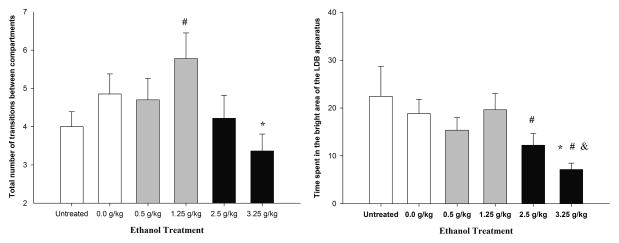
Figure 4 (left panel) depicts locomotion scores in the LDB (i.e., total number of transitions between the two compartments) as a function of ethanol treatment. The ANOVA yielded a significant main effect of treatment (F5,222 = 2.63, p < 0.05). Animals given 1.25 g/kg ethanol exhibited significantly greater locomotion than untreated animals. When compared to vehicle-treated animals, animals given 3.25 g/kg ethanol showed significantly lower locomotion scores. Animals treated with 2.5 or 0.5 g/kg ethanol exhibited similar locomotion scores as control animals.
Figure 4.
(Left panel) Total number of transitions between compartments and (Right panel) time spent in the bright area in the light-dark box test in 28-day-old male and female adolescent rats as a function of ethanol treatment (0.0 [vehicle], 0.5, 1.25, 2.5, and 3.25 g/kg ethanol or untreated [UT]). All of the subjects were tested 5–9 min or 10–14 min post-administration. The data were collapsed across sex (male and female) and order of testing. Across treatments, total number of transitions and time spent in the bright compartment was greater when the animals were first tested in the open field than when they were first tested in the elevated plus maze. The sex factor did not exert a significant main effect or interact with the remaining variables. The asterisk indicates a significant difference between a given group and the 0.0 g/kg ethanol group. The pound sign indicates a significance difference between a given group and the untreated control group. The ampersand sign indicates a significant difference between adolescents treated with 3.25 g/kg ethanol and animals given 1.25 g/kg ethanol. The vertical bars indicate SEM.
The ANOVA of the time spent in the bright compartment of the LDB (s) revealed significant main effects of treatment (F5,222 = 2.45, p < 0.05) and order of testing (F5,222 = 16.44, p < 0.001). Across treatments, the time spent in the bright compartment was greater when the animals were first tested in the OF than when they were first tested in the EPM (21.80 ± 2.47 vs. 9.95 ± 1.41). Order of testing did not significantly interact with ethanol treatment. Perhaps more importantly, the post hoc comparisons indicated that untreated animals and animals given 0.0, 0.5, or 1.25 g/kg ethanol spent a similar percentage of time in the bright compartment. Animals given 2.5 g/kg ethanol spent as much as time in the bright area as vehicle-treated controls, but they spent significantly less time in that area than untreated controls. Moreover, adolescents treated with 3.25 g/kg ethanol spent significantly less time in the bright compartment than untreated animals, vehicle-treated controls, and animals given 1.25 g/kg ethanol (Figure 4, right panel). This result suggests that 3.25 g/kg ethanol may have exerted an anxiogenic effect in the LDB test. No significant main effect of sex or significant interactions involving sex were observed.
3.4. Correlation analyses between EPM, OF, and LDB scores
Table 1 depicts Pearson correlation scores between the EPM, OF, and LDB measures. The analyses conducted in the overall sample of subjects indicated that the total duration of locomotion (s) in the OF and total number of arm entries in the EPM were moderately but significantly and positively correlated (r = 0.27). Greater motor activity in one test was associated with greater activity in the other test. Furthermore, locomotion in the OF for the overall sample of subjects was significantly associated with closed arm entries (r = 0.30) but not open arm entries (r = 0.07) in the EPM. This result appears to indicate that closed arm entries is a better index of activity in the EPM than the total number of entries, although in previous studies the measure most often used to assess activity has been total arm entries [27]. Closed arm entries appeared to be less affected by the anxiety associated with exploring the open arms. Consistent with this possibility, the percentage of open arm entries in the EPM was significantly and positively correlated with the number of open arm entries (r = 0.92) and significantly and negatively correlated with closed arm entries (r = −0.24) in the EPM.
Table 1.
Correlations among behavioral scores gathered in Open-field, Elevated Plus Maze Tests and Light.Dark Box
|
|
1. OA entries
|
2. CA entries
|
3. OA entries (%)
|
4. EPM Locomotion
|
5. OF Locomotion
|
6. Time spent on the center OF (%)
|
7. LDB Locomotion
|
8. Time spent on the bright side of LDB
|
|---|---|---|---|---|---|---|---|---|
| General | ||||||||
| 1. | 1.00 | |||||||
| 2. | −0.02 | 1.00 | ||||||
| 3. | 0.93* | −0.24* | 1.00 | |||||
| 4. | 0.61* | 0.77* | 0.40* | 1.00 | ||||
| 5. | 0.07 | 0.30* | −0.02 | 0.27* | 1.00 | |||
| 6. | 0.05 | 0.08 | 0.03 | 0.10 | 0.12 | 1.00 | ||
| 7. | 0.35* | 0.10 | 0.32* | 0.31* | −0.14 | −0.02 | 1.00 | |
| 8. | 0.17 | 0.02 | 0.17 | 0.12 | −0.16 | −0.03 | 0.64* | 1.00 |
|
| ||||||||
| UT | ||||||||
| 1. | 1.00 | |||||||
| 2. | −0.13 | 1.00 | ||||||
| 3. | 0.93* | −0.32 | 1.00 | |||||
| 4. | 0.39 | 0.86* | 0.18 | 1.00 | ||||
| 5. | −0.17 | 0.13 | −0.15 | 0.03 | 1.00 | |||
| 6. | 0.21 | −0.08 | 0.27 | 0.04 | 0.11 | 1.00 | ||
| 7. | 0.00 | 0.27 | −0.01 | 0.23 | −0.21 | −0.12 | 1.00 | |
| 8. | −0.03 | 0.18 | −0.03 | 0.14 | 0.01 | −0.03 | 0.29 | 1.00 |
|
| ||||||||
| 0.0 g/kg | ||||||||
| 1. | 1.00 | |||||||
| 2. | −0.29 | 1.00 | ||||||
| 3. | 0.95* | −0.48* | 1.00 | |||||
| 4. | 0.25 | 0.85* | 0.03 | 1.00 | ||||
| 5. | 0.03 | 0.16 | −0.03 | 0.16 | 1.00 | |||
| 6. | −0.10 | 0.11 | −0.13 | 0.04 | 0.05 | 1.00 | ||
| 7. | 0.41 | 0.10 | 0.31 | 0.35 | −0.02 | 0.03 | 1.00 | |
| 8. | 0.28 | 0.06 | 0.20 | 0.23 | −0.12 | 0.17 | 0.89* | 1.00 |
|
| ||||||||
| 0.5 g/kg | ||||||||
| 1. | 1.00 | |||||||
| 2. | −0.39 | 1.00 | ||||||
| 3. | 0.96* | −0.54* | 1.00 | |||||
| 4. | 0.42 | 0.66* | 0.24 | 1.00 | ||||
| 5. | −0.13 | 0.23 | −0.11 | 0.10 | 1.00 | |||
| 6. | −0.14 | 0.04 | −0.16 | −0.05 | 0.01 | 1.00 | ||
| 7. | 0.30 | −0.06 | 0.28 | 0.17 | −0.28 | 0.00 | 1.00 | |
| 8. | 0.29 | −0.01 | 0.27 | 0.21 | −0.24 | 0.04 | 0.96* | 1.00 |
|
| ||||||||
| 1.25 g/kg | ||||||||
| 1. | 1.00 | |||||||
| 2. | 0.03 | 1.00 | ||||||
| 3. | 0.94* | −0.18 | 1.00 | |||||
| 4. | 0.66* | 0.76* | 0.47* | 1.00 | ||||
| 5. | −0.03 | 0.36 | −0.13 | 0.23 | 1.00 | |||
| 6. | 0.12 | −0.06 | 0.13 | 0.04 | −0.18 | 1.00 | ||
| 7. | 0.54* | 0.25 | 0.52* | 0.53* | −0.06 | 0.18 | 1.00 | |
| 8. | 0.44* | 0.14 | 0.45* | 0.38 | −0.15 | 0.16 | 0.87* | 1.00 |
|
| ||||||||
| 2.5 g/kg | ||||||||
| 1. | 1.00 | |||||||
| 2. | −0.16 | 1.00 | ||||||
| 3. | 0.94* | −0.33 | 1.00 | |||||
| 4. | 0.68* | 0.61* | 0.51* | 1.00 | ||||
| 5. | −0.13 | 0.11 | −0.18 | −0.03 | 1.00 | |||
| 6. | 0.04 | −0.13 | 0.07 | −0.07 | 0.10 | 1.00 | ||
| 7. | 0.37 | 0.28 | 0.27 | 0.53* | −0.15 | −0.04 | 1.00 | |
| 8. | 0.36 | 0.19 | 0.29 | 0.44* | −0.24 | −0.01 | 0.93* | 1.00 |
|
| ||||||||
| 3.25 g/kg | ||||||||
| 1. | 1.00 | |||||||
| 2. | 0.16 | 1.00 | ||||||
| 3. | 0.94* | 0.00 | 1.00 | |||||
| 4. | 0.64* | 0.86* | 0.48* | 1.00 | ||||
| 5. | 0.34 | 0.32 | 0.25 | 0.42 | 1.00 | |||
| 6. | 0.29 | 0.11 | 0.29 | 0.23 | 0.18 | 1.00 | ||
| 7. | 0.14 | −0.02 | 0.12 | 0.05 | −0.15 | −0.09 | 1.00 | |
| 8. | 0.08 | −0.10 | 0.10 | −0.04 | −0.24 | −0.10 | 0.94* | 1.00 |
Note 1: OA = open arms, CA = closed arms, OF = open-field, EPM = elevated plus maze, LDB = light-dark box. Note 2: significant correlations (p < .00625) are expressed in bold and denoted by an asterisk and mirrored coefficients have been deleted. Note 3: OA, CA and % OA were registered in the EPM. EPM locomotion refers to total number of open and closed arm entries. OF locomotion indicates total amount of time (s) exhibiting forward locomotion in the apparatus. Number of transfers between compartments was considered an index of locomotion in the LDB.
One of the objectives of the correlational approach was to determine whether greater locomotion in the OF, particularly after ethanol administration, is associated with a lower anxiety response in the EPM or LDB. The association between locomotion (s) in the OF and percentage of open arm entries in the EPM was null (r = −0.02) for the overall sample and not significant (r ≤ 0.25) in the ethanol-treated groups or in the untreated group. Similarly, locomotion in the OF was not significantly associated with the time spent in the bright area of the LDB or number of entries into the open arms of the EPM in any of the groups. In the overall sample and across groups, time spent on the center of the OF (%) was not significantly associated with the measures gathered at EPM or LDB, nor was significantly associated with locomotion in the OF. Overall, these results suggest that ethanol-induced motor activation in the OF did not result from the ethanol-induced amelioration of anxiety.
In the overall sample of subjects, locomotion in the LDB was significantly associated with the total number of arm entries, percentage of open arm entries, and number of open arm entries in the EPM (r = 0.31, 0.32, and 0.35, respectively) and the time spent in the bright side of the LDB (r = 0.64). As could be expected, locomotion (i.e., total number of arm entries) in the EPM was significantly associated with the percentage of arm entries (r = 0.40).
The total number of arm entries and percentage of open arm entries in the EPM were significantly associated across all groups (r = 0.93–0.96). The total number of closed arm entries was significantly correlated with overall locomotion scores in the EPM in the overall dataset (r = 0.77) and in each particular group (r = 0.60–0.86). The total number of open arm entries was significantly correlated with overall locomotion scores in the EPM in the overall dataset (r = 0.61) and in the groups treated with the ethanol doses of 1.25, 2.5, and 3.25 g/kg (r = 0.60, 0.68, and 0.64, respectively).
The time spent in the bright side of the LDB was significantly correlated with locomotion in the LDB (r = 0.65–0.96) in all of the groups, with the exception of the untreated group. The time spent in the bright side of the LDB was also significantly correlated with the percentage of open arm entries in the overall dataset and in the 1.25 g/kg ethanol group (r = 0.45), and with locomotion in the EPM (r = 0.44). Locomotion in the LDB was also significantly associated with locomotion in the EPM in the overall sample of subjects (r = 0.31), and in the 1.25 and 2.5 g/kg ethanol groups (both r = 0.53). It was also significantly associated with closed arm entries in the EPM in subjects given 1.25 g/kg ethanol (r = 0.54) and in the overall population (r = 0.36) and with the percentage of open arm entries in the EPM in subjects given 1.25 g/kg ethanol (r = 0.52).
3.5. Ethanol, sucrose, and overall fluid intake as a function of ethanol treatment on PD28
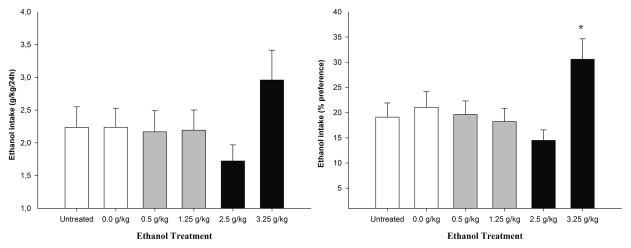
Figure 5 depicts absolute ethanol intake (g/kg) and the percentage of ethanol intake as a function of ethanol treatment during the behavioral assessments. Visual inspection suggests that treatment with 3.25 g/kg ethanol on PD28 affected later ethanol intake. As depicted in the right panel of Figure 5 and confirmed by the ANOVA (significant main effect of ethanol treatment, F5,263 = 3.29, p < 0.01) and subsequent post hoc tests, the percentage of ethanol intake was significantly higher in animals given 3.25 g/kg ethanol than in all of the remaining groups. Subjects given 3.25 g/kg ethanol also exhibited higher absolute ethanol consumption (g/kg) than their counterparts. The ANOVA of g/kg of ethanol consumed, however, indicated that the effect of ethanol did not reach significance (p > 0.15). There were no significant main effects attributable to sex or to order of testing or significant interactions comprising these factors.
Figure 5.
(Left panel) Ethanol consumption (g/kg/24h) and (Right panel) preference (%) in 29-day-old male and female adolescent rats as a function of ethanol treatment received on PD28 (0.0 [vehicle], 0.5, 1.25, 2.5, and 3.25 g/kg ethanol or untreated [UT]). After behavioral screening on PD28, all of the animals underwent a 24-h ethanol intake test, in which they had simultaneous access to three 25-ml graduated glass tubes filled with tap water, 1% v/v sucrose, or 5% v/v ethanol. The data were collapsed across sex. The sex factor did not exert a significant main effect or interact with the remaining variables. The asterisk indicates a significant difference between a given group and the 0.0 g/kg ethanol group. The vertical bars indicate SEM.
Interestingly, treatment with 3.25 g/kg ethanol significantly decreased sucrose intake. The ANOVAs indicated a significant effect of ethanol treatment on PD28 in terms of both ml/100 g of body weight and percent preference (F5,263 = 3.09 and F5,263 = 3.68, respectively; p < 0.01). The post hoc tests indicated that animals given 3.25 g/kg ethanol exhibited significantly less sucrose intake than subjects in the remaining groups. The mean values (ml/100 g and percent preference) and standard errors for each treatment group were the following: untreated (20.65 ± 1.14 ml/100 g and 64.34% ± 3.00%), 0.0 g/kg ethanol (18.93 ± 1.18 ml/100 g, 62.72% ± 3.49%), 0.5 g/kg ethanol (17.94 ± 1.20 ml/100 g, 61.04% ± 3.64%), 1.25 g/kg ethanol (20.81 ± 1.06 ml/100 g, 66.60% ± 2.96%), 2.5 g/kg ethanol (20.52 ± 1.18 ml/100 g, 66.51% ± 3.10%), and 3.5 g/kg (15.12 ± 1.60 ml/100 g, 49.07% ± 3.81%).
The ANOVA indicated that ethanol treatment on PD28 did not alter overall fluid consumption (ml/100 g). The mean values and standard errors for each group were the following: untreated (31.70 ± 0.94), 0.0 g/kg ethanol (29.67 ± 1.05), 0.5 g/kg ethanol (28.87 ± 1.04), 1.25 g/kg ethanol (31.38 ± 1.14), 2.5 g/kg ethanol (30.58 ± 1.01), and 3.5 g/kg ethanol (27.39 ± 2.36).
Neither sex nor order of testing exerted a significant main effect or interaction with the remaining variables in any of the variables measured during the intake test.
3.6. Univariate and multivariate analyses of intake patterns in control animals (i.e., untreated or vehicle-treated)
Multiple regression
Predictive variables were simultaneously entered into the multiple regression model. The analysis indicated that ethanol consumed (g/kg) in the 24-h ethanol intake test was significantly explained by the set of predictive variables. Exploratory and anxiety-like behaviors in the OF, EPM, and LDB accounted for 22% of the variability in intake scores (R2 = 0.22, R2a = 0.14, both p < 0.05). Inspection of the individual correlation scores indicated that the percentage of open arm entries in the EPM, percent time spent in the center of the OF, and frequency of rearing behavior in the OF significantly predicted ethanol intake scores (β = 0.23, −0.26, and 0.25, respectively). The remaining estimations of the partial, individual relationships between the predictive variables and ethanol intake did not achieve significance. A description of the multiple regression model is presented in Table 2.
Table 2.
Multiple regression (MR) results. Variables were simultaneously added into the model. The regular and adjusted multiple correlation coefficient (R2 and R2a) indicate the percent of variance explained; and the adjusted version decreases with the number of predictive variables introduced in the equation. Significant effects (p<0.05) at the individual or multivariate levels are indicated in bold.
| Variables entered
|
Dependent variable: Ethanol intake (g/kg) R2 = 0.22, Adjusted R2 = 0.14, p < 0.01. |
||
|---|---|---|---|
| Beta
|
t
|
P level
|
|
|
Frequency of rearing in OF
|
0.25
|
2.31
|
0.02
|
|
Forward Locomotion in OF
|
−0.07
|
−0.64
|
0.52
|
|
Time in the center of OF (%)
|
−0.26
|
−2.49
|
0.02
|
|
Locomotion in EPM
|
−0.01
|
−0.08
|
0.94
|
|
Open arm entries in EPM (%)
|
0.23
|
2.14
|
0.03
|
|
Locomotion in LDB
|
−0.19
|
−1.58
|
0.12
|
|
Time spent in the bright area of LDB
|
0.18
|
1.60
|
0.11
|
| Variables entered
|
Dependent variable: sucrose intake (ml/100 g) R2 = 0.07, Adjusted R2 = −0.02, p > 0.50. |
||
|---|---|---|---|
| Beta
|
t
|
P level
|
|
|
Frequency of rearing in OF
|
−0.21
|
−1.82
|
0.07
|
|
Forward Locomotion in OF
|
0.05
|
0.39
|
0.70
|
|
Time in the center of OF (%)
|
−0.02
|
−0.20
|
0.84
|
|
Locomotion in EPM
|
0.09
|
0.72
|
0.47
|
|
Open arm entries in EPM (%)
|
0.05
|
0.38
|
0.71
|
|
Locomotion in LDB
|
0.13
|
1.08
|
0.28
|
|
Time spent in the bright area of LDB
|
0,05
|
0.44
|
0.66
|
The same set of predictive variables did not significantly explain sucrose intake (ml/100 g) during the 24-h intake test (R2 = 0.07, R2a = −0.02, both p < 0.05). Inspection of the β values for each predictive variable indicated a lack of a significant association between the individual variables and sucrose intake (all p > 0.05).
3.7. Ethanol intake in high-, medium-, and low-anxiety responders
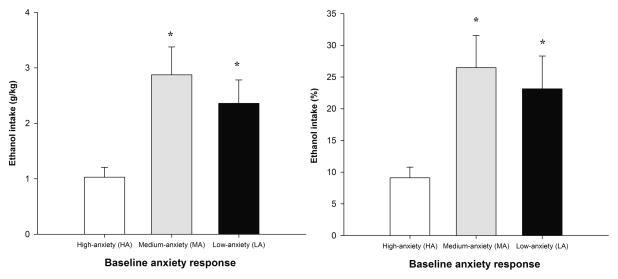
The overall sample of control subjects (n = 80) was classified into three groups (low-, medium-, and high-anxiety responders [LA, MA, and HA, respectively]) as a function of the percentage of open arm entries in the EPM. The ANOVAs of ethanol intake and percent ethanol preference across animals yielded significant main effects as a function of the selection criteria (F2,57 = 6.02, p < 0.01, and F2,57 = 4.61, p < 0.05, respectively). The post hoc tests indicated that HA adolescents preferred the ethanol solution less and drank less absolute ethanol when compared with MA and LA groups. The latter two groups exhibited similar absolute ethanol drinking and preference. Ethanol intake (g/kg) and percent preference among groups are depicted in Figure 6 and Table 3. The ANOVAs indicated that the differences observed as a function of baseline anxiety response were specific to ethanol intake and preference. Neither absolute, percent sucrose intake nor total fluid intake were significantly different between the HA, MA, and LA groups (Table 3).
Figure 6.
Ethanol consumption (g/kg/24h) and preference (%) in control subjects (n = 80; animals given 0.0 g/kg ethanol or untreated during behavioral assessment) classified as high-, medium-, or low-anxiety responders (HA, MA, and LA, respectively). The selection criteria resulted from the use of a quartile-split procedure for the percentage of open arms entries in the elevated plus maze. The sex factor did not exert a significant main effect or interact with the remaining variables. The asterisk indicates a significant difference between the LA and MA groups and the HA group. The vertical bars indicate SEM.
Table 3.
Overall Fluid intake (ml/100 g) and sucrose absolute (ml/100 g) and percent (%) intake during a three-bottle choice intake in adolescent rats classified as a high, medium or low anxiety responders, as function of their percent number of entries into the open arms of the elevated plus maze (EPM, left section); or as a function of the percent time spent on the center area of an open field (OF, right section of the table).
| Dependent variable
|
Percent number of entries into the open arms of the EPM
|
Percent time spent on the center of the OF
|
||||
|---|---|---|---|---|---|---|
| High Anxiety Group
|
Medium Anxiety Group
|
Low Anxiety Group
|
High Anxiety Group
|
Medium Anxiety Group
|
Low Anxiety Group
|
|
|
Overall Fluid Intake (ml/100g)
|
29.70±1.25
|
30.18±1.53
|
29.51±1.63
|
29.45±1.52
|
30.84±1.43
|
29.46±1.49
|
|
Sucrose Intake (ml/100g)
|
21.00±1.56
|
18.30±2.04
|
19.67±1.68
|
18.65±1.92
|
22.23±1.47
|
21.12±1.69
|
|
Sucrose Preference (%)
|
70.46±4.15
|
58.19±5.85
|
65.64±4.59
|
61.75±5.37
|
71.99±3.47
|
70.32±4.88
|
|
Ethanol Intake (g/kg/24h)
|
1.03±0.17
|
2.91±0.50
|
2.24±0.42
|
2.52±0.48
|
1.86±0.39
|
1.26±0.30
|
| Ethanol Preference (%) | 9.09±1,59 | 26.58±5.06 | 22.01±5.04 | 24.56±5.44 | 14.76±2.83 | 13.46±3.82 |
Control subjects (n = 80) were also classified as high-, medium-, and low-anxiety responders (HA1, MA1, and LA1, respectively) as a function of the percent time spent in the central area of the OF. The ANOVAs indicated similar levels of absolute ethanol intake and the percentage of ethanol preference in the HA1, MA1, and LA1 groups (F2,57 = 2.51, p > 0.05, and F2,57 = 2.11, p > 0.10, respectively). The ANOVAs also revealed that these subjects exhibited statistically similar levels of sucrose intake (ml/100 g and percent preference) and did not differ in terms of overall fluid intake (all p > 0.05). See Table 3 for ethanol intake, sucrose intake, and overall fluid intake in the high-, medium-, and low-anxiety responders classified according to patterns of central area exploration in the OF.
4. Discussion
The present study focused on ethanol-related behaviors during adolescence, a developmental stage in which ethanol experimentation and escalation usually occurs [28]. The aims of the present study were to (a) assess the relationship between measures of ethanol-induced activity and anxiolysis in the OF, EPM, and LDB, (b) analyze ethanol intake as a function of previous ethanol exposure, and (c) analyze associations between ethanol intake and behavioral responsiveness in these apparatus.
The administration of ethanol in rats and mice apparently induces psychomotor activity [16, 29] that is akin to the stimulant effects observed in human subjects [30]. The drug may also reduce the animal’s innate fear of open spaces and facilitate exploration because of its anxiolytic effects. We assessed the motor-stimulant and anxiolytic effects of a range of ethanol doses 5–19 min post-administration in three different tests. The dose-response curves yielded by these tests helped determine whether enhanced ethanol-induced activation in the OF reflects the anxiolytic or stimulant effects of the drug. Drug-induced motor stimulation in the OF was exhibited after 1.25, 2.5, and 3.5 g/kg ethanol administration but not after the lowest ethanol dose (0.5 g/kg). The analysis of EPM scores revealed a greater percentage of open-arm entries in animals given 0.5, 1.25, or 2.5 g/kg ethanol compared with controls. These doses and the 3.25 g/kg dose also increased locomotion (i.e., total number of arm entries in both arms) in the EPM. Therefore, one could argue that the apparent anxiolytic effect of ethanol in the EPM reflected motor stimulation induced by the drug. Separate analyses of the number of entries into the open and closed arms revealed that the ethanol-induced enhancement of activity in animals given 0.5 and 1.25 g/kg ethanol was specific for the open arms. The animals given 3.25 g/kg ethanol exhibited a greater number of arm entries than control counterparts but only in the closed arms. Locomotion was distributed evenly across both arms in animals given 2.5 g/kg ethanol.
Interestingly, the 2.5 g/kg ethanol dose significantly enhanced locomotion in the OF but lacked reliable anxiolytic effects in the EPM. Specifically, the 2.5 g/kg dose increased absolute exploration in the open arms of the EPM, but this effect was driven by a general increase in motor activity instead of by a specific reduction of fear for open spaces. Moreover, the highest 3.25 g/kg dose exerted a significant stimulant effect in the OF but was devoid of any anxiolytic effect in the EPM. Animals given this high dose of ethanol also spent less time in the bright area of the LDB than control counterparts. This suggests that 3.25 g/kg ethanol may have induced an anxiogenic effect in the LDB test. Animals given this high dose, however, also exhibited a significant suppression of overall activity (i.e., number of transitions between compartments) in the LDB test. This raises the possibility that the lower time spent in the bright compartment after 3.25 g/kg could simply have been a byproduct of a delayed depressant effect of the highest dose. The LDB test occurred late during the postadministration curve, at minutes 15–19. Another caveat is that, unlike the plus-maze and open field, order of testing was not counterbalanced for LDB. It is likely that behavior during this third test was strongly influenced by the previous two tests.
Overall, these results suggest that the enhanced exploration of the OF after 2.5 and 3.25 g/kg ethanol reflects a motor-stimulating effect that appears to be relatively independent of any anxiolytic consequences. The 1.25 g/kg dose appeared to induce motor stimulation in the OF and anxiolytic effects in the EPM but the effects were relatively independent. In the EPM, the 1.25 g/kg ethanol dose increased the number of arm entries only in the open sections of the apparatus. The 0.5 g/kg ethanol dose, in turn, exerted significant anxiolytic effects in the EPM and lacked stimulating effects in the OF. Moreover, when compared to the vehicle-treated condition, none of the lower ethanol doses (i.e., 0.5, 1.25 and 2.5 g/kg) were effective in increasing time spent in the center of the OF.
Positive correlations were observed between locomotion scores in the OF and EPM and between locomotion in the EPM and total number of compartment transitions in the LDB. Greater locomotion in one apparatus appeared to be associated with greater locomotion in the other tests. Similarly, ambulation in the OF was positively correlated with closed arm entries but not open arm entries in the EPM. These correlations are consistent with studies that employed factor analysis and found that ambulation in the OF and closed-arm entries and total entries in the EPM were represented by a common factor known as “locomotor/exploratory activity” [27, 31, 32]. Another aim of the correlational approach was to determine whether greater locomotion in the OF, particularly after ethanol administration, was associated with a lower anxiety response in the EPM or LDB. No associations were found between these behaviors. These results further support the hypothesis that the motor-activating effects of ethanol in the OF reflect a stimulant rather than an anxiolytic effect. In other words, these results indicate that the OF is a valid assay for the measurement of ethanol-induced stimulation. This information is valuable when considering that there has been substantial controversy as to the validity of the test [12].
An important outcome was that adolescents that were treated with the highest ethanol dose (3.25 g/kg) exhibited a significantly greater percent preference for ethanol than the remaining groups when tested in a 24-h three-bottle choice test. Previous studies indicated that ethanol is non-metabolically eliminated through perspiration, breath, salivation, and urine [33], and that the perception of ethanol’s odor during the intoxication can alter subsequent responsiveness to ethanol. A study [17] reported heightened ethanol intake and orofacial responsiveness to ethanol in infant rats that were given 3.0 g/kg ethanol at the end of the first week of life. A replication with slightly older animals on PD14-15 found ethanol aversion [34]. The present study adds new information, indicating that adolescence may be a developmental stage where chemosensory learning during ethanol intoxication leads to an enhanced predisposition to ethanol intake.
The present study lacked a temporally precise measurement of intake (e.g. lickometer, or more frequent measurements). It cannot be suggested, therefore, that animals ingested enough ethanol to induce pharmacologically relevant blood alcohol concentrations. Under the present circumstances it is more likely that ethanol intake was mainly driven by orosensory (taste/odor) factors. We cannot exclude the possibility, however, that enhanced ethanol drinking in the 3.25 g/kg group was driven by the negative effects of ethanol hangover. Following the administration of 3.0–4.0 g/kg ethanol, adult rats exhibit rebound hyperthermia [35], and adolescent but not adult rats exhibit alterations in slow-wave sleep [36]. Although we did not measure or observe overt signs of withdrawal, higher ethanol drinking in the present study may have been promoted by similar lingering aversive effects of high-ethanol dosing.
Ethanol was offered in a low 1% sucrose solution. The enhanced preference in these ethanol-treated animals may have reflected greater sucrose preference. However, this did not appear to be the case. Animals that received 3.25 g/kg ethanol actually drank less sucrose than the remaining animals. This indicates that the rewarding effect of sucrose or its value as a caloric supplement were unrelated to the significantly greater ethanol intake in the 3.25 g/kg ethanol group. A decrease in the intake of a highly palatable sweet solution following acute [37] or chronic [38] stress exposure has been considered to reflect a negative emotional state (i.e., anhedonia). The combination of greater ethanol intake and the suppression of sucrose consumption as observed in the present study resembles results found in rats experiencing negative emotional states as a consequence of prolonged neonatal maternal separation [38].
The multivariate regression analysis in the present study significantly accounted for 22% of the variability observed in ethanol intake scores in subjects that were drug-free during the behavioral assessment. Rats with a higher frequency of rearing in the OF, higher percentage of open arm entries in the EPM, and lower propensity to enter the central area of the OF exhibited greater ethanol intake. Rearing in a novel environment has long been considered a measure of exploratory behavior [40] that predicts the propensity to engage in the consumption of ethanol [41] and other drugs [42]. Reduced exploration of the central area of the OF likely reflects greater innate anxiety and, therefore, a higher predisposition to consume ethanol as a way to mitigate this state [43]. The positive relationship between the percentage of open arm entries and ethanol intake was unexpected. This variable, however, may also reflect a higher level of risk taking or novelty seeking, which have been consistently related to ethanol and drug intake [44, 45]. An intriguing study [46] found that 35-day old and 61-day old mice (referred to as juvenile and adults, respectively) exhibited, as expected, open arm avoidance in an elevated plus-maze. In contrast, EPM exploration in 48-day old mice (referred to as adolescents) was evenly distributed between both arms. The adolescents exhibited as much risk-assessment behaviors (i.e., stretched–attend posture towards the open arm) as the other age groups. These data suggested that the adolescents perceived the open space as anxiety inducing, yet they exhibited significant novelty seeking that made them explore and spend time in the unprotected sections of the apparatus. The study also indicated that, at certain ages, the EPM might be measuring novelty seeking rather than anxiety and that measurement of multiple dependent variables helps understand the psychobiological meaning of the test.
The percentage of open arm entries in the EPM and percent time spent in the central area of the OF were selected to differentiate adolescents that remained ethanol-naive during the behavioral tests into high-, medium-, and low-anxiety responders. Subjects that exhibited relatively less time in the center of the OF were expected to consume and prefer significantly more ethanol than their counterparts that exhibited more time in the center of the OF. The anxiolytic effects of ethanol have been proposed to be one of the components that mediate initial consumption of the drug by ameliorating the unpleasant states associated with anxiety, fear, and stress [43, 47]. Such a result, however, was not observed. No differences in ethanol intake were observed among groups classified based on a long or short time spent in the center of the OF. With regard to the percentage of open arm entries in the EPM and consistent with the positive association found in the multiple regression analysis between this variable and ethanol intake, rats classified as high-anxiety responders in the EPM (i.e., that exhibited a relatively lower number of OA entries) consumed significantly less ethanol than rats that were classified as low- or medium-anxiety responders in this apparatus.
The present results add to accumulating evidence that indicates that the relationship between anxiety states and ethanol consumption yields complex and sometimes contradictory results. An association between increased anxiety states and ethanol abuse has been inferred in human studies [48] and animal studies [43, 49, 50], but several preclinical reports indicated an opposite relationship [51, 52, 53]. For example, it has been found that selectively bred low-anxiety behavior (LAB) rats drank more than high-anxiety behavior (HAB) counterparts [52]. Similarly, exposure to nociceptive stress in rodents has been found to increase, decrease, or have no effect on ethanol intake [54].
Notably, the conditions under which the relationship between ethanol intake and anxiety was investigated in rats have usually involved long-term ethanol self-administration [24, 55]. In these studies, ethanol consumption and preference were not established until the animals underwent several intake sessions, previously initiated drug intake [16, 56], or were exposed to stress [57, 58, 59]. It has been suggested that animals require several intake sessions to learn the anxiety-ameliorating effects of ethanol intake [60]. A caveat of the present study was the use of a single 24-h intake test. Sensitivity to the drug would likely differ as a function of innate anxiety only after several intake sessions. Animals were tested from post-administration minute 5 to minute 19. The rationale for choosing this interval was that ethanol’s stimulatory effects diminish over time; an effect that is more pronounced in rats, which only exhibited ethanol-induced locomotor stimulation when tested during the rising phase of the blood ethanol curve. By counterbalancing EPM and OF tests and restricting tests to the first 20 min postadministration we aimed at minimizing the effects of time course of intoxication on the expression of the drug’s motor effects. This confounding effect, however, cannot be completely dismissed, particularly when interpreting the results of the LDB test. This test was not counterbalanced and occurred at the end of testing, when it was more likely that doses that initially produced an activating effect produced a depressant (or weaker activating) effect. It is likely that the dose-response curve for the LDB test would have been significantly different if the test had been conducted immediately after the injection.
Despite previous studies that reported sex-related differences in ethanol intake [15] and the stimulating effects of ethanol [39], the present results were fairly similar across males and females. This study was conducted at the beginning of the adolescent stage. At PD28 adolescent-typical behaviors (e.g., increased socialization) are well established and striking brain changes (e.g., pruning of dopamine receptors in mesolimbic areas) begin [61]. It has been observed that ethanol exposure on PD28 increased ethanol intake during late adolescence, but exposure on PD31 did not [16]. It seems that the earlier stages of adolescence are critical periods in terms of sensitivity to alcohol and other drugs. It is conceivable that testing this early during the adolescent stage could have accounted for the lack of sex differences in the present study.
An important limitation of this study was the lack of automatic behavior recording in the OF. A recent study [62] has presented a software-based system that provides high-throughput in conjunction with detailed analysis of center vs. peripheral locomotion in the OF. This system discriminates four different patterns of behaviors in the center area of the arena. It can also be considered a limitation that the percentage of total variance explained by the multiple regression model was relatively low (i.e., 78% of total variance remains unexplained). It is noteworthy, however, that a significant prediction was achieved by a model that incorporated only behavioral variables. Future studies should take advantage of these findings and expand the model through the addition of neural, hormonal or genetic predictors. Moreover, the percentage of total variance explained by the model was similar to that found in other studies that aimed at predicting ethanol intake as a function of behavioral variables (e.g., [63]).
Overall, the present results provide new information about the effect of acute ethanol administration on motor-stimulant and anxiety-related behaviors and consumption of the drug. Ethanol exerts both stimulating and anxiolytic effects, and the present data support the hypothesis that these effects are likely mediated by distinct underlying mechanisms for most of the doses tested. High-ethanol administration exerts a potent stimulatory effect and increases later ethanol preference. The stimulatory effect is reliably detected by the open field test and seems to be unrelated to potential anxiolytic effects. Important new information is that a significant amount of the variability in ethanol intake scores was explained as a function of behavioral scores inherent to the OF and EPM. This result helps characterize subpopulations of adolescents that may be at risk for initiating alcohol drinking. This method could be used in future studies to differentiate between adolescents that quickly engage in alcohol self-administration from those that maintain alcohol drinking low despite similar levels of drug availability. The present model could be expanded and refined by the addition of neural, hormonal and genetic predictors.
Research Highlights.
Ethanol-induced motor activation in the open-field at 1.25, 2.5 and 3.25 g/kg dose.
Ethanol-induced anxiolysis in elevated plus maze at 0.5, 1.25 and 2.5 g/kg dose.
At some doses ethanol-induced motor activation was independent of anxiolysis.
Adolescents given 3.25 g/kg drank less sucrose and more ethanol than controls.
Behavior in the OF, EPM and LDB explained 22% of ethanol intake scores.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol’s motivational effects. Alcohol Res Health. 2000;24:85–2. [PMC free article] [PubMed] [Google Scholar]
- 2.Nizhnikov ME, Pautassi RM, Truxell E, Spear NE. Opioid antagonists block the acquisition of ethanol-mediated conditioned tactile preference in infant rats. Alcohol. 2009;43:347–58. doi: 10.1016/j.alcohol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pautassi RM, Godoy JC, Spear NE, Molina JC. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin Exp Res. 2002;26:644–54. [PubMed] [Google Scholar]
- 4.Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163(3):890–7. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciccocioppo R, Panocka I, Froldi R, Quitadamo E, Massi M. Ethanol induces conditioned place preference in genetically selected alcohol-preferring rats. Psychopharmacology (Berl) 1999;141:235–41. doi: 10.1007/s002130050830. [DOI] [PubMed] [Google Scholar]
- 6.Karadayian AG, Busso MJ, Feleder C, Cutrera RA. Alterations in affective behavior during the time course of alcohol hangover. Behav Brain Res. 2013;253:128–38. doi: 10.1016/j.bbr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Fukushiro DF, Josino FS, Saito LP, Berro LF, Morgado F, Frussa-Filho R. Acute and chronic ethanol differentially modify the emotional significance of a novel environment: implications for addiction. Int J Neuropsychopharmacol. 2012;15(8):1109–20. doi: 10.1017/S1461145711001283. [DOI] [PubMed] [Google Scholar]
- 8.Quoilin C, Didone V, Tirelli E, Quertemont E. Ontogeny of the stimulant and sedative effects of ethanol in male and female Swiss mice: gradual changes from weaning to adulthood. Psychopharmacology (Berl) 2010;212:501–12. doi: 10.1007/s00213-010-1971-z. [DOI] [PubMed] [Google Scholar]
- 9.Pautassi RM, Nizhnikov ME, Spear NE. Assessing appetitive, aversive, and negative ethanol-mediated reinforcement through an immature rat model. Neurosci Biobehav Rev. 2009;33:953–74. doi: 10.1016/j.neubiorev.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer PJ, Meshul CK, Phillips TJ. Ethanol- and cocaine-induced locomotion are genetically related to increases in accumbal dopamine. Genes Brain Behav. 2009;8:346–55. doi: 10.1111/j.1601-183X.2009.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S. Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology. 2004;172:264–70. doi: 10.1007/s00213-003-1647-z. [DOI] [PubMed] [Google Scholar]
- 12.Stanford SC. The open field test: reinventing the wheel. J Psychopharmacol. 2007;21(2):134–35. doi: 10.1177/0269881107073199. [DOI] [PubMed] [Google Scholar]
- 13.Arias C, Mlewski EC, Molina JC, Spear NE. Ethanol induces locomotor activating effects in preweanling Sprague–Dawley rats. Alcohol. 2009;43:13–23. doi: 10.1016/j.alcohol.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acevedo MB, Pautassi RM, Spear NE, Spear LP. Age-dependent effects of stress on ethanol-induced motor activity in rats. Psychopharmacology (Berl) 2013;230:389–98. doi: 10.1007/s00213-013-3163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- 16.Acevedo MB, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM. High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence. Dev Psychobiol. 2010;52:424–40. doi: 10.1002/dev.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chotro MG, Arias C. Ontogenetic difference in ethanol reinforcing properties: the role of the opioid system. Behav Pharmacol. 2007;18(7):661–6. doi: 10.1097/FBP.0b013e3282f00754. [DOI] [PubMed] [Google Scholar]
- 18.Pilatti A, Caneto F, Garimaldi JA, Vera BD, Pautassi RM. Contribution of Time of Drinking Onset and Family History of Alcohol Problems in Alcohol and Drug Use Behaviors in Argentinean College Students. Alcohol Alcohol. 2013 doi: 10.1093/alcalc/agt176. [DOI] [PubMed] [Google Scholar]
- 19.National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 20.Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30(2):141–50. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 21.Arias C, Pautassi RM, Molina JC, Spear NE. A comparison between taste avoidance and conditioned disgust reactions induced by ethanol and lithium chloride in preweanling rats. Dev Psychobiol. 2010;52(6):545–57. doi: 10.1002/dev.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 23.Ottoni EB. EthoLog 2.2 -a tool for the transcription and timing of behavior observation sessions. Behavior Research Methods Instruments, & Computers. 2000;32(3):446–49. doi: 10.3758/bf03200814. [DOI] [PubMed] [Google Scholar]
- 24.Spanagel R, Hölter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol. 1999;34(2):231–43. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- 25.Fabio MC, Nizhnikov ME, Spear NE, Pautassi RM. Binge ethanol intoxication heightens subsequent ethanol intake in adolescent, but not adult, rats. Dev Psychobiol. 2013 doi: 10.1002/dev.21101. [DOI] [PubMed] [Google Scholar]
- 26.Da Silva GE, Vendruscolo LF, Takahashi RN. Effects of ethanol on locomotor and anxiety-like behaviors and the acquisition of ethanol intake in Lewis and spontaneously hypertensive rats. Life Sci. 2005;77(6):693–706. doi: 10.1016/j.lfs.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 27.File SE. Animal models of different anxiety states. Adv Biochem Psychopharmacol. 1995;48:93–113. [PubMed] [Google Scholar]
- 28.Pilatti A, Godoy JC, Brussino SA, Pautassi RM. Patterns of substance use among Argentinean adolescents and analysis of the effect of age at first alcohol use on substance use behaviors. Addict Behav. 2013;(12):2847–50. doi: 10.1016/j.addbeh.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Camarini R, Pautassi RM, Méndez M, Quadros IM, Souza-Formigoni ML, Boerngen-Lacerda R. Behavioral and neurochemical studies in distinct animal models of ethanol’s motivational effects. Curr Drug Abuse Rev. 2010;3(4):205–21. doi: 10.2174/1874473711003040205. [DOI] [PubMed] [Google Scholar]
- 30.Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology (Berl) 2001;57(1):20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- 31.Ramos A, Berton O, Mormède P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav Brain Res. 1997;85(1):57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 32.Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52(2):297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- 33.Winger G, Hofmann FG, Woods JH. The Biomedical Aspects. 3. New York: Oxford University Press; 1992. A Handbook on Drug and Alcohol Abuse. [Google Scholar]
- 34.Molina JC, Chotro MG. Acute alcohol intoxication paired with aversive reinforcement: ethanol odor as a conditioned reinforcer in rat pups. Behav Neural Biol. 1989;52(1):1–19. doi: 10.1016/s0163-1047(89)90122-2. [DOI] [PubMed] [Google Scholar]
- 35.Spiers DE, Fusco LE. Delayed thermoregulatory changes in the immature rat following a single injection of ethanol. Alcohol Clin Exp Res. 1992;16(1):41–7. doi: 10.1111/j.1530-0277.1992.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 36.Ehlers CL, Desikan A, Wills DN. Developmental differences in EEG and sleep responses to acute ethanol administration and its withdrawal (hangover) in adolescent and adult Wistar rats. Alcohol. 2013;47(8):601–10. doi: 10.1016/j.alcohol.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosellini RA, DeCola JP, Plonsky M, Warren DA, Stilman AJ. Uncontrollable shock proactively increases sensitivity to response-reinforcer independence in rats. J Exp Psychol Anim Behav Process. 1984;10(3):346–59. [PubMed] [Google Scholar]
- 38.Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158(4):366–73. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- 39.Kawakami SE, Quadros IM, Takahashi S, Suchecki D. Long maternal separation accelerates behavioural sensitization to ethanol in female, but not in male mice. Behav Brain Res. 2007;3;184(2):109–16. doi: 10.1016/j.bbr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 40.Van Abeelen JH. Genetic analysis of behavioural responses to novelty in mice. Nature. 1975 Mar 20;254(5497):239–41. doi: 10.1038/254239a0. [DOI] [PubMed] [Google Scholar]
- 41.Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162(3):333–8. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- 42.Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology. 2013 Apr 29; doi: 10.1016/j.neuropharm.2013.04.033. pii:S0028-3908(13)00179-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spanagel R, Montkowski A, Allingham K, Shoaib M, Holsboer K, Landgraf R. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology. 1995;122:369–73. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- 44.Nagoshi CT, Wilson JR, Rodriguez LA. Impulsivity, sensation seeking, and behavioral and emotional responses to alcohol. Alcohol Clin Exp Res. 1991;15(4):661–7. doi: 10.1111/j.1530-0277.1991.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 45.Stojek M, Fischer S. Impulsivity and motivations to consume alcohol: a prospective study on risk of dependence in young adult women. Alcohol Clin Exp Res. 2013;37(2):292–9. doi: 10.1111/j.1530-0277.2012.01875.x. [DOI] [PubMed] [Google Scholar]
- 46.Macri S, Adriani W, Chiarotti F, Laviola G. Risk taking during exploration of a plus-maze is greater in adolescent than in juvenile or adult mice. Animal Behaviour. 2002;64:541–546. [Google Scholar]
- 47.Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytiä P, Merlo-Pitch E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- 48.Kushner MG, Sher KJ, Beitman BD. The relation between alcohol problems and the anxiety disorders. Am J Psychiatry. 1990;147:685–95. doi: 10.1176/ajp.147.6.685. [DOI] [PubMed] [Google Scholar]
- 49.Bahi A. Individual differences in elevated plus-maze exploration predicted higher ethanol consumption and preference in outbred mice. Pharmacol Biochem Behav. 2013;105:83–8. doi: 10.1016/j.pbb.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Möller C, Wiklund L, Thorsell A, Hyytia P, Heilig M. Decreased measures of experimental anxiety in rats bred for high alcohol preference. Alcohol Clin Exp Res. 1997;21:656–60. [PubMed] [Google Scholar]
- 51.McMillen BA, Means LW, Matthews JN. Comparison of the alcohol-preferring P rat to the Wistar rat in behavioral tests of impulsivity and anxiety. Physiol Behav. 1998;63:371–75. doi: 10.1016/s0031-9384(97)00442-3. [DOI] [PubMed] [Google Scholar]
- 52.Henniger MS, Spanagel R, Wigger A, Landgraf R, Hölter SM. Alcohol self-administration in two rat lines selectively bred for extremes in anxiety-related behavior. Neuropsychopharmacology. 2002;26(6):729–36. doi: 10.1016/S0893-133X(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 53.Schuckit MA, Hesselbrock V. Alcohol dependence and anxiety disorders: what is the relationship? Am J Psychiatry. 1994;151:1723–34. doi: 10.1176/ajp.151.12.1723. [DOI] [PubMed] [Google Scholar]
- 54.Pautassi RM, Camarini R, Quadros IM, Miczek KA, Israel Y. Genetic and environmental influences on ethanol consumption: perspectives from preclinical research. Alcohol Clin Exp Res. 2010;34(6):976–87. doi: 10.1111/j.1530-0277.2010.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27:1048–54. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- 56.Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal of Neurochemistry. 2009;108:920–31. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- 57.Gomez JL, Lewis MJ, Luine VN. The interaction of chronic restraint stress and voluntary alcohol intake: effects on spatial memory in male rats. Alcohol. 2012;46(5):499–504. doi: 10.1016/j.alcohol.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pohorecky LA. Psychosocial stress and chronic ethanol ingestion in male rats: effects on elevated plus maze behavior and ultrasonic vocalizations. Physiol Behav. 2008;94(3):432–47. doi: 10.1016/j.physbeh.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcoholism: Clinical and Experimental Research. 2005;29:1139–45. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- 60.Samson HH, Pfeffer AO, Tolliver GA. Oral ethanol self-administration in rats: models of alcohol-seeking behavior. Alcohol Clin Exp Res. 1988;12(5):591–8. doi: 10.1111/j.1530-0277.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 61.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 62.Lipkind D, Sakov A, Kafkai N. New replicable anxiety-related measures of wall vs. center behavior of mice in the open field. J Applied Physiol. 2014;97(1):347–359. doi: 10.1152/japplphysiol.00148.2004. [DOI] [PubMed] [Google Scholar]
- 63.Schramm-Sapyta NL, Kingsley MA, Rezvani AH, Propst K, Swartzwelder HS, Kuhn C. Early Ethanol Consumption Predicts Relapse-Like Behavior in Adolescent Male Rats. Alcohol Clin Exp Res. 2008;32(5):754–762. doi: 10.1111/j.1530-0277.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]