Abstract
Murine retroviruses which encode c-myb proteins that have either complete or truncated carboxy (C) termini were used to infect haemopoietic cells from murine fetal liver. Using an agar colony assay, we could show that infection with the virus encoding the truncated protein resulted in the persistence of colony-forming cells well beyond the short period for which such cells are present in uninfected cultures. The resultant colonies failed to give rise to cell lines; however, clonal cell lines occasionally emerged from the original infected liquid cultures. The virus which encoded a myb protein with a complete C-terminus was virtually inactive in the colony assay; surprisingly, however, this virus could enhance proliferation in liquid cultures and has led to the generation of at least one cell line. In addition to demonstrating 'activation' of c-myb by C-terminal truncation, our results imply that an unaltered c-myb protein can also contribute to cellular transformation and that a second event is required to establish myb-transformed cells as a permanent cell line.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowtell D. D., Cory S., Johnson G. R., Gonda T. J. Comparison of expression in hemopoietic cells by retroviral vectors carrying two genes. J Virol. 1988 Jul;62(7):2464–2473. doi: 10.1128/jvi.62.7.2464-2473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M. F., Kukowska-Latallo J. F., Westin E., Smith M., Prochownik E. V. Constitutive expression of a c-myb cDNA blocks Friend murine erythroleukemia cell differentiation. Mol Cell Biol. 1988 Feb;8(2):884–892. doi: 10.1128/mcb.8.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Metcalf D., Nicola N. A., Burgess A. W., Walker F. Malignant transformation of a growth factor-dependent myeloid cell line by Abelson virus without evidence of an autocrine mechanism. Cell. 1985 Jul;41(3):677–683. doi: 10.1016/s0092-8674(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Cook W. D. Thymocyte subsets transformed by Abelson murine leukemia virus. Mol Cell Biol. 1985 Feb;5(2):390–397. doi: 10.1128/mcb.5.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Cory S., Sobieszczuk P., Holtzman D., Adams J. M. Generation of altered transcripts by retroviral insertion within the c-myb gene in two murine monocytic leukemias. J Virol. 1987 Sep;61(9):2754–2763. doi: 10.1128/jvi.61.9.2754-2763.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Gough N. M., Dunn A. R., de Blaquiere J. Nucleotide sequence of cDNA clones of the murine myb proto-oncogene. EMBO J. 1985 Aug;4(8):2003–2008. doi: 10.1002/j.1460-2075.1985.tb03884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Ramsay R. G., Johnson G. R. Murine myeloid cell lines derived by in vitro infection with recombinant c-myb retroviruses express myb from rearranged vector proviruses. EMBO J. 1989 Jun;8(6):1767–1775. doi: 10.1002/j.1460-2075.1989.tb03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., von Kirchbach A., Beug H. Characterization of the hematopoietic target cells of AEV, MC29 and AMV avian leukemia viruses. Exp Cell Res. 1981 Feb;131(2):331–343. doi: 10.1016/0014-4827(81)90236-6. [DOI] [PubMed] [Google Scholar]
- Hapel A. J., Vande Woude G., Campbell H. D., Young I. G., Robins T. Generation of an autocrine leukaemia using a retroviral expression vector carrying the interleukin-3 gene. Lymphokine Res. 1986 Fall;5(4):249–254. [PubMed] [Google Scholar]
- Ibanez C. E., Lipsick J. S. Structural and functional domains of the myb oncogene: requirements for nuclear transport, myeloid transformation, and colony formation. J Virol. 1988 Jun;62(6):1981–1988. doi: 10.1128/jvi.62.6.1981-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. R., Gonda T. J., Metcalf D., Hariharan I. K., Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989 Feb;8(2):441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Nature of cells forming erythroid colonies in agar after stimulation by spleen conditioned medium. J Cell Physiol. 1978 Mar;94(3):243–252. doi: 10.1002/jcp.1040940302. [DOI] [PubMed] [Google Scholar]
- Kanter M. R., Smith R. E., Hayward W. S. Rapid induction of B-cell lymphomas: insertional activation of c-myb by avian leukosis virus. J Virol. 1988 Apr;62(4):1423–1432. doi: 10.1128/jvi.62.4.1423-1432.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Ramsay G., Bishop J. M., Moscovici M. G., Moscovici C., McGrath J. P., Levinson A. D. The product of the retroviral transforming gene v-myb is a truncated version of the protein encoded by the cellular oncogene c-myb. Cell. 1983 Jun;33(2):345–355. doi: 10.1016/0092-8674(83)90416-6. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Gazzolo L., Moscovici M. G. Focus assay and defectiveness of avian myeloblastosis virus. Virology. 1975 Nov;68(1):173–181. doi: 10.1016/0042-6822(75)90159-2. [DOI] [PubMed] [Google Scholar]
- Mucenski M. L., Taylor B. A., Ihle J. N., Hartley J. W., Morse H. C., 3rd, Jenkins N. A., Copeland N. G. Identification of a common ecotropic viral integration site, Evi-1, in the DNA of AKXD murine myeloid tumors. Mol Cell Biol. 1988 Jan;8(1):301–308. doi: 10.1128/mcb.8.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina Y., Nakagoshi H., Imamoto F., Gonda T. J., Ishii S. Trans-activation by the c-myb proto-oncogene. Nucleic Acids Res. 1989 Jan 11;17(1):107–117. doi: 10.1093/nar/17.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overell R. W., Watson J. D., Gallis B., Weisser K. E., Cosman D., Widmer M. B. Nature and specificity of lymphokine independence induced by a selectable retroviral vector expressing v-src. Mol Cell Biol. 1987 Oct;7(10):3394–3401. doi: 10.1128/mcb.7.10.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci P. G., Lanfrancone L., Brathwaite M. D., Wolman S. R., Dalla-Favera R. Amplification of the c-myb oncogene in a case of human acute myelogenous leukemia. Science. 1984 Jun 8;224(4653):1117–1121. doi: 10.1126/science.6585957. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Radke K., Beug H., Kornfeld S., Graf T. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell. 1982 Dec;31(3 Pt 2):643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- Rosson D., Reddy E. P. Nucleotide sequence of chicken c-myb complementary DNA and implications for myb oncogene activation. Nature. 1986 Feb 13;319(6054):604–606. doi: 10.1038/319604a0. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Rettenmier C. W., Sherr C. J. Introduction of a human colony stimulating factor-1 gene into a mouse macrophage cell line induces CSF-1 independence but not tumorigenicity. Blood. 1988 May;71(5):1218–1225. [PubMed] [Google Scholar]
- Shen-Ong G. L., Morse H. C., 3rd, Potter M., Mushinski J. F. Two modes of c-myb activation in virus-induced mouse myeloid tumors. Mol Cell Biol. 1986 Feb;6(2):380–392. doi: 10.1128/mcb.6.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stober-Grässer U., Lipsick J. S. Specific amino acid substitutions are not required for transformation by v-myb of avian myeloblastosis virus. J Virol. 1988 Mar;62(3):1093–1096. doi: 10.1128/jvi.62.3.1093-1096.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Cleveland J. L., Askew D. S., Rapp U. R., Ihle J. N. Insertion and truncation of c-myb by murine leukemia virus in a myeloid cell line derived from cultures of normal hematopoietic cells. J Virol. 1987 Jul;61(7):2339–2343. doi: 10.1128/jvi.61.7.2339-2343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Ihle J. N., Lavu S., Reddy E. P. Truncation of the c-myb gene by a retroviral integration in an interleukin 3-dependent myeloid leukemia cell line. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5010–5014. doi: 10.1073/pnas.83.14.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
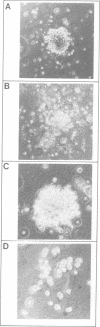
- Wong P. M., Chung S. W., Nienhuis A. W. Retroviral transfer and expression of the interleukin-3 gene in hemopoietic cells. Genes Dev. 1987 Jun;1(4):358–365. doi: 10.1101/gad.1.4.358. [DOI] [PubMed] [Google Scholar]
- Yung Y. P., Moore M. A. Long-term in vitro culture of murine mast cells. III. Discrimination of mast cells growth factor and granulocyte-CSF. J Immunol. 1982 Sep;129(3):1256–1261. [PubMed] [Google Scholar]