Cell-to-cell adhesion as well as the interaction of cells with the extracellular matrix are key phenomena in different physiological and pathological conditions, including embryogenesis, blood coagulation, lymphocyte homing, immune response, angiogenesis, metastasis, thrombosis and inflammation1, 2. Thus, it has been widely proposed that cell adhesion molecules are an important therapeutic target in a wide array of diseases with high impact on public health, including atherosclerosis, thromboembolic disorders, cancer, graft rejection and autoimmune inflammatory conditions1, 2. However, anti-adhesion therapy with either biological agents (mainly blocking monoclonal antibodies, mAb's) or chemical inhibitors (mainly synthetic peptides) has not yet fulfilled these expectations and has not been devoid of undesirable effects3, 4. In this context, the research team of Gupta at the University of Miami, recently published an interesting article employing an alternative approach for the pharmacological inhibition of leukocyte extravasation, thus blocking the inflammatory phenomenon5. According to this report, novel pharmacological agents (“leukadherins”) effectively inhibit the inflammatory phenomenon, with no apparent toxicity, which may constitute a major step ahead in this area.
The interaction between leukocytes and endothelial cells (EC), an essential step in the process of extravasation of these cells to an inflammatory focus, is mainly mediated by cell adhesion receptors that belong to the selectin, integrin and immunoglobulin superfamilies1, 2, 3. According to the sequential model of leukocyte extravasation (Figure 1)6, activated endothelial cells favor their initial interaction with leukocytes (tethering or first step) through selectins (CD62L, CD62P and CD62E) and their ligands, a phenomenon that is followed by the rolling of leukocytes on endothelium (second step). Then, leukocytes slow their motion and transiently remain adhered to the EC (firm adhesion, third step), which is followed by their polarization and migration through the EC monolayer (extravasation, fourth step), mainly by a paracellular route (through the EC junctions). The firm adhesion of leukocytes to endothelium is mainly mediated by LFA-1 and Mac-1 integrins (also called CD11a/CD18 and CD11b/CD18), which during the rolling are able to activate, changing their conformation and significantly increasing their affinity for their different ligands expressed by EC cells (mainly ICAM-1 and -2). In addition, it has been shown that α4β1 and α4β7 integrins are also able to mediate both the initial and late steps of EC-leukocyte interaction3, 6. As expected, in order to extravasate leukocytes must exert a very dynamic regulation of their adhesiveness to endothelium mediated by integrins, allowing their detachment from the luminal side of EC cells. In this regard, Kuijpers et al demonstrated, more than 15 years ago, that the freezing of the α4β1 integrin in an activated state promotes the adhesion of eosinophils to endothelium but inhibits their extravasation7.
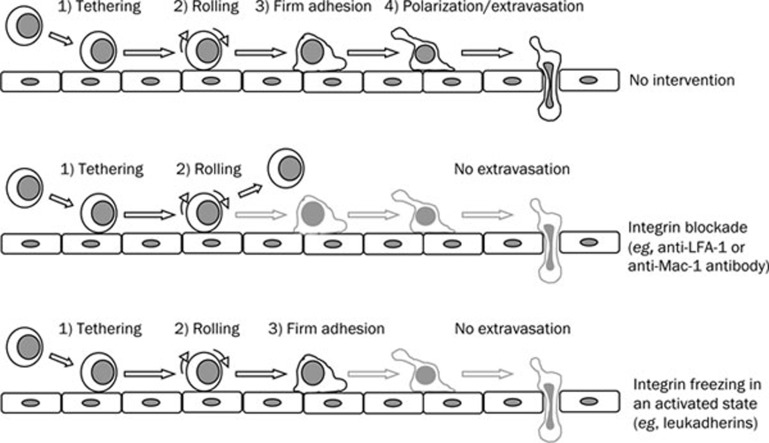
Figure 1.
The migration of leukocytes towards an inflammatory focus is a sequential process that includes the tethering of leukocytes with endothelial cells (1st step), the rolling of leukocytes on endothelium (2nd step), the transient firm adhesion of leukocytes (3rd step), and their polarization and extravasation (4th step), reaching thus the inflamed tissue (upper panel). When LFA-1 or Mac-1 integrins are blocked with antibodies of synthetic peptides, the firm adhesion of leukocytes is inhibited and these cells return to the bloodstream (middle panel), but when these integrins are induced to activate, increasing their avidity for their endothelial ligands (ICAM-1, -2), leukocytes remain firmly adhered to endothelium, with no progression to the 4th step or extravasation (lower panel). As stated in the text, leukadherins exert the latter effect, and are able to inhibit the inflammatory phenomenon.
In the work of the research team of Gupta5, three synthetic compounds called leukadherins-1 to -3 were identified by their ability to increase the adhesion of K562 cells expressing Mac-1 to fibrinogen, a ligand of this integrin. By using different experimental approaches and by in silico analysis, these authors concluded that leukadherins bind to the A domain of Mac-1, inducing a high-affinity conformation of this integrin. Accordingly, leukadherins increased the adhesion of leukocytes mediated by Mac-1 but inhibited their migration and extravasation in vivo. Furthermore, leukadherins exerted a significant therapeutic effect in three different models of inflammatory disease (peritonitis, vascular neointimal thickening and glomerulonephritis), with no apparent toxicity on leukocytes or endothelium, nor evidence of side-effects. Interestingly, additional experiments showed that leukadherins were more effective than a blocking anti-Mac-1 mAb. Finally, by using a transgenic zebrafish model of neutrophil recruitment, these authors demonstrated that the effect of leukadherins was transient and reversed by their removal. It was concluded that leukadherins act as Mac-1 agonists, increasing the adhesion of leukocytes but blocking their transendothelial migration and inhibiting thus the inflammatory phenomenon.
As stated above, it has been widely proposed that the anti-adhesion therapy is a rational and effective approach for the treatment of inflammatory conditions1, 2, 3, 4. As a result of intensive work over a long time, two anti-adhesion biological agents were approved and marketed in 2003–2004, natalizumab (a blocking mAb directed against the common chain of the α4β1 and α4β7 integrins) for the therapy of multiple sclerosis and Crohn's disease, and efalizumab (a blocking mAb directed against the chain of LFA-1) for the treatment of moderate-to-severe chronic plaque psoriasis. However, mainly due to the increased risk of progressive multifocal leukoencephalopathy (PML), natalizumab and efalizumab were withdrawn from the market (2005 and 2009). Natalizumab returned to the market (2006), but only for the therapy of the relapsing-remitting form of multiple sclerosis. With the exception of three inhibitors of the gpIIb/IIIa (CD41/CD61) platelet integrin (abxicimab, integrilin and tirofiban, used for the therapy of thromboembolic disorders), no other anti-integrin agents have reached the market, mainly due to their lack of effect. Thus, it seems very important to generate novel approaches for the therapy of inflammatory diseases based on modulation of cell adhesion receptors. In this regard, the work of Gupta et al is an interesting step ahead in this area. However, two important issues should be addressed during the long trip of leukadherins to the market: the possible consequences of their prolonged administration and the potential cytotoxic effect of leukocytes on EC, as a result of the sustained interaction of these cells induced by the leukadherins. In addition, the possibility of an enhanced risk of PML remains as an important matter. Nevertheless, the results of the work of Gupta et al strongly suggest that leukadherins do not promote EC damage and that are well tolerated. In addition, these results indicate that these agents could be more effective than the traditional blockade of integrins. In this regard, it seems evident that the effect of leukadherins could be mediated through the activation of only a fraction of Mac-1 molecules per cell, whereas the effect of blocking antibodies requires their binding to a large fraction of molecules of this integrin. Therefore, this work, relied on an old idea7, seems to represent a significant step ahead in the therapy of inflammatory diseases based on the modulation of cell adhesion receptors.
References
- Frenette PS, Wagner DD. Adhesion molecules — Part 1. N Engl J Med. 1996;334:1526–9. doi: 10.1056/NEJM199606063342308. [DOI] [PubMed] [Google Scholar]
- Frenette PS, Wagner DD. Adhesion molecules — Part II: Blood vessels and blood cells. N Engl J Med. 1996;335:43–5. doi: 10.1056/NEJM199607043350108. [DOI] [PubMed] [Google Scholar]
- Barreiro O, Martín P, González-Amaro R, Sánchez-Madrid F. Molecular cues guiding inflammatory responses. Cardiovasc Res. 2010;86:174–82. doi: 10.1093/cvr/cvq001. [DOI] [PubMed] [Google Scholar]
- González-Amaro R, Mittelbrunn M, Sánchez-Madrid F. Therapeutic anti-integrin (alpha4 and alphaL) monoclonal antibodies: two-edged swords. Immunology. 2005;116:289–96. doi: 10.1111/j.1365-2567.2005.02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiguel D, Faridi MH, Wei C, Kuwano Y, Balla KM, Hernandez D, et al. Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Sci Signal. 2011;4:ra57. doi: 10.1126/scisignal.2001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Kuijpers TW, Mul EP, Blom M, Kovach NL, Gaeta FC, Tollefson V, et al. Freezing adhesion molecules in a state of high-avidity binding blocks eosinophil migration. J Exp Med. 1993;178:279–84. doi: 10.1084/jem.178.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]