Abstract
Aim:
To study the effects of Ganoderma lucidum polysaccharides (Gl-PS) on methotrexate (MTX)-induced small intestinal damage in mice and the underlying mechanisms.
Methods:
BALB/c mice were used for in vivo study. The mice were administered with Gl-PS (50, 100, or 200 mg/kg, ig) for 10 d, and injected with MTX (50 mg/kg, ip) on d 7 and 8 to induce intestinal damage, and then sacrificed on d 11 for morphological study and tissue malondialdehyde (MDA) and superoxide dismutase (SOD) measurements. Before sacrificing, blood samples were collected to analyze immunoglobulin A (IgA). Rat intestinal IEC-6 cells were used for in vitro study. Cell proliferation and migration were assessed using MTT method and an in vitro wounding model, respectively. Transforming growth factor β (TGFβ) protein expression was determined using ELISA assay. Ornithine decarboxylase (ODC) and c-Myc mRNA expression profiles were determined using RT-PCR.
Results:
MTX treatment caused severe mucosal damage, significantly increased small intestine MDA levels, and decreased SOD and serum IgA levels in BALB/c mice. Gl-PS (100 and 200 mg/kg) markedly reversed the MTX effects. In IEC-6 cells, Gl-PS (0.1, 1, and 10 μg/mL) significantly stimulated the cell proliferation. Furthermore, Gl-PS (10 μg/mL) significantly stimulated the cell migration. In addition, Gl-PS (10 and 20 μg/mL) significantly increased the expression of ODC and c-Myc mRNAs. However, Gl-PS (up to 20 μg/mL) had no effect on the expression of TGFβ protein.
Conclusion:
The results suggest that Gl-PS protects small intestine against MTX-induced injury via induction of epithelial cell proliferation and migration.
Keywords: Ganoderma lucidum polysaccharides, chemotherapy, methotrexate, small intestine injury, IEC-6 cell line
Introduction
The intestinal mucosa barrier (IMB), which is the first line of defense against a hostile environment, is composed of a single layer of columnar epithelium with inter-epithelial tight junctions. Intestinal mucositis is a clinical term that is used to describe the side effects of cancer chemotherapy on the intestinal mucosa surface, resulting from dysfunction of the IMB. Typical symptoms are bloating, abdominal pain and diarrhea. Approximately 40%–100% of cancer patients undergoing chemotherapy develop mucositis1, 2, 3, 4, and 50% of these patients require a reduction in the dose of chemotherapy or occasional cessation of treatment. Mucositis is occasionally fatal. However, no effective treatment for mucositis has been developed.
In recent years, researchers have focused on traditional Chinese medicine for its therapeutic effects and low toxicity. Ganoderma lucidum (Leyss, ex Fr) Karst (Gl) has been widely used to promote health and longevity in China for thousands of years. Ganoderma lucidum polysaccharides (Gl-PS) comprise the critical biological activity components of Gl. Gl-PS have been reported to prevent oxidative damage5, protect the liver, and reduce serum glucose levels without causing toxicity6, 7. In addition, Gl-PS modify biological and immune responses8.
Unfortunately, little attention has been paid to the effects of Gl-PS on gastrointestinal mucosal function. In this study, we used a murine model of intestinal damage that was induced using methotrexate (MTX), which is a folate antagonist, to evaluate the protective role of Gl-PS in IMB. The intestinal epithelial cell line IEC-6 was also used as an in vitro wounding model to evaluate the role of Gl-PS on epithelial cell proliferation and cell restitution to elucidate the possible action mechanisms of Gl-PS in the treatment of IMB.
Materials and methods
Materials
Ganoderma lucidum polysaccharides (Gl-PS) were isolated from boiling water extracts of fruit bodies of Ganoderma lucidum (Leyss, ex Fr) Karst followed by ethanol precipitation, dialysis and protein depletion using the Sevag method. Gl-PS is glycopeptide with a molecular weight of 584 900. The ratio of polysaccharides to peptides is 93.61%: 6.49%. The polysaccharides consisted of D-rhamnose, D-xylose, D-fructose, D-galactose, D-mannose and D-glucose with a molar ratio of 0.793:0.964:2.944:0.167:0.389:7.94. The polysaccharides were linked together by β-glycosidic linkages. Gl-PS is a hazel-colored water-soluble powder that was kindly provided by the Fuzhou Institute of Green Valley Bio-Pharm Technology.
Inbred female BALB/c mice (7–8 weeks old) were purchased from the Department of Experimental Animals at the Health Science Centre at Peking University in Beijing, China. The animals were housed in environmentally controlled conditions with 12-h light/12-h dark cycles and were allowed free access to water and standard laboratory chow.
IEC-6 cells were obtained from the Cell Bank of Peking Union Medical College (Beijing, China). IEC-6 cells were routinely maintained in the presence of Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, USA) containing 5% inactivated fetal calf serum (FCS), 10 μg/mL insulin, 2.8 g/L sodium bicarbonate, 100 U/mL penicillin G sodium and 100 μg/mL streptomycin sulfate.
MTX was purchased from Zhejiang Hengrui Pharmaceutical Co, Ltd. Malondialdehyde (MDA) and superoxide dismutase (SOD) analysis kits were purchased from the Nanjing Jiancheng Biology Research Center. The TGFβ ELISA kit was purchased from Wuhan Boster Bio-engineering Co, Ltd. Primary, and secondary antibodies for the measurement of IgA were obtained from Sigma Chemical Co.
Murine MTX-induced enteritis model
The animals were randomly assigned to five groups that contained nine animals each. Mice received ig once daily for 10 consecutive days of (1) low-dose Gl-PS (50 mg/kg), (2) intermediate-dose Gl-PS (100 mg/kg), (3) high-dose Gl-PS (200 mg/kg), (4) vehicle (ie, sterile physiological saline), which was used as a model control, and (5) vehicle (ie, sterile physiological saline) as a normal control. With the exception of group (5), the mice were injected intraperitoneally (ip) with MTX (50 mg/kg) on the 7th d and 8th d. Two days after completing the course of MTX (the 11th d), the mice were sacrificed by cervical dislocation. Subsequently, the intestinal tissue samples were harvested and prepared for histological studies. The other segments of the intestine were removed to determine MDA and SOD levels. Before the mice were sacrificed, blood samples were obtained from each animal to perform biochemical analyses (plasma IgA level). The experiments were approved by the Animal Care and Research Ethics Committee of the Peking University Health Science Center.
Intestinal morphology and histopathology
Tissue samples of the intestine (0.5 cm) were obtained at a distance of 15 cm from the pylorus of each animal following laparotomy. Imaging using light microscopy (LM) and transmission electronic microscopy (TEM) was performed for morphological and histopathological studies. The tissue sections were stained with hematoxylin and eosin for imaging using LM.
Measurement of MDA and SOD levels
Quantitative MDA and SOD measurements were performed with tissues that were obtained from the intestine using the MDA and SOD analysis kits.
Measurement of total immunoglobulin A
A sandwich ELISA technique was used as previously described9.
Determination of IEC-6 cell proliferation
IEC-6 cells (3×104) were seeded into 96-well plates (Costar, USA) in the presence of DMEM containing 5% FCS. The cultures were treated with different concentrations of Gl-PS. After 44 h of incubation at 37 °C and 5% CO2, a colorimetric MTT assay was performed as previously described10. The cultures were incubated with tetrazolium salt thiazolyl blue (20 μL) at a concentration of 5 mg/mL for another 4 h. The cell supernatants were discarded, and the cells were lysed using dimethyl sulfoxide (DMSO). The metabolization of MTT directly correlates with the cell number and was quantitated by measuring the absorbance at 570 nm (reference wavelength 450 nm) using a microplate-reader.
Determination of ODC and c-Myc mRNA expression profiles using RT-PCR analysis
The mRNA expression profiles of ODC and c-Myc in IEC-6 cells were evaluated using reverse transcription polymerase chain reaction (RT-PCR) with the Access RT-PCR system (Takara, Japan) according to the manufacturer's procedures. IEC-6 cells that were treated with or without Gl-PS were harvested after 12 h of incubation. The total RNA was extracted using TRIzol Reagent (Invitrogen, USA). The concentration of RNA was spectrophotometrically quantified. The cDNA was synthesized using the Advantage RT-for-PCR kit protocol (Promega, USA). Diluted aliquots from these reactions were subsequently used as templates for PCR.
Commercial primers to rat glyceraldehyde 3-phosphate-dehydrogenase (GAPDH) (sense, 5′-GCCAAGGTCATCCATGACAAC-3′ and antisense, 5′-GTCCACCACCCTGTTGCTGTA-3′), rat ODC (sense, 5′-TGCTTGACATTGGTGGTG-3′ and antisense, 5′-TTCTCATCTGGCTTGGGT-3′) and rat c-Myc (sense, 5′-GCTCGCCCAAATCCTGTA-3′ and antisense, 5′-ACCCTGCCACTGTCCAAC-3′) were provided by Shanghai Sangon Biological Engineering Technology and Service Co, Ltd (Beijing, China) were used to generate products of 498 bp, 355 bp and 385 bp, respectively. The PCR reactions included 0.4 μmol/L of each primer, 0.2 mmol/L dNTPs, 1×PCR-Buffer with 15 mmol/L MgCl2 and 2 U/μL Taq polymerase (Takara, Japan). The PCR cycling protocol was as follows: 45 s at 94 °C, 45 s at 60 °C (GAPDH), 57 °C (ODC), or 59 °C (c-Myc) and 2 min at 72 °C. This protocol was performed for 24 (GAPDH)/26 (ODC)/26 (c-Myc) cycles and included an initial 5-min denaturation at 94 °C and a final 10-min extension at 72 °C. The performed cycles of PCR was chosen to ensure the exponential amplification of all specific cDNA products. The PCR-amplified samples were electrophoresed using 1.5% agarose gels, stained with ethidium bromide, visualized via UV transillumination and quantified via densitometry scanning using AlphaEaseFC V4.0.0 software (Alpha Innotech Corp). ODC and c-Myc expression profiles were normalized relative to that of GAPDH.
Monolayer wounding and measurement of epithelial cell restitution
IEC-6 cell restitution assays were performed using a modified version of a previously described technique11, 12. IEC-6 cells were plated in six-well polystyrene plates (Costar) with normal growth medium and allowed to reach confluence. A denuded epithelial wound was created in a standardized fashion by scraping the IEC-6 monolayers with a 200-μL pipet tip. After the scraping, the cells were washed twice with D-Hanks' buffer to remove residual cell debris. The wounded monolayers were cultured for 42 h in DMEM containing 2% FCS in the presence or absence of Gl-PS. Wound areas were viewed under the microscope at various time points after wounding and photographed with an Olympus IX71 microscope. The denuded wound area (ROI, region of interest) was quantified using LEICA QWIN software (Germany). Two wound areas per well were analyzed. Each group was tested in triplicate, and at least three independent experiments were performed. Restitution was calculated as the migration ratio using the following equation: (ROI0 h–ROI42 h)/ROI0 h×100%. The data are expressed as the mean value±SD (standard deviation) representing at least three independent experiments.
IEC-6 cell supernatant collection and determination of TGFβ levels
The collection of IEC-6 cell supernatants was performed as previously described13. IEC-6 cells were plated in 6-well culture dishes at a density of 2.5×105 cells/dish. After 24 h, the medium was replaced with medium that had been supplemented with different concentrations of Gl-PS. IEC-6 monolayers were wounded using a 200-μL pipet tip as previously described. The cells were washed twice with D-Hanks' buffer and subsequently incubated with fresh medium that had been supplemented with Gl-PS. After 24 h, the cell supernatants were collected. Secretion of TGFβ from supernatants was determined using a sandwich ELISA kit according to the manufacturer's protocol.
Statistical analysis
The data were analyzed using one-way ANOVA followed by the least-significant difference (LSD) test. P values that were below 0.05 were considered statistically significant.
Results
Morphological and histological results
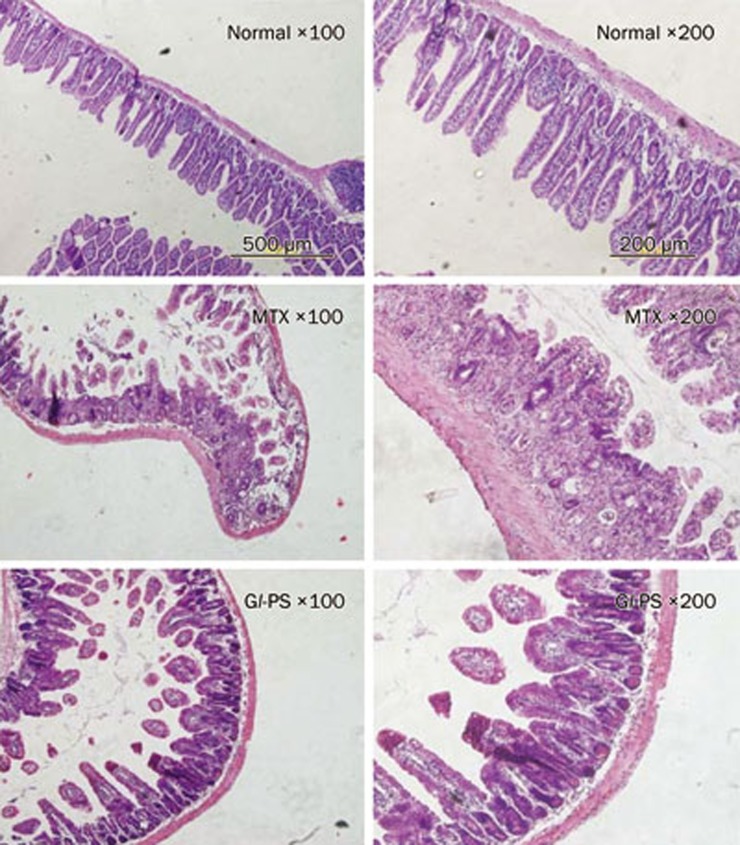
As shown in Figure 1, Gl-PS did not perturb the morphological and histological features of the jejunum in mice. All tissue sections of the intestine of the control group were normal. In the tissue sections of the MTX-treated mice, we observed villus shortening, variable degrees of fusion, epithelial atrophy, a decreased number of crypt cells, crypt loss and the development of abscesses in crypts. In addition, we noted an inflammatory infiltration in the lamina propria. The number of goblet cells was significantly decreased in the villi and the crypts. Although the histopathological features in the MTX+Gl-PS 100 mg/kg-treated group were similar to the findings in the MTX-treated group, the total small intestine damage in the MTX+Gl-PS 100 mg/kg-treated group was less than that of the MTX-treated group.
Figure 1.
Morphology of the murine jejunum under magnification of 100 and 200. Normal: group of normal control; MTX: group of MTX model; Gl-PS: group of 100 mg/kg Gl-PS under MTX stress. These photographs are representative examples of a group of nine mice.
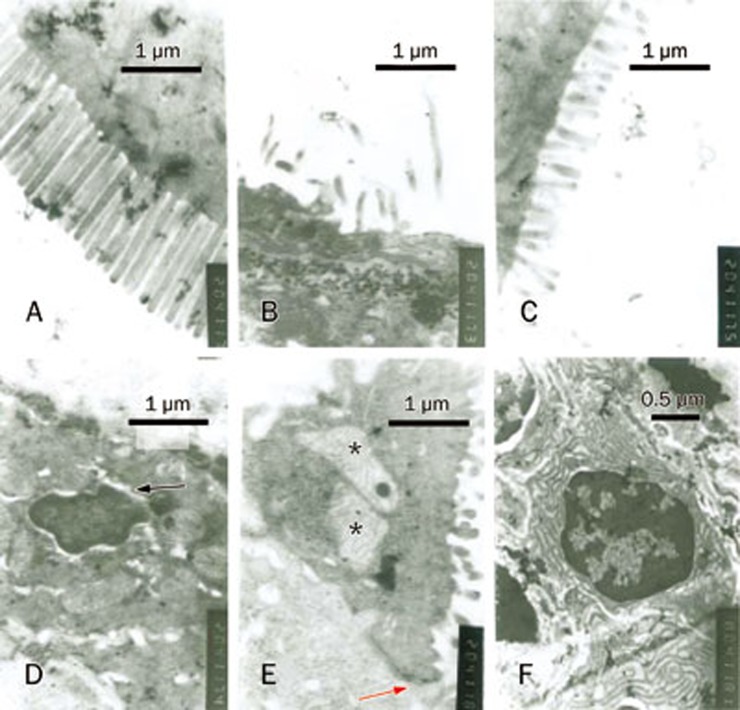
Examination of the intestinal ultrastructure revealed that the microvilli in the normal control group were long, abundant and neatly arranged, whereas the microvilli in the MTX-treated group were reduced, shortened and disordered. Some of the microvilli were ablated. Swelling of the nuclear membrane and mitochondria was observed. The intestinal ultrastructural changes in the MTX+Gl-PS 100 mg/kg-treated group were ameliorated compared to those in the MTX-treated group (Figure 2).
Figure 2.
Changes of intestinal ultrastructure. (A) normal control group. (B, D, E) MTX model group. (C, F) group of 100 mg/kg Gl-PS under MTX stress. The arrow (D) indicates swelling of nuclear membrane. The arrow (D, E) indicates tight junction. * indicates swelling of mitochondria.
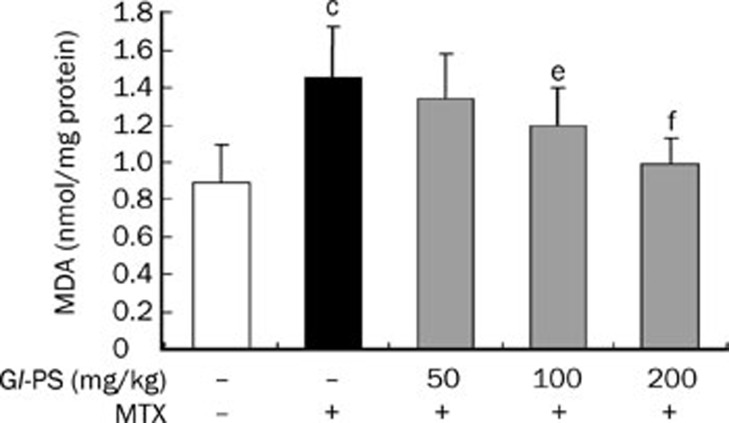
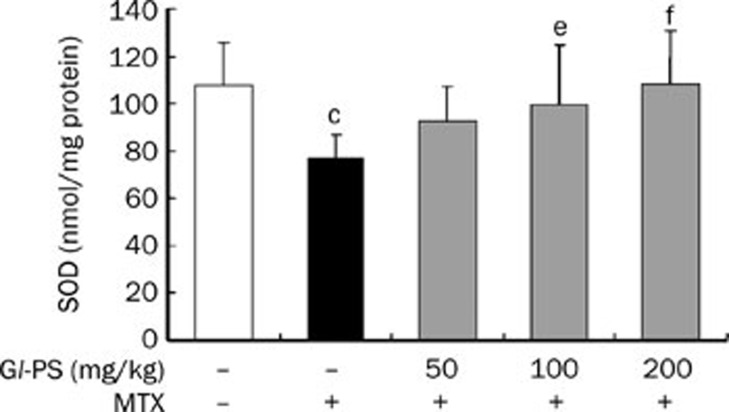
Gl-PS decreased MDA levels but increased SOD levels in mice intestine
We showed that the concentration of MDA in the intestine of mice in the MTX-treated group was significantly higher than that in the normal control group (P<0.001). However, we observed decreases in MDA concentration in MTX+Gl-PS groups in a dose-dependent manner (Figure 3). However, the level of SOD in the MTX model group was down-regulated compared to that in the control group (P<0.01). MTX-induced decreases in SOD responded to Gl-PS treatment in a dose-dependent manner (P<0.05) (Figure 4).
Figure 3.
Effect of Gl-PS on MDA content in supernatant homogenate for intestine in MTX-induced mice. n=9. Mean±SD. cP<0.01 vs normal control. eP<0.05, fP<0.01 vs MTX model.
Figure 4.
Effect of Gl-PS on SOD content in supernatant homogenate of intestine in MTX induced mice. n=9. Mean±SD. cP<0.01 vs normal control. eP<0.05, fP<0.01 vs MTX model.
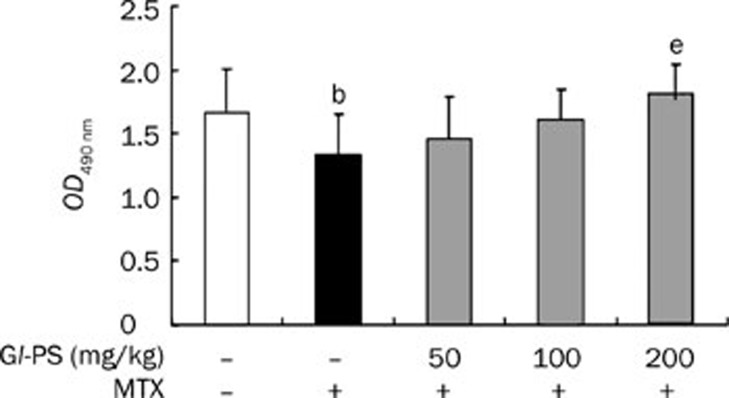
The concentration of IgA in serum
The concentration of total serum IgA was measured using an ELISA. MTX treatment significantly decreased the total serum IgA concentration compared to the normal control (P<0.05). Gl-PS treatment elevated the concentration of IgA in a dose-dependent manner (Figure 5).
Figure 5.
Effect of Gl-PS on serum IgA level in MTX-induced mice. IgA level show by OD490 value. n=7. Mean±SD. bP<0.05 vs normal. eP<0.05 vs MTX model.
Effect of Gl-PS on the proliferation of IEC-6 cells
The cells were incubated with different concentrations of Gl-PS (0.1, 1, and 10 μg/mL) for 48 h. As shown in Table 1, Gl-PS significantly promoted the proliferation of IEC-6 cells in a dose-dependent manner compared to the control. The proliferation rate was 34% higher in cells that were treated with 10 μg/mL Gl-PS compared to that in control cells.
Table 1. Effects of Gl-PS on IEC-6 cells proliferation. n=8. Mean±SD. bP<0.05, cP<0.01 vs DMEM.
| Group | OD value (570 nm) |
|---|---|
| DMEM | 0.626±0.072 |
| Gl-PS 0.1 μg/mL | 0.725±0.080b |
| Gl-PS 1 μg/mL | 0.751±0.075c |
| Gl-PS 10 μg/mL | 0.840±0.077c |
Gl-PS modulated mRNA expression profiles of ODC and c-Myc in IEC-6 cells
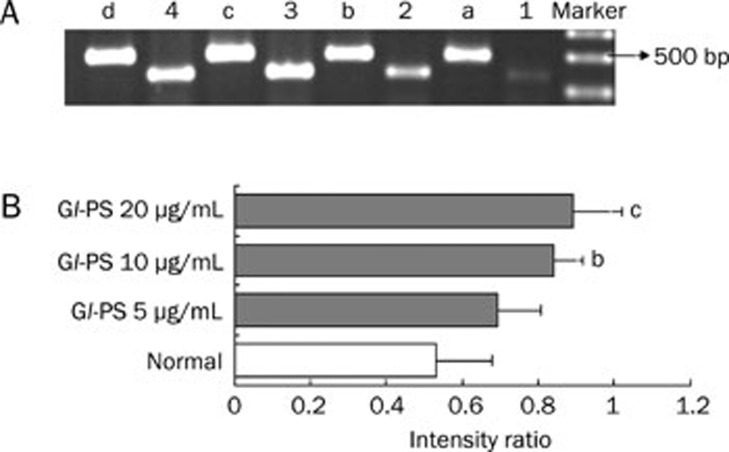
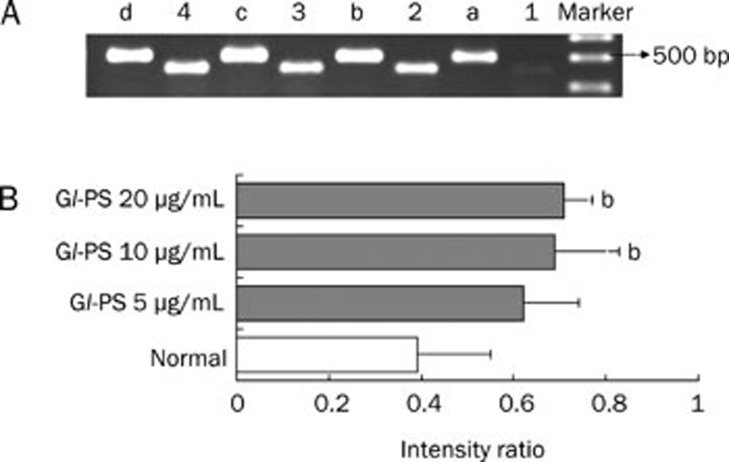
Semiquantitative RT-PCR was used to determine the effect of Gl-PS on ODC and c-Myc mRNA expression profiles in IEC-6 cells. As shown in Figures 6 and 7, ODC and c-Myc mRNAs were expressed in IEC-6 cells. Gl-PS (10 and 20 μg/mL) treatment significantly up-regulated the expression of ODC and c-Myc mRNAs compared with that in the normal control group (Figures 6 and 7).
Figure 6.
The mRNA expression of ODC and GAPDH in IEC-6 cells by RT-PCR. (A) 1–4: IEC-6 cells were treated with 0, 5, 10, and 20 μg/mL Gl-PS and it is mRNA expression of ODC. a–d: IEC-6 cells were treated with 0, 5, 10, and 20 μg/mL Gl-PS and it is mRNA expression of GAPDH. (B) PCR products were quantified by densitometric scanning and ODC expression was normalized relative to the steady-state expression of GAPDH used as internal control (intensity ratio: ODC to GAPDH). Values represent means±SD from three independent experiments. bP<0.05, cP<0.01 vs normal.
Figure 7.
The mRNA expression of c-Myc and GAPDH in IEC-6 cells by RT-PCR. (A) 1–4: IEC-6 cells were treated with 0, 5, 10, and 20 μg/mL Gl-PS and it is mRNA expression of c-Myc. a–d: IEC-6 cells were treated with 0, 5, 10, and 20 μg/mL Gl-PS and it is mRNA expression of GAPDH. (B) Quantification of RT-PCR data. n=3. bP<0.05 vs normal control.
Gl-PS enhanced IEC-6 cell restitution
As shown in Table 2, Gl-PS caused a dose-dependent enhancement of intestinal epithelial cell restitution in a well-established wounding model with IEC-6 cell monolayers. We detected a 36% increase in the restitution of IEC-6 cells that were treated with 10 μg/mL Gl-PS compared to that of control cells.
Table 2. The effect of Gl-PS on IEC-6 cells wound restitution. n=8. Mean±SD. bP<0.05, cP<0.01 vs DMEM.
| Group | OD value (570 nm) |
|---|---|
| DMEM | 0.486±0.054 |
| Gl-PS 0.1 μg/mL | 0.544±0.062 |
| Gl-PS 1 μg/mL | 0.587±0.046b |
| Gl-PS 10 μg/mL | 0.661±0.089c |
Effects of Gl-PS on TGFβ levels in IEC-6 cell culture supernatants
Recent evidence has supported a central role for TGFβ in the process of intestinal epithelial restitution14. Therefore, the production of TGFβ was measured to determine the possible involvement of this cytokine in Gl-PS-mediated restitution. No significant effect was observed on the production of TGFβ following Gl-PS treatment (Table 3).
Table 3. Effects of Gl-PS on TGFβ level in IEC-6 cells culture supernatants. Values represent means (±SD) from three independent experiments performed in duplicate.
| Group | TGFβ (pg/mL) |
|---|---|
| DMEM | 9.602±0.001 |
| Gl-PS 5 μg/mL | 9.603±0.001 |
| Gl-PS 10 μg/mL | 9.605±0.001 |
| Gl-PS 20 μg/mL | 9.607±0.002 |
Discussion
The intestinal epithelial barrier, including the biotic, mechanical and immunity barriers, is a crucial barrier against pathogen infection. Previous studies have not demonstrated an effective method to prevent IMB damage15. It is necessary to develop natural medicines that are effective in treating IMB dysfunction and intercurrent diseases. In the current report, the effects of Gl-PS on the intestinal epithelial barrier were investigated.
Chemotherapy commonly produces structural damage to the intestinal mucosa in cancer patients16 and causes side effects such as severe enterocolitis. In agreement with previous studies17, the results of the present study reveal that MTX induced small intestinal injury, which is characterized by villus shortening and fusion, epithelial atrophy, crypt loss, inflammatory infiltration of the lamina propria, and goblet cell depletion. Gl-PS treatment reduced intestinal mucosal damage in this study and reduced gastric mucosal lesions in a previous study18.
Increased oxidative stress and decreased antioxidant defenses have been demonstrated in intestinal mucosal biopsies of patients with MTX-induced damage to the small intestinal epithelium. These damages may be reduced by antioxidant agents. As previously reported, protective effects of various antioxidants, such as curcumin19, aged garlic extract20 and N-acetylcysteine17, have been shown in MTX-induced small intestinal damage. MDA is frequently used in the measurement of lipid peroxide levels and correlates with the degree of lipid peroxidation. SOD levels correlate with the elimination of free radicals. Gl-PS have been widely used as antioxidants in vivo and in vitro21, 22, 23. Therefore, in the present study, we investigated whether Gl-PS inhibit MTX-induced small intestine damage via decreasing MDA levels and increasing SOD levels. We showed that the MDA level in the small intestinal mucosa of MTX-treated mice was remarkably increased, suggesting that MTX treatment caused oxidative damage and lipid peroxidation in the intestinal mucosa. MTX-induced increases in MDA levels were attenuated after Gl-PS administration (Figure 3). These findings may indicate that Gl-PS protect intestinal tissue against MTX-induced lipid peroxidation. The present study also demonstrated that the MTX-induced downregulation of SOD was inhibited by Gl-PS treatment in the intestinal mucosa of mice (Figure 4). These results suggest that the effects of Gl-PS may be achieved via its antioxidant and free radical-eliminating activities.
Immunoglobulin A (IgA) is an important component of the intestinal immunological barrier and is the most abundant immunoglobulin at the mucosal surface where it plays crucial role in mucosal protection24. The protective barrier of the gastrointestinal system is impaired in IgA deficiency, and IgA-deficient individuals tend to develop gastrointestinal infections25, 26. Gl-PS are well-known modulators of the immune system27, 28. In this study, we found that MTX-treated mice displayed a reduced level of serum IgA. This result indicates that mucosal immune barrier dysfunction occurs during MTX-induced intestine damage. Gl-PS restored the level of IgA, suggesting that Gl-PS bolstered intestinal immunity.
Observations over the past several years have demonstrated the ability of the gastrointestinal tract to rapidly restore the continuity of the surface epithelium after extensive destruction. Three different phases have been identified. First, epithelial cells that are adjacent or just beneath the injured surface migrate into the wound to cover the denuded area, which is a process that has been termed epithelial restitution. Secondly, epithelial cell proliferation takes place to replenish the decreased cell pool. Finally, maturation and differentiation of epithelial cells enable the epithelium to maintain its functional activities12. The initial mechanism contributing to rapid resealing of epithelial defects after mucosal injury is the migration of viable epithelial cells from the wound margin into the denuded area, which is a process that does not require cell proliferation29. The data presented in this study revealed that Gl-PS at a concentration of 10 μg/mL augmented the migration of intestinal epithelial cells in an in vitro model that mimicked the early cell division-independent stages of epithelial restitution. When Gl-PS were added immediately after wounding, they significantly increased intestinal epithelial cell migration in a dose-dependent manner. Although the exact mechanism of restitution has not been elucidated, the cytokine TGFβ has been shown to play an important role in the stimulation of cell migration after wounding. Some studies have revealed that the intestinal epithelial cell restitution process is stimulated via a TGFβ-dependent pathway30, 31. However, in other cases, the intestinal epithelial cell restitution was independent of TGFβ32. In our study, we found that Gl-PS did not affect TGFβ expression in IEC-6 cells after wounding. These results may indicate that Gl-PS stimulate restitution possibly through a TGFβ-independent pathway.
Epithelial cell proliferation, which is another essential mechanism to mediate resealing of mucosal wounds in the intestine, was substantially promoted by Gl-PS treatment in our investigation. This effect was dose-dependent, and maximal effects were detected at a concentration of 10 μg/mL Gl-PS.
The polyamines are a group of ubiquitously distributed organic cations that are intimately involved in the regulation of gastrointestinal mucosal growth33. ODC, which is a pyridoxal phosphate-dependent enzyme, is the first rate-limiting enzyme for the biosynthesis of polyamines. The extent of the ODC mRNA expression correlates with the cell proliferation. Treatment of gastrointestinal origin cells with difluoromethylornithine, which is a suicide substrate inhibitor of ODC and induces depletion of intracellular polyamines, inhibits proliferation34, 35. In addition, induction of the ODC gene may play an important role in the signaling pathways that are associated with several oncogenes. Transformation by activated ras, v-src and myc appears to be tightly coupled to ODC gene expression and polyamine accumulation36, 37, 38. ODC is a transcriptional target of c-Myc39, which has a central role in the proliferation of normal cells. Following mitogenic stimulation of quiescent cells, c-Myc is rapidly induced and remains elevated, suggesting that it is required for continuous cell growth40. In the present study, an increase in c-Myc and ODC gene expression (Figure 6 and 7) was observed in IEC-6 cells 12 h after exposure to Gl-PS. c-Myc and ODC gene expression were coincident to the stimulatory effect on cell proliferation (Table 1).
In summary, the present study demonstrated that Gl-PS reduced MTX-induced intestinal toxicity. We demonstrated that Gl-PS increased the antioxidation and intestinal immunity in vivo, accelerated wound repair by stimulating cell proliferation and migration in vitro, and up-regulated c-Myc and ODC mRNA expression. However, Gl-PS had no effects on TGFβ levels. Altogether, we demonstrated a new protective effect of Gl-PS on the intestinal barrier via induction of epithelial cell proliferation and migration. The present study may provide a pharmacological basis for the clinical use of Gl-PS in preventing enteritis in patients receiving chemotherapeutic agents. Previous studies have shown that cell differentiation also plays an important role in protecting the intestinal barrier. However, the effect of Gl-PS on cell differentiation was not assessed in this study. Therefore, further investigation is required using molecular markers such as sucrase-isomaltase and alkaline phosphatase.
Author contribution
Li-hua CHEN, Wei-dong LI, and Z hi-bin LIN designed the research; Li-hua CHEN performed the research; Wei-dong LI and Zhi-bin LIN contributed new analytical reagents and tools; Li-hua CHEN and Wei-dong LI analyzed the data; and Li-hua CHEN wrote the paper.
Acknowledgments
The authors are grateful to Dr Mei-hua BAO for her critical reading of the manuscript.
References
- Gupta G, Agarwala S, Thulkar S, Shukla B, Bakhshi S. Jejunal stricture: a rare complication of chemotherapy in pediatric gastrointestinal B-cell non-Hodgkin lymphoma. J Pediatr Hematol Oncol. 2011;33:e69–71. doi: 10.1097/MPH.0b013e3181ef0402. [DOI] [PubMed] [Google Scholar]
- Nicoletto MO, Dalla Palma M, Donach ME, Gusella M, Cappetta A, Shams M, et al. Positive experience of intraperitoneal chemotherapy followed by intravenous chemotherapy in heavily pretreated patients with suboptimal residual ovarian cancer and primary peritoneal cancer. Tumori. 2010;96:918–25. [PubMed] [Google Scholar]
- Prisciandaro LD, Geier MS, Butler RN, Cummins AG, Howarth GS. Evidence supporting the use of probiotics for the prevention and treatment of chemotherapy-induced intestinal mucositis. Crit Rev Food Sci Nutr. 2011;51:239–47. doi: 10.1080/10408390903551747. [DOI] [PubMed] [Google Scholar]
- Van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010;6:e1000878. doi: 10.1371/journal.ppat.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You YH, Lin ZB. Protective effects of Ganoderma lucidum polysaccharides peptide on injury of macrophages induced by reactive oxygen species. Acta Pharmaco Sin. 2002;23:787–91. [PubMed] [Google Scholar]
- Zhang GL, Wang YH, Ni W, Teng HL, Lin ZB. Hepatoprotective role of Ganoderma lucidum polysaccharide against BCG-induced immune liver injury in mice. World J Gastroenterol. 2002;8:728–33. doi: 10.3748/wjg.v8.i4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HN, He JH, Yuan L, Lin ZB. In vitro and in vivo protective effect of Ganoderma lucidum polysaccharides on alloxan-induced pancreatic islets damage. Life Sci. 2003;73:2307–19. doi: 10.1016/s0024-3205(03)00594-0. [DOI] [PubMed] [Google Scholar]
- Zhu XL, Lin ZB. Modulation of cytokines production, granzyme B and perforin in murine CIK cells by Ganoderma lucidum polysaccharides. Carbohydrate Polymers. 2006;63:188–97. [Google Scholar]
- Ha CL. The inhibitory effect of the Chinese herb Ganoderma lucidum mycelium on gut immunoglobulin A responses to cholera toxin in mice. Nutr Res. 2003;23:691–701. [Google Scholar]
- Mossman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Zou L, Sato N, Kone BC. α- Melanocyte stimulating hormone protects against H2O2-induced inhibition of wound restitution in IEC-6 cells via a Syk kinase and NF-κB dependent mechanism. Shock. 2004;22:453–9. doi: 10.1097/01.shk.0000142255.15759.de. [DOI] [PubMed] [Google Scholar]
- Jung S, Fehr S, Harder-d'Heureuse J, Wiedenmann B, Dignass AU. Corticosteroids impair intestinal epithelial wound repair mechanisms in vitro. Scand J Gastroenterol. 2001;36:963–70. doi: 10.1080/003655201750305495. [DOI] [PubMed] [Google Scholar]
- Ruthig DJ, Meckling-Gill KA. N-3 and n-6 fatty acids stimulate restitution by independent mechanisms in the IEC-6 model of intestinal wound healing. J Nutr Biochem. 2002;13:27–35. doi: 10.1016/s0955-2863(01)00192-9. [DOI] [PubMed] [Google Scholar]
- Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factorβ. Gastroenterology. 1993;105:1323–32. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- Daniel C, Baumgart, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685–94. doi: 10.1097/00075197-200211000-00012. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Morgan RJ, Mills PR, Nelson LM, Toner PG, Soukop M, et al. Functional and structural changes of the human proximal small intestine after cytotoxic therapy. J Clin Pathol. 1985;38:265–70. doi: 10.1136/jcp.38.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciralik H, Bulbuloglu E, Cetinkaya A, Kurutas EB, Celik M, Polat A. Effects of N-acetylcysteine on methotrexate-induced small intestinal damage in rats. Mt Sinai J Med. 2006;73:1086–92. [PubMed] [Google Scholar]
- Gao Y, Zhou S, Wen J, Huang M, Xu A. Mechanism of the antiulcerogenic effect of Ganoderma lucidum polysaccharides on indomethacin-induced lesions in the rat. Life Sci. 2002;72:731–45. doi: 10.1016/s0024-3205(02)02301-9. [DOI] [PubMed] [Google Scholar]
- Song WB, Wang YY, Meng FS, Zhang QH, Zeng JY, Xiao LP, et al. Curcumin protects intestinal mucosal barrier function of rat enteritis via activation of MKP-1 and attenuation of p38 and NF-κB activation. PLoS One. 2010;5:1–11. doi: 10.1371/journal.pone.0012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yüncü M, Eralp A, Celik A. Effect of aged garlic extract against methotrexate-induced damage to the small intestine in rats. Phytother Res. 2006;20:504–10. doi: 10.1002/ptr.1896. [DOI] [PubMed] [Google Scholar]
- Sudheesh NP, Ajith TA, Ramnath V, Janardhanan KK. Therapeutic potential of Ganoderma lucidum (Fr) P Karst against the declined antioxidant status in the mitochondria of post-mitotic tissues of aged mice. Clin Nutr. 2010;29:406–12. doi: 10.1016/j.clnu.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Liu W, Wang H, Pang X, Yao W, Gao X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int J Biol Macromol. 2010;46:451–7. doi: 10.1016/j.ijbiomac.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Cherian E, Sudheesh NP, Janardhanan KK, Patani G. Free-radical scavenging and mitochondrial antioxidant activities of Reishi-Ganoderma lucidum (Curt: Fr) P Karst and Arogyapacha-Trichopus zeylanicus Gaertn extracts. J Basic Clin Physiol Pharmacol. 2009;20:289–307. doi: 10.1515/jbcpp.2009.20.4.289. [DOI] [PubMed] [Google Scholar]
- Kadaoui KA, Corthésy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment. J Immunol. 2007;179:7751–7. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30:10–6. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeq P. Update on mucosal immunoglobulin A in gastrointestinal disease. Curr Opin Gastroenterol. 2010;26:554–63. doi: 10.1097/MOG.0b013e32833dccf8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Tang Q, Zhou C, Jia W, Da Silva L, Nguyen LD, et al. GLIS, a bioactive proteoglycan fraction from Ganoderma lucidum, displays anti-tumour activity by increasing both humoral and cellular immune response. Life Sci. 2010;87:628–37. doi: 10.1016/j.lfs.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Huang SQ, Li JW, Wang Z, Pan HX, Chen JX, Ning ZX. Optimization of alkaline extraction of polysaccharides from Ganoderma lucidum and their effect on immune function in mice. Molecules. 2010;15:3694–708. doi: 10.3390/molecules15053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten MJ, Ito S. Morphology and electrophysiology of guinea pig gastric mucosal repair in vitro. Am J Physiol. 1983;244:G171–82. doi: 10.1152/ajpgi.1983.244.2.G171. [DOI] [PubMed] [Google Scholar]
- Strauch ED, Wang JY, Bass BL. Bile salt stimulates intestinal epithelial cell migration through TGFbeta after wounding. J Surg Res. 2001;97:49–53. doi: 10.1006/jsre.2001.6110. [DOI] [PubMed] [Google Scholar]
- Bulut K, Meier JJ, Ansorge N, Felderbauer P, Schmitz F, Hoffmann P, et al. Glucagon-like peptide 2 improves intestinal wound healing through induction of epithelial cell migration in vitro-evidence for a TGF-β-mediated effect. Regul Pept. 2004;121:137–43. doi: 10.1016/j.regpep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Paclik D, Lohse K, Wiedenmann B, Dignass AU, Sturm A. Galectin-2 and -4, but not galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism. Inflamm Bowel Dis. 2008;14:1366–72. doi: 10.1002/ibd.20499. [DOI] [PubMed] [Google Scholar]
- Seidel ER, Scemama JL. Gastrointestinal polyamines and regulation of mucosal growth. Nutrit Biochem. 1997;8:104–11. [Google Scholar]
- Ye YN, Liu ES, Shin VY, Koo MW, Li Y, Wei EQ, et al. A mechanistic study of proliferation induced by Angelica sinensis in a normal gastric epithelial cell line. Biochem Pharmacol. 2001;61:1439–48. doi: 10.1016/s0006-2952(01)00625-6. [DOI] [PubMed] [Google Scholar]
- Lefevre PL, Palin MF, Chen G, Turecki G, Murphy BD. Polyamines are implicated in the emergence of the embryo from obligate diapause. Endocrinology. 2011;152:1627–39. doi: 10.1210/en.2010-0955. [DOI] [PubMed] [Google Scholar]
- Hölttä E, Sistonen L, Alitalo K. The mechanism of ornithine decarboxylase deregulation in c-Ha-ras oncogene-transformed NIH 3T3 cells. J Biol Chem. 1988;263:4500–7. [PubMed] [Google Scholar]
- Guerrero I, Pellicer A, Alitalo K. Dissociation of c-fos from ODC expression and neuronal differentiation in a PC12 subline stably transfected with an inducible n-ras oncogene. Biochem Biophys Res Commun. 1988;150:1185–92. doi: 10.1016/0006-291x(88)90754-1. [DOI] [PubMed] [Google Scholar]
- Celano P, Baylin SB, Giardello FM, Nelkin BD, Casero RA. Effect of polyamine depletion on c-myc expression in human colon carcinoma cells. J Biol Chem. 1988;263:5491–4. [PubMed] [Google Scholar]
- Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target for c-Myc. Proc Natl Acad Sci U S A. 1993;90:7804–8. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G, Littlewood TD. The role of c-myc in cell growth. Curr Opin Genet Dev. 1993;3:44–9. doi: 10.1016/s0959-437x(05)80339-9. [DOI] [PubMed] [Google Scholar]