Abstract
Aim:
To investigate the effects of wogonin (5,7-dihydroxy-8-methoxyflavone) extracted from Scutellaria baicalensis Georgi (S baicalensis) on lipotoxicity-induced apoptosis of vascular smooth muscle cells (VSMCs) and the underlying mechanisms.
Methods:
Cultured VSMCs were used. Apoptosis of VSMCs was induced by palmitate (0.75 mmol/L), and detected using TUNEL assay. The expression levels of protein and phosphorylated protein were measured using Western blot analysis.
Results:
Treatment of VSMCs with wogonin (10, 25 and 50 μmol/L) significantly attenuated the apoptosis and endoplasmic reticulum (ER) stress induced by palmitate in concentration- and time-dependent manners. Wogonin (50 μmol/L) decreased palmitate-induced reactive oxygen species (ROS) generation. The ER stress inhibitor 4-phenyl butyric acid (5 mmol/L) significantly decreased palmitate-induced apoptotic cells, and occluded the anti-apoptotic effect of wogonin (25 μmol/L). Wogonin (10, 25 and 50 μmol/L) significantly reduced the intracellular diacylglycerol (DAG) accumulation and expression levels of phosphorylated PKCs in palmitate-treated VSMCs.
Conclusion:
Our results suggest that wogonin inhibits lipotoxicity-induced apoptosis of VSMCs via suppressing the intracellular DAG accumulation and subsequent inhibition of PKC phosphorylation. Wogonin has therapeutic potential for the prevention and treatment of atherosclerosis.
Keywords: wogonin, atherosclerosis, vascular smooth muscle cells, apoptosis, endoplasmic reticulum stress, palmitate, diacylglycerol (DAG), PKC
Introduction
Diabetes mellitus is a major contributor to cerebrovascular and cardiovascular disease morbidity and mortality worldwide. Increased free fatty acid (FFA) levels in the plasma caused lipotoxicity, which is a hallmark of diabetes mellitus and leads to an increased risk of atherosclerosis and cardiovascular diseases1, 2, 3. Strong evidence has suggested that elevated FFA levels in the plasma enhance the intracellular accumulation of diacylglycerol (DAG), which leads to the activation of protein kinase Cs (PKCs), inhibitor kappaB kinase (IKK), or c-Jun N-terminal kinase (JNK), resulting in atherosclerotic plaque development and instability4, 5. Therefore, the plasma FFA and intracellular DAG levels represent selective targets to prevent atherosclerosis.
Vascular smooth muscle cells (VSMCs) play a pivotal role in the initiation and early progression of atherosclerosis and plaque rupture6, 7. Due to the important function of synthesizing components of the fibrous cap in plaques, VSMCs are responsible for promoting plaque stability in advanced atherosclerotic lesions. Published evidence has shown that VSMC apoptosis precipitates a number of deleterious consequences in atherosclerosis, such as plaque rupture7, 8, 9, 10, 11, 12. Therefore, anti-apoptotic therapies for VSMCs may benefit the prevention and treatment of atherosclerosis8, 10.
Scutellaria baicalensis Georgi (S baicalensis) is a traditional Chinese herb (Baikal Skullcap) that is widely used for the treatment of inflammation, infection, cancer, hypertension, and cardiovascular disease13, 14. Wogonin (5,7-dihydroxy-8-methoxyflavone) is a major bioactive component of the flavonoids from S baicalensis. Pharmacological findings have highlighted the therapeutic potential regarding the use of plant-derived wogonin to modulate endothelial cell and VSMC function for the prevention and treatment of atherosclerosis15, 16, 17. However, the underlying mechanism is poorly understood.
In this study, our results show that wogonin attenuates lipotoxicity-induced apoptosis and inhibits endoplasmic reticulum (ER) stress by suppressing intracellular DAG accumulation, which perturbs the DAG/PKC pathway in cultured VSMCs. This novel finding supplies evidence for the potential administration of wogonin for the treatment of atherosclerosis.
Materials and methods
Materials and reagents
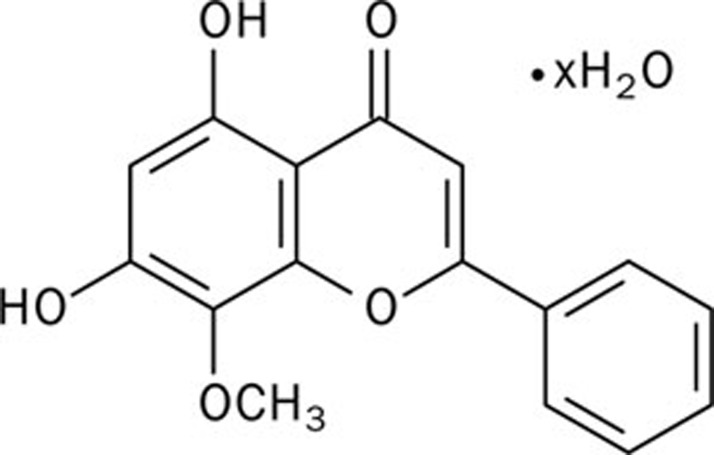
Biochemical reagents were obtained from the following sources: palmitic acid, tunicamycin, wogonin (C16H12O5) (Figure 1), 4-phenyl butyric acid (4-PBA) from Sigma (St Louis, MO, USA); the anti-CHOP antibody, anti-cleaved caspase-3 antibody, anti-cleaved caspase-6 antibody, anti-eIF2α antibody, anti-phosphorylated-eIF2α Ser51 antibody, anti-Bcl-2 antibody, anti-Bax antibody, PKC isoform antibody sampler kit, and phosphorylated-PKC isoform antibody sampler kit from Cell Signaling Technology; and secondary antibodies that were conjugated to alkaline phosphatase from Promega. The APO-BrdUTM TUNEL Assay Kit was purchased from Invitrogen. The ELISA Kit for DAG was obtained from Uscn Life Science, Inc (Wuhan, China).
Figure 1.
Chemical structure of wogonin.
Cell culture and treatment
Vascular smooth muscle cells (SV40LT-SMC Clone HEP-SA, ATCC CRL-2018™) were cultured in growth medium (Dulbecco's modified Eagle's medium with 4 mmol/L L-glutamine, which was adjusted to contain 1.5 g/L sodium bicarbonate and 4.5 g/L glucose, 0.2 mg/mL G418, and 10% bovine calf serum). A10 VSMCs (ATCC CRL-1476™) were incubated in DMEM (ATCC 30-2002) containing 10% FBS and 1.0 g/L sodium bicarbonate. The culture conditions were 37.0 °C, 95% O2, and 5% CO2. For treatment, the cells were serum-starved for 6 h and then incubated with palmitate and/or wogonin for the desired exposure time. The preparation of palmitate was as described in our previous study18. Briefly, palmitic acid was dissolved in ethanol, mixed with 20% BSA and then incubated overnight at 4 °C. The solution was filtered, stored at −20 °C and used within 2 weeks. The same concentration of ethanol was mixed with 20% BSA and used as a control.
Western blot analysis
The cells were lysed with lysis buffer (50 mmol/L Hepes, pH 7.6, 150 mmol/L NaCl, 1% Triton X-100, 10 mmol/L NaF, 20 mmol/L sodium pyrophosphate, 20 mmol/L β-glycerol phosphate, 1 mmol/L sodium orthovanadate, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 mmol/L phenylmethanesulfonyl fluoride). The cell lysates were incubated on ice for 10 min and then centrifuged at 14 000×g for 10 min at 4 °C. The supernatants were mixed with equal volumes of 2×SDS-PAGE sample loading buffer. After heating at 95 °C for 4 min, the proteins were separated using a SDS-PAGE gel, transferred to a nitrocellulose membrane, and detected with the aforementioned antibodies.
Apoptosis determination
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL) was performed to detect cells undergoing apoptosis according to the manufacturer's protocol. Briefly, VSMCs were treated with palmitate and/or wogonin, washed with cold PBS three times and then fixed with 1% of paraformaldehyde on ice for 1 h. After washing three times with PBS, the cells were treated with 70% ethanol and incubated at −20 °C for 24 h. The cells were washed with wash buffer 3 times and incubated in DNA-labeling solution (including terminal deoxynucleotidyl transferase enzyme and BrdU triphosphate) at 37 °C for 1 h. The cells were rinsed with PBS, collected by centrifugation, and incubated in anti-BrdU-staining mix for 45 min at room temperature. The apoptotic nuclei containing nicked DNA were stained brown. To calculate the apoptosis rate, 1000 nuclei were identified in 20 random high power fields per slide.
Extraction and measurement of DAG
Total DAG levels were measured using an ELISA Assay Kit (Uscn Life Science Inc, Wuhan, China) according to the manufacturer's protocol. Briefly, the serum-starved VSMCs (1×106 cells/well in 6-well dishes) were incubated with or without different doses of palmitate and/or wogonin for the desired time. Total cell lipids were extracted with chloroform:methanol (1:2, v/v) after centrifugation at 5000×g for 2 min19. The lower chloroform phase was analyzed for DAG contents.
Measurement of reactive oxygen species
Intracellular reactive oxygen species (ROS) production was measured using the method of Shaw et al20. Briefly, VSMCs were plated in a 24-well plate at a density of 2×104 cells/well in DMEM, serum-starved for 6 h, and treated with or without different doses of palmitate and/or wogonin for the desired time. The cells were washed with modified Eagle's medium without phenol red and incubated in the dark for 10 min in Krebs-Ringer solution containing 50 μmol/L DCHF diacetate. Changes in fluorescence intensity were determined using an Flx-800 microplate fluorescence reader (Bio-Tek Instruments) at excitation and emission wavelengths at 485 and 528 nm, respectively19, 20.
Statistical analysis
Three independent experiments were performed with each sample in triplicate. The data were expressed as the mean±SEM. Statistical analysis was performed using analysis of variance followed by Student's t-test for paired data. P<0.05 was considered significant. The figures are representative of at least three independent experiments with similar results.
Results
Effects of wogonin on palmitate-induced apoptosis
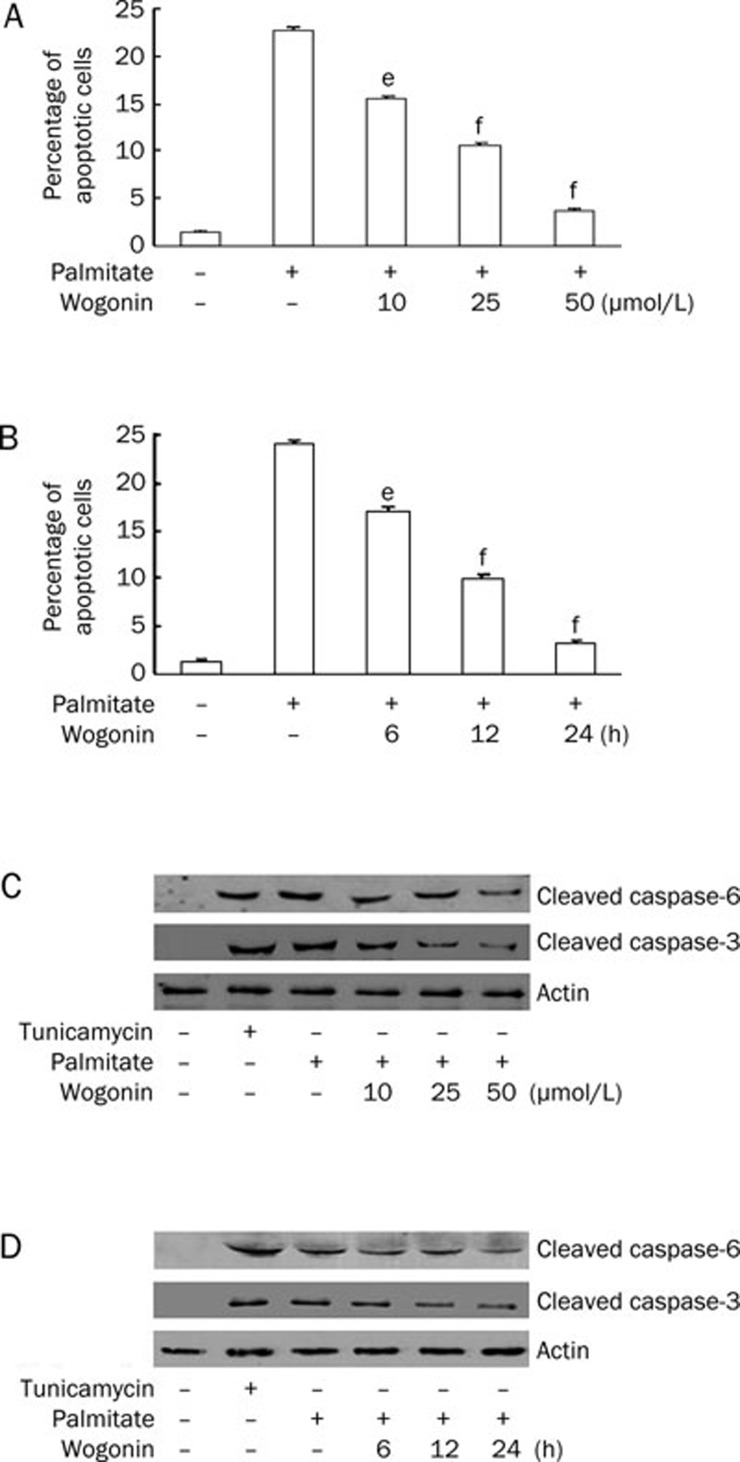
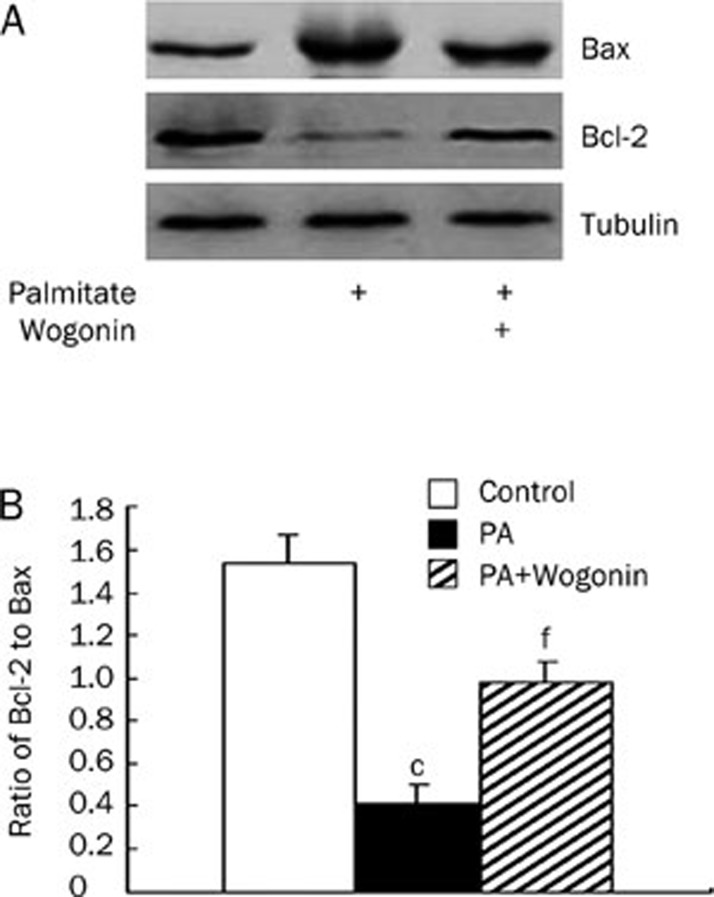
Wogonin modulates endothelial cell and VSMC functions to promote its anti-atherosclerotic effects15, 16, 17. Considering the pivotal roles of VSMC apoptosis7, 8, 9, 10, 11, 12 and the elevated plasma FFA levels4, 5 in atherosclerotic plaque ruptures, we investigated the potential effects of wogonin on palmitate-induced VSMC apoptosis. The serum-starved VSMCs were treated with different doses of wogonin for the desired time in the presence of 0.75 mmol/L palmitate for 24 h. As shown in Figure 2A and 2B, wogonin significantly attenuated the palmitate-induced apoptosis in a dose- and time-dependent manner. We evaluated the expression levels of cleaved caspase-3 and caspase-6 because activation of the caspase-3 pathway is a hallmark of apoptosis21, 22, 23. We found that wogonin inhibited the expression of cleaved caspase-3 and caspase-6 in a dose- and time-dependent manner in VSMCs that were pre-treated with 0.75 mmol/L palmitate (Figure 2C and 2D). The anti-apoptotic Bcl-2 and pro-apoptotic Bax proteins are among many key regulators of apoptosis. Therefore, we investigated the effects of wogonin on these regulatory proteins. We treated A10 VSMCs with 0.75 mmol/L palmitate for 12 h followed by treatment with 25 μmol/L wogonin for an additional 12 h. We found that wogonin restored Bcl-2 expression and decreased Bax expression, which consequently restored the ratio of Bcl-2 to Bax (Figure 3). These data suggest that wogonin protects VSMCs from palmitate-induced apoptosis.
Figure 2.
Wogonin prevented palmitate-induced apoptosis. VSMCs were cultured in serum-free medium for 6 h and treated with or without 0.75 mmol/L of palmitate for 12 h, followed by a second treatment with or without 10, 25, and 50 μmol/L of wogonin for an additional 12 h (A and C) or 25 μmol/L of wogonin for 6, 12, and 24 h (B and D). (A and B) The effects of wogonin on the percentage of apoptotic cells. Apoptotic cells were visualized using TUNEL methods. eP<0.05; fP<0.01 compared with the palmitate-treated group. (C and D) The effects of wogonin on cleaved caspase-3 and caspase-6 expression. In total, 5 μg/mL of tunicamycin (18 h) was used as a positive control. The expression levels of cleaved caspase-3 and caspase-6 were detected using Western blotting. The figures are representative of at least three independent experiments with similar results.
Figure 3.
Wogonin restored the ratio of Bcl-2 to Bax. A10 VSMCs were cultured in serum-free medium for 6 h and treated with 0.75 mmol/L of palmitate (PA) for 12 h, followed by a second treatment with or without 25 μmol/L of wogonin for an additional 12 h. (A) Bcl-2 and Bax protein expression. (B) Bar graph for the ratio of Bcl-2 to Bax. Western blot was performed to detect the expression levels of Bcl-2 and Bax. The figures are representative of at least three independent experiments with similar results. cP<0.01 compared with the control group. fP<0.01 compared with the palmitate-treated group.
Effects of wogonin on palmitate-induced ER stress
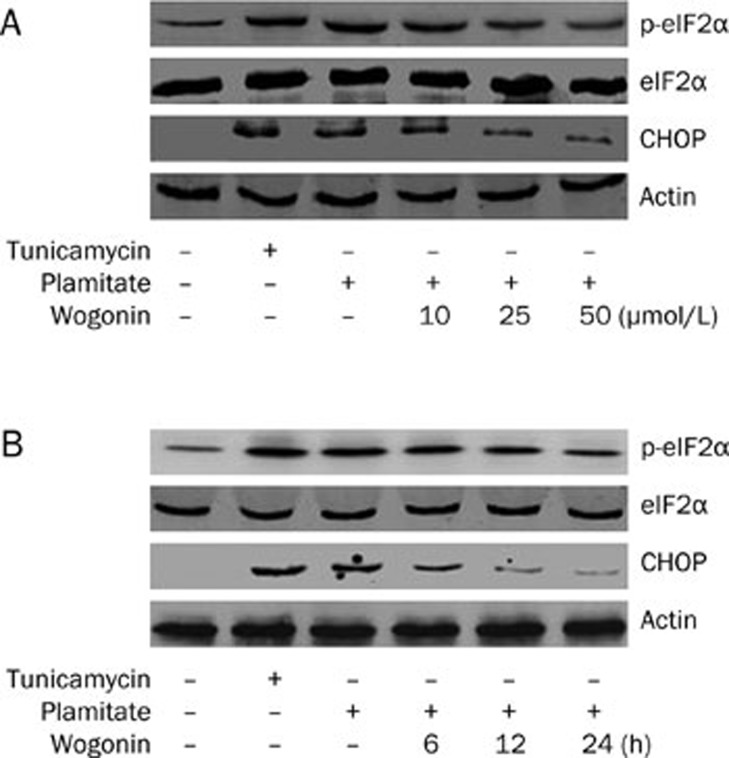
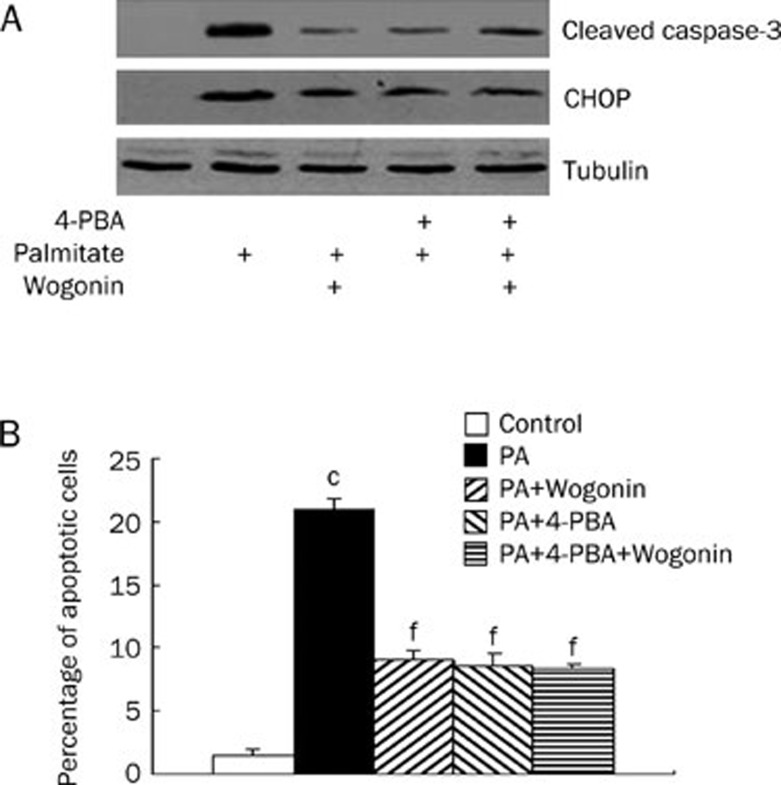
To identify the signaling pathway that initialized apoptosis, VSMCs were serum-starved by culturing in serum-free medium. Serum-starved VSMCs were treated with wogonin in a dose- and time-dependent manner in culture medium with 0.75 mmol/L palmitate. The levels of CHOP expression and eIF2α phosphorylation for these cells were analyzed using the Western blot analysis as stated under the Materials and methods. As shown in Figure 4A and 4B, palmitate-induced CHOP expression and eIF2α phosphorylation were suppressed by wogonin in a dose- and time-dependent manner similar to that observed in wogonin-mediated suppression of cleaved caspase-3 and caspase-6 expression and apoptosis. Apoptosis is initialized by extrinsic (activated by death ligands), intrinsic (mitochondrial pathway), or ER stress pathways21, 22, 23. We treated VSMCs to discover the potential anti-apoptotic effects of wogonin so that we could determine whether ER stress mediated the inhibitory effects of wogonin on apoptosis. 4-PBA (5 mmol/L) was used to inhibit ER stress in A10 VSMCs that were treated with 0.75 mmol/L palmitate and/or 25 mmol/L wogonin. We found that administration of 4-PBA significantly decreased the number of apoptotic cells in A10 VSMCs that were treated with palmitate and that wogonin treatment did not further enhance the inhibitory effects (Figure 5). These data suggest that palmitate-induced apoptosis and wogonin-mediated protection against apoptosis are mediated by ER stress. Taken together, these data indicate that wogonin promotes anti-apoptotic effects via inhibition of ER stress.
Figure 4.
Wogonin inhibited palmitate-induced ER stress. VSMCs were cultured in serum-free medium for 6 h and treated with or without differential doses of wogonin for the indicated time in the presence of 0.75 mmol/L of palmitate or 10 μg/mL of the ER inducer tunicamycin for 24 h. (A) Dose course. In total, 10, 25, or 50 μmol/L of wogonin was added to the culture medium for 24 h. (B) Time course. In total, 50 μmol/L of wogonin was added to the culture medium for 6, 12, or 24 h. Western blot was performed to detect the expression levels of CHOP, elF2α, and phosphorylated elF2α. The figures are representative of at least three independent experiments with similar results.
Figure 5.
The effects of combined treatment of 4-PBA and wogonin on apoptosis. A10 VSMCs were cultured in serum-free medium for 6 h and treated with or without 4-PBA (5 mmol/L) for 1 h, followed by treatment with or without 25 μmol/L of wogonin for 12 h in the presence of 0.75 mmol/L of palmitate (PA) for 24 h. (A) CHOP and cleaved caspase-3 protein expression. (B) Bar graph for percentage of apoptotic cells. Western blot was performed to detect the expression levels of CHOP and cleaved caspase-3. The figures are representative of at least three independent experiments with similar results. cP<0.01 compared with the control group; fP<0.01 compared with the palmitate-treated group.
Effects of wogonin on palmitate-induced ROS generation
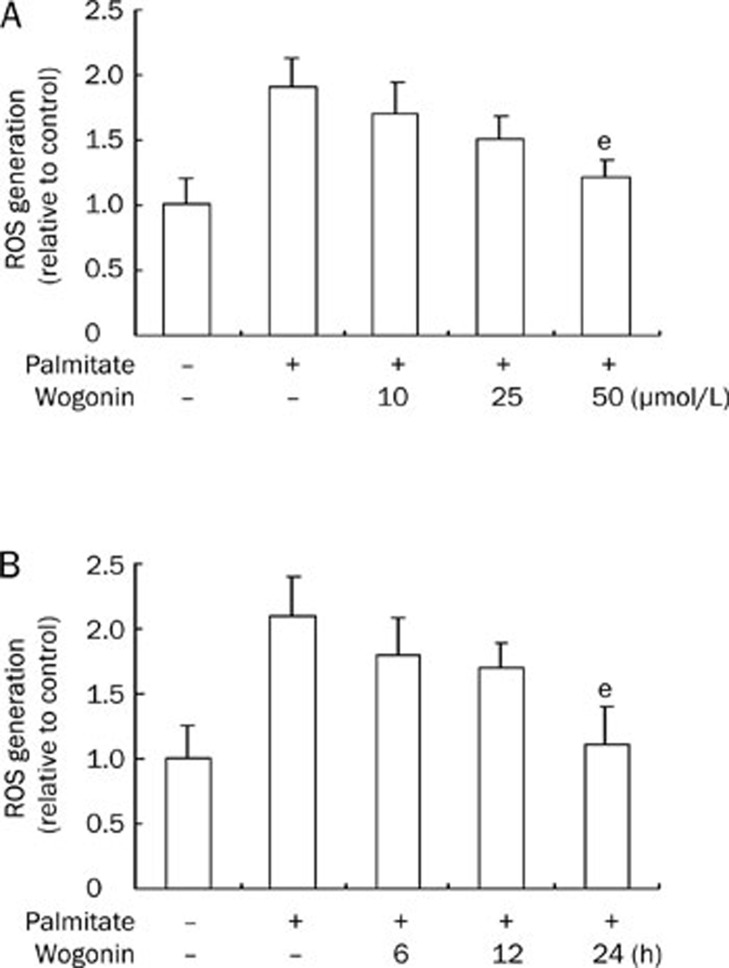
Over-generation of ROS is responsible for apoptosis in FFA-treated cells24, 25, 26, 27. We addressed whether wogonin has any effects on palmitate-mediated ROS generation. Serum-starved VSMCs were treated with wogonin in a dose- and time-dependent manner in culture medium with 0.75 mmol/L palmitate and evaluated to measure ROS generation. As shown in Figure 6A and 6B, ROS generation was increased in cells that were treated with palmitate and suppressed after a high dose (50 μmol/L) of wogonin treatment (P<0.05) and long exposure time (24 h) (P<0.05). These results indicate that the inhibition of ROS generation by wogonin is not a major mechanism for its anti-apoptotic effects.
Figure 6.
Wogonin suppressed palmitate-induced ROS generation. VSMCs were cultured in serum-free medium for 6 h and treated with or without different doses of wogonin for the indicated time in the presence of 0.75 mmol/L of palmitate for 24 h. (A) Dose course. In total, 10, 25, or 50 μmol/L of wogonin was added to the culture medium for 24 h. (B) Time course. In total, 50 μmol/L of wogonin was added to the culture medium for 6, 12, or 24 h. The production of ROS was determined as described in the Materials and methods section. eP<0.05, fP<0.01 compared with the palmitate-treated group.
Effects of wogonin on the DAG/PKC pathway
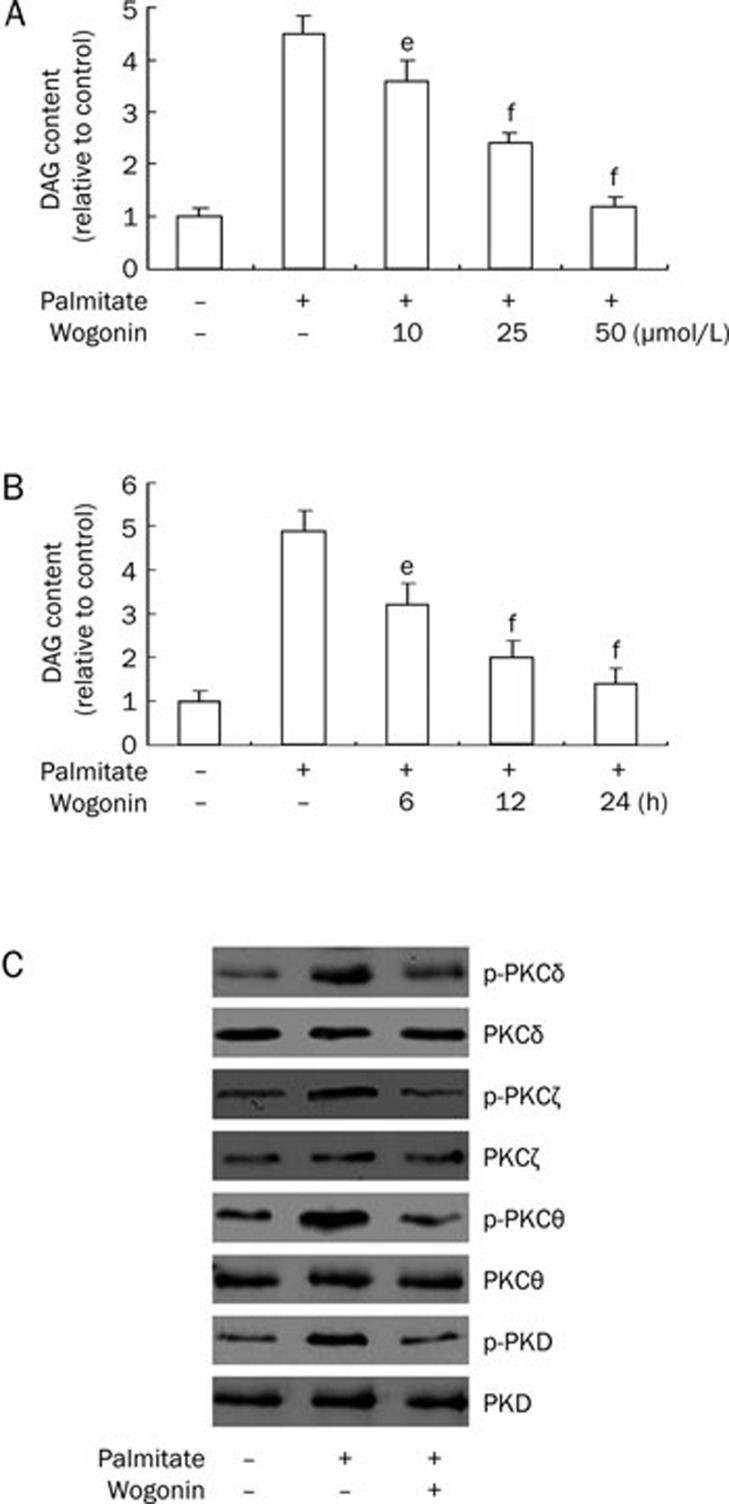
Accumulating evidence suggests that the DAG/PKC pathway contributes to deleterious consequences in cells, including apoptosis in response to FFA administration28, 29, 30, 31, 32, 33, 34, 35. Based on this evidence, we hypothesized that wogonin-mediated suppression of apoptosis might counter this mechanism and improve cell survival. To confirm this hypothesis, serum-starved VSMCs were treated with wogonin in a dose- and time-dependent manner in culture medium with 0.75 mmol/L of palmitate and evaluated to measure intracellular levels of DAG. As expected, palmitate significantly increased the intracellular contents of DAG, which were suppressed by wogonin administration (Figure 7A and 7B). In addition, the administration of wogonin at a dose of 25 μmol/L for 24 h inhibited palmitate-induced PKC phosphorylation for multiple PKC-isozymes (Figure 7C). These observations suggest that wogonin inhibits the DAG/PKC pathway, and attenuates apoptosis when combined with palmitate treatment.
Figure 7.
Wogonin ameliorated intracellular DAG accumulation and attenuated PKC phosphorylation. (A and B) VSMCs were cultured in serum-free medium for 6 h and treated with or without 0.75 mmol/L of palmitate for 12 h, followed by a second treatment with or without 10, 25, and 50 μmol/L of wogonin for an additional 12 h (A) or 25 μmol/L of wogonin for 6, 12, and 24 h (B). DAG content in the cells was determined as described in the Materials and methods section. eP<0.05, fP<0.01 compared with the palmitate-treated group. (C) VSMCs were cultured in serum-free medium for 6 h and treated with or without 25 μmol/L of wogonin for 12 h in the presence of 0.75 mmol/L of palmitate for 24 h. Western blot analysis was performed to detect the expression levels of total and phosphorylated PKC isoforms. The figures are representative of at least three independent experiments with similar results.
Discussion
In this study, we demonstrate a protective effect of wogonin on palmitate-induced VSMC apoptosis. Palmitate-induced apoptosis of VSMCs was attenuated by wogonin administration in a dose- and time-dependent manner. The administration of wogonin inhibits the DAG/PKC pathway by downregulating intracellular DAG accumulation and attenuating ER stress.
It is well documented that obese and diabetic patients have increased FFA levels, which induce atherosclerotic vascular disease. Previous studies have shown that even minute increases in plasma FFA may initiate early vascular abnormalities that promote atherosclerosis and cardiovascular disease (CVD)1. The most abundant saturated fatty acid in the plasma is palmitate. Studies have indicated that palmitate is involved in atherogenesis by increasing the extent of plaque formation (or plaque score)36 and inducing extracellular matrix alterations37. In this study, we found that the administration of palmitate induced VSMC apoptosis (Figure 2). VSMCs synthesize components of the fibrous cap in plaques. Therefore, VSMC apoptosis may result in plaque ruptures7, 8, 9, 10, 11, 12. Our results indicate a potential mechanism for FFA-induced plaque rupture.
Apoptosis can be initialized through an extrinsic pathway that is activated via death ligands, the intrinsic pathway (mitochondrial pathway), or the ER stress pathway that converges on the activation of caspase-321, 22, 23. Our results confirm that palmitate significantly increases CHOP protein expression and eIF2α phosphorylation (Figure 4).
We detected the increased expression of cleaved caspase-3 and caspase-6 (Figure 2), and the decreased ratio of anti-apoptotic protein Bcl-2 to pro-apoptotic protein Bax (Figure 3). Furthermore, the ER stress inhibitor 4-PBA prevented palmitate-induced apoptosis (Figure 5), which indicated that palmitate-induced apoptosis of VSMCs was mediated by ER stress. These data are congruent with previous studies showing that palmitate is a potent inducer of ER stress38. Recent evidence shows that atherosclerosis is associated with ER dysfunction and the accumulation of unfolded proteins39, 40, 41, 42, 43. Suppressing ER-stress signaling significantly attenuates accelerated atherogenesis40. The results of the current study suggest that increased FFA levels enhance ER stress, which may be an important risk factor in the induction of VSMC apoptosis in atherosclerosis.
Wogonin has recently been shown to be toxic in malignant cells but has no or little toxicity in normal cells44, 45, 46. Wogonin selectively induces apoptosis in tumor cells but has protective effects on glucocorticoid-induced apoptosis in normal rat thymocytes46. This selective antitumor function is largely due to its abilities to reduce inflammation, scavenge oxidative radicals, attenuate NF-ĸB activity, inhibit several genes that are important for regulation of the cell cycle, suppress COX-2 gene expression, block NO, and prevent viral infections13, 47, 48, 49. Our results demonstrate that wogonin attenuates palmitate-induced apoptosis of VSMCs in a dose- and time-dependent manner (Figure 2), which is accompanied by the suppression of ER stress (Figure 4). When ER stress was inhibited by 4-PBA, the administration of wogonin did not enhance its protective effects on apoptosis (Figure 5). Therefore, these data confirm that the inhibitory effects of wogonin on apoptosis are mediated by the suppression of ER stress.
ER and oxidative stresses are common manifestations in cells that are treated with FFA. The effect of FFA on ROS production has been examined in numerous cell types50, 51. ER stress and ROS are proposed to be involved in cell death. Nevertheless, their relative involvements in the processes leading to cell apoptosis are not well elucidated. Research groups have confirmed that over-generation of intracellular ROS might induce apoptosis in endothelial cells, beta cells, and retinal pericytes24, 25, 26, 27. In contrast, some data shows that apoptosis may not be caused by increased ROS in VSMCs and neonatal cardiomyocytes that are incubated with palmitate52, 53. Our results show that palmitate increases ROS generation (Figure 6). Although the administration of wogonin at doses of 10 μmol/L and 25 μmol/L significantly reduced apoptosis (Figure 2), no effects where observed on ROS generation (Figure 6). These results suggest that palmitate-induced ROS generation may not be the major cause of apoptosis in VSMCs.
Furthermore, we found that palmitate increased the intracellular accumulation of DAG and consequently enhanced PKC phosphorylation (Figure 7). This result coincides with previous studies showing that saturated non-esterified fatty acids stimulate an increase in de novo intramuscular synthesis and accumulation of DAG31, 32 and the subsequent activation of PKCs28, 29, 30, 33, 34, 35. Our results show that pre-treatment with wogonin significantly reduces the intracellular DAG levels and attenuates PKC phosphorylation (Figure 7). Because activated PKCs may contribute to altered cellular functions such as regulating signaling function of the ER54, 55 and enhancing ROS production51, our data suggest that the DAG/PKC pathway mediates palmitate-induced apoptosis and over-generated ROS production in VSMCs, and that wogonin ameliorates palmitate-induced ER stress by inhibiting the DAG-PKC pathway.
In conclusion, we provide novel evidence that wogonin attenuates lipotoxicity-induced apoptosis by suppressing intracellular DAG accumulation and inhibiting PKC phosphorylation in cultured VSMCs. However, further studies are necessary to determine whether the administration of wogonin will protect in vivo VSMCs from apoptosis and the subsequent rupture of advanced atherosclerotic plaques. Additional research into wogonin is also required to investigate the underlying mechanisms that suppress intracellular DAG accumulation in VSMCs.
Author contribution
Yu-min LIU, Chang-hua WANG, and Jun-jian ZHANG designed the research; Xiong WANG, Ahmed NAWAZ, and Zhao-hong KONG performed the research; Yan HONG contributed new reagents and analytic tools; Yu-min LIU and Yan HONG analyzed the data; and Chang-hua WANG and Jun-jian ZHANG wrote the paper.
Abbreviations
DAG, diacylglycerol; DMEM, Dulbecco's modified Eagle's medium; ER, endoplasmic reticulum; FBS, fetal bovine serum; FFA, free fatty acid; PKC, protein kinase C; ROS, reactive oxygen species; VSMC, vascular smooth muscle cell; PBS, phosphate-buffered saline.
Acknowledgments
We would like to thank Levi FROKE and Michael FREITAG (University of South Dakota, USA) for editing the manuscript for English grammar.
References
- Mathew M, Tay E, Cusi K. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol. 2010;9:9. doi: 10.1186/1475-2840-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade W, Schmit J, Collins M, Gade J. Beyond obesity: the diagnosis and pathophysiology of metabolic syndrome. Clin Lab Sci. 2010;23:51–61. [PubMed] [Google Scholar]
- Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am. 2008;37:635–46. doi: 10.1016/j.ecl.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S, März W. Free fatty acids as a cardiovascular risk factor. Clin Chem Lab Med. 2008;46:429–34. doi: 10.1515/CCLM.2008.118. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Steffens S, Mach F. Insulin resistance: a proinflammatory state mediated by lipid-induced signaling dysfunction and involved in atherosclerotic plaque instability. Mediators Inflamm. 2008;2008:767623. doi: 10.1155/2008/767623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–9. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudijanto A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med Indones. 2007;39:86–93. [PubMed] [Google Scholar]
- Clarke M, Bennett M. The emerging role of vascular smooth muscle cell apoptosis in atherosclerosis and plaque stability. Am J Nephrol. 2006;26:531–5. doi: 10.1159/000097815. [DOI] [PubMed] [Google Scholar]
- Clarke M, Bennett M. Defining the role of vascular smooth muscle cell apoptosis in atherosclerosis. Cell Cycle. 2006;5:2329–31. doi: 10.4161/cc.5.20.3383. [DOI] [PubMed] [Google Scholar]
- Stoneman VE, Bennett MR. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin Sci (Lond) 2004;107:343–54. doi: 10.1042/CS20040086. [DOI] [PubMed] [Google Scholar]
- Newby AC, Libby P, van der Wal AC. Plaque instability: the real challenge for atherosclerosis research in the next decade. Cardiovasc Res. 1999;41:321–2. [PubMed] [Google Scholar]
- Bennett MR. Apoptosis of vascular smooth muscle cells in vascular remodelling and atherosclerotic plaque rupture. Cardiovasc Res. 1999;41:361–8. doi: 10.1016/s0008-6363(98)00212-0. [DOI] [PubMed] [Google Scholar]
- Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Huang Y, Tsang SY, Yao X, Chen ZY. Biological properties of baicalein in cardiovascular system. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:177–84. doi: 10.2174/1568006043586206. [DOI] [PubMed] [Google Scholar]
- Lee SO, Jeong YJ, Yu MH, Lee JW, Hwangbo MH, Kim CH, et al. Wogonin suppresses TNF-alpha-induced MMP-9 expression by blocking the NF-kappaB activation via MAPK signaling pathways in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2006;351:118–25. doi: 10.1016/j.bbrc.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Chang YL, Shen JJ, Wung BS, Cheng JJ, Wang DL. Chinese herbal remedy wogonin inhibits monocyte chemotactic protein-1 gene expression in human endothelial cells. Mol Pharmacol. 2001;60:507–13. [PubMed] [Google Scholar]
- Huang HC, Wang HR, Hsieh LM. Antiproliferative effect of baicalein, a flavonoid from a Chinese herb, on vascular smooth muscle cell. Eur J Pharmacol. 1994;251:91–3. doi: 10.1016/0014-2999(94)90447-2. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu M, Riojas RA, Xin X, Gao Z, Wu J, et al. Protein Kinase C {theta} (PKC{theta})-dependent phosphorylation of PDK1 at Ser504 and Ser532 contributes to palmitate-induced insulin resistance. J Biol Chem. 2009;284:2038–44. doi: 10.1074/jbc.M806336200. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818–29. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- Shaw S, Wang X, Redd H, Alexander GD, Isales CM, Marrero MB. High glucose augments the angiotensin II-induced activation of JAK2 in vascular smooth muscle cells via the polyol pathway. J Biol Chem. 2003;278:30634–41. doi: 10.1074/jbc.M305008200. [DOI] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165:347–56. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–94. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Kim JE, Kim YW, Lee IK, Kim JY, Kang YJ, Park SY. AMP-activated protein kinase activation by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) inhibits palmitate-induced endothelial cell apoptosis through reactive oxygen species suppression. J Pharmacol Sci. 2008;106:394–403. doi: 10.1254/jphs.fp0071857. [DOI] [PubMed] [Google Scholar]
- Cai Y, Martens GA, Hinke SA, Heimberg H, Pipeleers D, Van de Casteele M. Increased oxygen radical formation and mitochondrial dysfunction mediate beta cell apoptosis under conditions of AMP-activated protein kinase stimulation. Free Radic Biol Med. 2007;42:64–78. doi: 10.1016/j.freeradbiomed.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Iverson SL, Orrenius S. The cardiolipin-cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch Biochem Biophys. 2004;423:37–46. doi: 10.1016/j.abb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Okamoto T, Amano S, Inagaki Y, Koga M, Choei H, et al. Palmitate-induced apoptosis of microvascular endothelial cells and pericytes. Mol Med. 2002;8:179–84. [PMC free article] [PubMed] [Google Scholar]
- Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16:400–2. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55:498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006;27:317–23. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lee JS, Pinnamaneni SK, Eo SJ, Cho IH, Pyo JH, Kim CK, et al. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol. 2006;100:1467–74. doi: 10.1152/japplphysiol.01438.2005. [DOI] [PubMed] [Google Scholar]
- Gaster M, Rustan AC, Beck-Nielsen H. Differential utilization of saturated palmitate and unsaturated oleate: evidence from cultured myotubes. Diabetes. 2005;54:648–56. doi: 10.2337/diabetes.54.3.648. [DOI] [PubMed] [Google Scholar]
- Yu HY, Inoguchi T, Kakimoto M, Nakashima N, Imamura M, Hashimoto T, et al. Saturated non-esterified fatty acids stimulate de novo diacylglycerol synthesis and protein kinase c activity in cultured aortic smooth muscle cells. Diabetologia. 2001;44:614–20. doi: 10.1007/s001250051668. [DOI] [PubMed] [Google Scholar]
- Way KJ, Katai N, King GL. Protein kinase C and the development of diabetic vascular complications. Diabet Med. 2001;18:945–59. doi: 10.1046/j.0742-3071.2001.00638.x. [DOI] [PubMed] [Google Scholar]
- Lee IK, Koya D, Ishi H, Kanoh H, King GL. d-Alpha-tocopherol prevents the hyperglycemia induced activation of diacylglycerol (DAG)-protein kinase C (PKC) pathway in vascular smooth muscle cell by an increase of DAG kinase activity. Diabetes Res Clin Pract. 1999;45:183–90. doi: 10.1016/s0168-8227(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Ebbesson SO, Roman MJ, Devereux RB, Kaufman D, Fabsitz RR, Maccluer JW, et al. Consumption of omega-3 fatty acids is not associated with a reduction in carotid atherosclerosis: the Genetics of Coronary Artery Disease in Alaska Natives study. Atherosclerosis. 2008;199:346–53. doi: 10.1016/j.atherosclerosis.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Lee M, Ostergren-Lundén G, Wallin B, Moses J, Bondjers G, Camejo G. Fatty acids cause alterations of human arterial smooth muscle cell proteoglycans that increase the affinity for low-density lipoprotein. Arterioscler Thromb Vasc Biol. 2006;26:130–5. doi: 10.1161/01.ATV.0000191659.94059.62. [DOI] [PubMed] [Google Scholar]
- Rho MC, Ah Lee K, Mi Kim S, Sik Lee C, Jeong Jang M, Kook Kim Y, et al. Sensitization of vascular smooth muscle cell to TNF-alpha-mediated death in the presence of palmitate. Toxicol Appl Pharmacol. 2007;220:311–9. doi: 10.1016/j.taap.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Kedi X, Ming Y, Yongping W, Yi Y, Xiaoxiang Z. Free cholesterol overloading induced smooth muscle cells death and activated both ER- and mitochondrial-dependent death pathway. Atherosclerosis. 2009;207:123–30. doi: 10.1016/j.atherosclerosis.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Khan MI, Pichna BA, Shi Y, Bowes AJ, Werstuck GH. Evidence supporting a role for endoplasmic reticulum stress in the development of atherosclerosis in a hyperglycaemic mouse model. Antioxid Redox Signal. 2009;11:2289–98. doi: 10.1089/ars.2009.2569. [DOI] [PubMed] [Google Scholar]
- Cheng WP, Hung HF, Wang BW, Shyu KG. The molecular regulation of GADD153 in apoptosis of cultured vascular smooth muscle cells by cyclic mechanical stretch. Cardiovasc Res. 2008;77:551–9. doi: 10.1093/cvr/cvm057. [DOI] [PubMed] [Google Scholar]
- Chin TY, Lin HC, Kuo JP, Chueh SH. Dual effect of thapsigargin on cell death in porcine aortic smooth muscle cells. Am J Physiol Cell Physiol. 2007;292:C383–95. doi: 10.1152/ajpcell.00069.2006. [DOI] [PubMed] [Google Scholar]
- Werstuck GH, Khan MI, Femia G, Kim AJ, Tedesco V, Trigatti B, et al. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes. 2006;55:93–101. [PubMed] [Google Scholar]
- Baumann S, Fas SC, Giaisi M, Müller WW, Merling A, Gülow K, et al. Wogonin preferentially kills malignant lymphocytes and suppresses T-cell tumor growth by inducing PLC{gamma}1- and Ca2+-dependent apoptosis. Blood. 2008;111:2354–63. doi: 10.1182/blood-2007-06-096198. [DOI] [PubMed] [Google Scholar]
- Lee DH, Kim C, Zhang L, Lee YJ. Role of p53, PUMA, and Bax in Wogonin induced apoptosis in human cancer cells. Biochem Pharmacol. 2008;75:2020–33. doi: 10.1016/j.bcp.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto R, Sugahara C, Suzuki C, Nagase I, Takamura Y, Yoshikawa A, et al. Wogonin prevents glucocorticoid-induced thymocyte apoptosis without diminishing its anti-inflammatory action. J Pharmacol Sci. 2007;104:355–65. doi: 10.1254/jphs.fp0061501. [DOI] [PubMed] [Google Scholar]
- Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–56. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med. 1997;22:749–60. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- Lambertucci RH, Hirabara SM, Silveira Ldos R, Levada-Pires AC, Curi R, Pithon-Curi TC. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J Cell Physiol. 2008;216:796–804. doi: 10.1002/jcp.21463. [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Aoki T, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–45. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- Mattern HM, Hardin CD. Vascular metabolic dysfunction and lipotoxicity. Physiol Res. 2007;56:149–58. doi: 10.33549/physiolres.930899. [DOI] [PubMed] [Google Scholar]
- Hickson-Bick DL, Sparagna GC, Buja LM, McMillin JB. Palmitate-induced apoptosis in neonatal cardiomyocytes is not dependent on the generation of ROS. Am J Physiol Heart Circ Physiol. 2002;282:H656–64. doi: 10.1152/ajpheart.00726.2001. [DOI] [PubMed] [Google Scholar]
- Pino SC, O'Sullivan-Murphy B, Lidstone EA, Thornley TB, Jurczyk A, Urano F, et al. Protein kinase C signaling during T cell activation induces the endoplasmic reticulum stress response. Cell Stress Chaperones. 2008;13:421–34. doi: 10.1007/s12192-008-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kim DH, Boo JH, Kim YH, Park IS, Mook-Jung I. ER stress-induced caspase-12 activation is inhibited by PKC in neuronal cells. Apoptosis. 2005;10:407–15. doi: 10.1007/s10495-005-0814-6. [DOI] [PubMed] [Google Scholar]