Abstract
Objective
To test the hypothesis that self-collected urine could be used to detect high-risk human papillomavirus (HPV) DNA with sensitivity and specificity comparable to those of standard cervical testing.
Methods
Women attending a gynecology clinic for evaluation of abnormal cytology were recruited. Fifty-two participants (21–60 years of age) collected urine samples, and clinicians collected cervical brush samples. When appropriate, cervical biopsies were obtained during colposcopy. HPV detection and typing were performed on DNA extracts from each sample, using commercial reagents for L1 consensus polymerase chain reaction (PCR) and type-specific hybridization. HPV 16 viral load was determined by quantitative PCR in HPV 16-positive samples. A diagnostic test analysis was conducted for urine samples.
Results
Fifty paired samples were analyzed, with 76% agreement between samples. The 12 discrepant pairs were all urine negative/cervix positive. The most common HPV types detected were 16, 51, 53, and 62. The urine test correctly identified 100% of the uninfected and 65% of the infected patients.
Conclusion
The results indicate that HPV DNA detection using urine is less sensitive than cervical sampling in a population with abnormal cytology. Further exploration is warranted to determine clinical utility when other options are unavailable.
Keywords: Cancer screening, Cervical cancer, Human papillomavirus, Urine samples
1. Introduction
Human papillomavirus (HPV) is a double-stranded DNA virus that infects the deeper layers of skin and the inner mucosal lining of organs. Of more than 100 known HPV types, 40 preferentially infect the stratified squamous epithelium of the mucosa of the cervix and the vagina, and the skin of the vulva, the penis, and the perianal areas [1]. Persistent infection develops in 10%–20% of HPV-positive women, with shedding of HPV DNA from the genital tract occurring for 24 or more months after infection.
Cervical cancer is the fifth most commonly diagnosed cancer among women in Puerto Rico [2]. This malignancy is preventable and curable if detected early. Screening for cervical cancer has reduced the number of cases in high-resource countries, including Puerto Rico. However, populations with limited access to healthcare need easier and more effective methods of prevention and treatment [3,4]. Although the prophylactic HPV vaccine is 100% effective in the prevention of severe dysplasia due to HPV 16 and HPV 18, it might not protect women from severe dysplasia caused by other high-risk HPV types or women who are already infected with HPV 16 or HPV 18. Therefore, screening will be necessary for the next several decades [1].
Access to a gynecologist and compliance with screening are barriers to the success of cervical cancer prevention programs. It has been shown that HPV assays can be performed on vaginal cells as well as epithelial cells recovered by the centrifugation of voided urine [5]. The correlation between urine and the cervical detection of HPV as reported in the literature varies. Urine-based tests might facilitate HPV detection in women who do not have easy access to a gynecologist, and given their non-invasive nature should be attractive to all patients [6]. Such tests can also be used in epidemiologic studies in which pelvic exams may be impractical. In 2008, Fambrini et al. [7] found excellent concordance (96.6%) in the detection of high-risk HPV between urine and cervical samples. The long-term goal is to develop a new, better screening method (one that analyzes urine) for detecting HPV-related malignancies in high-risk populations.
The aim of the present study was to evaluate urine testing for high-risk HPV in a high-risk population using the linear array (LA) assay, which is a research test yielding type-specific results with high analytic sensitivity. Our hypothesis was that urine self-sampling can be used in the detection and quantification of high-risk HPV DNA and can be accomplished with levels of sensitivity and specificity comparable to those attained with standard cervical testing.
2. Materials and methods
Fifty-two women attending a series of gynecology clinics at the University of Puerto Rico, San Juan, USA, from September 1, 2011, to June 30, 2012, were recruited. Women were invited to participate if they were attending the gynecology clinics for evaluation of an abnormal Pap smear, were sexually active but not pregnant, were 21–60 years of age, had an intact uterus, and resided in Puerto Rico. Women were not eligible to participate in the study if they had any cognitive or physical impairment that prevented informed participation, were pregnant, were outside the 21–60-year age group, had no uterus, or did not reside in Puerto Rico. Patients over 60 years of age were excluded to avoid false-positive cytology results secondary to atrophic changes, but without missing the second peak of HPV infection that may occur around 55 years of age. Approval from the institutional review board Ethics Committee at the University of Puerto Rico Medical Sciences Campus was obtained prior to the study. Participants provided signed consent at enrollment.
Participants collected a first-void urine sample at home on the day of their second study visit and brought it to the clinic at ambient temperature. Clinic personnel mixed the sample and prepared 2 3-mL aliquots. At the time of pelvic examination during the second study visit, a clinician collected a sample from the participant's cervix under speculum visualization using a cervical brush (Digene HC2 DNA Collection Device; Qiagen, Valencia, CA, USA); the sample was then stored in Specimen Transport Medium (STM, Qiagen). Colposcopically directed cervical biopsies obtained for diagnostic purposes were processed and interpreted at local pathology laboratories. All specimens were stored at –20 °C and shipped on dry ice to the Centers for Disease Control and Prevention (CDC), Atlanta, USA, for further processing. DNA was extracted using the MagNA Pure LC System (Roche Applied Science, Indianapolis, IN, USA). For urine, 1 aliquot was thawed and centrifuged to collect a cell pellet, which was resuspended in 150 μL of TE buffer and processed with MagNA Pure DNA Isolation Kit 1 (Roche Applied Science). The cervical STM sample was thawed and resuspended, and a 150-μL aliquot was mixed with 85 μL of bacterial lysis buffer and 15 μL of Proteinase K from MagNA Pure DNA Isolation Kit 3 (Roche Applied Science). The emulsion was incubated at 65 °C for 1 hour and then processed with the MagNA Pure System, following the manufacturer's specification. All DNA extracts were eluted in a final volume of 100 μL. A water blank was processed with every sample batch as a negative control.
HPV typing was performed with the Roche Linear Array HPV Genotyping Test (Roche Diagnostics, Indianapolis, IN, USA). A 10-μL aliquot of extract was used with 40 μL of sterile water as sample template. BeeBlot (Bee Robotics, Gwynedd, UK) instruments were used for automation of the hybridization and wash steps of the line blot assay; otherwise, the assay was performed according to the manufacturer's protocol. A positive control (50 IU of HPV 16) and a no-template sample were processed with every LA assay. Samples with ambiguous results for HPV 52 (caused by the cross-hybridizing XR probe of the LA) were tested with an additional real-time polymerase chain reaction (PCR) assay specific for this type [8].
The amount of genomic DNA extracted from all of the specimens, and the HPV 16 viral copy numbers in all of the sample pairs that were positive for this type were determined by quantitative PCR (qPCR). In an additional assay, an external plant-based template was performed to measure the PCR inhibitors in all of the DNA extracts. In all reactions, 10 μL of the extracted DNA was used as template. The primers and conditions for the 3 qPCR assays are detailed elsewhere [9].
An interviewer-based questionnaire was administered after sample collection and colposcopy. Data about demographic characteristics, sexual history, and toxic habits were stored without identifiers in an Epi Info version 3.5.1 (CDC) database.
Normally distributed data were summarized as mean ± SD, and non-normally distributed data were presented as median (interquartile range). Basic crude associations with severity of disease were tested via Wilcoxon rank-sum test for medians; t test was used to compare means; χ2 or Fisher exact test was used to compare proportions. gDNA yield, HPV 16 copy number, and Ct values from the inhibitor assay were analyzed via paired t test, considering a P value less than 0.05 to be significant.
3. Results
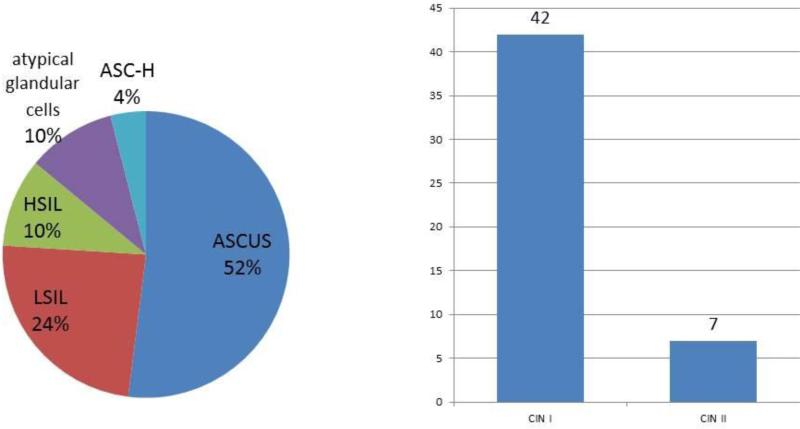
The study population consisted of 52 women with a mean age of 35 years who were recruited after an abnormal cytology result (Figure 1). The sociodemographic and reproductive characteristics for the overall study population, categorized according to the severity of disease (low risk and high risk, corresponding to cervical intraepithelial neoplasia [CIN] 1 and CIN 2/3, respectively), are presented in Table 1. The distribution of abnormal cervical cytology results in the 52 participants is shown in Figure 2. The most common result was atypical squamous cells of undetermined significance (52%), followed by low-grade squamous intraepithelial lesion (24%). Results were obtained from 51 of the 52 participants (1 biopsy sample was lost after being sent to the local pathology laboratory, so the result was not obtained for that patient), and 2 patients were excluded from analysis owing to inadequacy for HPV analysis. Of the 49 cervical biopsies included in the analysis, 86% (42/49) were diagnosed as CIN 1 and 14% as CIN 2 (7/49) (Figure 3).
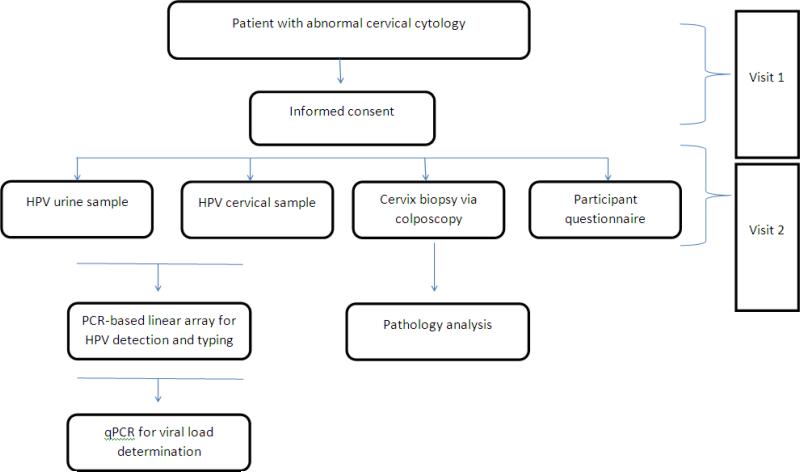
Figure 1.
Study design. Abbreviations: HPV, human papillomavirus; PCR, polymerase chain reaction; qPCR, quantitative polymerase chain reaction.
Table 1.
Sociodemographic and reproductive characteristics of the study populationa
| Characteristic | Overall (n=52) | Low risk (n=42) | High risk (n=7) | P valued |
|---|---|---|---|---|
| Age, yb | 35 ± 9.9 | 35 ± 9.7 | 31 ± 6.9 | 0.35 |
| Number of sexual partnersc | 3 (3–3) | 3 (1–4) | 3 (3–4) | 0.23 |
| Age at first coitus, yb | 18 ± 4.7 | 18 ± 5.0 | 16 ± 2.9 | 0.21 |
| Smoking history | ||||
| Smoked/smokes | 15 (28.9) | 9 (21.4) | 5 (71.4) | 0.02 |
| Has never smoked | 37 (71.1) | 33 (78.6) | 2 (28.6) | |
| Current smoking status | ||||
| Smokes | 5 (9.6) | 2 (4.8) | 3 (42.9) | 0.02 |
| Doesn't smoke | 47 (90.4) | 40 (95.2) | 4 (57.1) | |
| History of STDs | ||||
| Yes | 14 (26.9) | 9 (21.4) | 4 (57.1) | 0.07 |
| No | 38 (73.1) | 33 (78.6) | 3 (42.9) | |
| HPV vaccinated | ||||
| Yes | 1 (1.9) | 1 (2.4) | 0 (0.0) | >0.99 |
| No | 50 (98.0) | 41 (97.6) | 7 (100.0) | |
| Annual Papanicolaou test | ||||
| Yes | 36 (70.6) | 32 (78.0) | 2 (28.6) | 0.02 |
| No | 15 (29.4) | 9 (22.0) | 5 (71.4) |
Abbreviations: CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; STD, sexually transmitted disease.
Categorized according to severity of disease (low [CIN 1] and high [CIN 2/3] risk). Values are given as mean ± SD, median (interquartile range), or number (percentage) unless otherwise indicated.
Data are normally distributed.
Data are non-normally distributed.
P values are from Wilcoxon rank-sum test (for medians); t test (for means); and either χ2 or Fisher exact test (for proportions) where numbers in the cells are <5.
Figure 2.
Distribution of abnormal cervical cytology results (n=52) and cervical biopsies (n=49). Abbreviations: ASC-H, atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion; ASCUS, atypical squamous cells of undetermined significance; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
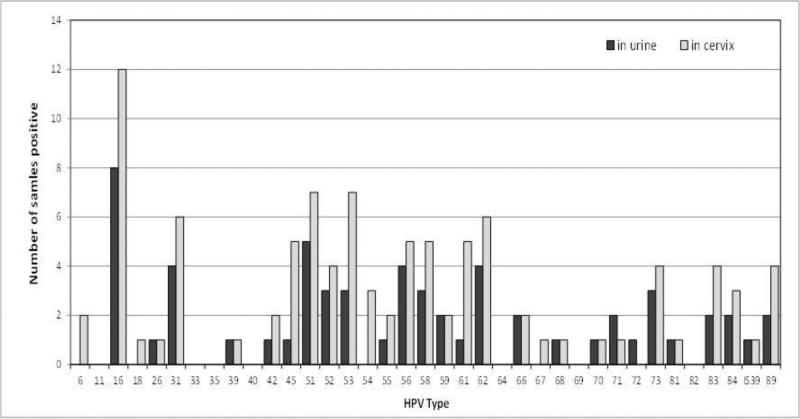
Figure 3.
Frequency of individual human papillomavirus (HPV) types as detected in urine samples and cervical extracts.
Of the cervical samples, 35 (67%) were positive for any HPV and 17 (33%) were negative. Twenty-two (42%) of the matching urine samples were positive and 28 (54%) were negative; the test results for another 2 (4%) were inadequate (Table 2). If restricted to samples with adequate results (n=50), the overall agreement on HPV status was 73%.
Table 2.
Diagnostic agreement
| Cervical extracts |
||||
|---|---|---|---|---|
| HPV positive | HPV negative | Inadequate | Sum | |
| Urine extracts | ||||
| HPV positive | 22 | 0 | 0 | 22 |
| HPV negative | 12 | 16 | 0 | 28 |
| Inadequate | 1 | 1 | 0 | 2 |
| Sum | 35 | 17 | 0 | 52 |
Abbreviation: HPV, human papillomavirus.
In 12 cervical samples, multiple HPV types (2–6) were detected, with a mean of 2.8 types per positive sample. Of the urine samples, 7 had multiple types (2–7), with a mean of 3.1 types per positive sample. Figure 3 shows the type-specific frequency of detection. If we consider only the 14 types present in clinical assays, the concordant pairs would be 35/50 (70%; 18 urine negative/cervix negative and 17 urine positive/cervix positive) and the discordant pairs would be 15/50 (30%; 1 urine positive/cervix negative and 14 urine negative/cervix positive).
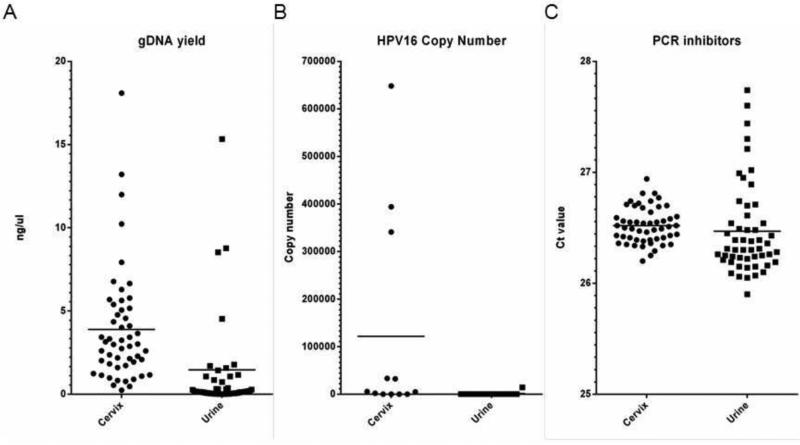
Quantitative PCR analysis showed that the gDNA yield was significantly higher in the cervical extracts (P=0.003). For the 12 women for whom HPV 16 was detected in the cervical sample, the HPV 16 copy number was determined in both sample pairs (Figure 4). The cervical sample had a greater number of copies in all cases but the difference was not statistically significant overall (P=0.076). Amplification of the external template showed identical Ct values in the presence of cervical (mean, 26.52) and urine (mean, 26.46) extracts, indicating that inhibitors did not cause any difference in PCR efficiency (P=0.366). Results from the qPCR assays are shown in Figure 4.
Figure 4.
Scatter plots showing results for gDNA yields (A), human papillomavirus (HPV) 16 copy number per μL (B), and Ct values for the external template (inhibitor assay) (B), as measured by quantitative polymerase chain reaction (PCR) assays. Median values are indicated by vertical bars in each plot.
4. Discussion
The results from the present pilot study confirm that, while HPV detection in urine is possible (50/52 [96%] yielding adequate results), the detection sensitivity is significantly lower than that in paired cervical samples. The cervical samples of 35 patients were positive for HPV, while only 22 of the urine samples from these patients also tested positive. A finding against urine testing is that there were 4 cases (8%) of discordant (urine negative/cervix positive) samples, which were consistent with CIN 2 on cervical biopsy. Assuming that LA testing of cervical cells is a gold standard and that these numbers are representative, urine testing would result in a reduction of approximately 35% in HPV detection sensitivity. An additional 39 HPV types were found in the positive cervical extracts. These included a total of 19 different genotypes (Figure 3), indicating that these discrepancies were not type specific.
The quantity of genomic and HPV 16 DNA in the extracts clearly indicated that the observed sensitivity difference was caused by significantly lower yields from the urine samples. While PCR inhibitors in nucleic acid extracts from urine have been reported [10], there was no evidence of this in the present study because urine DNA did not have a different effect on the amplification efficiency of the external templates. Payan et al. [11] reported a very good concordance of HPV detection in paired urine/cervical samples, with a κ test at 93%. Consistent with the present results, they also reported a marked difference in the HPV viral load—with the viral load of cervical samples 50-fold higher than that of urine, and explaining some of the discordant cases with low viral loads in urine. However, in certain populations with very limited access to healthcare, a urine sample can be more feasible than a pelvic exam or even vaginal self-sampling, providing information that may not be obtained otherwise. Those patients for whom high-risk HPV is detected in urine can be evaluated further with pelvic examination to exclude advanced cervical disease.
A limitation of the present study was its small sample size and the fact that no CIN 3 lesions were present in the cervical biopsies. Therefore, the sample was not representative of the general population. Larger studies would be necessary to confirm the numbers, and the observed differences may be different in other populations. Increasing either the amount of urine in the extraction process or the concentration of template DNA in the HPV assay might also be considered in order to decrease the loss of sensitivity. However, the cell pellets derived from the 3-mL aliquots were rather large, and the viscosity of the lysed cells can probably be considered an indication of the limit for the DNA extraction protocol used.
The conclusions regarding the main objective of the study—to evaluate the HPV testing of women using urine instead of cervical sampling—may be situation dependent. By most standards, a 35% loss of sensitivity would probably be unacceptable in terms of collecting meaningful data on HPV prevalence. However, if cervical sampling were impossible owing to study logistics, religious restriction in the population, or other reasons, the advantages of privacy and noninvasiveness of the sampling method may outweigh the existing shortcomings, and urine may be acceptable as an alternative specimen for HPV testing.
Synopsis.
Urine-based human papillomavirus DNA detection is feasible, especially in a research setting and among special populations, but the process needs further optimization and standardization.
Acknowledgments
The project was supported by the following grants: R25RR017589 and U54 RR026139 from the National Center for Research Resources; R25MD007607 and U54MD 007587 from the National Institute on Minority Health and Health Disparities; and 8U54MD007587-03 (RCMI Clinical and Translational Research award) from the University of Puerto Rico Medical Sciences Campus. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicts of interest.
References
- 1.Vorsters A, Micalessi I, Bilcke J, Ieven M, Bogers J, Van Damme P. Detection of human papillomavirus DNA in urine. A review of the literature. Eur J Clin Microbiol Infect Dis. 2012;31(5):627–40. doi: 10.1007/s10096-011-1358-z. [DOI] [PubMed] [Google Scholar]
- 2.Puerto Rico Department of Health Central Cancer Registry of Puerto Rico. http://www.salud.gov.pr/rcancer.
- 3.Pérez-Irizarry J, Nazario-Delgado CM, De la Torre T, Ortiz-Ortiz KJ, Torres-Cintrón M, Figueroa-Vallés NR. Incidence trends of cervical cancer in Puerto Rico, 1987-2004. P R Health Sci J. 2010;29(4):364–71. [PubMed] [Google Scholar]
- 4.Datta SD, Koutsky LA, Ratelle S, Unger ER, Shlay J, McClain T, et al. Human papillomavirus infection and cervical cytology in women screened for cervical cancer in the United States, 2003-2005. Ann Intern Med. 2008;148(7):493–500. doi: 10.7326/0003-4819-148-7-200804010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson DL, Womack SD, Peralta L, Zenilman JM, Feroli K, Maehr J, et al. Concordance of human papillomavirus in the cervix and urine among inner city adolescents. Pediatr Infect Dis J. 2000;19(8):722–8. doi: 10.1097/00006454-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Tanzi E, Bianchi S, Fasolo MM, Frati ER, Mazza F, Martinelli M, et al. High performance of a new PCR-based urine assay for HPV-DNA detection and genotyping. J Med Virol. 2013;85(1):91–8. doi: 10.1002/jmv.23434. [DOI] [PubMed] [Google Scholar]
- 7.Fambrini M, Penna C, Pieralli A, Bussani C, Fallani MG, Andersson KL, et al. PCR detection rates of high risk human papillomavirus DNA in paired self-collected urine and cervical scrapes after laser CO2 conization for high-grade cervical intraepithelial neoplasia. Gynecol Oncol. 2008;109(1):59–64. doi: 10.1016/j.ygyno.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Onyekwuluje JM, Steinau M, Swan DC, Unger ER. A real-time PCR assay for HPV52 detection and viral load quantification. Clin Lab. 2012;58(1–2):61–6. [PubMed] [Google Scholar]
- 9.Steinau M, Patel SS, Unger ER. Efficient DNA extraction for HPV genotyping in formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2011;13(4):377–81. doi: 10.1016/j.jmoldx.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huggett JF, Novak T, Garson JA, Green C, Morris-Jones SD, Miller RF, et al. Differential susceptibility of PCR reactions to inhibitors: an important and unrecognised phenomenon. BMC Res Notes. 2008;1:70. doi: 10.1186/1756-0500-1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payan C, Ducancelle A, Aboubaker MH, Caer J, Tapia M, Chauvin A, et al. Human papillomavirus quantification in urine and cervical samples by using the Mx4000 and LightCycler general real-time PCR systems. J Clin Microbiol. 2007;45(3):897–901. doi: 10.1128/JCM.02022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]