Abstract
Aim:
To examine the protective effects of scutellarin (Scu) on rats with learning and memory deficit induced by β-amyloid peptide (Aβ).
Methods:
Fifty male Wistar rats were randomly divided into 5 groups: control, sham operation, Aβ, Aβ+Scu, and Aβ+piracetam groups. Aβ25–35 was injected into the lateral ventricle (10 μg each side). Scu (10 mg/2 mL) or piracetam (10 mg/2 mL was intragastrically administered per day for 20 consecutive days following Aβ treatment. Learning and memory was assessed with Morris water maze test. The protein and mRNA levels of nicotinic acetylcholine receptor (nAChR) α4, α7, and β2 subunits in the brain were examined using Western blotting and real-time PCR, respectively. The activities of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) in the brain and plasma were measured using Ellman's colorimetric method.
Results:
In Aβ group, the escape latency period and first platform cross was significantly increased, and the total number of platform crossings was significantly decreased, as compared with the control and the sham operation groups. Both Scu and piracetam treatment significantly reduced the escape latency period and time to cross platform, and increased the number of platform crosses, but there were no significant differences between Aβ+Scu and Aβ+piracetam groups. In Aβ group, the protein levels of nAChR α4 and α7 subunits in the cerebral cortex were significantly decreased by 42%–47% and 58%–61%, respectively, as compared to the control and the sham operation groups. Scu treatment caused upregulation of α4 and α7 subunit proteins by around 24% and 30%, respectively, as compared to Aβ group, but there were no significant differences between Aβ+Scu and Aβ+piracetam groups. The protein level of nAChR β2 subunit had no significant difference among different groups. The mRNA levels of nAChR α4, α7, and β2 subunits were not significantly changed. In Aβ group, the activities of AChE and BuChE in the brain were significantly increased, but were significantly decreased in the plasma, as compared to the control and the sham operation groups. Scu or piracetam treatment restored the activities in brain and plasma nearly to the levels in the control group.
Conclusion:
The results suggest that Scu may rescue some of the deleterious effects of Aβ, possibly by stimulating nAChR protein translation and regulating cholinesterase activity.
Keywords: scutellarin, piracetam, β-amyloid peptide, learning and memory, cholinesterase, nicotinic acetylcholine receptor
Introduction
Herba Erigerontis (HE), a Chinese medicinal herb derived from Erigeron breviscapus (vant) Hand-Mazz, has been used to effectively treat brain and cardiovascular disorders1. Flavonoids, a large group of natural compounds found in HE, have been considered as substitutes for estrogen2 and proven to have neuroprotective effects3, 4, 5, 6. Interestingly, scutellarin (Scu) is a major component of flavonoids in HE (over 40% of the total flavonoids: 5,6,4′,7-glucuronyl oxyflavone). Several basic research and clinical studies have shown that Scu plays an important role in combating neurotoxicity1, 7, 8.
Alzheimer's disease (AD) is one of the most devastating diseases of the central nervous system (CNS). Neuropathologically, it is characterized by amyloid plaques composed primarily of β-amyloid peptide (Aβ) aggregates, neurofibrillary tangles comprised of hyperphosphorylated tau protein, and extensive neuronal loss that is particularly pronounced in the cholinergic system9. Aβ, a protein fragment derived from sequential proteolytic cleavage by β- and γ-secretases on amyloid precursor protein (APP), is one of the key events leading to neuronal dysfunction and cognitive decline in the progression of AD10. It has been hypothesized that the altered processing of APP results in the accumulation and aggregation of neurotoxic forms of Aβ11 that induces neurotoxic effects via signaling cascades12.
The cholinergic neurotransmitter system in the brain is critical for the processing of information related to cognitive function13. Indeed, AD dysfunction is marked by conspicuous decreases in choline acetyltransferase activity and acetylcholine (ACh) release, significant loss of cholinergic neurons and depletion of cholinergic acetylcholine receptors9, 14. Within the cholinergic system, nicotinic acetylcholine receptors (nAChRs), which are members of the superfamily of ligand-gated ion channels, are important components involved in a wide range of brain activities and functions, including cognitive enhancement and neuroprotection15. Within the last decade, experimental evidence has accumulated supporting nAChRs as direct therapeutic targets to improve cognitive function and slow neurodegenerative progression in AD patients16. Accordingly, some of the interventions to prevent or treat the development and progression of AD have focused on enhancing cholinergic transmission, either through increasing ACh synthesis or inhibiting the activities of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE), enzymes responsible for ACh hydrolysis17, 18.
At present, there are no effective pharmacological interventions that prevent or stall AD. In researching alternative therapeutic agents against the disease, HE was found to inhibit aggregation of Aβ19, and Scu may prevent neuronal death induced by Aβ3. In this study, we investigated the potential mechanisms underlying the effects of Scu on learning and memory, expression of nAChRs and the activity of ChE by using the dementia model of rats produced by intracerebroventricular (icv) injection of Aβ.
Materials and methods
Preparation of Scu
HE, whole grass of the perennial Erigeron breviscapus (vant) Hand-Mazz, was collected from the Leisan County, Guizhou Province in China, and the identity of plant materials was verified morphologically. A voucher specimen was deposited in Guiyang Medical College (accession number EB20070730). Plant material was sun-dried and ground.
Scu was derived from extraction of HE, and its purity above 95% was determined by ultra performance liquid chromatography (UPLC). Each killogram of air-dried powder produced from whole HE was decocted with 10 L of water 3 times for 30 min each, for a total time of 1.5 h. Individual batches of the decoction were combined, filtered, and concentrated to obtain a relative density (or specific gravity) of 1.10 (50 °C) and then adjusted to an ethanol content of 55% with 95% ethanol. The resulting mixture was stirred for 30 min and then kept at 50 °C for 12 h. The filtrate thus obtained was first concentrated under reduced atmospheric pressure to yield a relative density of 1.11 (50 °C) and then adjusted to pH 2 with hydrochloric acid. The mixture was kept at 55 °C for 6 h, filtered, and finally vacuum-dried to yield Scu used in subsequent studies.
Chemicals
The chemical reagents including Aβ25–35, mouse monoclonal anti-β-actin antibody, and AChE and BuChE were purchased from Sigma-Aldrich Co (St Louis, MO, USA). Goat polyclonal anti-α4 and -β2 antibodies, mouse monoclonal anti-α7 antibody, and secondary anti-goat and anti-mouse IgG conjugated with horseradish peroxidase were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA). Piracetam was purchased from the Hangzhou Minsheng Pharmaceutical Group Co (Hangzhou, China). Hyper Performance Chemiluminescence film and ECL Plus reagent were obtained from Amersham (Uppsala, Sweden). Real-time PCR reagents were obtained from Promega Co (Madison, WI, USA) and the Taqman probe was from Applied Biosystems Co (Foster City, CA, USA).
Aβ aggregation
Aβ25–35 was dissolved in 10% dimethyl sulfoxide (DMSO) to a final concentration of 5 μg/μL (4.7 mmol/L), and the solution was incubated at 37 °C for 3 d to induce a conformational transformation from a soluble form to insoluble β-sheets, thus increasing the neurotoxicity of the peptide.
Experimental animals
Male Wistar rats [Grade II; Certificate No: SCXK (Q) 200220001], 300–350 g, were purchased from the Experimental Animal Center in Guizhou province, China, and the experiments were approved by the regional ethical committee for animal studies in Guizhou. The rats were acclimated for one month in a controlled housing facility (humidity 30%–55% and temperature 22–25 °C) prior to use in experiments.
Surgery and drug administration
Fifty rats with similar learning and memory ability as determined by the Morris water maze test were randomly divided into 5 groups: control (no operation), sham operation, learning and memory deficit model (Aβ icv), Aβ+Scu and Aβ+piracetam (which received Aβ icv before treatment with Scu or piracetam) groups. Ten animals were used for each group. During the study, the rats were given water and food ad libitum. The learning and memory deficit model with AD-like dementia was produced by bilateral icv injection with Aβ25–35. Rats were anesthetized with chloral hydrate (0.3 g/kg, ip) and placed in a stereotaxic apparatus. Referring to the atlas of Paxinos and Watson20, the coordinates were located for lateral ventricle injection (anterior-posterior, 3.0 mm; medial-lateral, 2.0 mm; dorsal-ventral, 3.3 mm from the bregma). Two microliters of Aβ25–35 (10 μg) were gradually delivered into each lateral ventricle via a microsyringe with a stainless steel needle within 5 min. The sham operation was identical except sterile saline was used in place of Aβ25–35 solution. No procedure was performed on the control group. The rats in the Aβ+Scu or Aβ+piracetam group were treated with the same protocol as the learning and memory deficit model, but five days after icv the animals were additionally treated with 10 mg/2 mL per day of Scu or piracetam by intragastric administration (ig) for 20 consecutive days. At the end of the experiment, the rats were assessed with the Morris water maze test and then sacrificed by femoral artery exsanguination. Blood samples were collected, and brains, including whole cerebral cortices, were removed and stored at −80 °C for later analysis.
Examination of spatial learning and memory
Spatial learning and memory was evaluated with the Morris water maze test21. As described previously22, each rat was placed facing the pool from different quadrants at the start of a trial and subjected to 4 trials each day with a 30-min interval of rest between trials for a training period of 4 d. Their movement was monitored with Videotrack Software (View Point). During the navigation test, the time required to locate the escape platform (escape latency) was determined. The 4 trials on each individual day were averaged for statistical analysis. Furthermore, on d 5, the platform was removed, and the time that the animal first crossed the platform site and the total number of crosses within 60 s were recorded. All of these behavioral tests were conducted in a quiet environment with subdued lighting.
Protein levels of α4, α7, and β2 nAChR subunits detected by Western blotting
The cerebral cortices were homogenized, and the protein concentrations of the resulting supernatant were determined22. The proteins in the solubilized membrane fraction recovered in the supernatant were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. For the quantification of nAChR subunits and β-actin, the PVDF membranes were incubated with appropriate antibodies for corresponding proteins: nAChR subunit α4 (1:300), α7 (1:500) and β2 (1:300) and β-actin (1:10000) at 4 °C overnight. After washing, the membranes were incubated with HRP-conjugated anti-goat IgG (1:5000) or anti-mouse IgG (1:3000), respectively, for 60 min. Finally, these membranes were incubated in ECL Plus reagent for 5 min and the signals were visualized by exposure to Hyper Performance Chemiluminescence film. For the calculation of Western blotting results, we used β-actin (a housekeeping gene) as an internal standard. The resultant values (as compared with β-actin) were expressed as a percentage of the average value for controls.
The mRNA levels of α4, α7, and β2 nAChR subunits determined by real-time PCR
Total RNA in the cerebral cortices was isolated by TRIzol reagents (Invitrogen, Carlsbad, CA, USA). cDNA was generated from 3 μg of total RNA by utilizing a cDNA synthesis kit (Promega, Madison, WI, USA)23, and the reaction mixture was incubated in a Mastercycler 5700 (Eppendorf, Hamburg, Germany) at 37 °C for 1 h, then at 70 °C for 15 min and at 4 °C for 1 min. The primers and probes for α4 (Rn 00577436), α7 (Rn 00563223), β2 (Rn 00570733) nAChR subunits, and β-actin (4352931E; as an internal standard) were purchased from Applied Biosystems (Foster City, CA, USA) (Table 1). Reactions were performed with the Universal TaqMan 2X PCR master mix (Applied Biosystems, Foster City, USA) in a 20 μL reaction volume. The detailed procedure was performed as described in the manufacturer's protocol. After an initial denaturation at 50 °C for 120 s, 40 cycles were performed consisting of 95 °C for 600 s, 55 °C for 15 s followed by a final extension at 60 °C for 60 s. The experiments were repeated at least three times. The results are expressed as the ratio of detected signal for each nAChR subunit to the β-actin signal, and the CT values were calculated as percentages of the controls (as 100%).
Table 1. Sequences of PCR primers and product length.
| Gene | Primers sequences | Length |
|---|---|---|
| nAChR α4 | CTCAGCTCATTGACGTGGACGAGAA | 65 |
| nAChR α7 | TGCTGCACGTGTCCCTGCAAGGCGA | 72 |
| nAChR β2 | ACCAGAGTGTGAGGGAGGACTGGAA | 63 |
| β-actin | CCTTCCTTCCTGGGTATGGAATCCT | 91 |
AChE and BuChE activities in brain and plasma
The cerebral cortical tissues were homogenized 1:9 (w/v) of 0.9% saline. The resulting brain homogenate supernatants were diluted with PBS by 1:15, and plasma was diluted 1:13. Activities of AChE and BuChE in these solutions were determined by an improved Ellman's colorimetric method24, employing acetylthiocholine (ATC) and butyrylthiocholine (BTC), respectively, as substrates for the reactions. Activities were calculated with the following formula:
The data were normalized to the amount of protein measured by the Lowry method, using the Bio-Rad DC protein assay and bovine serum albumin as the standard.
Statistical analysis
The results were expressed as the mean±SD. Groups were assessed for significant differences with analysis of variance (ANOVA) and Tukey-Kramer post-hoc tests using SPSSv11.5 software (SPSS Inc, USA). P<0.05 was considered statistically significant.
Results
Learning and memory dysfunction in response to Aβ treatment
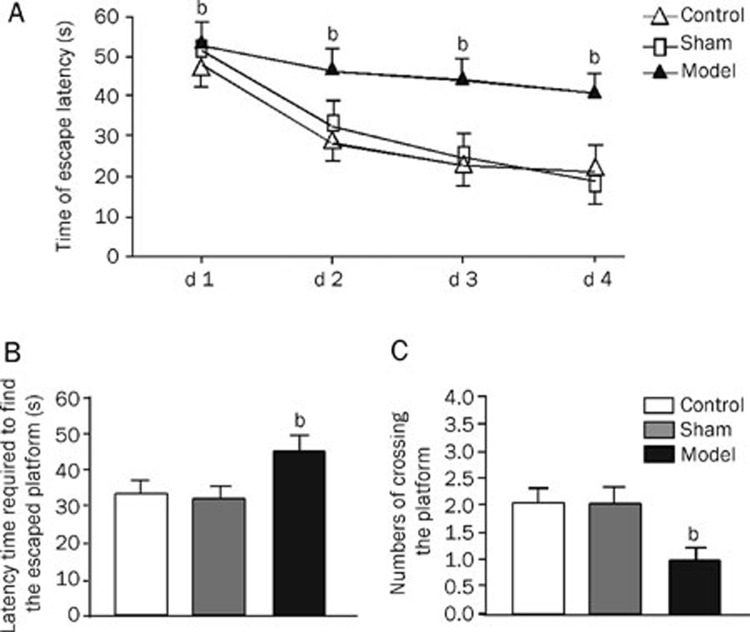
Rats given Aβ icv had increased values for the escape latency period and first platform cross, and the total number of platform crossings was significantly lower than the control and the sham operation groups (Figure 1).
Figure 1.
Spatial learning and memory of rats with cerebroventricular injection of Aβ and the rats as sham operation and control. Spatial learning and memory were tested in a Morris Water Maze involving a circular pool 180 cm in diameter. Following four trials daily for 4 d, the average escaping latency of rats in different groups during training session was conducted (A); on d 5 the platform was removed, and latency trials (B) and the number of times the central platform was crossed was counted (C). Values in the bar graphs are means±SD of 10 rats. bP<0.05 as compared to normal groups and sham operation groups, as determined by an analysis of the variance ANOVA with Tukey-Kramer using SPSS15.0 software.
Effect of Scu on the rats treated with Aβ
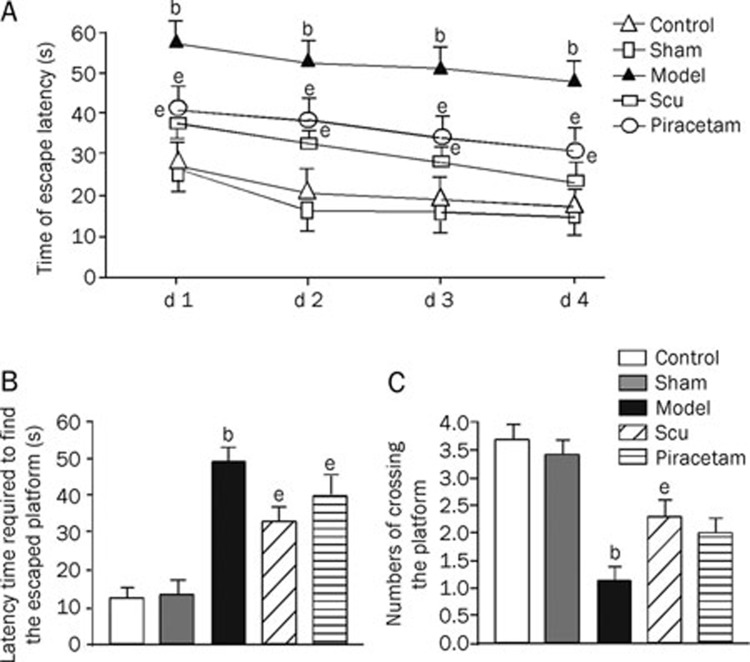
Compared to the learning and memory deficit group, rats additionally treated with Scu showed decreases in escape latency period and time to cross platform, as well as a higher number of platform crosses. There were no significant differences between Aβ+Scu and Aβ+piracetam treatment groups (Figure 2).
Figure 2.
Effects of Scu and piracetam on spatial learning and memory of rats treated previously with Aβ injection. Spatial learning and memory were tested in a Morris Water Maze involving a circular pool 180 cm in diameter. Following four trials daily for 4 d, the average escaping latency of rats in different groups during training session was conducted (A); on d 5 the platform was removed, and latency trials (B) and the number of times the central platform was crossed was counted (C). Values in the bar graphs are means±SD of 10 rats. bP<0.05 as compared to normal groups and sham operation groups. eP<0.05 as compared to model groups, as determined by an analysis of the variance ANOVA with Tukey-Kramer using SPSS15.0 software.
Protein and mRNA levels of α4, α7, and β2 nAChR subunits
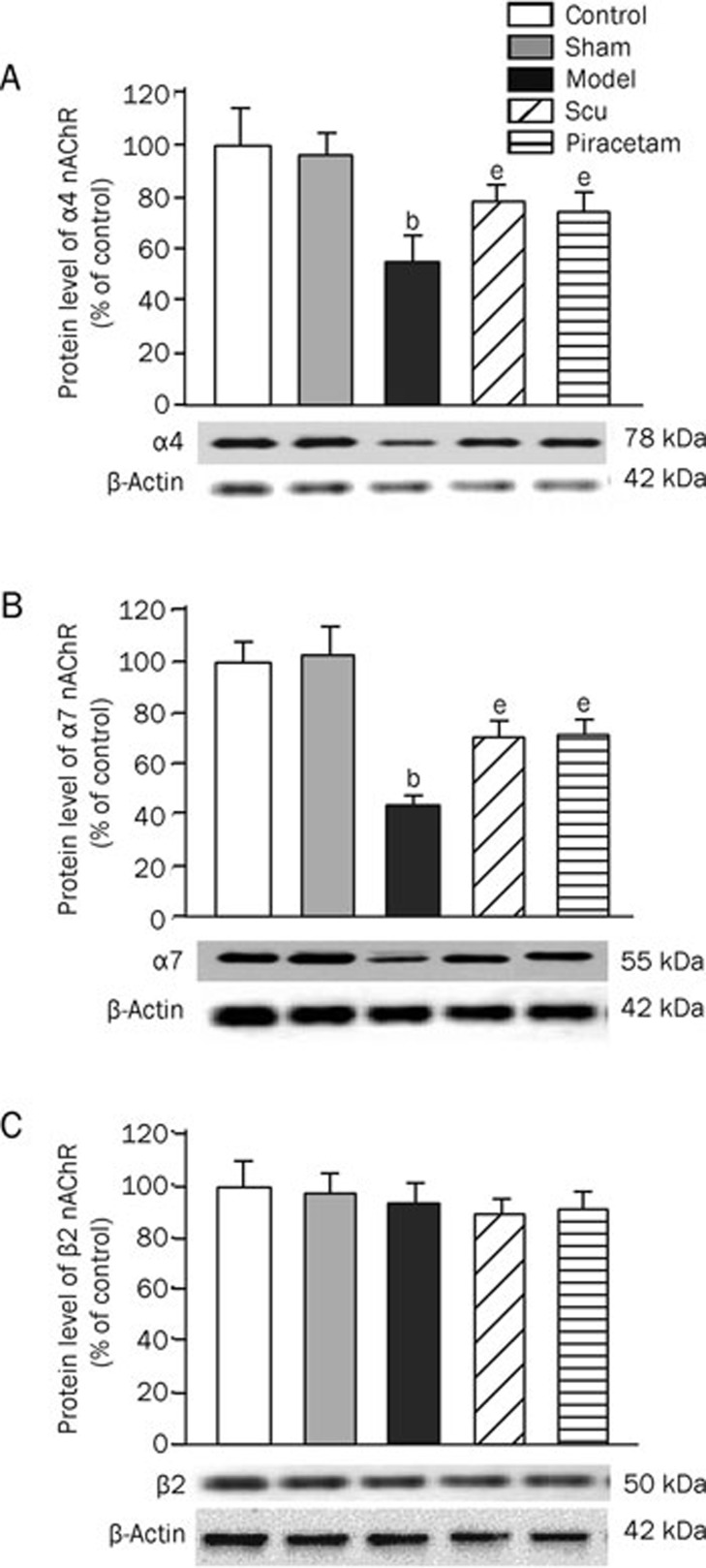
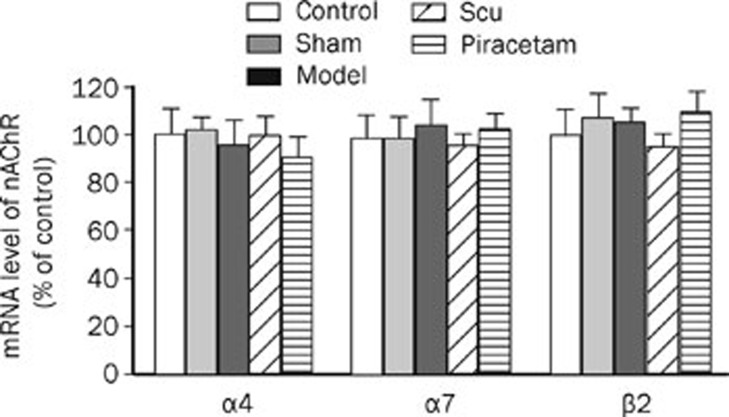
The protein levels of the α4 and α7 nAChR subunits in the cerebral cortex were significantly decreased by 42%–47% and 58%–61%, respectively, in the learning and memory deficit model group as compared to the control and the sham operation groups (Figure 3). Interestingly, Scu treatment resulted in an upregulation by 24% and 30% in α4 and α7 nAChR subunit proteins, respectively, as compared to the learning and memory deficit model group, while no significant difference of these receptor levels was observed when compared to the piracetam treatment. There was no change in β2 nAChR subunit protein level among different groups (Figure 3). No significant changes in the mRNA levels of α4, α7, and β2 nAChR subunits were observed (Figure 4).
Figure 3.
Expressions of α4, α7, and β2 nAChR subunits at protein level in the rat brains. The nAChR α4 (A), α7 (B), and β2 (C) subunit proteins in the rat brains as well as β-actin (as an internal standard) were measured by Western blotting. For the calculation of Western blotting results, β-actin (a house-keeping gene) was used as an internal standard, and the resultant values (as compared with β-actin) were expressed as a percentage of the average value for controls (as 100%). bP<0.05 as compared to normal group and sham operation group. eP<0.05 as compared to model group as determined by an analysis of the variance ANOVA with Tukey-Kramer using SPSS15.0 software.
Figure 4.
Expressions of α4, α7, and β2 nAChR subunits at mRNA level in the rat brains. The mRNAs of nAChR α4, α7, and β2 subunits as well as β-actin (as an internal standard) were measured by real-time PCR. The results are expressed as the ratio of detected signal for each mRNA of nAChR subunit to their respective β-actin signal and then the CT values were calculated as the percentages of controls (as 100%). The data were analyzed by an analysis of the variance ANOVA with Tukey-Kramer using SPSS15.0 software.
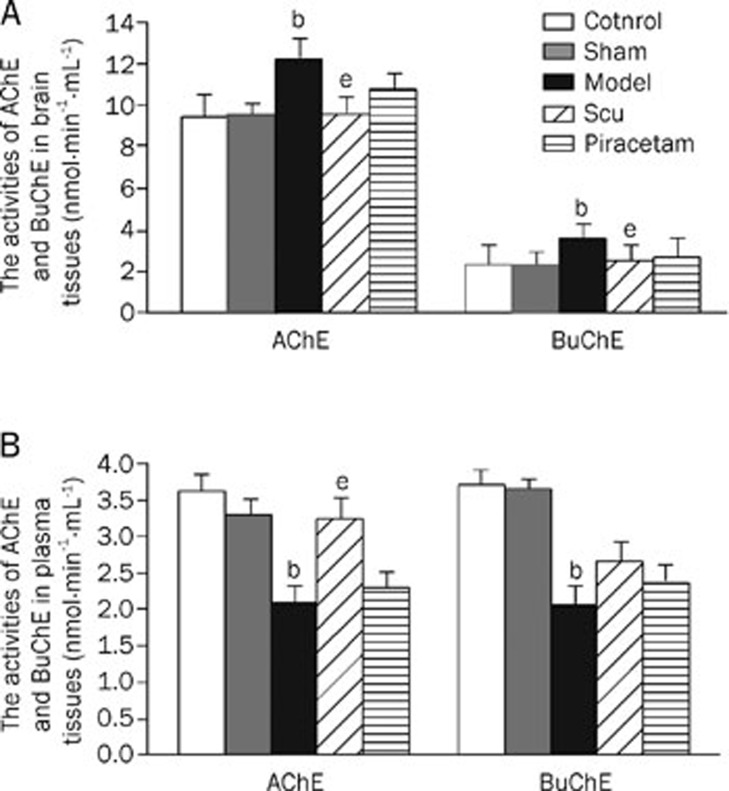
Activities of AChE and BuChE in brain and plasma
The activities of AChE and BuChE in the brain were significantly increased in the learning and memory deficit model group as compared to the control and the sham operation groups. However, the activities in both enzymes in plasma were significantly lower in the learning and memory deficit model group than those in the control and the sham operation groups. However, treatment by Scu or piracetam restored the activities in brain and plasma to near those of the control group (Figure 5).
Figure 5.
Activities of AChE and BuChE in the rat brains (A) and plasma (B). The activities of AChE and BuChE were determined by the improved Ellman's colorimetric method. bP<0.05 as compared to normal and sham operation groups. eP<0.05 as compared to model group as determined by an analysis of the variance ANOVA with Tukey-Kramer using SPSS15.0 software.
Discussion
Aβ, the major component in amyloid plaques of AD brains, is a 39- to 43-amino acid peptide derived from APP. Numerous groups have demonstrated that icv injection of several types of Aβ, including Aβ1–42, Aβ1–40, and Aβ25–35 (the neurotoxic domain of the full-length Aβ), into rats or mice impairs learning and memory25, 26, 27, 28. We have previously reported high levels of apoptosis in neuronal cultures induced by Aβ1–40 and Aβ25–35, while the reversed sequence Aβ35–25 had no effect29. In this study, we observed that the rats treated with Aβ25–35 icv exhibited impaired learning and memory, as demonstrated by increases in the escape latency period and the time required to pass the platform, and the decrease in the numbers of crossing the platform.
The nAChRs are ligand-gated ion channels consisting of five subunits that form a central, cation-permeable channel30. The nAChRs are generated from α (α2–α10) and β (β2–β4) subunits31 and the most abundant nAChR subtypes in brains are α4β2 and α732. A number of studies have indicated that deficient numbers of nAChRs play an important role in AD pathogenesis, specifically, a resultant decrease in α7, α4, and α3 nAChR subunit proteins, but the amount of β2 does not change in models of AD or AD brains33. Interestingly, changed α3 and β2 nAChR subunits, but not α4, were observed in the brains of patients with Parkinson disease34. For this study, goat polyclonal anti-α4 and -β2 antibodies and mouse monoclonal anti-α7 antibody were purchased from Santa Cruz Biotechnology Inc to detect the level of nAChR proteins. In our previous publications, the specific immunoreactivity of these antibodies has been well characterized and could identify the different nAChR subunits33. In this study, decreased expression of α4 and α7 nAChR subunits was observed in the brains of rats treated with Aβ, suggesting a toxic effect of Aβ on the receptors. Meanwhile, consistent with previous reports, no change occurred in β2 nAChR subunit35, in which the mechanism may be related to that the β subunit is considered as a structural rather than functional part and less influenced by Aβ35. In addition, despite differences in protein levels, we did not find any significant changes of α4, α7, or β2 nAChR subunit mRNA among the different groups, suggesting that the Aβ-mediated effect on nAChRs may be post-translational36.
ChEs are a group of serine hydrolases with two major forms, AChE and BuChE. The two enzymes differ in substrate specificity, kinetics and activity in different brain regions37, but they work synergistically to terminate ACh signaling by hydrolyzing, and thereby inactivating the transmitter. It has been proposed that AChE may interact with Aβ to promote fibrillogenesis and the deposition of amyloid plaques38, which might induce ACh downregulation and the degeneration or death of cholinergic neurons14. BuChE is biochemically related to AChE, and in particular, catalyzes the hydrolysis of ACh along with AChE, and thus serves as a co-regulator of cholinergic transmission39. Activities of both AChE and BuChE were reduced in plasma from AD patients. In our previous study, we also found the decreased activity of AChE in plasma of AD patients, which may reflect the altered central cholinergic function40. However, it has been reported that despite an overall decrease in the amount of AChE, the activity of the enzyme is increased around Aβ plaques in AD brains41, 42. This paradoxical finding may be due to the toxic Aβ affecting the catalysis of ACh. In this study, the activities of AChE and BuChE were significantly decreased in plasma but increased in brain tissues of the rats from the learning and memory deficit group, findings that correlate with previously reported results.
To date, there are no effective pharmacological interventions for AD, a fact that has stimulated interest in alternative therapies43, including traditional Chinese medicines44. HE is produced mainly in the Chinese regions of Hunan, Guizhou, Yunnan and Tibet and has been extensively used to treat brain injury, cardiovascular disorders and other diseases. It has been reported that a commercial herbal extract of HE, breviscapine (in which Scu is a major active principle), can improve learning and memory and protect against brain injury45, 46. In addition, flavones, one of the major components of HE, have been shown to possess neuroprotective effects9 and inhibit formation of reactive oxygen species47, 48, 49, 50. Furthermore, Scu can inhibit Aβ aggregation and prevent cell death induced by Aβ in cultured PC12 cells1, 3.
In this study, the rats were injected with Aβ to induce learning and memory dysfunction and then treated with Scu extracted from HE in order to investigate the effects of the compound on learning and memory function, expression of nAChRs and the activity of ChE. The results we obtained here showed that Scu rescued behavioral deficits induced by icv injected Aβ. This treatment also prohibited the decreased expression of α4 and α7 nAChR subunit proteins and recovered the activities of AChE and BuChE to normal levels in the AD animal model. Because the functions of nAChRs and ChE are believed to be integral to the processes of learning and memory, the results here indicate that Scu may stimulate cholinergic neuroprotective effects to ameliorate learning and memory dysfunction. Another possibility is that the positive effect of Scu on the cognitive deficits of the animals might be mediated via a direct or indirect effect through nAChRs against Aβ toxicity29, a hypothesis we plan to investigate in future studies.
Preclinical research suggests that piracetam, a neurotropic drug, may improve cognitive functions in AD patients51, possibly due to its ability to bind several central nervous system neurotransmitter receptors to protect against neuronal damage52. Therefore, we utilized the drug as a positive drug control in the study. The results here also showed that Scu and piracetam had similar effects. For some of the results, such as the rescue of cognitive deficits and enhanced activity of ChE, Scu was more effective than piracetam.
In summary, an acute Aβ treatment can induce learning and memory dysfunction in rats, inhibit expression of nAChR subunit proteins in the brain, and affect activity levels of AChE and BuChE in the brain or plasma. Importantly, Scu may reverse some of these outcomes. The results suggest that the mechanism concerning the protective effect of Scu on learning and memory deficit of rats induced by Aβ might involve stimulating expression of nAChRs and regulating activity of ChE, an insight that may provide an important therapeutic strategy for AD.
Author contribution
Li-li GUO performed experiments, analyzed data and wrote the paper; Zhi-zhong GUAN designed research, performed revisions, and approved the final version to be published; Yong-lin WANG carried out the isolation and the analysis of Scu.
Acknowledgments
This work was financed by grants from the National Natural Science Foundation (No 30870986), the Foundations in the Ministry of Science and Technology of China (2006DFA33530), and the Foundations in the Guizhou Province of China.
References
- Zhu JT, Choi RC, Li J, Xie HQ, Bi CW, Cheung AW, et al. Estrogenic and neuroprotective properties of scutellarin from erigeron breviscapus: a drug against postmenopausal symptoms and Alzheimer's disease. Planta Med. 2009;75:1489–93. doi: 10.1055/s-0029-1185776. [DOI] [PubMed] [Google Scholar]
- Miksicek RJ. Commonly occurring plant flavonoids have estrogenic activity. Mol Pharmacol. 1993;44:37–43. [PubMed] [Google Scholar]
- Zhu JT, Choi RC, Chu GK, Cheung AW, Gao QT, Li J, et al. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing beta-amyloid-induced cell death. J Agric Food Chem. 2007;55:2438–45. doi: 10.1021/jf063299z. [DOI] [PubMed] [Google Scholar]
- Wang CN, Chi CW, Lin YL, Chen CF, Shiao YJ. The neuroprotective effects of phytoestrogens on amyloid beta protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J Biol Chem. 2001;276:5287–95. doi: 10.1074/jbc.M006406200. [DOI] [PubMed] [Google Scholar]
- Kim H, Park BS, Lee KG, Choi CY, Jang SS, Kim YH, et al. Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J Agric Food Chem. 2005;53:8537–41. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, et al. Estrogen for Alzheimer's disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- Zhang HF, Hu XM, Wang LX, Xu SQ, Zeng FD. Protective effects of scutellarin against cerebral ischemia in rats: evidence for inhibition of the apoptosis-inducing factor pathway. Planta Med. 2009;75:121–6. doi: 10.1055/s-0028-1088368. [DOI] [PubMed] [Google Scholar]
- Lin LL, Liu AJ, Yu XH, Qin LP, Su DF. Protective effects of scutellarin and breviscapine on brain and heart ischemia in rat. J Cardiovasc Pharmacol. 2005;50:327–32. doi: 10.1097/FJC.0b013e3180cbd0e7. [DOI] [PubMed] [Google Scholar]
- Barrantes FJ, Borroni V, Vallés S. Neuronal nicotinic acetylcholine receptor-cholesterol crosstalk in Alzheimer's disease. FEBS Lett. 2010;584:1856–63. doi: 10.1016/j.febslet.2009.11.036. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Wirths O. Intracellular accumulation of amyloid-beta-a predictor for synaptic dysfunction and neuron loss in Alzheimer's disease. Front Aging Neurosci. 2010;10:2, 8. doi: 10.3389/fnagi.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmotto M, Giliberto L, Tamagno E, Tabaton M. Oxidative stress mediates the pathogenic effect of different Alzheimer's disease risk factors. Front Aging Neurosci. 2010;9:2, 3. doi: 10.3389/neuro.24.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Nishimura S, Kumagae Y, Kaneko I. In vivo conversion of racemized beta-amyloid ([D-Ser 26] A beta 1–40) to truncated and toxic fragments ([D-Ser 26]A beta 25–35/40) and fragment presence in the brains of Alzheimer's patients. J Neurosci Res. 2002;70:474–83. doi: 10.1002/jnr.10391. [DOI] [PubMed] [Google Scholar]
- Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness. Trends Neurosci. 1999;22:273–80. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- Mesulam M. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show. Learn Mem. 2004;11:43–9. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Lippiello P, Letchworth SR, Gatto GJ, Traina VM, Bencherif M. Ispronicline: a novel alpha4beta2 nicotinic acetylcholine receptor-selective agonist with cognition-enhancing and neuroprotective properties. J Mol Neurosci. 2006;30:19–20. doi: 10.1385/JMN:30:1:19. [DOI] [PubMed] [Google Scholar]
- Viegas C Jr, Bolzani Vda S, Barreiro EJ, Fraga CA. New anti-Alzheimer drugs from biodiversity: the role of the natural acetylcholinesterase inhibitors. Mini Rev Med Chem. 2005;5:915–26. doi: 10.2174/138955705774329546. [DOI] [PubMed] [Google Scholar]
- Kamal MA, Qu X, Yu QS, Tweedie D, Holloway HW, Li Y, et al. Tetrahydrofurobenzofuran cymserine, a potent butyrylcholinesterase inhibitor and experimental Alzheimer drug candidate, enzyme kinetic analysis. J Neural Transm. 2008;115:889–98. doi: 10.1007/s00702-008-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Yang HJ, Zhao J. Development of an assay for screening beta-amyloid aggregation inhibitors in vitro and study on inhibitive activity of fridelin. Chin Pharm J. 2005;40:1474–7. [Google Scholar]
- Paxinos G, Watson C. New York: Academic Press; 1982. The rat brain in stereotaxic coordinates. [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Liu RY, Gu R, Qi XL, Tang Z, Zhao Y, He Y, et al. Decreased nicotinic receptors and cognitive deficit in rats intracerebroventricularly injected with beta-amyloid peptide(1–42) and fed a high-cholesterol diet. J Neurosci Res. 2008;86:183–93. doi: 10.1002/jnr.21463. [DOI] [PubMed] [Google Scholar]
- An Y, Qi XL, Pei JJ, Tang Z, Xiao Y, Guan ZZ. Amyloid precursor protein gene mutated at Swedish 670/671 sites in vitro induces changed expression of nicotinic acetylcholine receptors and neurotoxicity. Neurochem Int. 2010;57:647–54. doi: 10.1016/j.neuint.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Ellman G, Courtney D, Andres V, Feather RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Miyashita H, Tsunekawa H, Mouri A, Kim HC, Saito K, et al. Effects of a novel cognitive enhancer, spiro[imidazo-[1,2-a]pyridine-3,2-indan]-2(3H)-one (ZSET1446), on learning impairments induced by amyloid-beta1–40 in the rat. J Pharmacol Exp Ther. 2006;317:1079–87. doi: 10.1124/jpet.105.098640. [DOI] [PubMed] [Google Scholar]
- Mazzola C, Micale V, Drago F. Amnesia induced by beta-amyloid fragments is counteracted by cannabinoid CB1 receptor blockade. Eur J Pharmacol. 2003;477:219–25. doi: 10.1016/j.ejphar.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Wang CN, Chi CW, Lin YL, Chen CF, Shiao YJ. The neuroprotective effects of phytoestrogens on amyloid beta protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J Biol Chem. 2001;276:5287–95. doi: 10.1074/jbc.M006406200. [DOI] [PubMed] [Google Scholar]
- Jhoo JH, Kim HC, Nabeshima T, Yamada K, Shin EJ, Jhoo WK, et al. Beta-amyloid (1–42)-induced learning and memory deficits in mice: involvement of oxidative burdens in the hippocampus and cerebral cortex. Behav Brain Res. 2004;155:185–96. doi: 10.1016/j.bbr.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Miao H, Tian JY, Unger C, Nordberg A, Zhang X. Suppressed expression of nicotinic acetylcholine receptors by nanomolar? amyloid peptides in PC12 cells. J Neural Transm. 2001;108:1417–33. doi: 10.1007/s007020100017. [DOI] [PubMed] [Google Scholar]
- Steven DB, Andrew KJ, Laurence AB, David BS. Nicotinic acetylcholine receptor signalling: roles in Alzheimer's disease and amyloid neuroprotection. Pharmacol Rev. 2009;61:39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Lindstrom JM. Nicotinic acetylcholine receptors of muscles and nerves: comparison of their structures, functional roles, and vulnerability to pathology. Ann N Y Acad Sci. 2003;998:41–52. doi: 10.1196/annals.1254.007. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer's disease. J Neurochem. 2000;74:237–43. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Nordberg A, Mousavi M, Rinne JO, Hellström-Lindahl E. Selective changes in the levels of nicotinic acetylcholine receptor protein and of corresponding mRNA species in the brains of patients with Parkinson's disease. Brain Res. 2002;956:358–66. doi: 10.1016/s0006-8993(02)03571-0. [DOI] [PubMed] [Google Scholar]
- Court J, Martin-Ruiz C, Piggott M. Nicotinic receptor abnormalities in Alzheimer's disease. Biol Psychiatry. 2001;49:175–84. doi: 10.1016/s0006-3223(00)01116-1. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S. Alzheimer's disease and acetylcholine receptors. Acta Neurobiol Exp. 2004;64:99–105. doi: 10.55782/ane-2004-1495. [DOI] [PubMed] [Google Scholar]
- Patocka J, Kuca K, Jun D. Acetylcholinesterase and butyrylcholinesterase-important enzymes of human body. Acta Medica (Hradec Kralove) 2004;47:215–28. [PubMed] [Google Scholar]
- Rees T, Hammond PI, Soreq H, Younkin S, Brimijoin S. Acetylcholinesterase promotes beta-amyloid plaques in cerebral cortex. Neurobiol Aging. 2003;24:777–87. doi: 10.1016/s0197-4580(02)00230-0. [DOI] [PubMed] [Google Scholar]
- Geula C, Darvesh S. Butyrylcholinesterase, cholinergic neurotransmission and the pathology of Alzheimer's disease. Drugs Today (Barc) 2004;40:711–21. doi: 10.1358/dot.2004.40.8.850473. [DOI] [PubMed] [Google Scholar]
- Zhang LJ, Xiao Y, Qi XL, Shan KR, Pei JJ, Kuang SX, et al. Cholinesterase activity and mRNA level of nicotinic acetylcholine receptors (alpha4 and beta2 Subunits) in blood of elderly Chinese diagnosed as Alzheimer's disease. J Alzheimers Dis. 2010;19:849–58. doi: 10.3233/JAD-2010-1283. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Asuncion Morán M. Cholinesterases within neurofibrillary tangles related to age and Alzheimer's disease. Ann Neurol. 1987;22:223–8. doi: 10.1002/ana.410220206. [DOI] [PubMed] [Google Scholar]
- Ulrich J, Meier-Ruge W, Probst A, Meier E, Ipsen S. Senile plaques: staining for acetylcholinesterase and A4 protein: a comparative study in the hippocampus and entorhinal cortex. Acta Neuropathol. 1990;80:624–8. doi: 10.1007/BF00307630. [DOI] [PubMed] [Google Scholar]
- Melnikova I. Therapies for Alzheimer's disease. Nat Rev Drug Discov. 2007;6:341–2. doi: 10.1038/nrd2314. [DOI] [PubMed] [Google Scholar]
- Howes MJ, Houghton PJ. Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav. 2003;75:513–27. doi: 10.1016/s0091-3057(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Liu JX, Liu Y, Chen XL, Zhao JJ, Song TS, Qian YH. Breviscapine improves functions of spatial learning and memory of focal cerebral ischemia rats. Zhong Yao Cai (Chinese) 2009;32:548–56. [PubMed] [Google Scholar]
- Xiong Z, Liu C, Wang F, Li C, Wang W, Wang J, et al. Protective effects of breviscapine on ischemic vascular dementia in rats. Biol Pharm Bull. 2006;29:1880–5. doi: 10.1248/bpb.29.1880. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang XL, Zhou LZ, Xu HB. Study on reactive oxygen species scavenging effects of scutellarin. J Chin Med Mat. 2002;25:491–3. [PubMed] [Google Scholar]
- Liu H, Yang XL, Wang Y, Tang XQ, Jiang DY, Xu HB. Protective effects of scutellarin on superoxide-induced oxidative stress in rat cortical synaptosomes. Acta Pharmacol Sin. 2003;24:1113–7. [PubMed] [Google Scholar]
- Xu W, Zha RP, Wang WY, Wang YP. Effects of scutellarin on PKC in PC12 cell injury induced by oxygen and glucose deprivation. Acta Pharmacol Sin. 2007;28:1573–9. doi: 10.1111/j.1745-7254.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- Hong H, Liu GQ. Protection against hydrogen peroxide-induced cytotoxicity in PC12 cells by scutellarin. Life Sci. 2004;74:2959–73. doi: 10.1016/j.lfs.2003.09.074. [DOI] [PubMed] [Google Scholar]
- Croisile B, Trillet M, Fondarai J, Laurent B, Mauguière F, Billardon M. Long-term and high-dose piracetam treatment of Alzheimer's disease. Neurology. 1993;43:301–5. doi: 10.1212/wnl.43.2.301. [DOI] [PubMed] [Google Scholar]
- Bering B, Muller WE. Interaction of piracetam with several neurotransmitter receptors in the central nervous system. Arzneim Forsch. 1985;35:1350–2. [PubMed] [Google Scholar]