Abstract
Aim:
To investigate the effects of high cholesterol diet on the development of osteoporosis and the underlying mechanisms in rats.
Methods:
Female Sprague-Dawley rats were randomly separated into 3 groups: (1) the high cholesterol fed rats were fed a high cholesterol diet containing 77% normal diet food, 3% cholesterol and 20% lard for 3 months; (2) ovariectomised (OVX) rats were bilaterally ovariectomised and fed a standard diet; and (3) the control rats were fed the standard diet. Bone mineral density (BMD) of the rats was measured using dual-energy X-ray absorptiometry. Serum levels of oestradiol (E2), osteocalcin (BGP) and carboxy-terminal collagen crosslinks (CTX) were measured using ELISA. Gene expression profile was determined with microarray. Mouse osteoblast cells (MC3T3-E1) were used for in vitro study. Proliferation, differentiation and oxidative stress of the osteoblasts were investigated using MTT, qRT-PCR and biochemical methods.
Results:
In high cholesterol fed rats, the femur BMD and serum BGP level were significantly reduced, while the CTX level was significantly increased. DNA microarray analysis showed that 2290 genes were down-regulated and 992 genes were up-regulated in this group of rats. Of these genes, 1626 were also down-regulated and 1466 were up-regulated in OVX rats. In total, 370 genes were up-regulated in both groups, and 976 genes were down-regulated. Some of the down-regulated genes were found to code for proteins involved in the transforming growth factor beta (TGF-β)/bone morphogenic protein (BMP) and Wnt signaling pathways. The up-regulated genes were found to code for IL-6 and Ager with bone-resorption functions. Treatment of MC3T3-E1 cells with cholesterol (12.5-50 μg/mL) inhibited the cell proliferation and differentiation in vitro in a concentration-dependent manner. The treatment also concentration-dependently reduced the expression of BMP2 and Cbfa1, and increased the oxidative injury in MC3T3-E1 cells.
Conclusion:
The results suggest a close correlation between hypercholesterolaemia and osteoporosis. High cholesterol diet increases the risk of osteoporosis, possible via inhibiting the differentiation and proliferation of osteoblasts.
Keywords: high cholesterol diet, hypercholesterolaemia, osteoporosis, osteoblasts, oxidative stress, bone morphogenic protein
Introduction
Osteoporosis is one of the most common bone diseases, affecting millions of people worldwide. Postmenopausal osteoporosis is a major health problem in women. Osteoporosis is associated with an increased risk of low-trauma fractures to the vertebral spine, femoral neck and distal radius that result in substantial morbidity. Even if the age-adjusted incidence of hip fracture remained stable, the estimated number of hip fractures globally will probably increase from 1.7 million in 1990 to an estimated 6.3 million in 20501.
Previous human clinical studies have found that hypercholesterolaemia is associated with a lower bone mineral density (BMD). It was reported that postmenopausal women with hypercholesterolaemia have a significantly higher serum bone-specific alkaline phosphatase (BAP) level compared with postmenopausal women with a normal lipid profile2. In addition, both BAP and the N-terminal telopeptide of type I collagen (NTx) are significantly and positively correlated with both serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) in women. Postmenopausal women who have never taken hormone replacement therapy (HRT) show significantly higher cholesterol levels and have lower BMD measurements in the lumbar spine than premenopausal women3. In a South Korean population-based study of 375 premenopausal and 355 postmenopausal rural women it was shown that levels of serum TC and LDL-C were inversely correlated with BMD4. Furthermore, plasma LDL-C and high-density lipoprotein cholesterol (HDL-C) levels have been shown to be inversely and positively correlated with BMD in both men and women, respectively5, 6. However, in some studies, serum triglyceride and HDL cholesterol were found to be correlated with BMD, but no relationship was seen between either total cholesterol or LDL and BMD7, 8. D'Amelio et al showed that HDL was significantly higher in osteoporotic patients than in controls and the risk of osteoporosis was significantly higher in women with high HDL9. The study provided evidence of the relationship between HDL, but not total cholesterol or LDL, and BMD in this cohort of normal-weight women.
Lipid-lowering drugs (statins) increase the BMD of the hip or femoral neck10, 11, 12, 13. However, some anti-osteoporotic drugs such as selective estrogen receptor modulators (SERMS, eg raloxifene) only moderately reduce serum levels of TC and LDL-C14, 15, 16. All of this evidence points to a clinical correlation between hyperlipidaemia and postmenopausal osteoporosis.
In this study, we investigated the effect of a high cholesterol diet on bone metabolism in rats. We also studied the effect of free cholesterol on the proliferation and differentiation of osteoblasts. We found that hypercholesterolemia in the rat was associated with a reduction of bone density, an increase in bone resorption and a reduction in bone formation. DNA microarray analysis showed that the bone morphogenic protein (BMP)/ transforming growth factor beta (TGF-β) and Wnt pathways, involved in bone formation, were altered by a high cholesterol diet in rats. Furthermore, in vitro studies showed that free cholesterol reduced the proliferation and differentiation of osteoblasts, and inhibited the expression of BMP2 and core binding factor alpha 1 (Cbfa1). Free cholesterol also increased the level of malondialdehyde (MDA) and decreased the activity of superoxidase dismutase (SOD) in osteoblasts.
Materials and methods
Animals and experimental treatment
Three-month-old female Sprague-Dawley rats (180–200 g, n=34) were obtained from the Shanghai Laboratory Animal Center. After a 1-week acclimatization, the rats were randomly separated into three groups. The high cholesterol fed rats were fed a high cholesterol diet containing 77% normal diet food, 3% cholesterol and 20% lard (n=12). Ovariectomised (OVX) rats were bilaterally ovariectomised and fed a standard diet. Aseptic surgery was performed via a 1–1.5-cm axillary midline incision and the ovaries were removed. Then the skin was stapled and washed with alcohol (n=12). The control group of rats (n=10) were fed a standard diet. All rats were kept at room temperature (21±2 °C) under a normal 12-h light: 12-h dark regime with good ventilation and convenient access to food and water. The experimental protocol was developed according to the institution's guideline for the care and use of laboratory animals.
Serum cholesterol and BMD measurement
After 3 months of treatment, the rats were sacrificed and blood was collected. The cholesterol concentration was measured by automated biochemistry equipment. Femurs and 1–4 lumbar vertebrae (LV) were also excised. The whole left femur and lumbar vertebrae (1–4) were used for further analysis. The bone mineral content and area was measured, and BMD was automatically calculated via dual-energy X-ray absorptiometry (HOLOGIC, MA, USA).
Serum E2, BGP, CTX, ALP measurement
The sera were collected for the ALP testing according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In brief, 50 μL serums was mixed with solution I and II and then incubated for 15 min at 37 °C. The chromogenic agent was added and the absorbance at 570 nm was measured. Rat sera were collected and the concentration of estradiol (E2, R&D, MN, USA), osteocalcin (BGP, Immunodiagnostic Systems Limited, Boldon, UK) and carboxy-terminal collagen crosslinks (CTX, Immunodiagnostic Systems Limited, Boldon, UK) were measured according to the description of manufacturers by ELISA. Serum (20 μL) was incubated with the first antibody specifically for each protein at room temperature for 1 h. Then the plate were washed with wash buffer and incubated with biotin-labeled antibody to each first antibody for 1 h at room temperature and washed again. Finally the chromogenic agent was added and incubated for 15 min and the reaction was stopped with H2SO4. Absorbance was read at 450 nm (reference wavelength 630 nm).
RNA extraction and microarray analysis
An Affymetrix rat genome 230 2.0 array was used, consisting of more than 31 000 probe sets, representing more than 30 000 transcripts and variants from over 28 000 well-substantiated rat genes. For gene expression analysis by microarray, a procedure from Affymetrix was followed. Total RNA from the proximal femurs was extracted using TRIzol (Invitrogen, CA, USA) and an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocols. All these experiments were performed by Shanghai Biochip Co Ltd (Shanghai, China).
Cell culture and drug administration
The mouse osteoblast cells (MC3T3-E1) were cultured in α-MEM medium (GIBCO BRL, CA, USA) supplemented with penicillin 100 IU/mL, streptomycin 100 IU/mL and 10% fetal bovine serum (GIBCO BRL, CA, USA). Cholesterol (Sigma, MO, USA) was dissolved in ethanol, and different concentrations were used for the experiment.
MTT assay
For the MTT (Sigma, MO, USA) assay, MC3T3-E1 cells at a density of 5×103 cells/well were inoculated in 96-well plates in the presence or absence of cholesterol. The concentrations of cholesterol were 0, 12.5, 25, and 50 μg/mL. After 72 h, 20 μL of 5 mg/mL MTT was added to each well and incubated for 5 h. The medium was removed and 150 μL DMSO was added to each well and incubated for 10 min. The absorbance of the culture plate was read at 570 nm. The inhibition ratio was calculated using the following formula: proliferation ratio=Mean Absorbance of Sample/Mean Absorbance of Control×100%.
ALP, SOD, and MDA measurement
The cell lysate were collected for the ALP, SOD, and MDA measurement according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The ALP measurement in cell lysate is the same with the serum ALP testing. In brief, for testing SOD, the cell lysate were incubated with solution I, II, III, and IV for 40 min at 37 °C. The chromogenic agent was added and the absorbance was read at 550 nm for SOD activity. For detecting MDA, the cell lysate were incubated with solution I, II and III for 40 min at 95 °C. The absorbance was read at 532 nm for MDA concentration.
Quantitative real-time PCR (qRT-PCR)
For the qRT-PCR assay, MC3T3-E1 cells at a density of 1×106 cells/well were inoculated in 6-well plates in the presence or absence of 12.5 μg/mL cholesterol. After 24 h, the cells were collected, and total RNA was extracted using TRIzol reagent (Invitrogen, CA, USA) according to manufacturer's instructions. The qRT-PCR was performed using One Step SYBR PrimeScriptTM RT-PCR Kit II (Takara, Tokyo, Japan) following the manufacturer's instructions. Primers for the qRT-PCR assay were as follows: Col1A1, forward 5′-TAC AGC ACG CTT GTG GAT G-3′, reverse 5′-TTG GGA TGG AGG GAG TTT A-3′ ALP, forward 5′ GAC GGT GAA CGG GAG AAC-3′, reverse 5′-CTC AGA ACA GGG TGC GTA G-3′ BMP2, forward 5′-GGA CTG CGG TCT CCT AA AG-3′ reverse 5′-CAG CCT CAA CTC AAA CTC G-3′ Cbfa1, forward 5′-AGA CAC AGA GCC TGT GGG-3′ reverse 5′-CTC TGG CTT GGA TTA GGG A-3′ β-actin, forward 5′-GAA ATC GTG CGT GAC ATT A-3′, reverse 5′-GGA GCC AGG GCA GTA ATC-3′. For quantification of gene expression changes, the relative quantification method was used.
Statistical analyses
Data were presented as the mean±standard deviation (SD). ANOVA and post-hoc analysis were used for comparison. All statistical analyses were performed using SPSS11.0.
Results
BMD was decreased in high cholesterol fed rats and OVX rats
After treatment with a high cholesterol diet containing 77% normal diet food, 3% cholesterol and 20% lard for 3 months, the level of cholesterol was increased, accompanied with an increase in body weight. The level of cholesterol was also increased in OVX rats fed with normal diets. There was no difference in the serum level of triglycerides, calcium and phosphorus between the three groups (Table 1).
Table 1. Effect of high cholesterol diet and OVX on weight, total cholesterol (TC), triglycerides (TG), calcium (Ca), phosphorus (P), ALP, osteocalcin (BGP), carboxy-terminal collagen crosslinks (CTX), estrogen (E2), and bone mineral density (BMD) level. Data are presented as the mean±SD. bP<0.05 vs Normal group.
| Normal (n=10) | High cholesterol fed rats (n=12) | OVX rats (n=12) | |
|---|---|---|---|
| Weight (g) | 311.00±21.95 | 439.17±65.76b | 320.83±28.87 |
| TC (mmol/L) | 1.87±0.39 | 3.17±1.06b | 2.04±0.7 |
| TG (mmol/L) | 0.76±0.17 | 0.58±0.18 | 0.71±0.26 |
| Ca (mmol/L) | 2.40±0.15 | 2.47±0.19 | 2.38±0.26 |
| P (mmol/L) | 4.16±2.13 | 4.47±1.68 | 4.64±1.98 |
| ALP (U/100 mL) | 16.15±0.68 | 12.34±0.46b | 20.41±0.71b |
| BGP (ng/mL) | 500.79±120.51 | 331.40±124.78b | 754.87±222.34b |
| CTX (ng/mL) | 62.49±31.31 | 85.55±30.82b | 95.77±36.96b |
| E2 (ng/L) | 4.44±0.33 | 4.54±0.39 | 4.05±0.31b |
| Femur BMD | 0.15±0.012 | 0.12±0.014b | 0.12±0.02b |
| Lumbar vertebrae BMD | 0.13±0.03 | 0.14±0.008 | 0.13±0.011 |
BMD in the femurs was significantly decreased in high cholesterol fed rats and OVX rats compared with controls (both P<0.01). ALP and BGP levels were reduced and the CTX level was increased in high cholesterol fed rats (both P<0.05). The intra- and inter-assay coefficient of variations (CV) of BGP were 4.7% and 5.6%, respectively; the CV of CTX were 4.1% and 5.3%, respectively. Estrogen concentration was reduced in OVX rats. High cholesterol diet had no effect on the estrogen concentration (Table 1), and the CV of E2 were 3.4% and 8.9%, respectively. It suggests that the high cholesterol diet reduces the BMD and bone formation and increases bone resorption in rats.
Gene expression profiles
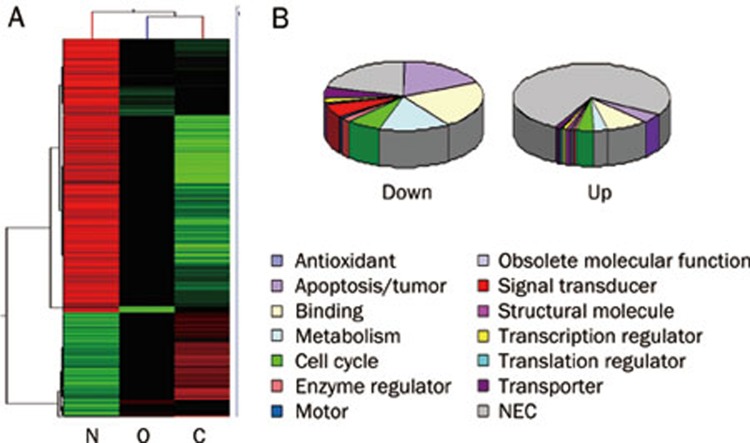
In high cholesterol fed rats, 2290 genes were down-regulated and 992 genes were up-regulated. In OVX rats, 1626 genes were down-regulated and 1466 genes were up-regulated. A comparative cluster analysis was carried out with the sets of differentially expressed genes to evaluate the correlation between the two groups of rats. In total, 370 genes were up-regulated in both groups, and 976 genes were down-regulated (Figure 1A). Genes with the most remarkable change in both groups include those coding for proteins involved in apoptosis, binding activity, metabolism, and the cell cycle. The change patterns for these genes were consistent in the two groups (Figure 1B).
Figure 1.
Gene expression profiles. Comparative analysis was performed to show the differentially expressed genes in high cholesterol fed and OVX rats. Cluster analysis of differentially expressed genes in high cholesterol fed rats and OVX rats. (B) The functional distribution of the differentially expressed genes in high cholesterol fed rats and OVX rats. N: normal rats; O: OVX rats with osteoporosis; C: high cholesterol fed rats.
GO and pathway analysis for gene expression
Some of the downregulated genes, Wnt5, β-catenin, Tgfbr, smad4, smad6, smad7, Bmpr2, and BMP6, are involved in the TGF-β/BMP2 and Wnt signalling pathway (Table 2). In addition, the up-regulated genes were found to code for IL-6 and Ager with bone-resorption functions.
Table 2. The expression of bone formation-related genes in the two groups.
| Gene name | Gene symbol | High cholesterol fed rats | OVX rats |
|---|---|---|---|
| Insulin-like growth factor binding protein 5 | Igfbp5 | −7.1 | −3.1 |
| Procollagen, Type XII, Alpha 1 | Col12a1 | −6.3 | −4.3 |
| Matrix metallopeptidase 8 | Mmp8 | −4.8 | −2.5 |
| Core binding factor beta | Cbfb | −4.6 | −2.4 |
| Caveolin | Cav | −4.6 | −3.7 |
| Transforming growth factor, beta receptor II | Tgfbr2 | −4.0 | −4.2 |
| Leptin receptor | Lepr | −4.0 | −2.1 |
| Wingless-type MMTV integration site 5A | Wnt5a | −3.8 | −1.0 |
| Bone morphogenetic protein 6 | Bmp6 | −3.8 | −1.4 |
| MAD homolog 6 (Drosophila) | Smad6 | −3.8 | −1.2 |
| Osteoglycin | Ogn | −3.7 | −1.9 |
| Bone morphogenic protein receptor, type II | Bmpr2 | −3.7 | −2.9 |
| MAD homolog 7 | Smad7 | −3.7 | −2.2 |
| Transforming growth factor, beta receptor III | Tgfbr3 | −3.7 | −3.2 |
| Insulin-like growth factor binding protein 7 | Igfbp7 | −3.1 | −1.0 |
| Catenin (Cadherin Associated Protein), beta 1 | Ctnnb1 | −3.1 | −1.4 |
| CD276 antigen | Cd276 | −3.1 | −3.1 |
| Parathyroid hormone receptor 2 | Pthr2 | −2.9 | −1.4 |
| Fibroblast growth factor receptor 2 | Fgfr2 | −2.9 | −1.2 |
| Fibroblast growth factor 9 | Fgf9 | −2.9 | −0.8 |
| Calcitonin receptor | Calcr | −2.8 | −2.6 |
| MAD homolog 4 (Drosophila) | Smad4 | −2.7 | −1.1 |
| Fibroblast growth factor 5 | Fgf5 | −2.5 | −3.7 |
| Procollagen, Type III, Alpha 1 | Col3a1 | −2.5 | −0.8 |
| MAD homolog 9 (Drosophila) | Smad9 | −2.3 | −0.1 |
| Matrix metallopeptidase 7 | Mmp7 | −2.3 | −3.0 |
| 3-Hydroxy-3-methylglutaryl-coenzyme A reductase | Hmgcr | −2.3 | −1.0 |
| MAD homolog 5 (Drosophila) | Smad5 | −2.2 | −2.0 |
| Interleukin 6 signal transducer | Il6st | −2.2 | −1.7 |
| Fibroblast growth factor 8 | Fgf8 | −2.1 | −0.7 |
| Apolipoprotein E | Apoe | −2.0 | −1.4 |
| Procollagen, Type I, Alpha 2 | Col1a2 | −1.8 | −0.4 |
| Cbp/P300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 1 | Cited1 | 2.1 | 0.9 |
| Interleukin 6 | IL6 | 2.2 | 3.0 |
| Interferon beta 1 | Ifnb1 | 3.1 | 1.3 |
| Advanced glycosylation end product-specific receptor | Ager | 4.6 | 4.2 |
The proliferation of osteoblasts in vitro
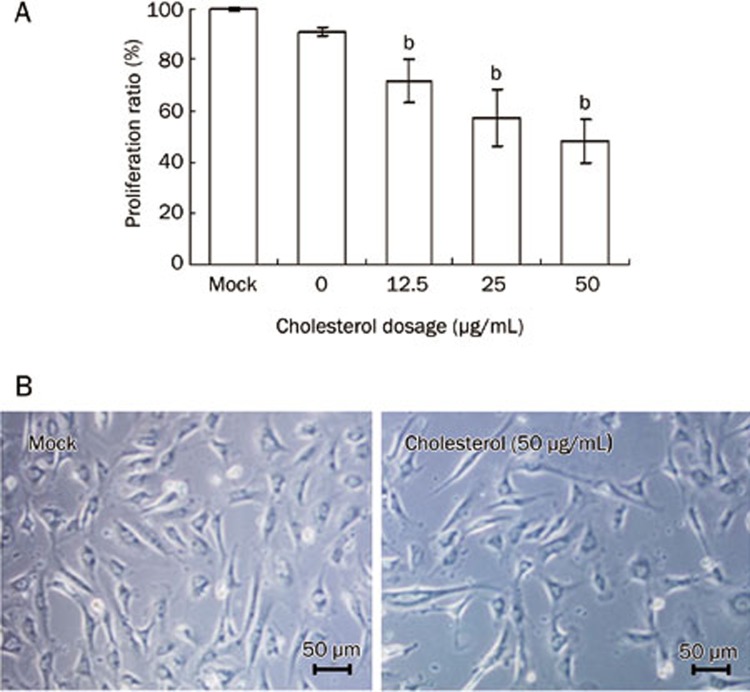
Free cholesterol at 12.5-50 μg/mL inhibited the proliferation and viability of MC3T3-E1 cells compared with control group (P<0.05, Figure 2A, 2B).
Figure 2.
MC3T3-E1 proliferation with the treatment of cholesterol. MC3T3-E1 cells were cultured in medium with 0, 12.5, 25, and 50 μg/mL of cholesterol for 72 h, cell proliferation was tested by MTT assay (A) and cell viability was observed by microscopy (B). Administration schedules are described in Materials and Methods. Data are shown as the mean±SD of eight wells for each group within one experiment, and it was repeated three times. bP<0.05 vs Mock.
The expression of ALP, collagen I, BMP2, and Cbfa1 genes in MC3T3-E1 cells
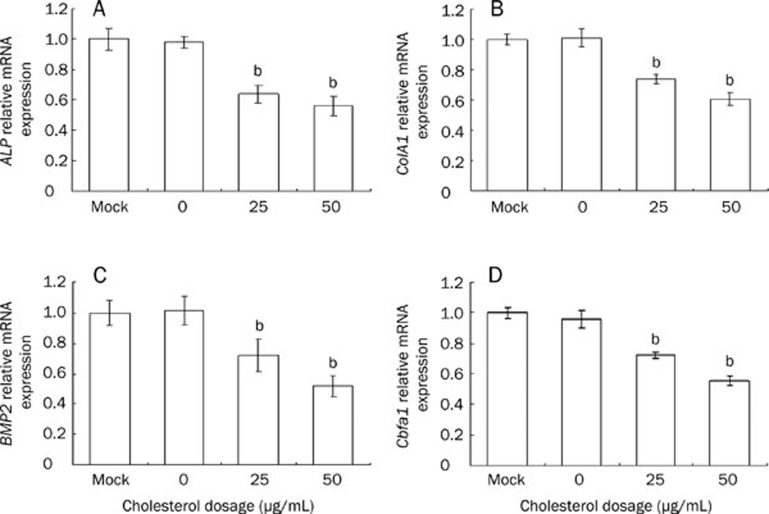
Cholesterol 25–50 μg/mL reduced the mRNA expression of ALP and collagen I, BMP2, and Cbfa1 compared with the control group (P<0.05, Figure 3).
Figure 3.
The expression of ALP, ColA1, BMP2, and Cbfa1 genes in MC3T3-E1 cells after the treatment of cholesterol. (A) ALP, (B) Collagen I, (C) BMP2 and (D) Cbfa1 were analyzed by real-time PCR. Data are shown as the mean±SD of three wells for each group within one experiment, and it was repeated for three times. bP<0.05 vs Mock.
The level of ALP, SOD, and MDA in MC3T3-E1 cells
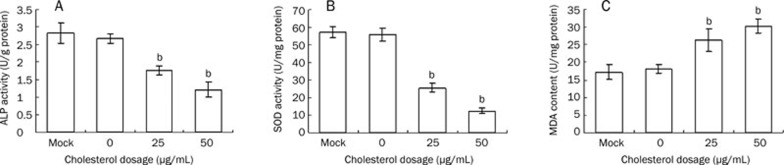
Cholesterol 50 μg/mL reduced the activity of ALP. Cholesterol treatment also reduced the activity of SOD and increased the level of MDA in vitro (Figure 4).
Figure 4.
The ALP, SOD activity and the level of MDA in MC3T3-E1 cells treated with cholesterol. Cells were collected after the treatment of 4 d and lysed with 0.1% triton X-100, and the lysate were used for ALP (A), SOD (B) and MDA (C) analysis. Data are shown as the mean±SD of eight wells for each group within one experiment, and it was repeated for three times. bP<0.05 vs Mock.
Discussion
In our clinical studies, we observed that postmenopausal women with hypercholesterolaemia showed increased bone turnover and lower BMD, which is consistent with the data reported in other clinical studies2, 3, 4. We thus hypothesise that a high cholesterol diet induces or promotes the development of osteoporosis. To confirm this hypothesis, we investigate the effects of high cholesterol diet on the development of osteoporosis and the underlying mechanisms. High cholesterol diet significantly increased the weight and the total cholesterol level in serum. BMD decreased in most rats fed with high cholesterol diet. The serum levels of ALP and BGP were decreased and CTX level was increased, indicating that high cholesterol diet increased bone loss. It not only promoted bone resorption, but also reduced bone formation. Interestingly, most OVX rats had developed hypercholesterolaemia, which was similar with the Gurer's study17. These data are consistent with our previous findings in clinic that a high cholesterol diet will increase the risk of osteoporosis.
Estrogen deficiency is critical to the pathogenesis of osteoporosis in postmenopausal women, as levels naturally decline, and this results in the imbalance between osteoclast-mediated bone resorption and bone formation18, 19. In our experiments, the estrogen level was reduced in the OVX rats, but not in high cholesterol diet fed rats. This suggests that the osteoporosis induced by the cholesterol diet was not the result of estrogen deficiency. High cholesterol fed rats had higher body weights, which is consistent with the view that obesity is related wtih osteoporosis. Kim20 showed that high percentage body fat and waist circumference correlated with low BMD and risk of a vertebral fracture. Obesity-reduced bone density was associated with activation of PPARgamma and suppression of Wnt/beta-catenin in rapidly growing male rats21.
Previous studies have shown that increased arterial calcification and blood lipid correlate with an increase in osteoporosis. Lipids have been shown to accumulate in bones of rats and blood vessels around bone in patients with osteoporosis22, 23. Osteoporosis has been associated with both atherosclerosis and vascular calcification24, 25, 26. Serum lipid levels may alter bone mineralization. In Xiao' study, a high fat diet induced the increased expression of genes involved in bone resorption and decreased expression of genes associated with bone formation27. In this study, in the high cholesterol fed rats there was also a decrease in the expression of genes involved in bone formation and an increase in the expression of genes associated with bone resorption. Genes involved in TGF-β/BMP and Wnt pathways promote the differentiation of mesenchymal stem cells into osteoblasts and facilitate the proliferation and maturation of osteoblasts. They also regulate the differentiation of osteoblasts, the secretion of bone matrix and mineralization of the bone matrix28, 29, 30. Some genes involved in these pathways were also down-regulated in high cholesterol fed rats, which will lead to a suppression of differentiation, proliferation and maturation of osteoblasts, as well as decreased bone formation. The expression of these genes was lower in high cholesterol diet fed rats than those in OVX rats.
Our in vitro studies also support a role for high blood lipids in the proliferation and differentiation of osteoblasts. Statins exerted a direct stimulatory effect on osteoblast cells31, 32 and simvastatin promoted osteoblast differentiation33. Parhami et al34 found that minimally oxidized low-density lipoprotein (MM-LDL) inhibited the differentiation of MC3T3-E1 cells. These studies suggest that higher levels of lipids inhibit the differentiation of osteoblasts but treatment with hypolipidemic drugs will recover the differentiation of osteoblasts. Free cholesterol inhibited the proliferation and differentiation of osteoblasts in vitro. The proliferation of MC3T3-E1 cells and the level of ALP and collagen I in osteoblasts were reduced by cholesterol. In addition, cholesterol reduced the expression of BMP2 and Cbfa1. Free cholesterol may inhibits the bone formation via the BMP2 pathway.
Oxidative status influences the pathophysiology of mineralised tissues35. The role of oxidative injury is one of the mechanisms for the high lipid-induced osteoporosis. In bone-derived preosteoblasts, oxidized low-density lipoprotein (oxLDL) and other bioactive oxidized lipids inhibit the expression of various markers of osteoblast differentiation34. Also OxLDL and oxidative products inhibit osteogenic differentiation of mesenchymal stem cells and preosteoblast in favour of an adipogenic differentiation36, 37. We found that free cholesterol inhibited bone formation, reduced the activity of SOD and increased the level of MDA, which indicated that the free cholesterol increased the oxidative injury in osteoblasts in vitro.
In conclusion, a close correlation between hypercholesterolaemia and osteoporosis was observed in our study. High cholesterol diet increased the bone resorption and reduced bone formation in rats, accompanied by a reduction in BMD. The results confirmed our hypothesis that a high cholesterol diet increases the risk of osteoporosis. Our in vitro experiments showed that free cholesterol inhibited the proliferation and differentiation of osteoblasts, and it reduced bone formation by decreasing BMP2 expression and increasing oxidative injury. High cholesterol diet increases the risk of osteoporosis, possibly via inhibiting the differentiation and proliferation of osteoblasts.
Author contribution
Li YOU designed research; Chuan-ling TANG and Ling PAN performed research; Zheng-yan SHENG contributed new analytical tools and reagents; Lin CHEN and Jin-yu CHEN analyzed data; Li YOU wrote the paper.
Acknowledgments
We thank Shanghai Biochip Co Ltd for their excellent technical assistance and appreciate the help of Dr Yan XIONG in the editing of the manuscript. This work was supported by Shanghai First People's Hospital, Shanghai Jiaotong University.
References
- Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–8. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- Majima T, Shimatsu A, Komatsu Y, Satoh N, Fukao A, Ninomiya K, et al. Increased bone turnover in patients with hypercholesterolemia. Endocr J. 2008;55:143–51. doi: 10.1507/endocrj.k07e-004. [DOI] [PubMed] [Google Scholar]
- Makovey J, Chen JS, Hayward C, Williams FM PN S. Association between serum cholesterol and bone mineral density. Bone. 2009;44:208–13. doi: 10.1016/j.bone.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Cui LH, Shin MH, Chung EK, Lee YH, Kweon SS, Park KS, et al. Association between bone mineral densities and serum lipid profiles of pre- and post-menopausal rural women in South Korea. Osteoporos Int. 2005;16:1975–81. doi: 10.1007/s00198-005-1977-2. [DOI] [PubMed] [Google Scholar]
- Adami S, Braga V, Zamboni M, Gatti D, Rossini M, Bakri J, et al. Relationship between lipids and bone mass in 2 cohorts of healthy women and men. Calcif Tissue Int. 2004;74:136–42. doi: 10.1007/s00223-003-0050-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, et al. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. 2002;49:211–7. doi: 10.1507/endocrj.49.211. [DOI] [PubMed] [Google Scholar]
- Dennison EM, Syddall HE, Aihie Sayer A, Martin HJ, Cooper C. Lipid profile, obesity and bone mineral density: the Hertfordshire Cohort Study. QJM. 2007;100:297–303. doi: 10.1093/qjmed/hcm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong IK, Cho SW, Kim SW, Choi HJ, Park KS, Kim SY, et al. Lipid profiles and bone mineral density in pre- and postmenopausal women in Korea. Calcif Tissue Int. 2010;87:507–12. doi: 10.1007/s00223-010-9427-3. [DOI] [PubMed] [Google Scholar]
- D'Amelio P, Di Bella S, Tamone C, Ravazzoli MG, Cristofaro MA, Di Stefano M, et al. HDL cholesterol and bone mineral density in normal-weight postmenopausal women: is there any possible association. Panminerva Med. 2008;50:89–96. [PubMed] [Google Scholar]
- Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Effects of statins on bone mineral density: a meta-analysis of clinical studies. Bone. 2007;40:1581–7. doi: 10.1016/j.bone.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Majima T, Komatsu Y, Fukao A, Ninomiya K, Matsumura T, Nakao K. Short-term effects of atorvastatin on bone turnover in male patients with hypercholesterolemia. Endocr J. 2007;54:145–51. doi: 10.1507/endocrj.k06-127. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Finkelstein JS, Wang PS, Avorn J. Statin lipid-lowering drugs and bone mineral density. Pharmacoepidemiol Drug Safety. 2005;14:219–26. doi: 10.1002/pds.984. [DOI] [PubMed] [Google Scholar]
- Lupattelli G, Scarponi AM, Vaudo G, Siepi D, Roscini AR, Gemelli F, et al. Simvastatin increases bone mineral density in hypercholesterolemic postmenopausal women. Metabolism. 2004;53:744–8. doi: 10.1016/j.metabol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Nanetti L, Camilletti A, Francucci CM, Vignini A, Raffaelli F, Mazzanti L, et al. Role of raloxifene on platelet metabolism and plasma lipids. Eur J Clin Invest. 2008;38:117–25. doi: 10.1111/j.1365-2362.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- Majima T, Komatsu Y, Shimatsu A, Satoh N, Fukao A, Ninomiya K, et al. Clinical significance of 1-year treatment with raloxifene on bone and lipid metabolism in Japanese postmenopausal women with osteoporosis. Endocr J. 2007;54:855–62. doi: 10.1507/endocrj.k06-208. [DOI] [PubMed] [Google Scholar]
- Dayspring T, Qu Y, Keech C. Effects of raloxifene on lipid and lipoprotein levels in postmenopausal osteoporotic women with and without hypertriglyceridemia. Metabolism. 2006;55:972–9. doi: 10.1016/j.metabol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Gurer G, Sendur OF, Aydeniz A. Serum lipid profile in postmenopausal women with osteoporosis or osteopenia. South Med J. 2006;99:95–6. doi: 10.1097/01.smj.0000197511.31712.23. [DOI] [PubMed] [Google Scholar]
- Shoback D. Update in osteoporosis and metabolic bone disorders. J Clin Endocrinol Metab. 2007;92:747–53. doi: 10.1210/jc.2007-0042. [DOI] [PubMed] [Google Scholar]
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–25. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, Shin DH, Lee SY, Im JA, Lee DC. Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med J. 2010;51:857–63. doi: 10.3349/ymj.2010.51.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Wu X, Tong Y, Blackburn ML, Shankar K, et al. Obesity reduces bone density associated with activation of PPARgamma and suppression of Wnt/beta-catenin in rapidly growing male rats. PLoS One. 2010;5:e13704. doi: 10.1371/journal.pone.0013704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhami F, Garfinkel A, Demer LL. Role of lipids in osteoporosis. Arterioscler Thromb Vasc Biol. 2000;20:2346–8. doi: 10.1161/01.atv.20.11.2346. [DOI] [PubMed] [Google Scholar]
- Rajendran KG, Chen SY, Sood A, Spielvogel BF, Hall IH. The anti-osteoporotic activity of amine-carboxyboranes in rodents. Biomed Pharmacother. 1995;49:131–40. doi: 10.1016/0753-3322(96)82606-0. [DOI] [PubMed] [Google Scholar]
- Hmamouchi I, Allali F, Khazzani H, Bennani L, El Mansouri L, Ichchou L, et al. Low bone mineral density is related to atherosclerosis in postmenopausal Moroccan women. BMC Public Health. 2009;9:388. doi: 10.1186/1471-2458-9-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998;62:209–13. doi: 10.1007/s002239900419. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Akishita M, de Souza AC, Nakamura T, Orimo H. Age-related loss of bone mass and aortic/aortic valve calcification — reevaluation of recommended dietary allowance of calcium in the elderly. Ann N Y Acad Sci. 1993;676:297–307. doi: 10.1111/j.1749-6632.1993.tb38743.x. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Cui J, Li YX, Shi YH, Le GW. Expression of genes associated with bone resorption is increased and bone formation is decreased in mice fed a high-fat diet. Lipids. 2010;45:345–55. doi: 10.1007/s11745-010-3397-0. [DOI] [PubMed] [Google Scholar]
- Yavropoulou MP, Yovos JG. The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones (Athens) 2007;6:279–94. doi: 10.14310/horm.2002.1111024. [DOI] [PubMed] [Google Scholar]
- Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-beta1 to the bone. Endocr Rev. 2005;26:743–74. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- Groeneveld EH, Burger EH. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol. 2000;142:9–21. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]
- Maeda T, Matsunuma A, Kurahashi I, Yanagawa T, Yoshida H, Horiuchi N. Induction of osteoblast differentiation indices by statins in MC3T3-E1 cells. J Cell Biochem. 2004;92:458–71. doi: 10.1002/jcb.20074. [DOI] [PubMed] [Google Scholar]
- Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–9. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- Maeda T, Matsunuma A, Kawane T, Horiuchi N. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun. 2001;280:874–7. doi: 10.1006/bbrc.2000.4232. [DOI] [PubMed] [Google Scholar]
- Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol Med. 2009;15:468–77. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–19. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- Parhami F, Jackson SM, Tintut Y, Le V, Balucan JP, Territo M, et al. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J Bone Miner Res. 1999;14:2067–78. doi: 10.1359/jbmr.1999.14.12.2067. [DOI] [PubMed] [Google Scholar]