Abstract
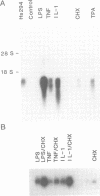
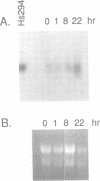
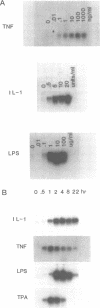
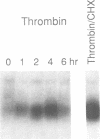
Melanoma growth stimulatory activity factor (MGSA) is a polypeptide which was initially isolated from Hs294 human melanoma cells. Its sequence is identical to the deduced amino acid sequence of the human gro cDNA, isolated from a human tumor cell line. MGSA stimulates the proliferation of malignant melanoma cells, but its function for normal cells has not been defined. Here we report that human umbilical vein endothelial cells are capable of synthesizing and secreting MGSA. The expression and secretion of MGSA are strongly induced by factors often involved in inflammation such as IL-1, TNF, LPS and thrombin. The induction of MGSA mRNA is dose and time dependent and is independent of new protein synthesis. This stimulation could be mimicked by TPA, suggesting that the action could be mediated through activation of protein kinase C. Furthermore, addition of MGSA to the endothelial cell cultures induces gro/MGSA gene expression, implying that an autocrine mechanism exists. Our data suggest that the protein encoded by gro/MGSA mRNA may play a role in inflammation and exert its effects on endothelial cells in an autocrine fashion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anisowicz A., Bardwell L., Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisowicz A., Zajchowski D., Stenman G., Sager R. Functional diversity of gro gene expression in human fibroblasts and mammary epithelial cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9645–9649. doi: 10.1073/pnas.85.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awbrey B. J., Hoak J. C., Owen W. G. Binding of human thrombin to cultured human endothelial cells. J Biol Chem. 1979 May 25;254(10):4092–4095. [PubMed] [Google Scholar]
- Barrett T. B., Gajdusek C. M., Schwartz S. M., McDougall J. K., Benditt E. P. Expression of the sis gene by endothelial cells in culture and in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6772–6774. doi: 10.1073/pnas.81.21.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg G. S., Pepper D. S., Chesterman C. N., Morgan F. J. Complete covalent structure of human beta-thromboglobulin. Biochemistry. 1978 May 2;17(9):1739–1744. doi: 10.1021/bi00602a024. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Segal G. M., Harlan J. M., Adamson J. W. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. doi: 10.1073/pnas.83.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. H., Herbosa G. J., Stiernberg J., Bergmann J. S., Gordon E. A., Scott D., Fenton J. W., 2nd Double-signal hypothesis for thrombin initiation of cell proliferation. Semin Thromb Hemost. 1986 Jul;12(3):231–240. doi: 10.1055/s-2007-1003559. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Miller J. W., Walz D. A. Structural and biological characteristics of connective tissue activating peptide (CTAP-III), a major human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1983 Feb;80(3):765–769. doi: 10.1073/pnas.80.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesterman C. N. Vascular endothelium, haemostasis and thrombosis. Blood Rev. 1988 Jun;2(2):88–94. doi: 10.1016/0268-960x(88)90029-x. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Collins T., Ginsburg D., Boss J. M., Orkin S. H., Pober J. S. Cultured human endothelial cells express platelet-derived growth factor B chain: cDNA cloning and structural analysis. Nature. 1985 Aug 22;316(6030):748–750. doi: 10.1038/316748a0. [DOI] [PubMed] [Google Scholar]
- Cotran R. S. American Association of Pathologists president's address. New roles for the endothelium in inflammation and immunity. Am J Pathol. 1987 Dec;129(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. D., Van Nostrand W. E., Farrell D. H., Campbell C. H. Interactions of serine proteases with cultured fibroblasts. J Cell Biochem. 1986;32(4):281–291. doi: 10.1002/jcb.240320405. [DOI] [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Garovoy M. R., Williams L. T. Thrombin stimulates c-sis gene expression in microvascular endothelial cells. J Biol Chem. 1986 Jul 25;261(21):9579–9582. [PubMed] [Google Scholar]
- Derynck R., Lindquist P. B., Lee A., Wen D., Tamm J., Graycar J. L., Rhee L., Mason A. J., Miller D. A., Coffey R. J. A new type of transforming growth factor-beta, TGF-beta 3. EMBO J. 1988 Dec 1;7(12):3737–3743. doi: 10.1002/j.1460-2075.1988.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Keim P. S., Farmer M., Heinrikson R. L. Amino acid sequence of human platelet factor 4. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2256–2258. doi: 10.1073/pnas.74.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Campbell I. L. Cytokines: an expanding network of immuno-inflammatory hormones. Mol Endocrinol. 1988 Dec;2(12):1151–1156. doi: 10.1210/mend-2-12-1151. [DOI] [PubMed] [Google Scholar]
- Holt J. C., Harris M. E., Holt A. M., Lange E., Henschen A., Niewiarowski S. Characterization of human platelet basic protein, a precursor form of low-affinity platelet factor 4 and beta-thromboglobulin. Biochemistry. 1986 Apr 22;25(8):1988–1996. doi: 10.1021/bi00356a023. [DOI] [PubMed] [Google Scholar]
- Hoshi H., McKeehan W. L. Brain- and liver cell-derived factors are required for growth of human endothelial cells in serum-free culture. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6413–6417. doi: 10.1073/pnas.81.20.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirik F. R., Podor T. J., Hirano T., Kishimoto T., Loskutoff D. J., Carson D. A., Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989 Jan 1;142(1):144–147. [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Low D. A., Cunningham D. D. A novel method for measuring cell surface-bound thrombin. Detection of iodination-induced changes in thrombin-binding affinity. J Biol Chem. 1982 Jan 25;257(2):850–858. [PubMed] [Google Scholar]
- Magnaldo I., Pouysségur J., Paris S. Thrombin exerts a dual effect on stimulated adenylate cyclase in hamster fibroblasts, an inhibition via a GTP-binding protein and a potentiation via activation of protein kinase C. Biochem J. 1988 Aug 1;253(3):711–719. doi: 10.1042/bj2530711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munker R., Gasson J., Ogawa M., Koeffler H. P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986 Sep 4;323(6083):79–82. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Handley D. A., Esmon C. T., Stern D. M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson Y., Hammacher A., Heldin C. H., Westermark B. Possible positive autocrine feedback in the prereplicative phase of human fibroblasts. Nature. 1987 Aug 20;328(6132):715–717. doi: 10.1038/328715a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Poncz M., Surrey S., LaRocco P., Weiss M. J., Rappaport E. F., Conway T. M., Schwartz E. Cloning and characterization of platelet factor 4 cDNA derived from a human erythroleukemic cell line. Blood. 1987 Jan;69(1):219–223. [PubMed] [Google Scholar]
- Richmond A., Balentien E., Thomas H. G., Flaggs G., Barton D. E., Spiess J., Bordoni R., Francke U., Derynck R. Molecular characterization and chromosomal mapping of melanoma growth stimulatory activity, a growth factor structurally related to beta-thromboglobulin. EMBO J. 1988 Jul;7(7):2025–2033. doi: 10.1002/j.1460-2075.1988.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A., Thomas H. G. Purification of melanoma growth stimulatory activity. J Cell Physiol. 1986 Dec;129(3):375–384. doi: 10.1002/jcp.1041290316. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988 Sep 1;141(5):1665–1669. [PubMed] [Google Scholar]
- Schleef R. R., Bevilacqua M. P., Sawdey M., Gimbrone M. A., Jr, Loskutoff D. J. Cytokine activation of vascular endothelium. Effects on tissue-type plasminogen activator and type 1 plasminogen activator inhibitor. J Biol Chem. 1988 Apr 25;263(12):5797–5803. [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sherry B., Cerami A. Cachectin/tumor necrosis factor exerts endocrine, paracrine, and autocrine control of inflammatory responses. J Cell Biol. 1988 Oct;107(4):1269–1277. doi: 10.1083/jcb.107.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K., LeRoy E. C., Grotendorst G. R. TGF-beta inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987 May 8;49(3):415–422. doi: 10.1016/0092-8674(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Roche N. S., Flanders K. C., Sporn M. B., Roberts A. B. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988 Jun 5;263(16):7741–7746. [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987 May 1;165(5):1316–1331. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms-Kretschmer K., Flax M. H., Cotran R. S. The fine structure of the vascular response in hapten-specific delayed hypersensitivity and contact dermatitis. Lab Invest. 1967 Sep;17(3):334–349. [PubMed] [Google Scholar]