Abstract
Light-activated opsins undergo carboxy-terminal phosphorylation, which contributes to the deactivation of their photoresponse. The photopigment melanopsin possesses an unusually long carboxy tail containing 37 serine and threonine sites that are potential sites for phosphorylation by a G-protein dependent kinase (GRK). Here, we show that a small cluster of six to seven sites is sufficient for deactivation of light-activated mouse melanopsin. Surprisingly, these sites are distinct from those that regulate deactivation of rhodopsin. In zebrafish, there are five different melanopsin genes that encode proteins with distinct carboxy-terminal domains. Naturally occurring changes in the same cluster of phosphorylatable amino acids provides diversity in the deactivation kinetics of the zebrafish proteins. These results suggest that variation in phosphorylation sites provides flexibility in the duration and kinetics of melanopsin-mediated light responses.
Melanopsin is an atypical vertebrate opsin involved in non-image-forming light functions such as photoentrainment of circadian rhythms, constriction of the pupil, suppression of pineal melatonin synthesis, direct regulation of sleep and arousal by light, regulation of mood, and learning.1−4 In mammals, melanopsin is expressed in a small subset of retinal ganglion cells, termed intrinsically photosensitive retinal ganglion cells (ipRGCs), that are important for luminance detection and integration of light information.5−8 Whereas mammals have only one melanopsin gene (opn4m), nonmammalian vertebrates (birds, amphibians, and fish) express two related groups of melanopsin genes: opn4m and opn4x. These are named on the basis of their similarity either to the mammalian form of the gene (opn4m) or to the gene first isolated from Xenopus laevis (opn4x).9 In zebrafish, there are five unique melanopsin genes that are expressed in many different cell types both in the retina and in the brain.10,11
All opsins are members of the G protein coupled receptor (GPCR) family. GPCR signaling endows cells with the ability to respond to a stimulus and to suppress the response rapidly, allowing dynamic stimulus detection. After activating heterotrimeric G proteins and initiating a signal transduction cascade, GPCRs use a stereotypical two-step mechanism for deactivation. The first step is phosphorylation of the carboxy-terminal tail of the receptor by a member of the G protein coupled receptor kinase family (GRK). This reduces the rate of G protein activation and also serves as a signal for the activation and binding of an arrestin molecule. In the second step, arrestin is activated by interaction with the phosphorylated amino acids in the carboxy tail and can then bind to the intracellular loops of the receptor to prevent any further G protein activation.12
Melanopsin, as a GPCR, undergoes light-dependent phosphorylation that is involved in deactivation of the photoresponse. However, it possesses an unusually long carboxy tail that contains 37 serine and threonine sites, any of which could be phosphorylated to mediate deactivation. Recently, we showed that when all putative phosphorylation sites are removed from the carboxy tail there is a severe deficit in the deactivation response of mouse melanopsin in HEK cells.13
To define the relevant phosphorylation sites within the carboxy tail, we undertook mutational and functional analysis of mouse melanopsin and characterized the naturally diverse proteins of zebrafish. Zebrafish melanopsin-related proteins show variation in their carboxy-terminal domain, which provides a naturally occurring example of changes in phosphorylatable amino acid residues that lead to changes in deactivation. Using these parallel approaches, we demonstrate that of the 37 phosphorylation sites a small cluster of six or seven sites in the proximal region of the carboxy tail is critical for mediating deactivation.
Materials and Methods
cDNA Constructs
In experiments using mouse melanopsin (opn4), the unmodified long isoform of wild-type mouse melanopsin (accession no. NP_038915) was used. For calcium imaging, both the long and short isoforms (accession no. NP_001122071) of wild-type melanopsin were used. Full-length cDNAs corresponding to the five zebrafish melanopsin genes10 were appended with sequences encoding the last eight amino acids of bovine rhodopsin (1D4 tag). All genes were cloned into the mammalian expression vector pMT3.14
Transfection and Kinetic Measurements of Melanopsin Activity Based on Fluorescent Ca2+ Imaging
HEK 293 cells were grown in DMEM (Gibco) supplemented with 10% fetal bovine serum (Gibco) and a penicillin/streptomycin cocktail (10 units/mL of penicillin and 10 μg/mL of streptomycin) and transfected in 12-well plates with 3 μg of DNA and 5 μL of TurboFect (Fermentas) in 200 μL of DMEM. All HEK293 cells used in this article are HEK293 obtained from ATCC, Manassas, VA. (ATCC no. CRL-1573).
Cells were harvested at 24 h post-transfection and reseeded into a 96-well plate at a density of 6 × 104 per well. One day later, cells were incubated with 20 mM 11-cis-retinal in the presence of the calcium-sensitive dye fluo-4 (Invtirogen) for 1 h. The cells were washed twice in Hank’s balanced salt solution (HBSS) in 20 mM HEPES buffer, and fluorescence measurements were taken at an excitation of 485 nm and emission of 525 nm every second for 1 min on a Tecan Infinite M200 microplate reader. Data were averaged and plotted in Microsoft Excel.
Construction of Truncated Mouse Melanopsin Mutants
Truncation mutants of the carboxy tail were constructed using PCR to insert a stop codon (TAG) at various locations within the mouse gene. The forward primer contained the restriction site EcoRI, whereas reverse primers contained a stop codon and a NotI restriction site. The truncated melanopsin PCR products were then digested with NotI and EcoRI FastDigest enzymes (Fermentas) and recloned into a similarly digested pMT3 expression vector. Sequences were verified by sequencing (GENEWIZ, Inc.) using plasmid-specific primers. Primer sequences are listed in Supporting Information Table 1.
Site-Directed Mutatgenesis
Point mutations eliminating putative phosphorylation sites were produced by site-directed mutagenesis. Individual serines and threonines were changed to alanine using primers containing the desired mutations (IDT DNA). High-fidelity polymerase (Pfx Turbo, Invitrogen) was used to minimize unwanted mutations. Mutations were confirmed by sequencing (GENEWIZ, Inc.).
Cassette Mutagenesis
To produce multiple mutations in the carboxy-tail region of the gene, two unique restriction sites (BlpI and XhoI) were introduced by site-directed mutagenesis on either side of the region of interest through silent mutations. Insertions were confirmed by sequencing using plasmid-specific primers (GENEWIZ, Inc.). A 98-base oligonucleotide was synthesized (IDT DNA) and made double-stranded by PCR amplification. Double-stranded DNA and the plasmid containing the melanopsin gene were digested with BlpI and XhoI FastDigest enzymes (Fermentas) for 30 min and separated on an agarose gel. Bands corresponding to the expected size were excised from the gel, recovered (Nucleospin ExtractII, Machery-Nagel), and ligated with T4 ligase (Promega). Ligated plasmid was transformed as above and subsequently sequenced (GENEWIZ, Inc.) to confirm insertion.
In Silico Identification of Potential Phosphorylation Sites
The group-based phosphorylation scoring (GPS) algorithm in the Group-Based Prediction System (2.0) was used to analyze and predict potential phosphorylaiton sites in the carboxy-tail region of mouse melanopsin (opn4). This program determines phosphorylation sites for families of kinases based on experimentally demonstrated sites. All known GRK family phosphorylation sites (84 at the time of program design) were used to train the program to find new sites for this family kinases.15
Results
Truncation of Mouse Melanopsin Defines a Carboxy-Terminal Region Controlling Deactivation
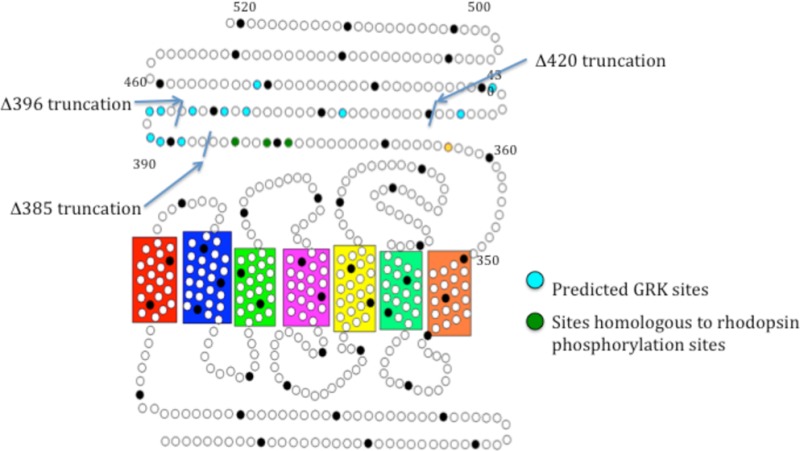
We used the group-based phosphorylation (GPS) scoring algorithm in the Group-Based Prediction System (2.0) to identify potential GRK phosphorylation sites.15 Using this method, 12 predicted phosphorylation sites were found distributed throughout the carboxy-tail domain (Figure 1). To determine if any of these sites are functionally relevant, we created three early termination mutations by inserting a stop codon at various locations in the carboxy tail of mouse melanopsin. The first mutation truncated the protein after amino acid 385 (Δ385) so that it contains only the phosphorylation sites homologous to those phosphorylated in rhodopsin during deactivation.16 The second mutation truncated the protein after amino acid 396 (Δ396), immediately following a large cluster of predicted sites, whereas the third truncated the protein after amino acid 420 (Δ420) (Figure 1). The signaling kinetics of these mutants was measured along with wild-type melanopsin and the previously characterized phospho-null melanopsin mutant13 using a functional calcium fluorescence assay (Figure 2).
Figure 1.
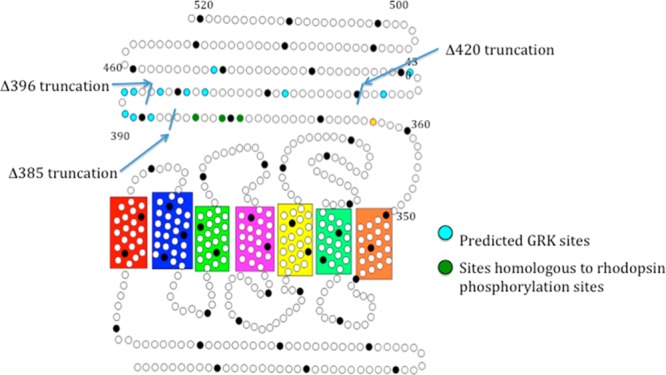
Secondary structure of mouse melanopsin. Each circle represents an amino acid, and every 10th amino acid is shaded black. Blue residues represent putative GRK phosphorylation sites (predicted by the group-based phosphorylation scoring (GPS) algorithm in GPS 2.0), green residues represent the homologous residues to those most often phosphorylated in rhodopsin, and the yellow dot represents a potential palmitoylation site.
Figure 2.
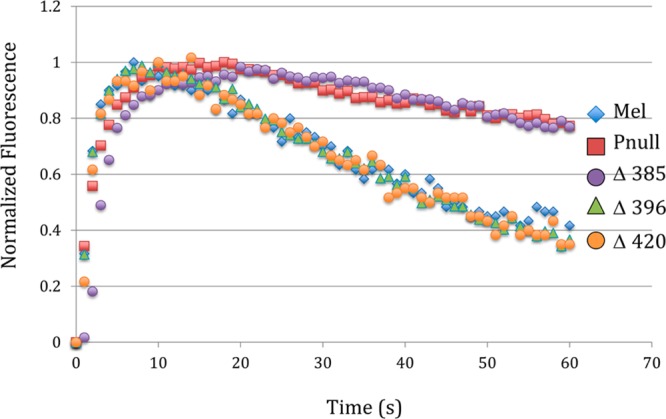
Calcium imaging of carboxy-tail-truncation mutants. Kinetics of the calcium response of a series of carboxy-tail-truncation mutants compared to wild-type melanopsin and a phospho-null mutant. The fluorescent Ca2+-imaging data presented in this article is normalized to facilitate comparison of different melanopsin constructs. Supporting Information Figure 1 demonstrates that the normalized kinetics are not a function of the expression levels of the heterologously expressed melanopsin gene.
The Δ385 mutant showed a severe defect in deactivation identical to the phospho-null construct. This finding demonstrates that, surprisingly, the phosphorylation sites homologous to those important for deactivation of rhodopsin are not sufficient for melanopsin deactivation. Neither Δ396 nor Δ420 had defective kinetics, implying that the region between amino acids 385 and 396 of the melanopsin carboxy tail is critical for the deactivation response (Figure 2). Within this region, there are six residues that can be phosphorylated and are arranged in pairs separated by one amino acid. Of these six sites, five were predicted GRK phosphorylation sites by GPS (Figure 1). Mutation of all six sites to alanine (S388A, T389A, S391A, S392A, S394A, and S395A) by cassette mutagenesis resulted in a phenotype equivalent to the phospho-null mutant (Figure 3). However, when each of the six sites was individually mutated to alanine by site-directed mutagenesis, alteration of the deactivation kinetics was not observed (Figure 4).
Figure 3.
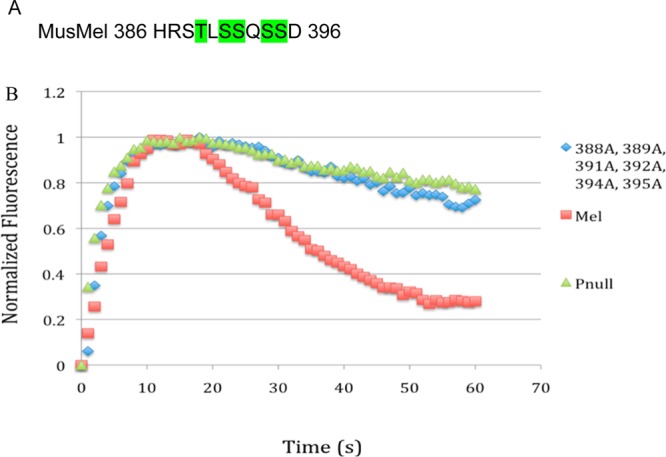
Sequence of deactivation control region. (A) Sites highlighted in green are predicted GRK family phosphorylation sites by the group-based phosphorylation algorithm. (B) Mutation of all phosphorylatable residues between amino acids 385 and 396 recapitulates the phospho-null phenotype.
Figure 4.
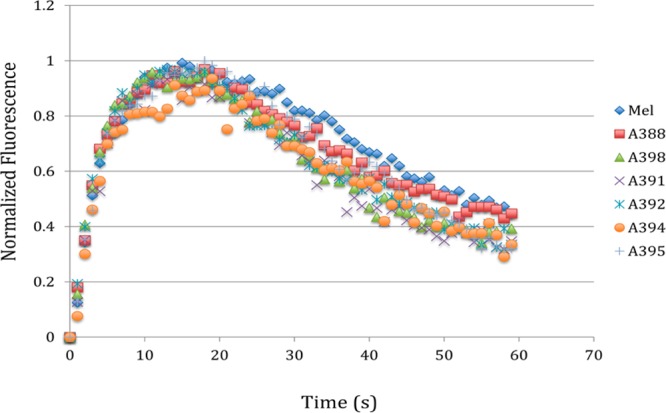
Single phosphorylation site mutagenesis of putative deactivation control region. Each serine and threonine within the identified control region was mutated individually to an alanine. Deactivation of each mutant was determined in a kinetic calcium assay and compared to wild-type melanopsin. None of the six single mutations had any effect on signaling kinetics.
Zebrafish Melanopsins Have Different Deactivation Kinetics
Recent work described five unique melanopsin genes expressed in zebrafish that are transcribed in diverse retinal cell types.10,11 Interestingly, examination of the carboxy-terminal regions of the corresponding proteins reveals variation in the key phosphorylation sites that we defined in mouse melanopsin (Figures 1–4). To test whether these naturally occurring mutations affect deactivation, the zebrafish proteins were heterologously expressed in HEK293 cells and assayed for their kinetic response. The five zebra fish melanopsin gene, opn4xa, opn4xb opn 4.1, opn4a, and opn4b, cDNAs were cloned into the mammalian expression vector pMT3 and heterologously expressed. We were able to express the opn4xa, opn 4.1, opn4a, and opn4b genes; however, opn4xb was not produced in HEK293 cells, as determined by western blot analyses (n = 3, data not shown), and was not analyzed in subsequent experiments.
When the four zebrafish genes were expressed in HEK293 cells and assayed for activity in the calcium-imaging assay, their gene products exhibited different deactivation kinetics. Deactivation of zebrafish Opn4a and Opn4b closely match with mouse melanopsin (Opn4), deactivating to 40% of their maximum fluorescence in 60 s (Figure 5). In contrast, Opn4xa and Opn4.1 were found to have greatly extended deactivation kinetics, mimicking the phospho-null melanopsin phenotype (Figure 5). To determine if the deactivation kinetics of zebrafish melanopsins correlate with the amino acid foot print of the carboxy-tail phosphorylation control region in mouse melanopsin (Figure 3A), the amino acid sequences of Opn4a, Opn4b, Opn4xa, and Opn4.1 were aligned and analyzed. Alignment of the four melanopsin zebrafish genes with mouse melanopsin demonstrated that there was broad conservation of sequence in the region of the carboxy tail that was defined as the region controlling deactivation kinetics (Figure 6). Zebrafish Opn4a and Opn4b (which more closely match the signaling kinetics of mouse melanopsin) share an identical pattern of phosphorylatable residues. In contrast, Opn4xa and Opn4.1 (which displayed delayed inactivation kinetics) are missing three or four serines and threonines in the important region of the carboxy tail that is necessary for the deactivation kinetics. These results suggest that naturally occurring variations in this region affect the kinetics of the light response mediated by each melanopsin protein.
Figure 5.
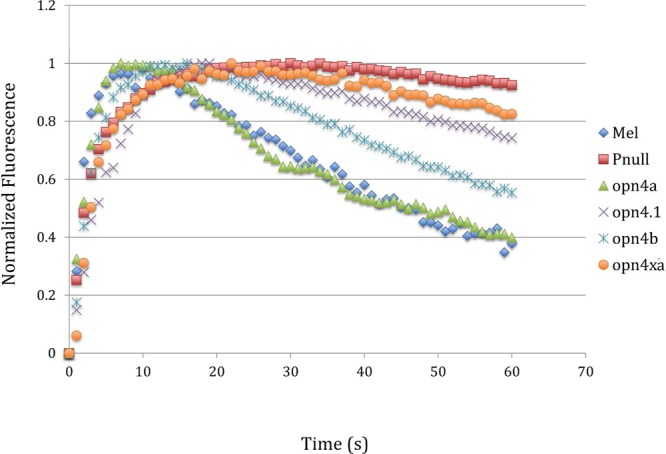
Kinetic calcium assay of zebrafish melanopsins expressed in HEK293 cells. Four of the five melanopsins found expressed in zebrafish were assayed for their deactivation kinetics. Opn4a and Opn4b were found to have similar deactivation kinetics to mouse melanopsin. Opn4.1 and Opn4xa were found to have extended deactivation kinetics matching the mouse melanopsin mutant lacking all carboxy-tail phosphorylaiton sites (phospho-null).
Figure 6.

Alignment of zebrafish melanopsins with mouse melanopsin. Alignment of the zebrafish and mouse melanopsin sequences in the identified control region. Shown in green are the phosphorylation sites that are the same as mouse melanopsin, whereas the sites that are divergent from mouse melanopsin are in red.
To directly test the importance of the variation in the amino acids in the carboxy-tail region of zebrafish melanopsin (amino acids 386–394), we created a mouse melanopsin gene with the same amino acid sequence of the zebrafish opn4.1, which shows slower deactivation kinetics. This mouse melanopsin mutant (S381A, S388A, and S394A) showed prolonged deactivation kinetics (Figure 7A), similar to the zebrafish protein. Remarkably, double mutant (S381A and S394A) mouse melanopsin does not alter the deactivation kinetics, indicating that three changes are necessary for the observed effect. Therefore, we defined this region of the carboxy tail of melanopsin as an important region in the regulation of melanopsin deactivation kinetics across species.
Figure 7.
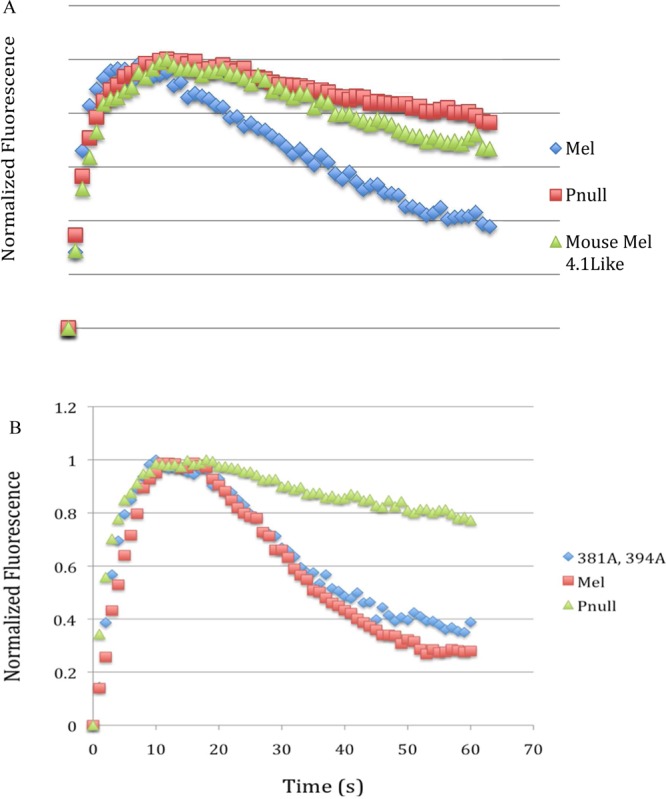
Kinetic calcium assay of mouse melanopsin constructed to mimick zebrafish melanopsin opn4.1. (A) Kinetic calcium imaging of a mutant of mouse melanopsin engineered to match the pattern of phosphorylatable sites found in slow deactivating zebrafish melanopsin. (B) Mutation of the two conserved changed residues between opn4.1 and opn4xa and mouse melanopsin has no effect on signaling.
Discussion
The temporal regulation of activated GPCRs is typically controlled by the phosphorylation of serines and threonines in the carboxy tail by a GRK and the subsequent activation and binding of an arrestin molecule. The initial phosphorylation of the carboxy tail reduces the rate of G protein activation, and the binding of arrestin further quenches G protein activation. In addition to quenching the activation of a G protein pathway, the binding of arrestin can also trigger additional signaling events such as receptor internalization. The regulation of the active lifetime of the vertebrate rod photoreceptor’s visual pigment rhodopsin by phosphorylation and the subsequent binding of arrestin-1 (visual arrestin) is the most thoroughly studied GPCR and visual pigment. In this study, we show that melanopsin, the photopigment of ipRGCs, utilizes distinct residues for the deactivation of the light signal in response to phosphorylation by GRK. This indicates a divergence of function between the visual pigment rhodopsin and melanopsin for the control of the deactivation kinetics. Specifically, we show that six residues are necessary and sufficient for the deactivation response of the melanopsin protein. Furthermore, we show that naturally occurring mutations in zebrafish that correspond to the region we defined in our mouse studies allow melanopsin proteins to modulate their deactivation kinetics. This could be evolutionary important because slower deactivation kinetics could lead to a much more prolonged light response that can be tailored toward the function that is driven by the melanopsin protein. As an example, measuring day length in the environment will benefit from a slower deactivation kinetics, whereas driving the pupillary light reflex may require faster shutoff properties of the light response.
We have previously shown that the deactivation of mouse melanopsin is mediated in a phosphorylation-dependent manner.13 However, the necessary sites of phosphorylation in the carboxy tail were not specifically determined. In the present study, we identified a cluster of serines and threonines in the region of amino acids 386–396 that is involved in controlling the deactivation response. This region contains six phosphorylatable sites arranged in three sets of pairs separated by a single amino acid (S388, T389, S391, S392, S394, and S395). There was no effect on the signaling kinetics when each site was mutated individually, implying that no particular site is critically important in the deactivation response.
Melanopsin is more similar to invertebrate rhodopsins. In Drosophila, the deactivation of the light response is not dependent on the carboxy tail of rhodopsin. This shows a divergence of function between melanopsin and Drosophila rhodopsins in the mechanisms for deactivation. However, we should mention that our studies were carried out in HEK cells, and to definitively confirm whether these residues play a role in vivo, we should eliminate these residues and determine the changes in the properties of the intrinsic light response in the ipRGCs as well as the behavioral outcome of such mutations.
Previous work has found that there are five distinct melanopsin genes expressed in the zebrafish, Danio rerio, of which four were functionally characterized here. Alignment of these gene sequences revealed that although these melanopsins are quite similar in the transmembrane region they have very little similarity in the carboxy tail except for two domains corresponding to the predicted eighth helix and to the region identified in the truncation mutants as being responsible for deactivation. Upon closer examination of the region between amino acids 386 and 396, there was some variation in the number and location of phosphorylatable residues among the zebrafish opsins. Opn4a and Opn4b are similar to mouse melanopsin in the location and spacing of the phosphorylatabe residue. In contrast, Opn4.1 and Opn4xa are missing two and three of the six sites, respectively, with another serine located just outside of the identified area at position 381 also being converted to a nonphosphorylatable residue. Opn4.1 lacks S388, S394, and S381, whereas Opn4xa lacks T389, S392, and S394 in addition to the loss of S381. The deactivation kinetics of the four expressed zebrafish melanopsin genes correlates with either wild-type mouse melanopsin or with the phosphorylation-defective mutant. Opn4a and Opn4b deactivate in the kinetic calcium assay with a time course similar to wild-type mouse melanopsin (Opn4). In contrast, the deactivation kinetics of Opn4.1 and Opn4xa are similar to the mouse melanopsin mutant lacking all phosphorylatable amino acids in the carboxy-tail region. In zebrafish melanopsin, we conclude that sites 381, S338, and S394 are required for rapid deactivation. There has been great debate about which sites and how many are required for deactivation of rhodopsin.17−20In vitro and in vivo work has shown that any phosphorylatable residue in the rhodopsin carboxy tail can be phosphorylated.18,21−23 Mass spectrometric analysis of murine rhodospin’s carboxy tail found that there were three amino sites that were most often phosphorylated, but all serines and threonines in the tail were substrates for GRK.16 Other work involving single photon responses of isolated transgenic mouse rods found that only three phosphorylation sites were necessary for deactivation regardless of the specific identity of the sites.18 More recently, Vishnivetskiy et al. used purified rhodopsin fractions with specific numbers of phosphorylated amino acids and determined that three phosphorylations were necessary to fully activate arrestin-1 for rhodopsin binding.20 If a similar mechanism is governing melanopsin deactivation, then the number of incorporated phosphates is important, but the specific identity of the modified amino acid is not.
The role of the five different zebrafish melanopsins in the retina has not yet been elucidated. It is intriguing to note that with the duplication of the melanopsin genes and diversification in expression there has also been a selection for variants in the region of carboxy tail that is necessary for melanopsin deactivation. This variation results in activated melanopsins having different active lifetimes. It will be interesting to determine if the active lifetime of the various zebrafish melanopsins correlate with their function in the various cell types in which this visual pigment is expressed.
In contrast to the zebrafish retina, the mammalian retina expresses one gene with two splice variants in five subtypes of intrinsically photosensitive ganglion cells.24,25 The light response in each of these five cell types has different deactivation kinetics, suggesting that the regulation of the deactivation reactions of melanopsin and the phototransduction cascade is varied. It has been suggested that variation is due to the difference in the rate of melanopsin deactivation and is due to the variation in the carboxy tail in the short and long melanopsin splice variants. The alternative splice variant only changes the distal region of the carboxy tail24 and not the more proximal control region that contains the amino acids identified here that are important for melanopsin deactivation. It therefore seems unlikely that these two isoforms have different deactivation kinetics. However, we tested the deactivation kinetics of the two mouse splice variants and observed no change in the activation or deactivation kinetics (Figure 8). These results suggested that the varied kinetics of the light response in the five subtypes of mouse iPRGs is not due to the difference melanopsin isoforms.
Figure 8.
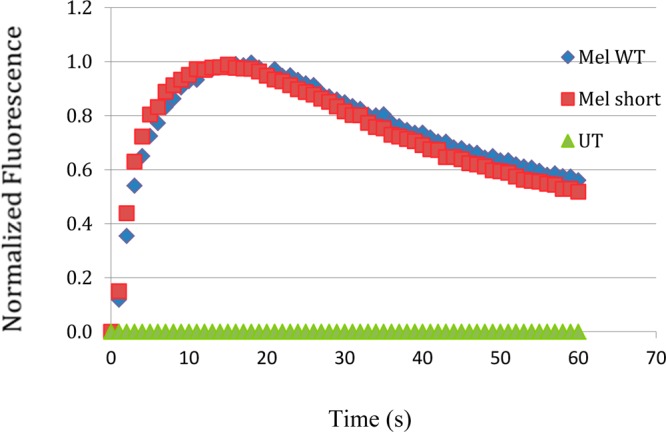
Calcium imaging of the two splice variants of mouse melanopsin. The long (Opn4L) (Mel WT) and short (Opn4S) (Mel short) isoforms of mouse melanopsins were assayed for their deactivation kinetics. They were found to have similar deactivation kinetics.
In summary, melanopsin is an atypical vertebrate visual pigment that is involved in regulating a large variety of physiological functions. In the mammalian retina, the variation in function seems to be mediated by the projection of the various retinal cell types and regulation of the phototransduction cascade. The deactivation of melanopsin does not appear to be regulated by changes to the carboxy terminal. In contrast, in nonmammalian vertebrates, there are two major families of melanopsin genes. In zebrafish, genes from both of these families have been duplicated, and there has been diversification in the carboxy terminus of these proteins. These zebrafish melanopsins have varied deactivation kinetics, and this may correlate with the visual function that these zebrafish isoforms drive.
Acknowledgments
We thank R. Crouch for the gift of 11-cis-retinal.
Glossary
Abbreviations Used
- DMEM
Dulbecco’s modified Eagle’s medium
- GPCR
G protein coupled receptor
- GPS
group-based phosphorylation scoring
- GRK
G-protein dependent kinase
- HBSS
Hank’s balanced salt solution
- HEK
human embryonic kidney cells
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- ipRGCs
intrinsically photosensitive retinal ganglion cells
- PCR
polymerase chain reaction
Supporting Information Available
Primers used to create the melanopsin mutants as well as data demonstrating that the inactivation kinetics observed using calcium imaging in transfected HEK cells is not a function of expression levels. This material is available free of charge via the Internet at http://pubs.acs.org.
This study was supported by grants from the National Science Foundation to P.R.R. (IOS0721608) and the National Eye Institute to P.R.R. and S.H. (R01EY019053). J.R.B. and E.C. were supported by an NIH training grant (T32 GM066706).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Panda S.; Provencio I.; Tu D. C.; Pires S. S.; Rollag M. D.; Castrucci A. M.; Pletcher M. T.; Sato T. K.; Wiltshire T.; Andahazy M.; Kay S. A.; Van Gelder R. N.; Hogenesch J. B. (2003) Melanopsin is required for non-image-forming photic responses in blind mice. Science 301, 525–527. [DOI] [PubMed] [Google Scholar]
- Lucas R. J.; Hattar S.; Takao M.; Berson D. M.; Foster R. G.; Yau K. W. (2003) Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science 299, 245–247. [DOI] [PubMed] [Google Scholar]
- Tsai J. W.; Hannibal J.; Hagiwara G.; Colas D.; Ruppert E.; Ruby N. F.; Heller H. C.; Franken P.; Bourgin P. (2009) Melanopsin as a sleep modulator: Circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4–/– mice. PLoS Biol. 7, e1000125-1–e1000125-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates T. A.; Altimus C. M.; Wang H.; Lee H. K.; Yang S.; Zhao H.; Kirkwood A.; Weber E. T.; Hattar S. (2012) Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 491, 594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do M. T.; Kang S. H.; Xue T.; Zhong H.; Liao H. W.; Bergles D. E.; Yau K. W. (2009) Photon capture and signalling by melanopsin retinal ganglion cells. Nature 457, 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T. M.; Chen S. K.; Hattar S. (2011) Intrinsically photosensitive retinal ganglion cells: Many subtypes, diverse functions. Trends Neurosci. 34, 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton T.; Buhr E.; Van Gelder R. N. (2012) Melanopsin and mechanisms of non-visual ocular photoreception. J. Biol. Chem. 287, 1649–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R. J. (2013) Mammalian inner retinal photoreception. Curr. Biol. 23, R125–133. [DOI] [PubMed] [Google Scholar]
- Bellingham J.; Chaurasia S. S.; Melyan Z.; Liu C.; Cameron M. A.; Tarttelin E. E.; Iuvone P. M.; Hankins M. W.; Tosini G.; Lucas R. J. (2006) Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 4, e254-1–e254-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos-Cruz V.; Blasic J.; Nickle B.; Robinson P. R.; Hattar S.; Halpern M. E. (2011) Unexpected diversity and photoperiod dependence of the zebrafish melanopsin system. PLoS One 6, e25111-1–e25111-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies W. I.; Zheng L.; Hughes S.; Tamai T. K.; Turton M.; Halford S.; Foster R. G.; Whitmore D.; Hankins M. W. (2011) Functional diversity of melanopsins and their global expression in the teleost retina. Cell. Mol. Life Sci. 68, 4115–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin A. B. (2008) G-protein-coupled receptor phosphorylation: where, when and by whom. Br. J. Pharmacol. 153, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasic J. R. Jr.; Brown R. L.; Robinson P. R. (2012) Light-dependent phosphorylation of the carboxy tail of mouse melanopsin. Cell. Mol. Life Sci. 69, 1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprian D. D.; Molday R. S.; Kaufman R. J.; Khorana H. G. (1987) Expression of a synthetic bovine rhodopsin gene in monkey kidney cells. Proc. Natl. Acad. Sci. U.S.A. 84, 8874–8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y.; Ren J.; Gao X.; Jin C.; Wen L.; Yao X. (2008) GPS 2.0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Mol. Cell. Proteomics 7, 1598–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A.; Craven K. B.; Niemi G. A.; Hurley J. B. (2002) Mass spectrometric analysis of the kinetics of in vivo rhodopsin phosphorylation. Protein Sci. 11, 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohguro H.; Van Hooser J. P.; Milam A. H.; Palczewski K. (1995) Rhodopsin phosphorylation and dephosphorylation in vivo. J. Biol. Chem. 270, 14259–14262. [DOI] [PubMed] [Google Scholar]
- Mendez A.; Burns M. E.; Roca A.; Lem J.; Wu L. W.; Simon M. I.; Baylor D. A.; Chen J. (2000) Rapid and reproducible deactivation of rhodopsin requires multiple phosphorylation sites. Neuron 28, 153–164. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J.; Lee K. A.; Niemi G. A.; Craven K. B.; Garwin G. G.; Saari J. C.; Hurley J. B. (2001) Multiple phosphorylation of rhodopsin and the in vivo chemistry underlying rod photoreceptor dark adaptation. Neuron 31, 87–101. [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy S. A.; Raman D.; Wei J.; Kennedy M. J.; Hurley J. B.; Gurevich V. V. (2007) Regulation of arrestin binding by rhodopsin phosphorylation level. J. Biol. Chem. 282, 32075–32083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden U.; Kuhn H. (1982) Light-dependent phosphorylation of rhodopsin: Number of phosphorylation sites. Biochemistry 21, 3014–3022. [DOI] [PubMed] [Google Scholar]
- Wilden U. (1995) Duration and amplitude of the light-induced cGMP hydrolysis in vertebrate photoreceptors are regulated by multiple phosphorylation of rhodopsin and by arrestin binding. Biochemistry 34, 1446–1454. [DOI] [PubMed] [Google Scholar]
- Brannock M. T.; Weng K.; Robinson P. R. (1999) Rhodopsin’s carboxyl-terminal threonines are required for wild-type arrestin-mediated quench of transducin activation in vitro. Biochemistry 38, 3770–3778. [DOI] [PubMed] [Google Scholar]
- Pires S. S.; Hughes S.; Turton M.; Melyan Z.; Peirson S. N.; Zheng L.; Kosmaoglou M.; Bellingham J.; Cheetham M. E.; Lucas R. J.; Foster R. G.; Hankins M. W.; Halford S. (2009) Differential expression of two distinct functional isoforms of melanopsin (Opn4) in the mammalian retina. J. Neurosci. 29, 12332–12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S.; Welsh L.; Katti C.; González-Menéndez I.; Turton M.; Halford S.; Sekaran S.; Peirson S. N.; Hankins M. W.; Foster R. G. (2012) Differential expression of melanopsin isoforms Opn4L and Opn4S during postnatal development of the mouse retina. PLoS One 7, e34531-1–e34531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


