Abstract
Aim:
To investigate the signaling pathways involved in thrombin-induced connective tissue growth factor (CTGF) expression in rat vascular smooth muscle cells (VSMCs).
Methods:
Experiments were preformed on primary rat aortic smooth muscle cells (RASMCs) and a rat VSMC line (A10). CTGF protein levels were measured using Western blotting. Luciferase reporter genes and dominant negative mutants (DNs) were used to investigate the signaling pathways mediating the induction of CTGF expression by thrombin.
Results:
Thrombin (0.3–3.0 U/mL) caused a concentration- and time-dependent increase in CTGF expression in both RASMCs and A10 cells. Pretreating A10 cells with the protease-activated receptor 1 (PAR-1) antagonist SCH79797 (0.1 μmol/L) significantly blocked thrombin-induced CTGF expression, while the PAR-4 antagonist tcY-NH2 (30 μmol/L) had no effect. The PAR-1 agonist SFLLRN-NH2 (300 μmol/L) induced CTGF expression, while the PAR-4 agonist GYPGQV-NH2 (300 μmol/L) had no effect. Thrombin (1 U/mL) caused time-dependent phosphorylation of c-Jun N-terminal kinase (JNK). Pretreating with the JNK inhibitor SP600125 (3–30 μmol/L) or transfection with DNs of JNK1/2 significantly attenuated thrombin-induced CTGF expression. Thrombin (0.3–3.0 U/mL) increased activator protein-1 (AP-1)-luciferase activity, which was inhibited by the JNK inhibitor SP600125. The AP-1 inhibitor curcumin (1–10 μmol/L) concentration-dependently attenuated thrombin-induced CTGF expression.
Conclusion:
Thrombin acts on PAR-1 to activate the JNK signaling pathway, which in turn initiates AP-1 activation and ultimately induces CTGF expression in VSMCs.
Keywords: thrombin, protease-activated receptor, activator protein-1 (AP-1), connective tissue growth factor, mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), vascular smooth muscle cell
Introduction
Thrombin, a serine protease, has been studied for its pleiotropic actions beyond hemostasis1. The biological actions of thrombin in tissues and cells are mostly transduced by the protease-activated receptors (PARs), a family of G protein-coupled receptors. At present, 4 different PARs (PAR1–4) have been cloned. PAR-1, PAR-3, and PAR-4 are activated by thrombin, whereas PAR-2 is activated by tryptase2. Thrombin is implicated in the process of vascular remodelling in atherosclerosis and restenosis3. Thrombin can stimulate the formation of collagen in a PAR-1-dependent mechanism in vascular smooth muscle cells (VSMCs)4. Connective tissue growth factor (CTGF) is a recently identified profibrotic agent. It is an immediate-early gene and belongs to the CCN family [Cyr61 (CCN1), CTGF (CCN2), Nov (CCN3), Wisp-1/elm1 (CCN4), Wisp-2/rCop1 (CCN5), and Wisp-3 (CCN6)] of growth factors5. The CTGF protein is a 38-kDa cysteine-rich, heparin-binding, secreted protein initially identified in the conditioned medium of cultured endothelial cells6. It is expressed by many human organs and is involved in various biological functions, including embryonic development, wound repair, and angiogenesis7. CTGF has been implicated in a variety of cardiovascular pathophysiological conditions. CTGF is overexpressed in human atherosclerotic lesions8. It has been proved to be a mediator of angiotensin II-induced fibrosis in VSMCs9. Transforming growth factor-β, endothelin-1, and homocysteine can regulate CTGF expression in VSMCs10, 11, 12. However, the role of thrombin in the induction of CTGF expression in VSMCs has not been reported. The promoter region of the human CTGF gene contains binding sites for multiple transcription factors. These transcription factors include activator protein-1 (AP-1), STAT, SMAD, basal control element (BCE) 1, NF-κB, specificity protein 1 (Sp1), and Elk-113, 14. Therefore, we hypothesized that thrombin can induce CTGF expression in VSMCs and its signaling pathways involve PAR-1, mitogen-activated protein kinases (MAPKs), and AP-1. In the present study, we demonstrated that thrombin acts on PAR-1 to activate the JNK signaling pathway, which in turn initiates AP-1 activation and ultimately induces CTGF expression in VSMCs.
Materials and methods
Materials
Thrombin (from bovine plasma), SCH79797, curcumin, actinomycin D (ActD), and cycloheximide (CHX) were purchased from Sigma-Aldrich (St Louis, MO, USA). SFLLRN-NH2 and GYPGQV-NH2 were purchased from Bachem Americas (Torrance, CA, USA). The human CTGF promoter (-747/+214) luciferase construct (pGL3-CTGF-Luc) was provided by Dr ML KUO (National Taiwan University, Taipei, Taiwan, China). JNK1 dominant-negative mutant (DN), JNK2DN15, and pcDNA were provided by Dr MC CHEN (Taipei Medical University, Taipei, Taiwan, China). pBK-CMV-LacZ (LacZ) was provided by Dr WW LIN (National Taiwan University, Taipei, Taiwan, China). Dulbecco's modified Eagle's medium (DMEM), fetal calf serum (FCS), penicillin/streptomycin, sodium pyruvate, L-glutamine, nonessential amino acids (NEAAs), and Lipofectamine Plus reagent were purchased from Invitrogen (Carlsbad, CA, USA). An antibody (Ab) specific for α-tubulin was purchased from Novus Biologicals (Littleton, CO, USA). Abs specific for CTGF, phospho-c-Jun N-terminal kinase (JNK), and anti-mouse, anti-rabbit, and anti-goat IgG-conjugated horseradish peroxidase were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). SP600125 and tcY-NH2 were purchased from Tocris Bioscience (Ellisville, MO, USA). The AP-1-luciferase plasmid was purchased from Stratagene (La Jolla, CA, USA). All materials for SDS-PAGE were purchased from Bio-Rad (Hercules, CA, USA). All other chemicals were obtained from Sigma-Aldrich.
Cell culture
Primary rat aortic smooth muscle cells (RASMCs) were obtained from Cell Applications, Inc (San Diego, CA, USA) and maintained in growth media at 37 °C in a humidified atmosphere containing 95% air and 5% CO2. Cells were used from passage 2 through passage 5. A VSMC line (A10) from the embryonic rat thoracic aorta was obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were grown in DMEM nutrient mixture containing 10% FCS, 2 mmol/L L-glutamine, 0.1 mmol/L NEAA, 1 mmol/L sodium pyruvate, 50 U/mL penicillin G, and 100 μg/mL streptomycin in a humidified 37 °C incubator with 5% CO2. Cells were used between passages 18 and 30 for all experiments. After reaching confluence, cells were seeded onto 6-cm dishes for cell transfection and immunoblotting and onto 12-well plates for cell transfection and luciferase assays.
Western blot analysis
Western blot analyses were performed as described previously16. In brief, A10 cells were cultured in 6-cm dishes. After reaching confluence, cells were treated with the vehicle and thrombin or pretreated with specific inhibitors as indicated followed by thrombin. Whole-cell lysates (50 μg) were subjected to SDS-PAGE and transferred onto a polyvinylidene difluoride membrane that was then incubated in TBST buffer (150 mmol/L NaCl, 20 mmol/L Tris-HCl, and 0.02% Tween 20; pH 7.4) containing 5% BSA. Proteins were visualized using specific primary Abs and then incubated with HRP-conjugated secondary Abs. The immunoreactivity was detected using the enhanced chemiluminescence (ECL) system according to the manufacturer's instructions. Quantitative data were obtained using a computing densitometer with scientific imaging systems (Kodak, Rochester, NY, USA).
Transfection and CTGF-luciferase assays
A10 cells (5×104 cells/well) were seeded onto 12-well plates, and were transfected the following day using Lipofectamine Plus with 0.5 μg of CTGF-luciferase plasmid, 0.8 μg of AP-1-luciferase plasmid, 1 μg of JNK1DN, or 1 μg of JNK2DN. Cells were also cotransfected with 0.2 μg of LacZ. After 6 h, the medium was aspirated and replaced with basal medium devoid of FCS overnight, and cells were stimulated with thrombin for another 16 h before being harvested. To assess the effects of the indicated inhibitors, drugs were added to cells 30 min before thrombin addition. Luciferase activity was determined and normalized on the basis of LacZ expression. The level of induction of luciferase activity was computed as the ratio of cells with and without stimulation.
Statistical analysis
Continuous variables are presented as the mean±SEM. Intergroup differences were analyzed by 1-way ANOVA for comparisons among 3 or more groups and the independent Student's t-test for comparisons between 2 groups. A probability value < 0.05 was regarded as significant.
Results
Thrombin induces CTGF expression
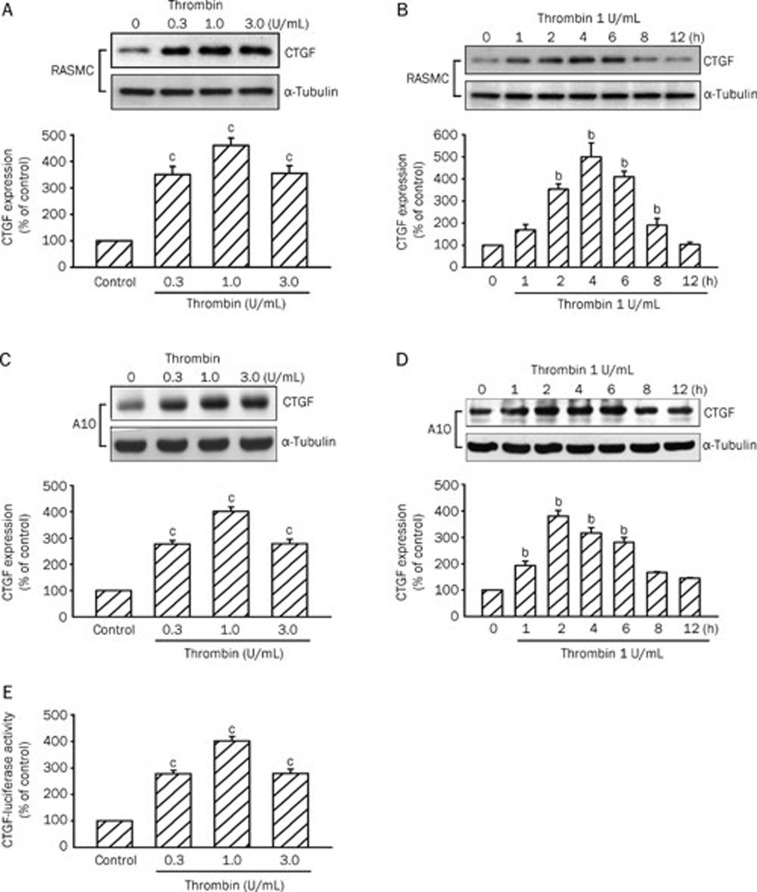
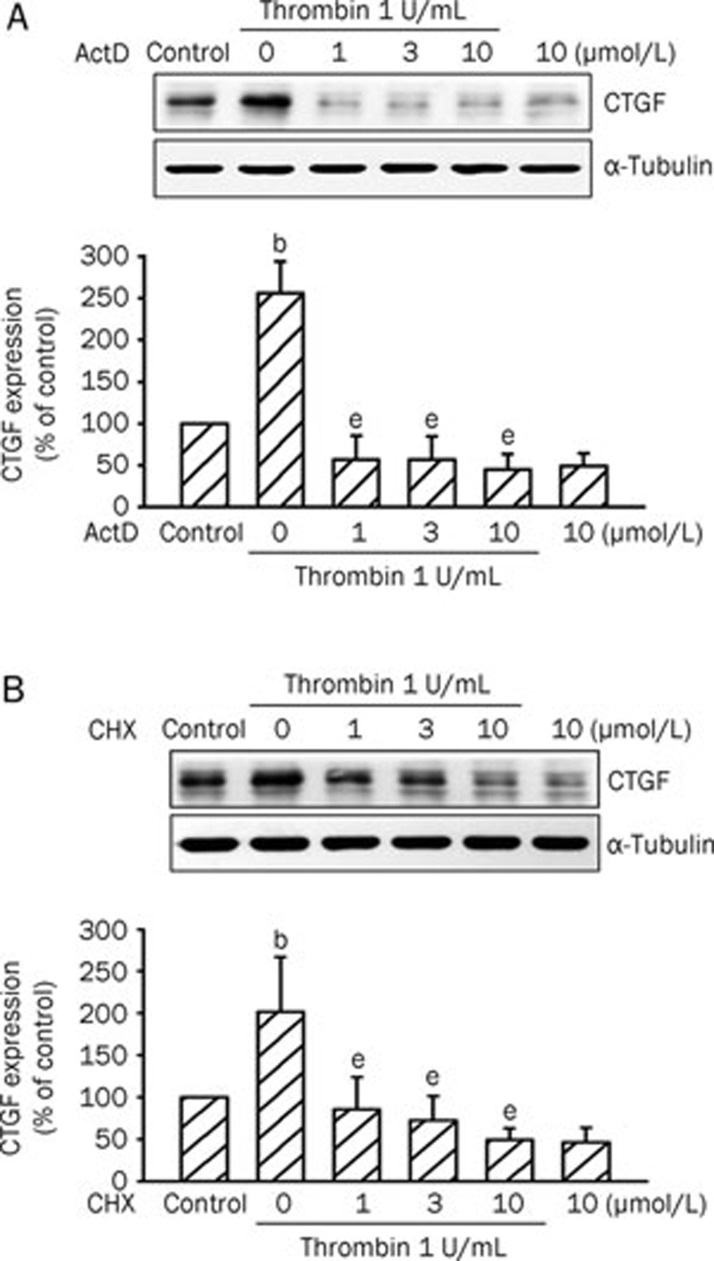
Incubation of the RASMC with thrombin (0.3–3 U/mL) for 4 h induced CTGF protein expression in a concentration-dependent manner, with maximum effects after 1 U/mL thrombin treatment (Figure 1A). The thrombin (1 U/mL)-induced increases in CTGF expression were time-dependent with a maximal effect at 4 h (Figure 1B). Incubation of the A10 cell line, a rat VSMC cell line, with thrombin (0.3–3 U/mL) for 2 h also induced CTGF protein expression in a concentration-dependent manner, with maximum effects after 1 U/mL thrombin treatment (Figure 1C). The thrombin (1 U/mL)-induced increases in CTGF expression were time-dependent (Figure 1D). The induction of CTGF protein began by 1 h after treatment, reached a maximum at 2 h, and then gradually diminished to 8 h after thrombin treatment (Figure 1D). Thrombin-induced CTGF expression obtained from A10 cells was similar to that of the primary RASMC response. Therefore, we used A10 cells in further studies. A10 cells were transiently transfected with a CTGF-luciferase plasmid. As shown in Figure 1E, A10 cells treated with thrombin (0.3–3 U/mL) for 16 h exhibited a 302%±19% (n=3) increase in CTGF-luciferase activity. In the following experiments, A10 cells were treated with 1 U/mL thrombin for 2 h. A10 cells were pretreated with either ActD (a transcriptional inhibitor) or CHX (a translational inhibitor) and then treated with 1 U/mL thrombin. As a result, thrombin-induced elevation of CTGF expression was almost completely inhibited by ActD (1, 3, and 10 μmol/L) and CHX (1, 3, and 10 μmol/L) (n=3 in each group) (Figures 2A and 2B). These results suggest that the increase in CTGF protein level in A10 cells responsive to thrombin was dependent on de novo transcription and translation.
Figure 1.
Thrombin-induced increases in CTGF expression and CTGF-luciferase activity in primary RASMCs and A10 cells. RASMCs were incubated with various concentrations of thrombin for 4 h (A) or with 1 U/mL thrombin for the indicated time intervals (B). A10 cells were incubated with various concentrations of thrombin for 2 h (C) or with 1 U/mL thrombin for the indicated time intervals (D). Cells were lysed and then immunoblotted with Abs specific for CTGF or α-tubulin. Data are presented as mean±SEM. n=3. bP<0.05, cP<0.01 vs basal level. (E) A10 cells were transiently transfected with 0.5 μg of CTGF-luciferase plasmid and 0.2 μg of LacZ for 6 h and then stimulated with 0.3–3 U/mL thrombin for 16 h. Cells were harvested for the luciferase activity assay. Data are presented as mean±SEM. n=3. cP<0.01 vs untreated cells.
Figure 2.
Effects of ActD and CHX on CTGF expression induced by thrombin. A10 cells were pretreated for 30 min with ActD (1–10 μmol/L) (A) or CHX (1–10 μmol/L) (B) and then stimulated with 1 U/mL thrombin for another 2 h. Cell lysates were prepared and then immunoblotted with Abs specific for CTGF or α-tubulin. Data are presented as mean±SEM. n=3. bP<0.05 vs control. eP<0.05 vs the thrombin treatment group.
Involvement of PAR-1 in thrombin-induced CTGF expression
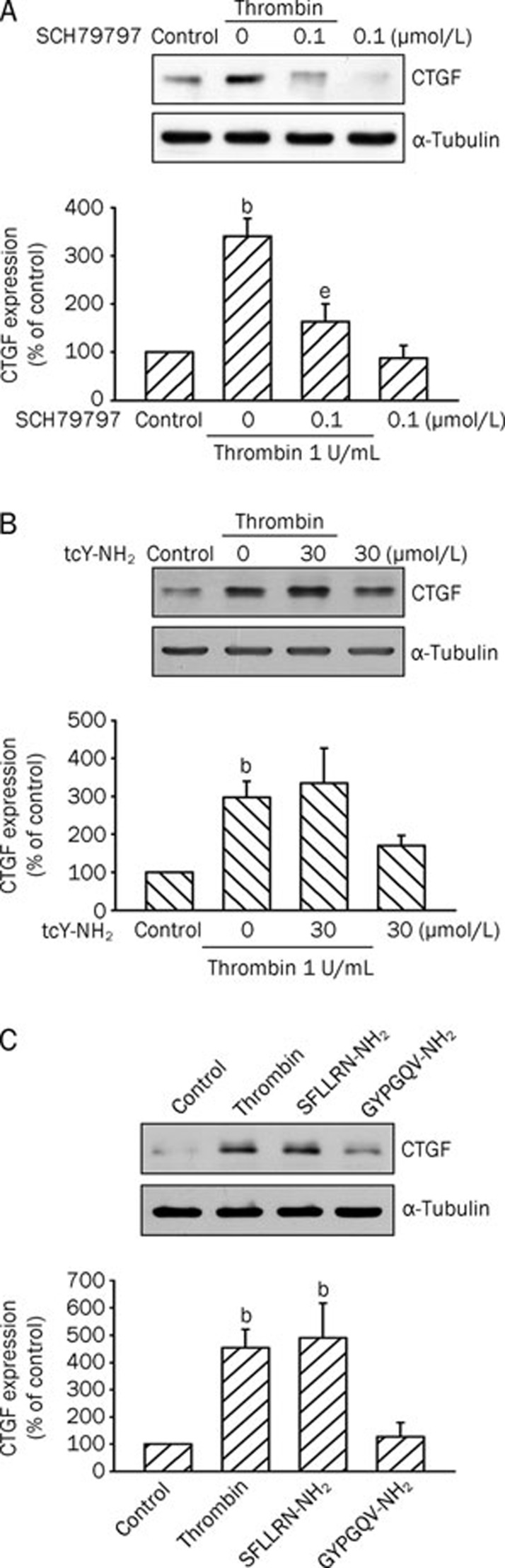
To identify the PARs involved in thrombin-induced CTGF expression, the PAR-1 antagonist SCH79797 and PAR-4 antagonist tcY-NH2 were tested. As shown in Figure 3A, pretreating A10 cells with SCH79797 (0.1 μmol/L) inhibited thrombin-induced CTGF expression by 83%±22%, while tcY-NH2 (30 μmol/L) had no effect (n=3; Figure 3B). Moreover, treatment of A10 cells with the PAR-1 agonist peptide SFLLRN-NH2 (300 μmol/L) also resulted in a 391%±117% (n=3) increase in CTGF expression, whereas the PAR-4 agonist peptide GYPGQV-NH2 (300 μmol/L) had no effect (n=3; Figure 3C). These results suggest that thrombin-mediated CTGF expression in A10 cells may occur via activation of PAR-1, but not PAR-4, signaling.
Figure 3.
Involvement of PAR-1 in thrombin-induced CTGF expression in A10 cells. Cells were pretreated with 0.1 μmol/L SCH79797 (A) or 30 μmol/L tcY-NH2 (B) for 30 min and then stimulated with 1 U/mL thrombin for another 2 h. Cell lysates were prepared and then immunoblotted with Abs specific for CTGF or α-tubulin. Data are presented as mean±SEM. n=3. bP<0.05 vs control; eP<0.05 vs the thrombin treatment group. (C) Cells were incubated with 1 U/mL thrombin, 300 μmol/L SFLLRN-NH2 (a PAR-1 agonist), or 300 μmol/L GYPGQV-NH2(a PAR-4 agonist) for 2 h. Cells were lysed and then immunoblotted with Abs specific for CTGF or α-tubulin. Data are presented as mean±SEM. n=3. bP<0.05 vs control.
JNK is involved in thrombin-induced CTGF expression
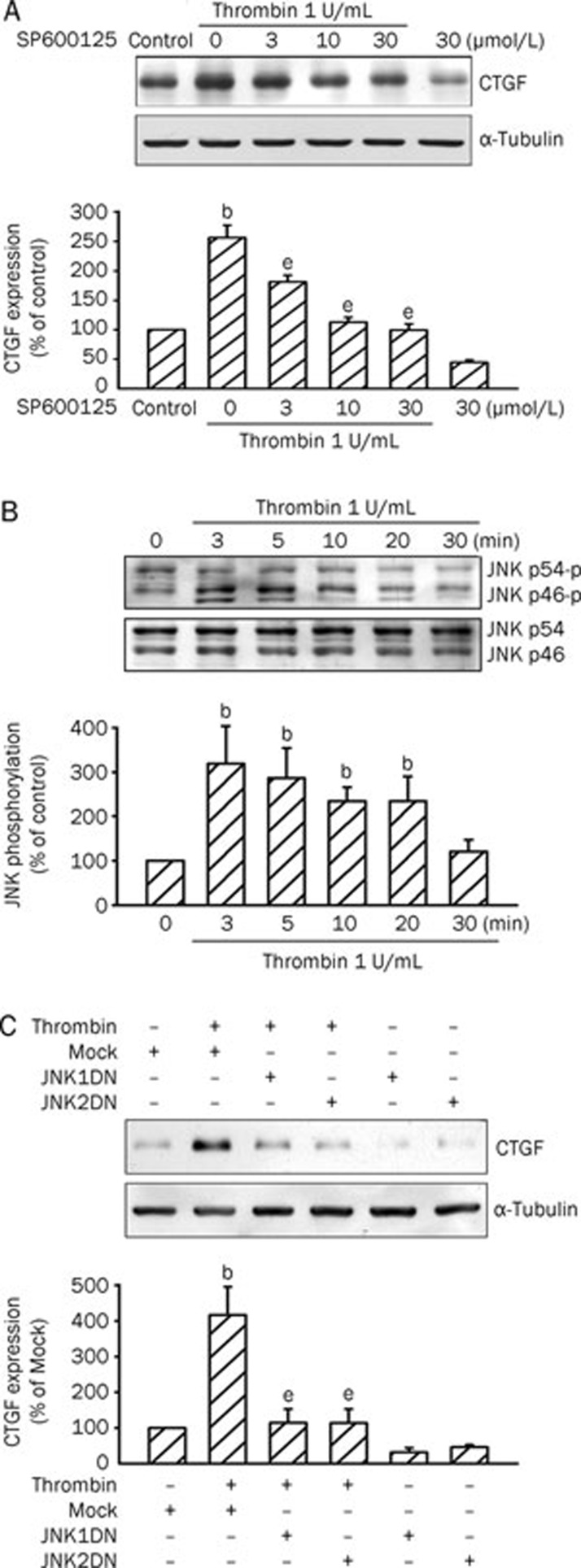
We next attempted to determine whether JNK signaling events are involved in thrombin-induced CTGF expression by using SP600125, a specific inhibitor of JNK17. As shown in Figure 4A, thrombin-induced CTGF expression was concentration-dependently attenuated by pretreating A10 cells with SP600125 (3–30 μmol/L). Pretreating A10 cells with 30 μmol/L SP600125 completely inhibited thrombin-induced CTGF expression (n=3). We then examined whether thrombin could activate JNK. Treating A10 cells with 1 U/mL thrombin resulted in a time-dependent phosphorylation of JNK. The phosphorylation of JNK was maximal at 3–5 min and returned to basal level after 30 min of thrombin treatment (Figure 4B). To further confirm that JNK mediates thrombin-induced CTGF expression JNK1DN and JNK2DN were used. As shown in Figure 4C, transfection of A10 cells with 1 μg of JNK1DN and JNK2DN, respectively, inhibited thrombin-induced CTGF expression by 86%±21% and 90%±25% (n=3).
Figure 4.
JNK is involved in thrombin-induced CTGF expression in A10 cells. (A) Cells were pretreated with various concentrations (3–30 μmol/L) of SP600125 for 30 min and then stimulated with 1 U/mL thrombin for another 2 h. Cells were lysed and then immunoblotted with Abs specific for CTGF or α-tubulin. Data are presented as mean±SEM. n=3. bP<0.05 vs control. eP<0.05 vs the thrombin treatment group. (B) Cells were treated with 1 U/mL thrombin for different time intervals. Cell lysates were prepared and then immunoblotted with Abs specific for phospho-JNK or JNK. Data are presented as mean±SEM. n=3. bP<0.05 vs basal level. (C) Cells were transiently transfected with 1 μg of JNK1DN or JNK2DN for 6 h and then stimulated with 1 U/mL thrombin for another 2 h. Cells were lysed and then immunoblotted with Abs specific for CTGF or α-tubulin. Data are presented as mean±SEM. n=3. bP<0.05 vs the mock group; eP<0.05 vs the thrombin treatment group.
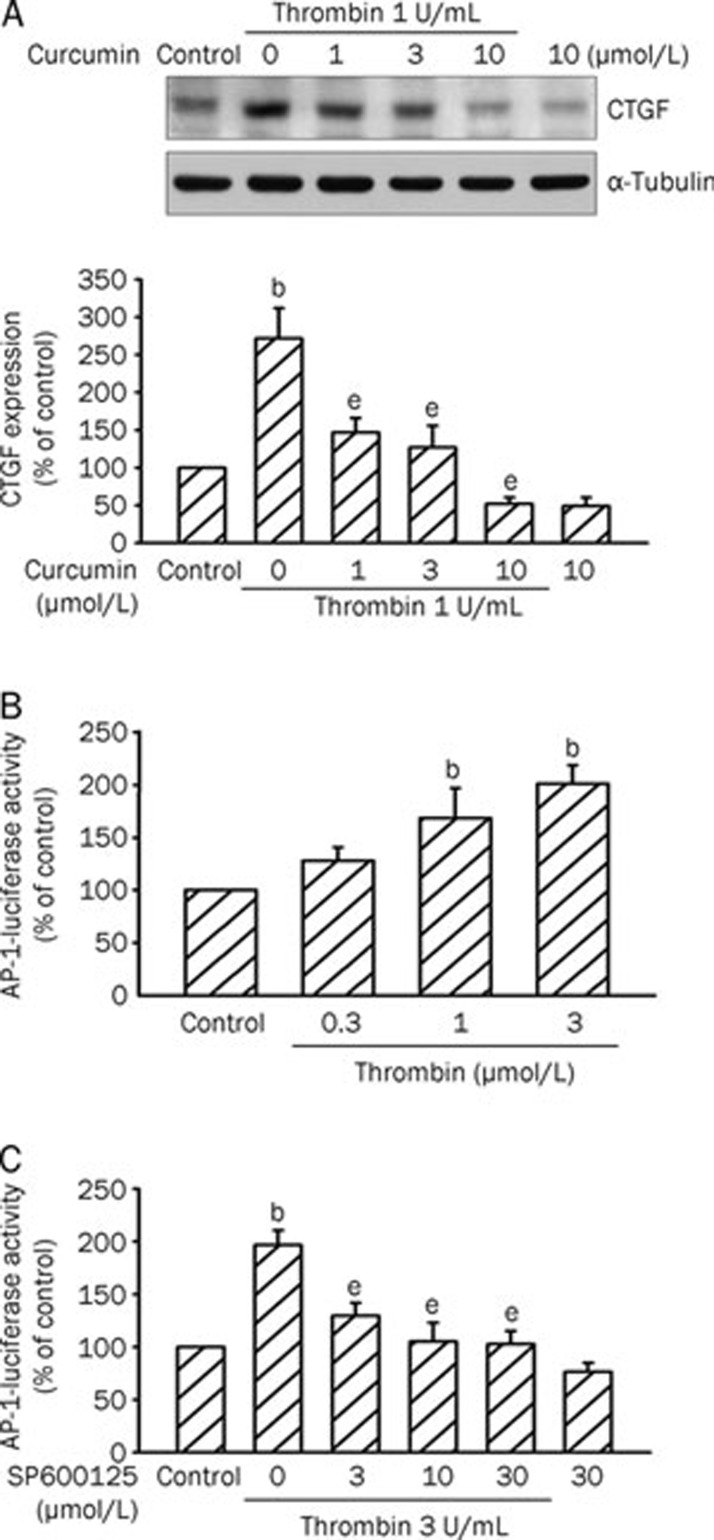
AP-1 mediates thrombin-induced CTGF expression
Next, we explored the role of AP-1 in thrombin-induced CTGF expression by using the AP-1 inhibitor curcumin18. As shown in Figure 5A, thrombin-induced CTGF expression was markedly attenuated by pretreating A10 cells with curcumin (1–10 μmol/L) in a concentration-dependent manner. Curcumin at 10 μmol/L completely suppressed thrombin-induced CTGF expression (n=3). To further confirm that AP-1 is involved in thrombin-induced CTGF expression, transient transfection was performed using the AP-1-luciferase reporter plasmids. Exposure to thrombin (0.3–3 U/mL) led to a concentration-dependent increase in AP-1-luciferase activity in A10 cells. There was a 101%±23% increase in AP-1-luciferase activity after treatment with 3 U/mL thrombin for 16 h (Figure 5B). To further confirm that thrombin-induced AP-1-luciferase activity occurs via JNK pathways, we used the JNK inhibitor. As shown in Figure 5C, pretreating A10 cells with SP600125 (3–30 μmol/L) exhibited decreases in thrombin-induced AP-1-luciferase activity (n=3 in each group).
Figure 5.
Involvement of AP-1 in thrombin-induced CTGF expression in A10 cells. (A) Cells were pretreated with various concentrations (1–10 μmol/L) of curcumin for 30 min and then stimulated with 1 U/mL thrombin for another 2 h. Cells were lysed and then immunoblotted with Abs specific for CTGF or α-tubulin. Data are presented as mean±SEM. n=3. bP<0.05 vs control; eP<0.05 vs the thrombin treatment group. (B) Cells were transiently transfected with 0.8 μg of AP-1-luciferase plasmid and 0.2 μg of LacZ for 4 h and then stimulated with 0.3–3 U/mL thrombin for 16 h. Cells were harvested for the luciferase activity assay. Data are presented as mean±SEM. n=3. bP<0.05 vs control. (C) Cells were transiently transfected with 0.8 μg of AP-1-luciferase plasmid and 0.2 μg of LacZ for 4 h and then were pretreated with various concentrations (3–30 μmol/L) of SP600125 for 30 min. The cells were then stimulated with 3 U/mL thrombin for 16 h. Cells were harvested for the luciferase activity assay. Data are presented as mean±SEM. n=3. bP<0.05 vs control. eP<0.05 vs the thrombin treatment group.
Discussion
In this study, we found for the first time that in VSMCs thrombin acts on PAR-1 to activate the JNK signaling pathway, which in turn initiates AP-1 activation and ultimately induces CTGF expression. Thrombin is a serine protease that is generated by cleavage of its inactive precursor prothrombin. Thrombin converts the monomer fibrinogen to insoluble fibrin, in addition to activating other clotting factors V, VIII, and XIII, thus facilitating thrombus formation19. However, more than 95% of thrombus-associated thrombin is formed after clotting is complete and is continuously released by mural thrombi20. Endothelial injury allows thrombin to have direct contact with the subendothelial VSMCs. Tissue factor presented by VSMCs can further trigger the formation of thrombin21. Therefore, subendothelial VSMCs may be exposed to high levels of thrombin continuously. Subsequently, activation of PAR-1 in VSMCs by thrombin causes the activation of several pathways, including calcium signaling, proliferation, cytoskeletal rearrangement, contraction, and extracellular matrix synthesis4, 22.
CTGF gene is highly conserved among species7. The CTGF primary translational product is more than 90% conserved in mammals23. The expression patterns of CTGF in RASMC and A10 cells were similar to that in human lung fibroblasts in our previous study15. The similar expression pattern of CTGF was also found in human umbilical vein smooth muscle cells12 and in human aortic smooth muscle cells24. Because the expression pattern was similar in rat and human VSMCs, we used A10 cells in this study that focused on the signaling pathways involving CTGF expression. CTGF has been suggested to play an important role in the development and progression of atherosclerosis through its paracrine effects25. CTGF is a mitogenic and chemotactic factor for VSMCs and stimulates extracellular matrix production26. CTGF also stimulates the expression of matrix metalloproteinase (MMP)-227. It is possible that CTGF overexpressed in advanced atherosclerotic plaques may contribute to plaque destabilization25. Atherosclerotic plaques are composed of a lipid-rich core, a cap of fibrous tissue, VSMCs, connective tissue extracellular matrix, and inflammatory cells. Plaque disruption may result in mural thrombi. Such thrombi may be the main contributor of progression of atherosclerosis28. In our present study, we found that thrombin could induce CTGF expression in VSMCs. This suggested that CTGF might play a role in the pathogenesis of atherothrombosis.
PARs play crucial roles in coagulation and vascular homeostasis29. Overexpression of PAR-1 has been found in the VSMCs from thickening intimas of human atherosclerotic arteries30. Although subtypes of thrombin-responsive PARs, PAR-1, PAR-3, and PAR-4, are present and functionally active in VSMCs, PAR-1 has the highest affinity for thrombin31. PAR-1 is the prototypic thrombin receptor and the main isoform involved in VSMC neointimal formation and restenosis in vivo32, whereas PAR-3 appears to function as a cofactor for PAR-433. In this study, we found that a PAR-1 antagonist (SCH79797) significantly inhibited thrombin-induced CTGF expression, while a PAR-4 antagonist (tcY-NH2) had no effect. We also demonstrated that a PAR-1 agonist (SFLLRN-NH2) induced CTGF expression, while a PAR-4 agonist (GYPGQV-NH2) had no effect. These results suggest that PAR-1, but not PAR-4, is responsible for thrombin-induced CTGF expression in A10 cells.
MAPKs, composed of ERK, JNK, and p38 MAPK, are serine/threonine kinases that play a critical role in cell differentiation, growth, apoptosis, and the regulation of various transcription factors and gene expression34. MAPKs are significantly activated in vascular tissues by hypertension, angiotensin II, or balloon injury35. MAPK also participate in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression36. The JNK cascade plays an important role in a variety of physiological and pathological processes such as cell apoptosis, the inflammatory response, and cytokine production37. Activation of JNK family activity is suggested to be involved in atherosclerosis. JNK activation was shown using atherosclerosis prone ApoE knockout mice and a high cholesterol diet38. JNK2 knockout mice were protected from the development of abdominal aortic aneurysm through a reduction in tissue breakdown and enhanced tissue repair39. In this study, we found that thrombin-induced CTGF expression was concentration-dependently attenuated by a JNK inhibitor (SP600125). Furthermore, thrombin caused a time-dependent phosphorylation of JNK. These results suggest that JNK is involved in thrombin-induced CTGF expression in VSMCs. This was further confirmed by transfection of A10 cells with JNK1DN and JNK2DN, which inhibited thrombin-induced CTGF expression. In addition, specific knockdown of JNK expression by using RNA interference would be also an appropriate method to study JNK signaling. A limitation of this study was that we did not perform RNA interference studies.
The promoter region of the human CTGF gene contains multiple transcription factor-binding sites, including those for AP-1, STAT, SMAD, BCE-1, NF-κB, Sp1, and Elk-113. AP-1 is one of the main transcription factors activated by MAPK, and it plays a central role in a variety of cellular responses40. In our previous report, we found that thrombin-induced CTGF expression required the JNK and AP-1 pathway in human lung fibroblasts15. In the present study, we demonstrated that AP-1 is involved in thrombin-induced CTGF expression in VSMCs by using an AP-1 inhibitor and the luciferase activity. We also demonstrated that thrombin-induced increase in AP-1-luciferase activity was inhibited by a JNK inhibitor. These results suggest that thrombin-induced AP-1 activation occurs via the JNK pathway. Nevertheless, one of the limitations of our study was that we did not directly assess the binding of AP-1 to CTGF promoter by using chromatin immunoprecipitation or electrophoretic mobility shift assay. In addition to this important JNK/AP-1 pathway, thrombin may act upon VSMCs through several other signaling pathways. Thrombin enhanced VSMC proliferation through epidermal growth factor receptor, ERK, and AP-1 pathways41. Thrombin also stimulated VSMC migration through an ROS-sensitive p38 MAPK pathway42.
Much evidence suggests that thrombin acts as a powerful modulator in the progression of atherosclerosis43. Overexpression of CTGF has been found in atherosclerotic carotid arteries and in the aortic wall from patient with thoracic aortic dissection8, 44. Based on the result of this study, together with evidence from clinical specimens, it might suggest that CTGF is one of the mediators in the progression of atherosclerosis44. There are many new and emerging antithrombotic agents including PAR-1 antagonists, thrombin inhibitors, etc45, 46. The direct thrombin inhibitor, dabigatran, could prevent thrombin-induced cleavage of the extracellular N-terminal domain of PAR-147. It may have clinical significance to study further the effects of the new antithrombotic agents on CTGF expression and atherosclerosis.
In conclusion, our results demonstrate for the first time that thrombin acts on PAR-1 to activate the JNK signaling pathway, which in turn initiates AP-1 activation and ultimately induces CTGF expression in VSMC. Our results provide a mechanism linking thrombin and the profibrotic protein CTGF and may provide an insight into the pathogenesis of atherothrombosis.
Author contribution
Wen-chin KO, Chien-huang LIN, and Bing-chang CHEN designed the research; Wen-chin KO and Bing-chang CHEN performed the experiments; Ming-jen HSU and Chia-ti TSAI performed some of the experiments; Wen-chin KO analyzed the data and wrote the article; and Chuang-ye HONG and Chien-huang LIN revised the article.
Acknowledgments
This study was supported by a grant (96CGH-TMU-03) from the Cathay General Hospital, Taipei, Taiwan, China.
References
- Schrör K, Bretschneider E, Fischer K, Fischer JW, Pape R, Rauch BH, et al. Thrombin receptors in vascular smooth muscle cells — function and regulation by vasodilatory prostaglandins. Thromb Haemost. 2010;103:884–90. doi: 10.1160/TH09-09-0627. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- Martorell L, Martinez-Gonzalez J, Rodriguez C, Gentile M, Calvayrac O, Badimon L. Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb Haemost. 2008;99:305–15. doi: 10.1160/TH07-08-0481. [DOI] [PubMed] [Google Scholar]
- Dabbagh K, Laurent GJ, McAnulty RJ, Chambers RC. Thrombin stimulates smooth muscle cell procollagen synthesis and mRNA levels via a PAR-1 mediated mechanism. Thromb Haemost. 1998;79:405–9. [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–4. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–94. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy. Matrix Biol. 2002;21:473–82. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- Cicha I, Yilmaz A, Klein M, Raithel D, Brigstock DR, Daniel WG, et al. Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2005;25:1008–13. doi: 10.1161/01.ATV.0000162173.27682.7b. [DOI] [PubMed] [Google Scholar]
- Ruperez M, Lorenzo O, Blanco-Colio LM, Esteban V, Egido J, Ruiz-Ortega M. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation. 2003;108:1499–505. doi: 10.1161/01.CIR.0000089129.51288.BA. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-β signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Vita J, Ruiz-Ortega M, Ruperez M, Esteban V, Sanchez-Lopez E, Plaza J, et al. Endothelin-1, via ETA receptor and independently of transforming growth factor-beta, increases the connective tissue growth factor in vascular smooth muscle cells. Circ Res. 2005;97:125–34. doi: 10.1161/01.RES.0000174614.74469.83. [DOI] [PubMed] [Google Scholar]
- Liu X, Luo F, Li J, Wu W, Li L, Chen H. Homocysteine induces connective tissue growth factor expression in vascular smooth muscle cells. J Thromb Haemost. 2008;6:184–92. doi: 10.1111/j.1538-7836.2007.02801.x. [DOI] [PubMed] [Google Scholar]
- Blom IE, van Dijk AJ, de Weger RA, Tilanus MGJ, Goldschmeding R. Identification of human ccn2 (connective tissue growth factor) promoter polymorphisms. Mol Pathol. 2001;54:192–6. doi: 10.1136/mp.54.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–80. [PubMed] [Google Scholar]
- Yu CC, Hsu MJ, Kuo ML, Chen RF, Chen MC, Bai KJ, et al. Thrombin-induced connective tissue growth factor expression in human lung fibroblasts requires the ASK1/JNK/AP-1 pathway. J Immunol. 2009;182:7916–27. doi: 10.4049/jimmunol.0801582. [DOI] [PubMed] [Google Scholar]
- Chen BC, Chang YS, Kang JC, Hsu MJ, Sheu JR, Chen TL, et al. Peptidoglycan induces nuclear factor-κB activation and cyclooxygenase-2 expression via Ras, Raf-1, and ERK in RAW 264.7 macrophages. J Biol Chem. 2004;279:20889–97. doi: 10.1074/jbc.M311279200. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin V, Kantor B, Weg V, Hartman M-L, Levi-Schaffer F. Tryptase activates the mitogen-activated protein kinase/activator protein-1 pathway in human peripheral blood eosinophils, causing cytokine production and release. J Immunol. 2002;169:2662–9. doi: 10.4049/jimmunol.169.5.2662. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100:148–52. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- Bretschneider E, Braun M, Fischer A, Wittpoth M, Glusa E, Schror K. Factor Xa acts as a PDGF-independent mitogen in human vascular smooth muscle cells. Thromb Haemost. 2000;84:499–505. [PubMed] [Google Scholar]
- Damiano BP, Derian CK, Maryanoff BE, Zhang HC, Gordon PA. RWJ-58259: a selective antagonist of protease activated receptor-1. Cardiovasc Drug Rev. 2003;21:313–26. doi: 10.1111/j.1527-3466.2003.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- Kang SW, Kim JL, Kwon GT, Lee YJ, Park JH, Lim SS, et al. Sensitive fern (Onoclea sensibilis) extract suppresses proliferation and migration of vascular smooth muscle cells inflamed by neighboring macrophages. Biol Pharm Bull. 2011;34:1717–23. doi: 10.1248/bpb.34.1717. [DOI] [PubMed] [Google Scholar]
- Game BA, He L, Jarido V, Nareika A, Jaffa AA, Lopes-Virella MF, et al. Pioglitazone inhibits connective tissue growth factor expression in advanced atherosclerotic plaques in low-density lipoprotein receptor-deficient mice. Atherosclerosis. 2007;192:85–91. doi: 10.1016/j.atherosclerosis.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Moussad EE-DA, Brigstock DR. Connective tissue growth factor: what's in a name. Mol Genet Metab. 2000;71:276–92. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- Fan WH, Karnovsky MJ. Increased MMP-2 expression in connective tissue growth factor over-expression vascular smooth muscle cells. J Biol Chem. 2002;277:9800–5. doi: 10.1074/jbc.M111213200. [DOI] [PubMed] [Google Scholar]
- Corti R, Hutter R, Badimon JJ, Fuster V. Evolving concepts in the triad of atherosclerosis, inflammation and thrombosis. J Thromb Thrombolysis. 2004;17:35–44. doi: 10.1023/B:THRO.0000036027.39353.70. [DOI] [PubMed] [Google Scholar]
- Leger AJ, Covic L, Kuliopulos A. Protease-activated receptors in cardiovascular diseases. Circulation. 2006;114:1070–7. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- Ku DD, Dai J. Expression of thrombin receptors in human atherosclerotic coronary arteries leads to an exaggerated vasoconstrictory response in vitro. J Cardiovasc Pharmacol. 1997;30:649–57. doi: 10.1097/00005344-199711000-00016. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. The cardiovascular actions of protease–activated receptors. Mol Pharmacol. 2005;67:2–11. doi: 10.1124/mol.104.003103. [DOI] [PubMed] [Google Scholar]
- Andrade-Gordon P, Derian CK, Maryanoff BE, Zhang H-C, Addo MF, Cheung W-m, et al. Administration of a potent antagonist of protease-activated receptor-1 (PAR-1) attenuates vascular restenosis following balloon angioplasty in rats. J Pharmacol Exp Ther. 2001;298:34–42. [PubMed] [Google Scholar]
- Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–13. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Kim S, Iwao H. Stress and vascular responses: mitogen-activated protein kinases and activator protein-1 as promising therapeutic targets of vascular remodeling. J Pharmacol Sci. 2003;91:177–81. doi: 10.1254/jphs.91.177. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Kim S, Izumi Y, Izumiya Y, Nakao T, Miyazaki H, et al. Role of JNK, p38, and ERK in platelet–derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler Thromb Vasc Biol. 2003;23:795–801. doi: 10.1161/01.ATV.0000066132.32063.F2. [DOI] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK) from inflammation to development. Curr Opin Cell Biol. 1998;10:205–19. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Ricci R, Sumara G, Sumara I, Rozenberg I, Kurrer M, Akhmedov A, et al. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 2004;306:1558–61. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Aoki H, Ikeda Y, Fujii K, Akiyama N, Furutani A, et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med. 2005;11:1330–8. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP–1 in cell proliferation and survival. Oncogene. 2001;20:2390–400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Tung WH, Wu CY, Wang HH, Lin CC, Wang TS, et al. Thrombin induces EGF receptor expression and cell proliferation via a PKC(δ)/c-Src-dependent pathway in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2009;29:1594–601. doi: 10.1161/ATVBAHA.109.185801. [DOI] [PubMed] [Google Scholar]
- Wang Z, Castresana MR, Newman WH. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2004;36:49–56. doi: 10.1016/j.yjmcc.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Borissoff JI, Spronk HMH, Heeneman S, ten Cate H. Is thrombin a key player in the 'coagulation-atherogenesis' maze. Cardiovasc Res. 2009;82:392–403. doi: 10.1093/cvr/cvp066. [DOI] [PubMed] [Google Scholar]
- Wang X, LeMaire SA, Chen L, Shen YH, Gan Y, Bartsch H, et al. Increased collagen deposition and elevated expression of connective tissue growth factor in human thoracic aortic dissection. Circulation. 2006;114:I200–5. doi: 10.1161/CIRCULATIONAHA.105.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Michel S. The mechanism of action of rivaroxaban — an oral, direct Factor Xa inhibitor — compared with other anticoagulants. Thromb Res. 2011;127:497–504. doi: 10.1016/j.thromres.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Leonardi S, Tricoci P, Becker RC. Thrombin receptor antagonists for the treatment of atherothrombosis: therapeutic potential of vorapaxar and E-5555. Drugs. 2010;70:1771–83. doi: 10.2165/11538060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Bogatkevich GS, Ludwicka-Bradley A, Silver RM. Dabigatran, a direct thrombin inhibitor, demonstrates antifibrotic effects on lung fibroblasts. Arthritis Rheum. 2009;60:3455–64. doi: 10.1002/art.24935. [DOI] [PMC free article] [PubMed] [Google Scholar]