Abstract
Aim:
CYP2C9 enzyme metabolizes numerous clinically important drugs. The aim of this study is to investigate the frequencies of CYP2C9 genotypes and the effects of selected alleles on losartan pharmacokinetics in a large sample of the Korean population.
Methods:
The CYP2C9 gene was genotyped in 1796 healthy Korean subjects. CYP2C9 alleles (CYP2C9*1, *2, *3 and *13 alleles) were measured using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay and direct sequencing assay. The enzymatic activity of each CYP2C9 genotype was evaluated using losartan as the substrate.
Results:
The frequencies of CYP2C9*1, *3 and *13 allele were 0.952 (95% confidence interval 0.945–0.959), 0.044 (95% CI 0.037–0.051) and 0.005 (95% CI 0.003–0.007), respectively. The frequencies of the CYP2C9*1/*1, *1/*3, *1/*13 and *3/*3 genotypes were 0.904 (95% CI 0.890–0.918), 0.085 (95% CI 0.072–0.098), 0.009 (95% CI 0.005–0.013) and 0.001 (95% CI 0.000–0.002), respectively. In the pharmacokinetics studies, the AUC0–∞ of losartan in CYP2C9*3/*3 subjects was 1.42-fold larger than that in CYP2C9*1/*1 subjects, and the AUC0–∞ of E-3174, a more active metabolite of losartan, in CYP2C9*3/*3 subjects was only 12% of that in CYP2C9*1/*1 subjects.
Conclusion:
The results confirmed the frequencies of CYP2C9 genotypes in a large cohort of Koreans, and detected the CYP2C9*3/*3 genotype. CYP2C9*3/*3 subjects metabolized much less losartan into E-3174 than CYP2C9*1/*1 subjects.
Keywords: CYP2C9, allele, genotype, Korean, pharmacokinetics, losartan
Introduction
The cytochrome P450 (CYP) 2C9 enzyme oxidizes many clinically important compounds, including drugs with narrow therapeutic indexes such as warfarin, tolbutamide, and phenytoin, as well as other common drugs such as glibenclamide, glimepiride, glipizide, losartan, irbesartan, torsemide, and many anti-inflammatory drugs1, 2. Genetic polymorphisms in enzymes that metabolize drugs are major determinants of variability in individual response. Thirty-five alleles of the CYP2C9 gene have been reported (http://www.cypalleles.ki.se/cyp2c9.htm) and three of these, CYP2C9*1, *2 and *3, are frequently identified in most populations. The CYP2C9*2 allele is the most common deleterious allele among people of European descent, with a frequency of 0.080 to 0.191. The CYP2C9*3 allele is less common (0.033–0.162)3. In contrast, the CYP2C9*2 allele is rare among East Asians3, 4, and CYP2C9*3 is more common than that in Europeans (0.007 to 0.060)5. In addition, CYP2C9*3 homozygotes are seldom detected in East Asian populations5. CYP2C9*3 has the lowest metabolic activity in vitro, while CYP2C9*2 has an intermediate enzyme activity, CYP2C9*1 the highest activity6. Individuals with mutant CYP2C9 variants may not metabolize drugs adequately, leading to drug toxicity. Therefore, drug doses must be adjusted according to genotype. The frequencies of CYP2C9 alleles vary between populations, information that is useful for clinical pharmacotherapy. The frequencies of CYP2C9 alleles and genotypes in the Korean population have been calculated5, 7, 8, 9, however, there are significant discrepancies in the reported CYP2C9*3 frequencies. Thus, we measured the CYP2C9 allele and genotype frequencies in a large Korean cohort, where we detected the CYP2C9*3/*3 genotype and analyzed the effects of the CYP2C9*3/*3 genotype on losartan pharmacokinetics.
Materials and methods
Subjects
We enrolled 1796 unrelated healthy Korean volunteers in this genotyping study. Written informed consent was obtained from all volunteers.
Genotyping tests
Genomic DNA was isolated from peripheral blood leukocytes using the Wizard® Genomic DNA Kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. Analyses of the CYP2C9*2, *3 and *13 alleles were performed using polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP), as described previously5. The CYP2C9*1 allele was assigned in the absence of other detectable alleles. The genotypes identified by PCR-RFLP were confirmed by sequence analysis. Exons and exon/intron junctions of the CYP2C9 gene were amplified as described with slight modifications5, 10. The PCR products were purified using a PCR purification kit (AxyPrep® PCR Clean-up Kit, Axygen Bioscience Inc, Union City, CA, USA) and sequenced on an ABI3730 automatic sequencer (Applied Biosystems Inc, Foster City, CA, USA) using a BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems Inc, Delaware, USA).
Protocol for pharmacokinetic studies
Thirteen healthy male Korean subjects with CYP2C9*1/*1 (n=12) or CYP2C9*3/*3 (n=1) genotypes were selected for a pharmacokinetic study of losartan. Although two subjects with CYP2C9*3/*3 were detected, one did not provide written informed consent. Thus, only one subject with CYP2C9*3/*3 was enrolled in the pharmacokinetic study.
The subjects were between 20 and 26 years old and had body mass indexes between 21 and 25 kg/m2. All subjects were healthy as defined by medical history, physical examination, and routine laboratory tests (blood chemistry, hematology, and urine analysis). The subjects were asked to refrain from ingesting medications, caffeine, grapefruit products, and alcoholic beverages and from smoking for at least 1 week before and during the study period. All subjects provided verbal and written informed consent after being given an explanation of the experimental procedures and purpose of the study. The institutional ethics committee of the School of Pharmacy, Sungkyunkwan University, Korea approved the study protocol. All procedures were performed in accordance with the recommendations of the Declaration of Helsinki on biomedical research involving human subjects.
On the day of the study, each subject received 50 mg of losartan potassium (Cozaar®, MSD-Korea, Seoul, Korea) orally with 240 mL of water after an overnight fast. The subjects maintained the fasting state for 4 h after receiving the drug. Before and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, and 24 h, venous blood samples (10 mL) were collected in heparinized tubes and centrifuged for 10 min at 3000 r/min. The plasma was separated and stored at -70 °C until needed.
Assay of losartan and E-3174 in plasma
CYP2C9 metabolizes losartan to a more active metabolite, E-317411. Thus, the losartan and E-3174 concentrations in the plasma were determined by HPLC with a fluorescence detector as previously reported with modifications12. Briefly, 1.0 mL of plasma, 150 ng of valsartan (IS), and 200 μL of 1 mol/L phosphoric acid were mixed in a glass tube, and extracted with 7 mL of methyl tert-butyl ether (MTBE) with constant vigorous stirring for 1 min. After centrifuging (2500 r/min for 10 min), the organic layer was transferred to another tube with 200 μL of 0.05 mol/L sodium hydroxide and stirred vigorously for 1 min. The samples were again centrifuged at 2500 r/min for 10 min. The aqueous layer was collected, and residual MTBE was removed by nitrogen evaporation. The sodium hydroxide layer was acidified with 50 μL of 0.2 mol/L phosphoric acid and mixed. The aqueous fraction was washed by adding 6 mL of n-hexane and mixing for 1 min. After centrifuging, the hexane was discarded, and residual n-hexane was removed by nitrogen evaporation. Methanol (150 μL) was added to 250 μL of the re-extracted water phase, and 100 μL of the resulting mixture was injected into the HPLC system. The HPLC system consisted of a Waters Model 515 HPLC pump, a Waters Model 717 Plus autosampler, a Waters 474 scanning fluorescence detector, and column oven (Waters, Milford, MA, USA). Separations were performed on a 5 μm Luna CN column (4.5 mm×250 mm; Phenomenex, Torrance, CA, USA). The mobile phase was 15 mmol/L phosphoric acid/acetonitrile (65:35, ν/ν) adjusted to pH 3.0 with 5 mol/L sodium hydroxide at 1 mL/min. The effluents were detected by fluorescence with excitation at 250 nm and emission at 380 nm. The standard curves for losartan and E-3174 were linear from 5 to 1000 ng/mL (r2>0.999). The mean accuracy for losartan and E-3174 were 90%–102% and 96%–101%, respectively. The coefficients of variation (within-day and between-day precisions) of losartan and E-3174 were <9% and 10%, respectively.
Pharmacokinetic analysis
The pharmacokinetic parameters of losartan and E-3174 were calculated by non-compartmental methods from the blood sampling times, maximum plasma concentration (Cmax), and time to reach Cmax (tmax) using the BA Calc 2007 analysis program (KFDA, Seoul, Korea). The area under the curve (AUC) for plasma concentration-time was calculated using the linear trapezoidal rule. The elimination rate constant (ke) was determined by linear regression analysis of the log-linear portion of the plasma concentration-time curve. The AUC from 0 to infinity (AUC0–∞) was calculated as AUC0–∞=AUC+Ct/ke(Ct being the final plasma concentration). The half-life (t1/2) was calculated as t1/2=ln 2/ke. The apparent oral clearance (CL/F) of losartan was calculated as CL/F=Dose/AUC0–∞.
Statistical analysis
Data were compiled according to the genotype and allele frequencies. The frequencies of each allele are reported with 95% confidence intervals. Hardy-Weinberg equilibrium was evaluated by comparing the genotype frequencies with the expected values using a contingency table χ2 test. Statistical significance was determined by χ2 test; a P-value less than 0.05 was considered significant. The pharmacokinetic data are expressed as mean±SD.
Results
Frequencies of CYP2C9 alleles and genotypes
The estimated frequencies of the CYP2C9 alleles and genotypes in the Korean population are summarized in Table 1. The genotype frequency distribution did not deviate significantly from Hardy-Weinberg equilibrium. CYP2C9*1 was the most common allele (0.952, 95% CI: 0.945–0.959). The most common variant allele was CYP2C9*3 (0.044, 95% CI: 0.037–0.051). The CYP2C9*1/*3 frequency in this study was more than four times higher than previously reported7(P<0.001, Table 2). The CYP2C9*13 frequency in our sample was 0.005 (95% CI: 0.003–0.007). There were 1624 subjects with the CYP2C9*1/*1 genotype (0.904, 95% CI: 0.890-0.918), 153 with the CYP2C9*1/*3 genotype (0.085, 95% CI: 0.072–0.098), 17 with the CYP2C9*1/*13 genotype (0.009, 95% CI: 0.005–0.013), and 2 with the CYP2C9*3/*3 genotype (0.001, 95% CI: 0.000-0.002)(Table 1). The genotype results from PCR-RFLP corresponded with the sequencing results (data not shown).
Table 1. CYP2C9 allele (A) and genotype (B) frequencies in a large Korean sample. The expected genotype frequencies were calculated from the allele frequencies using the Hardy-Weinberg equation.
| A | |||
|---|---|---|---|
| Allele | n (3592) | Frequency | 95% CI |
| CYP2C9*1 | 3418 | 0.952 | 0.945–0.959 |
| CYP2C9*2 | 0 | 0.000 | 0.000–0.000 |
| CYP2C9*3 | 157 | 0.044 | 0.037–0.051 |
| CYP2C9*13 | 17 | 0.005 | 0.003–0.007 |
| B | ||||
|---|---|---|---|---|
| Genotype | Number of subjects | Observed frequency | 95% CI | Expected frequency |
| CYP2C9*1/*1 | 1624 | 0.904 | 0.890–0.918 | 0.905 |
| CYP2C9*1/*3 | 153 | 0.085 | 0.072–0.098 | 0.083 |
| CYP2C9*1/*13 | 17 | 0.009 | 0.005–0.013 | 0.009 |
| CYP2C9*3/*3 | 2 | 0.001 | 0.000–0.002 | 0.002 |
| CYP2C9*3/*13 | 0 | 0.000 | 0.000–0.000 | 0.000 |
| CYP2C9*13/*13 | 0 | 0.000 | 0.000–0.000 | 0.000 |
Table 2. Comparisons of reported CYP2C9 allele frequencies in Koreans.
| n | CYP2C9 allele frequency | Reference | |||
|---|---|---|---|---|---|
| *1 | *2 | *3 | *13 | ||
| 3592 | 0.952 (0.945–0.959) | 0.000 (0.000–0.000) | 0.044 (0.037–0.051) | 0.005 (0.003–0.007) | Present study |
| 716 | 0.934 (0.916–0.952) | 0.000 (0.000–0.000) | 0.060 (0.043–0.077) | 0.006 (0.000–0.012) | 5 |
| 1148 | 0.989 (0.983–0.995) | 0.000 (0.000–0.000) | 0.011c (0.005–0.017) | ND | 7 |
| 590 | 0.947 (0.929–0.965) | 0.000 (0.000–0.000) | 0.051 (0.033–0.069) | 0.002 (0.000–0.006) | 13 |
Values in parentheses represent 95% confidence intervals; n=number of alleles; differences between frequency data were calculated using the chi-square test. ND=Not determined.
cP<0.01 between present and previous studies7 (95% CI on the difference 0.024–0.042).
The CYP2C9*3 allele frequency in our Korean sample was slightly (although not significantly) higher than in Chinese samples, and was significantly higher than in Japanese samples (P<0.01) (Table 3). The CYP2C9*13 allele frequency in our sample was slightly lower than in Chinese samples, and slightly higher than in Japanese samples, although these differences were not significant (Table 3).
Table 3. CYP2C9 allele frequencies in East Asian populations.
| Populations | n | CYP2C9 allele frequency | Reference | |||
|---|---|---|---|---|---|---|
| *1 | *2 | *3 | *13 | |||
| Korean | 4308 | 0.949 (0.942–0.956) | 0.000 (0.000–0.000) | 0.046 (0.040–0.052) | 0.005 (0.003–0.007) | Present study# |
| Chinese | 2174 | 0.964 | 0.000 | 0.036 | ND | 14 |
| 1008 | 0.967 | 0.000 | 0.033 | ND | 15 | |
| 788 | 0.963 | 0.001 | 0.036 | ND | 16 | |
| 400 | 0.975 | 0.000 | 0.025 | ND | 8 | |
| 338 | 0.967 | 0.000 | 0.033 | ND | 17 | |
| 230 | 0.983 | 0.000 | 0.017 | ND | 18 | |
| 204 | 0.951 | 0.000 | 0.049 | ND | 19 | |
| 796 | 0.958 | 0.001 | 0.041 | ND | 9 | |
| 658 | - | - | - | 0.006 | 20 | |
| 294 | - | - | - | 0.010 | 21 | |
| Sum of Chinese | 5938(952)* | 0.964 (0.959–0.969) | 0.003 (0.002–0.004) | 0.035 (0.030–0.040) | 0.007 (0.003–0.011) | |
| Japanese | 1000 | 0.966 | 0.000 | 0.034 | ND | 9 |
| 1448 | 0.968 | 0.000 | 0.032 | ND | 22 | |
| 1200 | 0.979 | 0.000 | 0.021 | ND | 23 | |
| 524 | 0.968 | 0.000 | 0.031 | 0.002 | 10 | |
| 436 | 0.979 | 0.000 | 0.021 | ND | 24 | |
| 400 | 0.965 | 0.000 | 0.035 | ND | 8 | |
| 280 | 0.982 | 0.000 | 0.018 | ND | 25 | |
| 236 | 0.970 | 0.000 | 0.030 | ND | 26 | |
| 246 | 0.955 | 0.000 | 0.045 | ND | 27 | |
| 294 | 0.993 | 0.000 | 0.007 | ND | 28 | |
| Sum of Japanese | 6064 (524)& | 0.972 (0.968–0.976) | 0.000 (0.000–0.000) | 0.028c (0.024–0.032) | 0.002 (0.000–0.005) | |
Values in parentheses represent 95% confidence intervals; n=number of alleles; differences between frequency data were calculated using the chi-square test. ND=Not determined.
#, Data were combined with a previous study5.
*,&, Values in parentheses represent the total numbers with the CYP2C9*13 allele.
cP<0.01 between present study and sum of Japanese (95% CI on the difference 0.010–0.026).
Pharmacokinetics of losartan
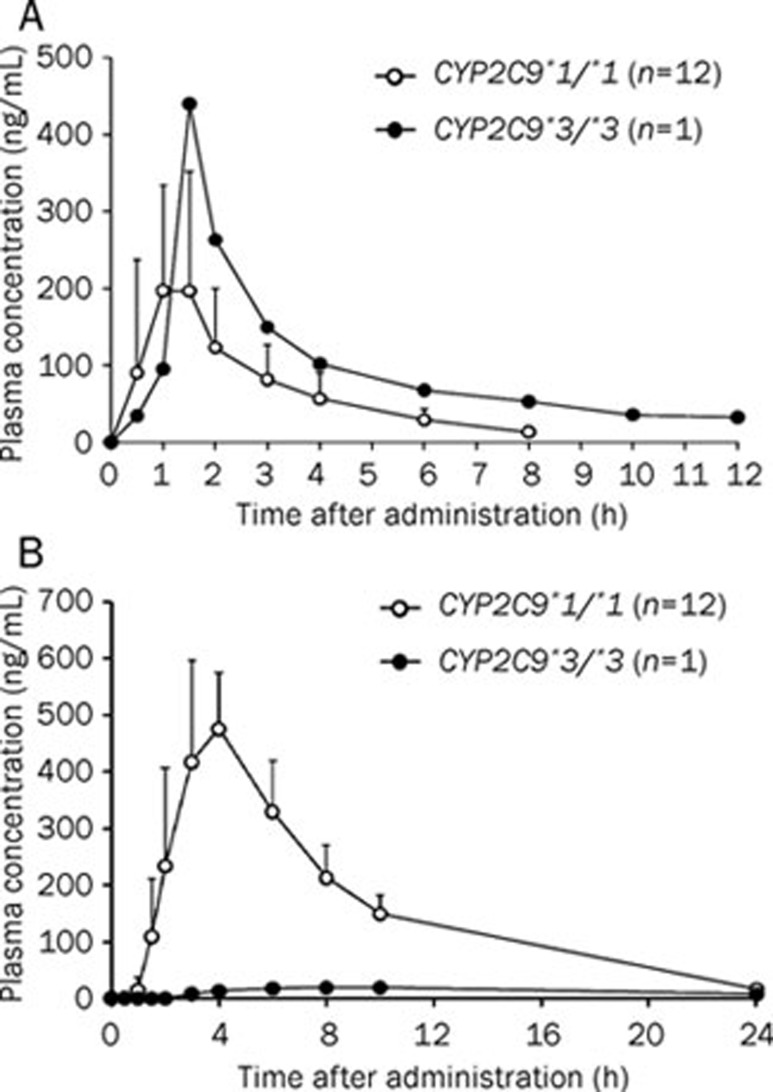
The losartan and E-3174 pharmacokinetic parameters were measured in one subject with the CYP2C9*3/*3 genotype, a rare genotype in Koreans (0.1%, Table 1). In this subject, the Cmax and AUC0–∞ of losartan were slightly higher and the CL/F was lower than in CYP2C9*1/*1 subjects, but losartan metabolism to E-3174 was almost completely blocked (Figure 1, Table 4).
Figure 1.
Plasma concentration-time profiles of losartan (A) and E-3174 (B) in subjects with the CYP2C9*1/*1 (n=12, open circles) or CYP2C9*3/*3 (n=1, closed circles) genotypes after administration of a single 50 mg oral dose of losartan.
Table 4. Pharmacokinetic parameters of oral losartan in subjects with the CYP2C9*1/*1 and CYP2C9*3/*3 genotypes. Mean±SD.
| Variable | CYP2C9*1/*1 (n=12) | CYP2C9*3/*3 (n=1) |
|---|---|---|
| Losartan | ||
| Cmax (ng/mL) | 235.1±98.4 (172.6, 297.6) | 440.2 |
| t1/2 (h) | 1.92±0.76 (1.44, 2.40) | 4.72 |
| CL/F (L/h) | 0.094±0.018 (0.082, 0.106) | 0.037 |
| AUC0-∞ (ng·h/mL) | 552.2±102.2 (487.3, 617.1) | 1334.9 |
| E-3174 | ||
| Cmax (ng/mL) | 524.3±84.1 (470.8, 577.8) | 19.1 |
| t1/2 (h) | 4.29±0.40 (4.04, 4.54) | 10.56 |
| AUC0-∞ (ng·h/mL) | 3471.9±466.2 (3175.7, 3768.1) | 400.9 |
Values in parentheses represent 95% confidence intervals. Cmax, maximum plasma concentration; AUC0-∞, area under the plasma concentration-time curve from time 0 to infinity; t1/2, elimination half-life; CL/F, apparent oral clearance; n, number of subjects.
Discussion
CYP2C9 catalyzes phase I metabolism for approximately 15%–20% of the drugs subject to this reaction. The CYP2C9 allelic variants CYP2C9*2, CYP2C9*3 and CYP2C9*13 code for enzymes with approximately 10%–40%, 5%–15% and 1%–12% of the activity of CYP2C9*1, respectively4, 10, 29. The CYP2C9*2 allele is the most common variant allele among people of European descent with a frequency of approximately 0.1324, 9, 16, 30. In contrast, the CYP2C9*2 allele is rare in East Asians3(Table 3). To date, the CYP2C9*2 allele has been detected in only two East Asian subjects, both Chinese with the CYP2C9*1/*2 genotype9, 16. We did not detect the CYP2C9*2 allele in our sample of 2154 Koreans (Table 3).
Functionally, the CYP2C9*3 allele has the lowest metabolic activity in vitro, while CYP2C9*2 lies between CYP2C9*3 and CYP2C9*16. Europeans have significant heterogeneity in the CYP2C9*2 allele frequency (ranging from 0.033 to 0.162), whereas the CYP2C9*3 allele is less common6. The CYP2C9*3 frequency in Koreans was previously reported as 0.0117, but we previously found it to be 0.0605. Because these results are so different, an additional study was needed. In addition, the first study found no difference in the CYP2C9*3 frequency between Korean and Japanese samples, but our previous report did5. In Korean population (2154 unrelated subjects), the CYP2C9*3 frequency was 0.044, significantly higher (P<0.001) than the mean frequency in Japanese (0.028), but similar to the frequency in Chinese (0.035). Because the CYP2C9 allele and genotype frequencies vary among studies, a large sample, such as ours, should reflect actual genotype frequencies. A recent study of 295 Koreans reported a CYP2C9*3 frequency similar to the one found in this study13, but the CYP2C9*13 frequency was lower. The CYP2C9*13 frequency in our study was 2.5-fold higher. Because the number of samples in this study (n=3592) was much higher than that in other study (n=590)13, this study may serve more reliable information on the frequencies of CYP2C9 alleles in Korean population.
The CYP2C9*13 allele was first identified in a Chinese sample, and the CYP2C9*3/*13 genotype confers a remarkable reduction in metabolic activity21. In this study, 17 of 1796 subjects had the CYP2C9*1/*13 genotype, while CYP2C9*13/*13 and *3/*13 were not found (Table 1). The CYP2C9*13 variant has impaired activity towards a number of substrates in vivo20, 29, 31, and has only been found in East Asians (Table 3). It is apparently absent from African-American, European, Hispanic, and Ashkenazi Jewish populations32.
In this study, two subjects were homozygous for CYP2C9*3/*3, but one did not provide written informed consent for inclusion in the pharmacokinetic study. We evaluated the enzymatic activity of the remaining CYP2C9*3/*3 homozygote using losartan. In the CYP2C9*3/*3 subject, the Cmax (187% of CYP2C9*1/*1) and AUC (242% of CYP2C9*1/*1) of losartan increased and the Cmax (3.6% of CYP2C9*1/*1) and AUC (11.5% of CYP2C9*1/*1) of E-3174 decreased compared with CYP2C9*1/*1 subjects. In the CYP2C9*3/*3 subject, E-3174 formation from losartan decreased markedly compared to CYP2C9*1/*1 subjects, therefore the AUC0–∞ was about 1/9th that of CYP2C9*1/*1 subjects. These results agree with a previous report from Sweden33. Although both losartan and E-3174 block angiotensin II receptors, E-3174 is at least 10-fold more potent than losartan34, and the clinical effects of losartan are mainly due to E-3174. Thus, losartan may have reduced antihypertensive effects in CYP2C9*3/*3 subjects than in CYP2C9*1/*1 subjects. Therefore, CYP2C9*3/*3 patients with hypertension might do well to take other hypertensive agents that are not metabolized by CYP2C9. In previous studies, losartan conversion to E-3174 was significantly reduced in the CYP2C9*1/*3 (50%–95% of that in CYP2C9*1/*1)20, 33, 35, 36 and *1/*13 genotypes (62% of that in CYP2C9*1/*1)20. Because the AUC0–∞ of E-3174 did not differ significantly between CYP2C9*1/*1 subjects and CYP2C9*3 or *13 heterozygotes, these genotypes did not affect the clinical effects of losartan. Because the CYP2C9*3/*3 genotype has almost no enzyme activity, the use of warfarin, phenytoin, and oral hypoglycemic agents might be hazardous1, 2, 6.
In summary, the CYP2C9*3 frequency in the Korean population was estimated to be 0.044 (95% CI 0.037–0.051) and the CYP2C9*13 frequency was estimated to be 0.005 (95% CI 0.003–0.007). Only four genotypes (CYP2C9*1/*1, *1/*3, *1/*13, and *3/*3) were found in a large Korean sample. CYP2C9*3/*3 subjects formed markedly less E-3174 from losartan than CYP2C9*1/*1 subjects, suggesting a profound reduction in antihypertensive effect of losartan in this genotype.
Acknowledgments
This study was supported by a grant (No 09172KFDA646) from Korea Food & Drug Administration in 2009–2010.
References
- Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998;45:525–38. doi: 10.1046/j.1365-2125.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz UI. Clinical relevance of genetic polymorphisms in the human CYP2C9 gene. Eur J Clin Invest. 2003;33 Suppl 2:23–30. doi: 10.1046/j.1365-2362.33.s2.6.x. [DOI] [PubMed] [Google Scholar]
- García-Martín E, Martínez C, Ladero JM, Agúndez JA. Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther. 2006;10:29–40. doi: 10.1007/BF03256440. [DOI] [PubMed] [Google Scholar]
- Scordo MG, Caputi AP, D'Arrigo C, Fava G, Spina E. Allele and genotype frequencies of CYP2C9, CYP2C19 and CYP2D6 in an Italian population. Pharmacol Res. 2004;50:195–200. doi: 10.1016/j.phrs.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bae JW, Kim HK, Kim JH, Yang SI, Kim MJ, Jang CG, et al. Allele and genotype frequencies of CYP2C9 in a Korean population. Br J Clin Pharmacol. 2005;60:418–22. doi: 10.1111/j.1365-2125.2005.02448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vivo and human data. Pharmacogenetics. 2002;12:251–63. doi: 10.1097/00008571-200204000-00010. [DOI] [PubMed] [Google Scholar]
- Yoon YR, Shon JH, Kim MK, Lim YC, Lee HR, Park JY, et al. Frequency of cytochrome P450 2C9 mutant alleles in a Korean population. Br J Clin Pharmacol. 2001;51:277–80. doi: 10.1046/j.1365-2125.2001.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrand SP, Sekiguchi K, Man MZ, Lin X, Tzeng RY, Teng CH, et al. Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther. 2008;84:347–61. doi: 10.1038/sj.clpt.6100482. [DOI] [PubMed] [Google Scholar]
- Man M, Farmen M, Dumaual C, Teng CH, Moser B, Irie S, et al. Genetic variation in metabolizing enzyme and transporter genes: comprehensive assessment in 3 major East Asian subpopulations with comparison to Caucasians and Africans. J Clin Pharmacol. 2010;50:929–40. doi: 10.1177/0091270009355161. [DOI] [PubMed] [Google Scholar]
- Maekawa K, Fukushima-Uesaka H, Tohkin M, Hasegawa R, Kajio H, Kuzuya N, et al. Four novel defective alleles and comprehensive haplotype analysis of CYP2C9 in Japanese. Pharmacogenet Genomics. 2006;16:497–514. doi: 10.1097/01.fpc.0000215069.14095.c6. [DOI] [PubMed] [Google Scholar]
- Stearns RA, Chakravarty PK, Chen R, Chiu SH. Biotransformation of losartan to its active carboxylic acid metabolite in human liver microsomes. Role of cytochrome P4502C and 3A subfamily members. Drug Metab Dispos. 1995;23:207–15. [PubMed] [Google Scholar]
- Ritter MA, Furtek CI, Lo MW. An improved method for the simultaneous determination of losartan and its major metabolite, EXP3174, in human plasma and urine by high-performance liquid chromatography with fluorescence detection. J Pharm Biomed Anal. 1997;15:1021–9. doi: 10.1016/s0731-7085(96)01948-6. [DOI] [PubMed] [Google Scholar]
- Lee HW, Lim MS, Lee J, Jegal MY, Kim DW, Lee WK, et al. Frequency of CYP2C9 variant alleles, including CYP2C9*13 in a Korean population and effect on glimepiride pharmacokinetics J Clin Pharm Ther 2011. doi: 10.1111/j.1365-2710.2010.01238.x [DOI] [PubMed]
- Hong X, Zhang S, Mao G, Jiang S, Zhang Y, Yu Y, et al. CYP2C9*3 allelic variant is associated with metabolism of irbesartan in Chinese population. Eur J Clin Pharmacol. 2005;61:627–34. doi: 10.1007/s00228-005-0976-8. [DOI] [PubMed] [Google Scholar]
- Yu BN, Luo CH, Wang D, Wang A, Li Z, Zhang W, et al. CYP2C9 allele variants in Chinese hypertension patients and healthy controls. Clin Chim Acta. 2004;348:57–61. doi: 10.1016/j.cccn.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Yang JQ, Morin S, Verstuyft C, Fan LA, Zhang Y, Xu CD, et al. Frequency of cytochrome P450 2C9 allelic variants in the Chinese and French populations. Fund Clin Pharmacol. 2003;17:373–6. doi: 10.1046/j.1472-8206.2003.00148.x. [DOI] [PubMed] [Google Scholar]
- Chen K, Wang R, Wen SY, Li J, Wang SQ. Relationship of P450 2C9 genetic polymorphisms in Chinese and the pharmacokinetics of tolbutamide. J Clin Pharm Ther. 2005;30:241–9. doi: 10.1111/j.1365-2710.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- Wang SL, Huang J, Lai MD, Tsai JJ. Detection of CYP2C9 polymorphism based on the polymerase chain reaction in Chinese. Pharmacogenetics. 1995;5:37–42. doi: 10.1097/00008571-199502000-00004. [DOI] [PubMed] [Google Scholar]
- Gaedigk A, Casley WL, Tyndale RF, Sellers EM, Jurima-Romet M, Leeder JS. Cytochrome P-4502C9 (CYP2C9) allele frequencies in Canadian Native Indian and Inuit populations. Can J Physiol Pharmacol. 2001;79:841–7. [PubMed] [Google Scholar]
- Li Z, Wang G, Wang LS, Zhang W, Tan ZR, Fan L, et al. Effects of the CYP2C9*13 allele on the pharmacokinetics of losartan in healthy male subjects. Xenobiotica. 2009;39:788–93. doi: 10.1080/00498250903134435. [DOI] [PubMed] [Google Scholar]
- Si D, Guo Y, Zhang Y, Yang L, Zhou H, Zhong D. Identification of a novel variant CYP2C9 allele in Chinese. Pharmacogenetics. 2004;14:465–9. doi: 10.1097/01.fpc.0000114749.08559.e4. [DOI] [PubMed] [Google Scholar]
- Yin T, Maekawa K, Kamide K, Saito Y, Hanada H, Miyashita K, et al. Genetic variations of CYP2C9 in 724 Japanese individuals and their impact on the antihypertensive effects of losartan. Hypertens Res. 2008;31:1549–57. doi: 10.1291/hypres.31.1549. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Hayashi H, Tashiro Y, Sakawa S, Moriwaki H, Akimoto T, et al. Effect of VKORC1-1639 G>A polymorphism, body weight, age, and serum albumin alterations on warfarin response in Japanese patients. Thromb Res. 2009;124:161–6. doi: 10.1016/j.thromres.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Nasu K, Kubota T, Ishizaki T. Genetic analysis of CYP2C9 polymorphism in a Japanese population. Pharmacogenetics. 1997;7:405–9. doi: 10.1097/00008571-199710000-00011. [DOI] [PubMed] [Google Scholar]
- Kimura M, Ieiri I, Mamiya K, Urae A, Higuchi S. Genetic polymorphism of cytochrome P-450s, CYP2C19, and CYP2C9 in a Japanese population. Ther Drug Monitor. 1998;20:243–7. doi: 10.1097/00007691-199806000-00001. [DOI] [PubMed] [Google Scholar]
- Obayashi K, Nakamura K, Kawana J, Ogata H, Hanada K, Kurabayashi M, et al. VKORC1 gene variations are the major contributors of variation in warfarin dose in Japanese patients. Clin Pharmacol Ther. 2006;80:169–78. doi: 10.1016/j.clpt.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Xie HG, Prasad H, Landau R, Kim RB, Cai WM, Ieiri I, et al. Frequency of the defective CYP2C9 variant alleles in different ethinc groups [abstract] Clin Pharmacol Ther. 2002;71:P102. [Google Scholar]
- Nakai K, Habano W, Nakai K, Fukushima N, Suwabe A, Moriya S, et al. Ethnic differences in CYP2C9*2 (Arg144Cys) and CYP2C9*3 (Ile359Leu) genotypes in Japanese and Israeli populations. Life Sci. 2005;78:107–11. doi: 10.1016/j.lfs.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Guo Y, Zhang Y, Wang Y, Chen X, Si D, Zhong D, et al. Role of CYP2C9 and its variants (CYP2C9*3 and CYP2C9*13) in the metabolism of lornoxicam in humans. Drug Metab Dispos. 2005;33:749–53. doi: 10.1124/dmd.105.003616. [DOI] [PubMed] [Google Scholar]
- Yasar U, Eliasson E, Dahl ML, Johansson I, Ingelman-Sundberg M, Sjöqvist F. Validation of methods for CYP2C9 genotyping: frequencies of mutant alleles in a Swedish population. Biochem Biophys Res Commun. 1999;254:628–31. doi: 10.1006/bbrc.1998.9992. [DOI] [PubMed] [Google Scholar]
- Bae JW, Choi CI, Jang CG, Lee SY. Effects of CYP2C9*1/*13 on the pharmacokinetics and pharmacodynamics of meloxicam. Br J Clin Pharmacol. 2011;71:550–5. doi: 10.1111/j.1365-2125.2010.03853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, Khasawneh R, Peter I, Kornreich R, Desnick RJ. Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics. 2010;11:781–91. doi: 10.2217/pgs.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasar U, Forslund-Bergengren C, Tybring G, Dorado P, Llerena A, Sjöqvist F, et al. Pharmacokinetics of losartan and its metabolite E-3174 in relation to the CYP2C9 genotype. Clin Pharmacol Ther. 2002;71:89–98. doi: 10.1067/mcp.2002.121216. [DOI] [PubMed] [Google Scholar]
- Sachinidis A, Ko Y, Weisser P, Meyer zu Brickwedde MK, Düsing R, Christian R, et al. EXP3174, a metabolite of losartan [MK 954, DuP 753] is more potent than losartan in blocking the angiotensin II-induced responses in vascular smooth muscle cells. J Hypertens. 1993;11:155–62. doi: 10.1097/00004872-199302000-00007. [DOI] [PubMed] [Google Scholar]
- Sekino K, Kubota T, Okada Y, Yamada Y, Yamamoto K, Horiuchi R, et al. Effect of the single CYP2C9*3 allele on pharmacokinetics and pharmacodynamics of losartan in healthy Japanese subjects. Eur J Clin Pharmacol. 2003;59:589–92. doi: 10.1007/s00228-003-0664-5. [DOI] [PubMed] [Google Scholar]
- Lee CR, Pieper JA, Hinderliter AL, Blaisdell JA, Goldstein JA. Losartan and E3174 pharmacokinetics in cytochrome P450 2C9*1/*1, *1/*2, and *1/*3 individuals. Pharmacotherapy. 2003;23:720–5. doi: 10.1592/phco.23.6.720.32187. [DOI] [PubMed] [Google Scholar]