Abstract
Aim:
To investigate the action of salvianolic acid A (SalA) on angiotensin II (Ang II)-induced proliferation of human umbilical vein endothelial cells (HUVECs) and the possible signaling pathways mediating this action.
Methods:
Cell proliferation was examined with MTT assay. The expression levels of Src phosphorylation (phospho-Src), Akt phosphorylation (phospho-Akt), and NADPH oxidase 4 (Nox4) in HUVECs were determined by Western blot. The production of reactive oxygen species (ROS) was estimated using fluorescence-activated cell sorting (FACS).
Results:
SalA (6.25–50 μmol/L) did not affect the viability of HUVECs. Treatment of HUVECs with Ang II (1 μmol/L) markedly increased the cell viability; pretreatment of HUVECs with SalA (12.5, 25 and 50 μmol/L) prevented Ang II-induced increase of the cell viability in a concentration-dependent manner. Treatment of HUVECs with Ang II (1 μmol/L) markedly up-regulated the protein expression levels of phospho-Src, phospho-Akt (473) and Nox4; pretreatment of HUVECs with SalA (12.5, 25 and 50 μmol/L) blocked all the effects in a concentration-dependent manner. Treatment of HUVECs with Ang II (1 μmol/L) dramatically increased ROS production in HUVECs; pretreatment of HUVECs with SalA (12.5, 25 and 50 μmol/L) blocked the ROS production in a concentration-dependent manner.
Conclusion:
SalA inhibits Ang II-induced proliferation of HUVECs via reducing the expression levels of phospho-Src and phospho-Akt (473), thereby attenuating the production of ROS.
Keywords: salvianolic acid A, human umbilical vein endothelial cells, angiotensin II, phospho-Src, phospho-Akt (473), NADPH oxidase 4, ROS
Introduction
Endothelial cell proliferation plays an important role in angiogenesis, as well as atherogenesis, postangioplasty restenosis and other inflammatory vascular diseases such as rheumatoid arthritis and proliferative diabetic retinopathy1, 2, 3. In atherosclerosis, the proliferation of endothelial cells triggers platelet aggregation, leukocyte adhesion and transmigration, and vascular smooth muscle cell proliferation1.
Angiotensin II (Ang II), one of the key components of the renin-angiotensin system, regulates a variety of physiological and pathological processes, including fluid homeostasis, aldosterone production, and renal function. It has recently been suggested that Ang II is not simply an autacoid with hemodynamic and renal actions but rather a biologically active mediator that acts directly on endothelial cells. Evidence is rapidly accumulating to support the theory that Ang II stimulates NADPH oxidase-dependent superoxide production in endothelial cells and that this is the main source of reactive oxygen species (ROS) in these cells4, 5, 6. NADPH oxidases (Noxs) are a family of multicomponent transmembrane enzymes that transport electrons across biological membranes to generate super-oxide, O2(–), by the reduction of oxygen7. A growing body of evidence has demonstrated that ROS production plays an important role in a variety of signal transduction pathways. ROS have been implicated in growth factor receptor signaling, as well as in the regulation of various transcription factors involved in cell proliferation, differentiation, and apoptosis8, 9, 10.
Src is a cytoplasmic protein tyrosine kinase, and the activation and recruitment of this protein to perimembranous signaling complexes has important implications for a cell's fate11. At present, there are at least 14 confirmed varieties of Src kinase. The 60 kDa c-Src isoform is the most widely expressed version in vascular endothelial cells12, and c-Src is the main modulator of NADPH oxidase-mediated superoxide anion production, which induces the vascular redox reaction13. Indeed, activation of the Src family kinases has been known to play an important role in cellular proliferation and migration, angiogenesis, and chemokine induction. Phosphoinositide 3-kinase (PI3K) and its downstream serine/threonine kinase Akt, also termed protein kinase B (PKB), play a central role in promoting the survival of a wide range of cell types. Akt is believed to play a crucial role in apoptosis, cell cycle regulation, angiogenesis, and tumor progression14, 15. Furthermore, Windham et al16 reported that Src activation contributes to the resistance of cancer cells to cell death through the PI3K/Akt pathway because increased Src kinase activity leads to increased Akt phosphorylation.
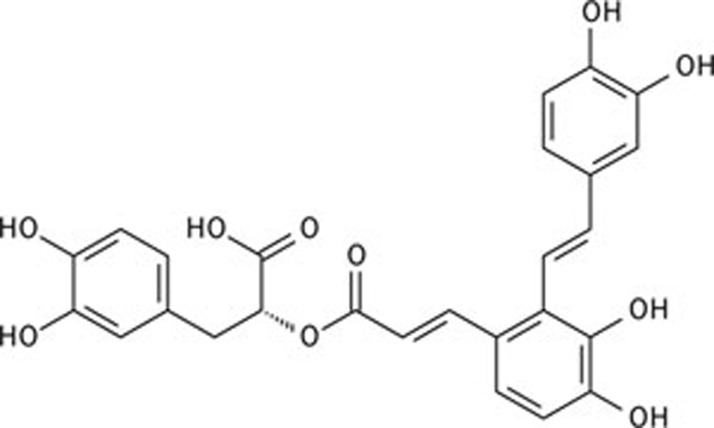
The dried root of Salvia miltiorrhiza Bunge (Danshen) is a popular traditional Chinese medicine that has been widely used in both Asian and Western countries for the treatment of various diseases, including cerebrovascular diseases, coronary artery disease, myocardial infarction, hepatitis, hemorrhage, diabetes mellitus, and menstrual abnormalities, for thousands of years. Salvianolic acids include salvianolic acid A (SalA), salvianolic acid B (SalB), rosmarinic acid, and other polyphenolic acids. SalA ((2R)-3-(3,4-dihydroxyphenyl)-2-[(E)-3-[2-[(E)-2-(3,4-dihydroxyphenyl) ethenyl]-3,4-dihydroxyphenyl]prop-2-enoyl]oxypropanoic acid, see Figure 1) is the main active constituent of S miltiorrhiza17. This water-soluble compound has been reported to possess a wide range of pharmacological effects, including anti-inflammatory, anticarcinogenic, antioxidant, estrogenic, antiplatelet, and antifibrotic activities18, 19, 20, 21. Investigative results from our laboratory showed that SalA also exhibited cardioprotective effects 22.
Figure 1.
The chemical structure of salvianolic acid A (SalA).
To date, little is known about the effects of SalA on vascular endothelial cells. The aims of this study, therefore, were to examine the inhibitory effect of SalA on the Ang II-induced proliferation of HUVECs and to further investigate the compound's effects on interrelated signaling pathways .
Materials and methods
Chemicals
SalA lyophilized powder (product number 20091201, purity>98%), was supplied by Qing Feng Pharmaceutical Products (Jiangxi, China). Ang II, DMSO, and MTT were obtained from Sigma Chemical Co (St Louis, MO, USA). SalA and Ang II were dissolved in distilled water to reach a concentration of 0.1 mmol/L and were stored at 4 °C, for use as soon as possible. F12K was supplied by Hyclone (Logan, Utah, USA). Endothelial cell growth factor (ECGS) was obtained from Sciencell (San Diego, USA). Fetal bovine serum (FBS) and 0.25% trypsin were purchased from Gibco (Grand Island, NY). DCFH-DA (2′,7′-dichlorofluorescin diacetate) and heparin were from Beyotime Institute of Biotechnology (Jiangsu, China). Antibodies for phospho-Akt (Ser-473) and phospho-Src were obtained from Cell Signaling Technology Inc (Danvers, MA, USA). Antibody for Nox4 was obtained from Proteintech Group (Chicago, IL, USA).
Cell culture
HUVECs were obtained from American Type Culture Collection (ATCC). The HUVECs were cultured in a medium containing F12K, 10% fetal bovine serum, 0.1 mg/mL heparin, and 0.03–0.05 mg/mL ECGS, at 37 °C and 5% humidified atmospheric CO2. The medium was refreshed every 2–3 d.
Cellular proliferation assay
Cellular proliferation was determined by MTT chromatometry, as originally described by Mosmann23. In brief, HUVECs were seeded in 96-well plates at a density of 5000 cells/well. First, the cells were treated with 0, 6.25, 12.5, 25, 50, 100, or 200 μmol/L SalA for 24 h and then, after treatment with either 12.5, 25, or 50 μmol/L SalA for 12 h, we treated the cells with 1 μmol/L Ang II for 24 h. Twenty microliters of MTT solution (5 mg/mL in PBS) were added to each well, and the plates were incubated for an additional 4 h at 37 °C. Then, the medium was removed, and wells were rinsed twice with PBS. To each well, 150 μL of DMSO was added at room temperature for 5 min to dissolve the formazan crystals, and then the optical density at 570 nm was determined with a Microplate Reader (Bio-Rad 550, Hercules, CA, USA). Each assay was performed in triplicate. Cellular proliferation is expressed as an index of percent viability (% viability), compared to the control cells.
Western blotting analysis for phospho-Akt, phospho-Src, and Nox4 expression
To investigate the mechanisms underlying the effects of SalA on the HUVEC proliferation induced by Ang II, the expression levels of Akt, Src, phospho-Akt, phospho-Src, and Nox4 in SalA-treated HUVECs were evaluated by Western blotting. The expression of β-actin was measured as an internal standard. After designated treatment, the HUVECs were lysed and centrifuged. The protein concentrations were quantitatively determined through the use of a modified Bradford assay (Bio-Rad, CA, USA). Equivalent amounts (40 μg) of protein samples were loaded and separated by 8% to 12% SDS-polyacrylamide gel electrophoresis and the proteins were then transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBST), the membranes were incubated with rabbit polyclonal primary antibody or mouse monoclonal antibody, at a dilution of 1:1000 overnight at 4 °C, against each of the following proteins: Akt, Src, phospho-Akt, phospho-Src, and Nox4. After washing, the membranes were incubated with the requisite secondary antibody at room temperature for 120 min. The protein bands were visualized with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate. The membranes were scanned, and the relative intensity of the bands was analyzed using Image J 3.0. Prestained markers were used for molecular mass determinations and were photographed after color development. The levels of proteins were determined using densitometry (Image J software), which allowed direct comparisons to be made between experimental sets. The optical density of the control group was 1 arbitrary densitometric unit.
Measurement of ROS production
To investigate the generation of ROS in HUVECs, DCFH-DA was used to determine the amount of intracellular ROS production. DCFH-DA can readily penetrate the cell membrane, where it can be rapidly de-esterified by membrane-bound esterases to yield free DCFH. On reaction with ROS, DCFH is oxidized to yield the fluorescent dichlorofluorescein (DCF). Thus, the intensity of the cellular fluorescence correlates with the amount of ROS formed in situ. For this assay, HUVECs were pre-incubated in 6-well plates for 24 h at 37 °C in culture medium. After pretreatment for 12 h with varying concentrations of SalA and for an additional 12 h with 1 μmol/L Ang II, the HUVECs were incubated with 10 μmol/L DCFH-DA for 30 min at 37 °C. After washing with PBS, the cells were collected in 500 μL PBS. The cell fluorescence intensity was measured at 490 nm excitation and 530 nm emission using a fluorescence-activated cell sorting (FACS) flow cytometer (Becton Dickinson, San Jose, CA, USA), as previously described24.
Statistical analyses
For each experiment, data are presented as mean±SEM. Statistical analysis was performed using GraphPad Prism 4.0. Statistical significance (P<0.05) for each variable was estimated by 1-way or 2-way analysis of variance, followed by Bonferroni post-hoc tests.
Results
Effects of SalA on Ang II-induced HUVEC proliferation
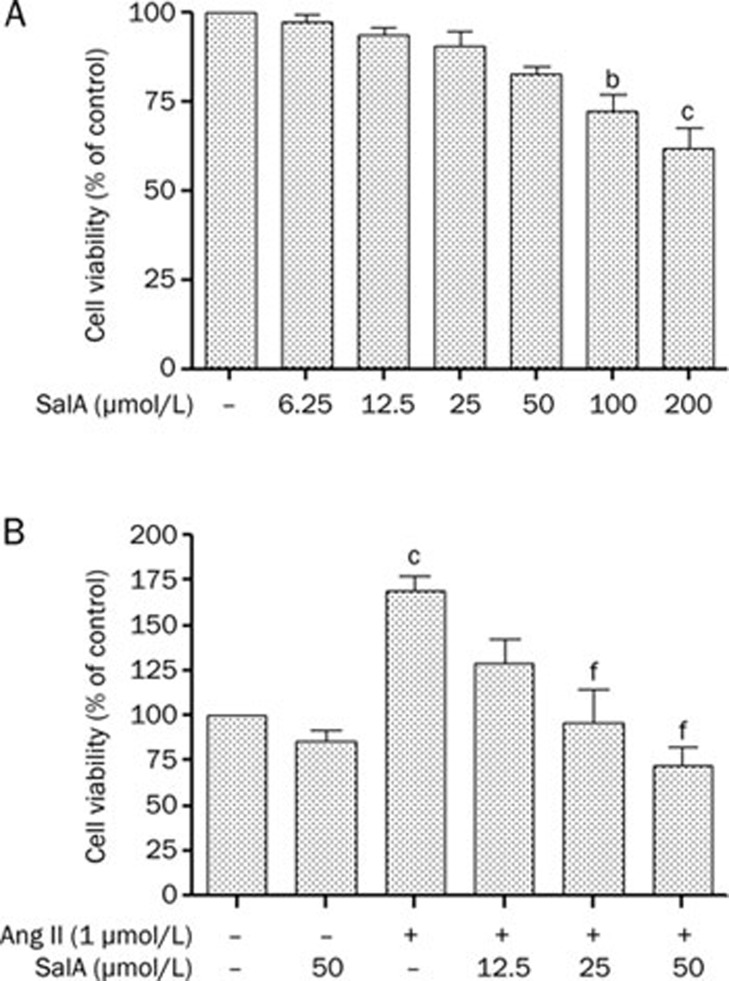
To examine the toxicity of SalA, cell viability was assessed using the MTT metabolism assay. Within the tested concentrations (6.25–200 μmol/L), cell viability was not significantly influenced by SalA at concentrations of 6.25–50 μmol/L. However, cell viability was significantly attenuated with both 100 μmol/L (P<0.05) and 200 μmol/L (P<0.01) doses of SalA in a concentration-dependent manner, as shown in Figure 2A. For our study, we chose to use SalA concentrations of 6.25, 12.5, 25, and 50 μmol/L. The MTT assay was also used to assess cellular proliferation. Ang II (1 μmol/L) significantly increased cell proliferation compared with the control group (P<0.01). However, compared with Ang II, pretreatment with 25 or 50 μmol/L SalA significantly inhibited cellular proliferation (P<0.01) (Figure 2B).
Figure 2.
Effects of SalA on proliferation of Ang II-induced HUVECs. (A) The toxicity of SalA (6.25–200 μmol/L) in HUVECs was measured using MTT metabolism assay after 24-h incubation. (B) Cells were pretreated with different concentrations of SalA (12.5, 25, and 50 μmol/L) for 12 h prior to incubation with of Ang II (1 μmol/L) for 24 h. Data represent means±SEM. n=3. bP<0.05, cP<0.01 vs control. fP<0.01 vs Ang II.
Effects of SalA on the expression levels of total and phosphorylated-Src
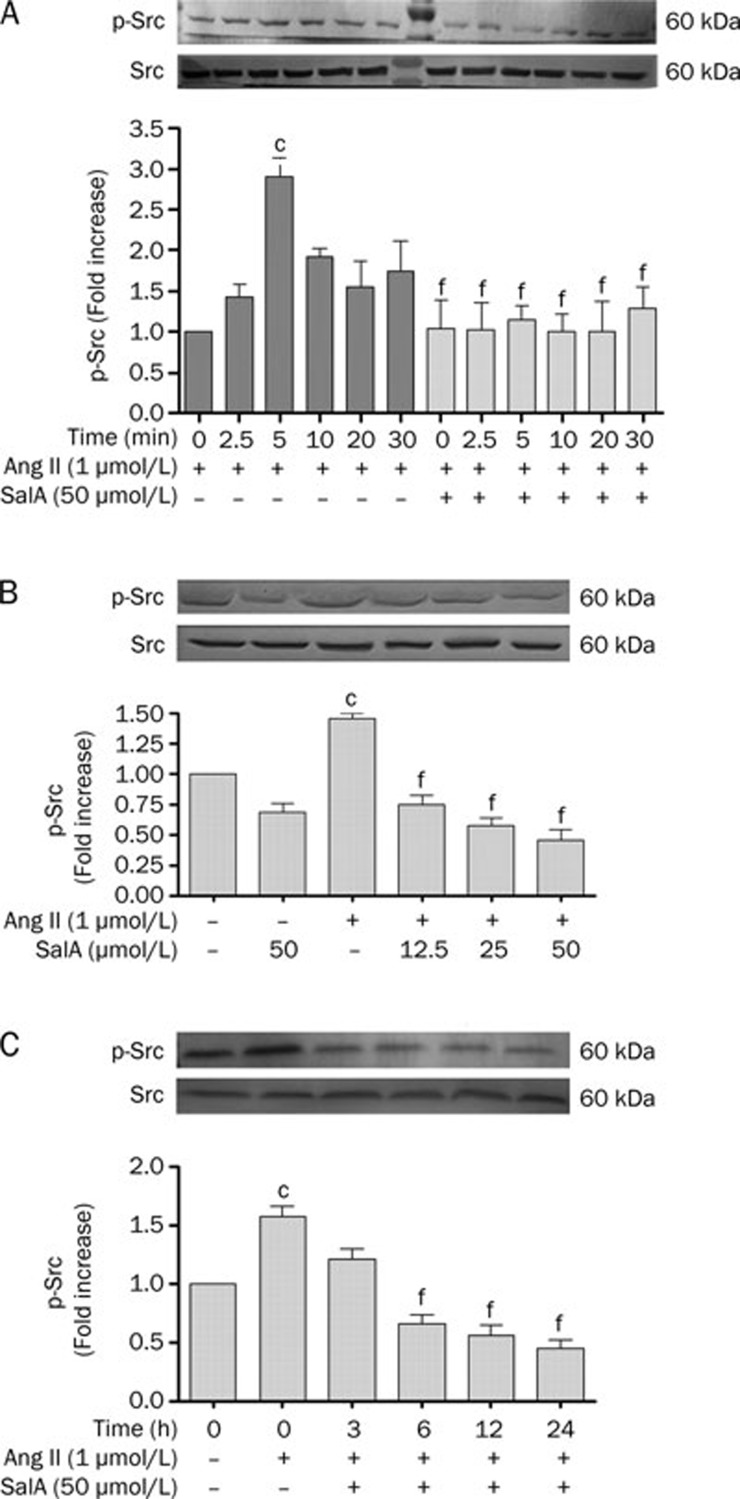
We incubated serum-deprived HUVECs with 1 μmol/L Ang II for 0, 2.5, 5, 10, 15, and 30 min. Ang II caused an increase in phospho-Src levels, starting 2.5 min after stimulation, with a maximal 2.91±0.23-fold increase relative to basal levels at 5 min (P<0.01). Thereafter, phospho-Src decreased rapidly (Figure 3A). When we pretreated serum-deprived HUVECs with 50 μmol/L SalA for 12 h, the levels of phospho-Src significantly decreased, compared with the group treated by Ang II alone (P<0.01) (Figure 3A).
Figure 3.
The effects of SalA on the expression levels of both total and phosphorylated src. (A) The time dependent effects of Ang II on src phosphorylation in HUVECs. Ang II (1 μmol/L) caused a time-dependent increase in phospho-src level, starting at 2.5 min after stimulation, with a maximal 2.91±0.23 fold basal after 5 min. Thereafter, phospho-src signaling decreased rapidly. (B) The effect of pretreatment with different doses SalA for 12 h on p-Src. Ang II increased the level of p-Src, whereas SalA down-regulated p-Src in a dose-dependent manner. (C) The effect of SalA on phosphor-src, petreated with 50 μmol/L SalA for 3, 6, 12, and 24 h downregulated phosphorylation of src in a time-dependent manner. Data represent mean±SEM. n=3. cP<0.01 vs control. fP<0.01 vs Ang II.
As shown in Figure 3B, Ang II significantly increased the phosphorylation of Src, whereas SalA (12.5, 25, or 50 μmol/L) downregulated phospho-Src in a dose-dependent manner. Figure 3C shows that, in serum-deprived HUVECs pretreated with 50 μmol/L SalA for varying lengths of time (3, 6, 12, or 24 h), the level of phospho-Src significantly decreased (P<0.01), whereas it increased in the Ang II-treated group compared to the control (P<0.01).
Effects of SalA on the expression levels of total Akt and phosphorylated Akt
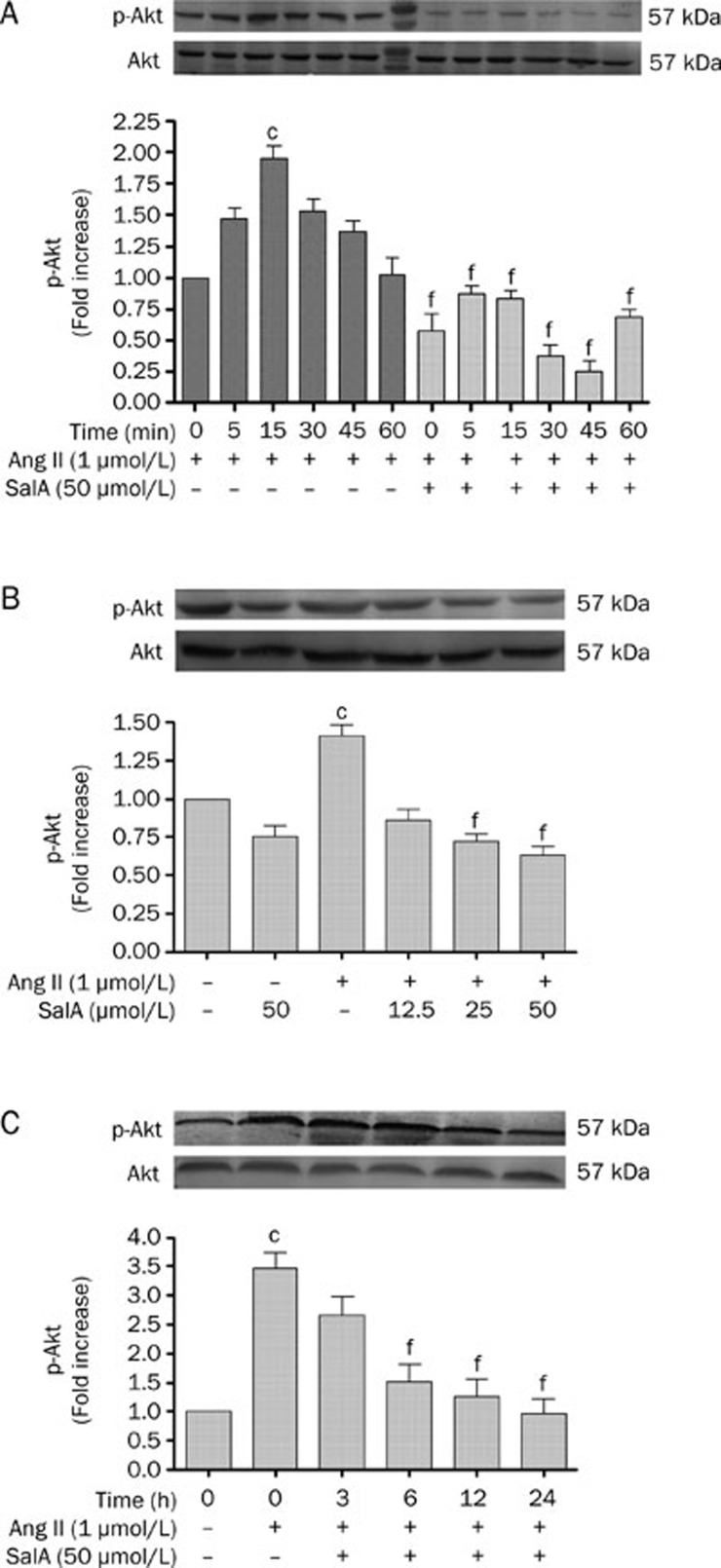
To examine whether SalA inhibits the proliferation of HUVECs, the expression of phospho-Akt was assessed by Western blot analysis (Figure 4). We incubated serum-deprived HUVECs with 1 μmol/L Ang II for 0, 5, 15, 30, 45, or 60 min and found that Ang II caused an increase in phospho-Akt levels, starting at 5 min post-stimulation, with a maximal 1.96±0.09 fold-increase over basal levels after 15 min (P<0.01). Thereafter, phospho-Akt signaling decreased rapidly (Figure 4A). When we pretreated serum-deprived HUVECs with 50 μmol/L SalA for 12 h, the levels of phospho-Akt were significantly reduced, when compared with the Ang II group (P<0.01) (Figure 4A).
Figure 4.
The effects of SalA on the expression levels of both total and phosphorylated Akt. (A) The time dependent effects of Ang II on Akt phosphorylation in HUVECs. Ang II (1 μmol/L) caused a time-dependent increase in phospho-Akt level, starting at 5 min post stimulation, with a maximal 1.96±0.09 fold basal after 15 min. Thereafter, phospho-Akt signaling decreased rapidly. (B) The effect of different doses SalA on phosphor-Akt. Ang II increased the level of phosphorylation of Akt, whereas pretreatment with SalA for 12 h downregulated phosphorylation of Akt in a dose-dependent manner. (C) The effect of different times SalA on phosphor-Akt. Ang II increased the level of phosphorylation of Akt, whereas SalA (3, 6, 12, and 24 h) down-regulated phosphorylation of Akt with in a time-dependent manner. Data represent means ±SEM. n=3. cP<0.01 vs Control. fP<0.01 vs Ang II.
As shown in Figure 4B, Ang II significantly increased the phosphorylation of Akt, whereas SalA (12.5, 25, or 50 μmol/L) downregulated Akt phosphorylation in a dose-dependent manner. Figure 4C showed that, in serum-deprived HUVECs pretreated with SalA (50 μmol/L) for different lengths of time (3, 6, 12, or 24 h), the level of phospho-Akt was significantly reduced (P<0.01), whereas the phosphorylation of Akt increased in the Ang II group, compared with the control (P<0.01).
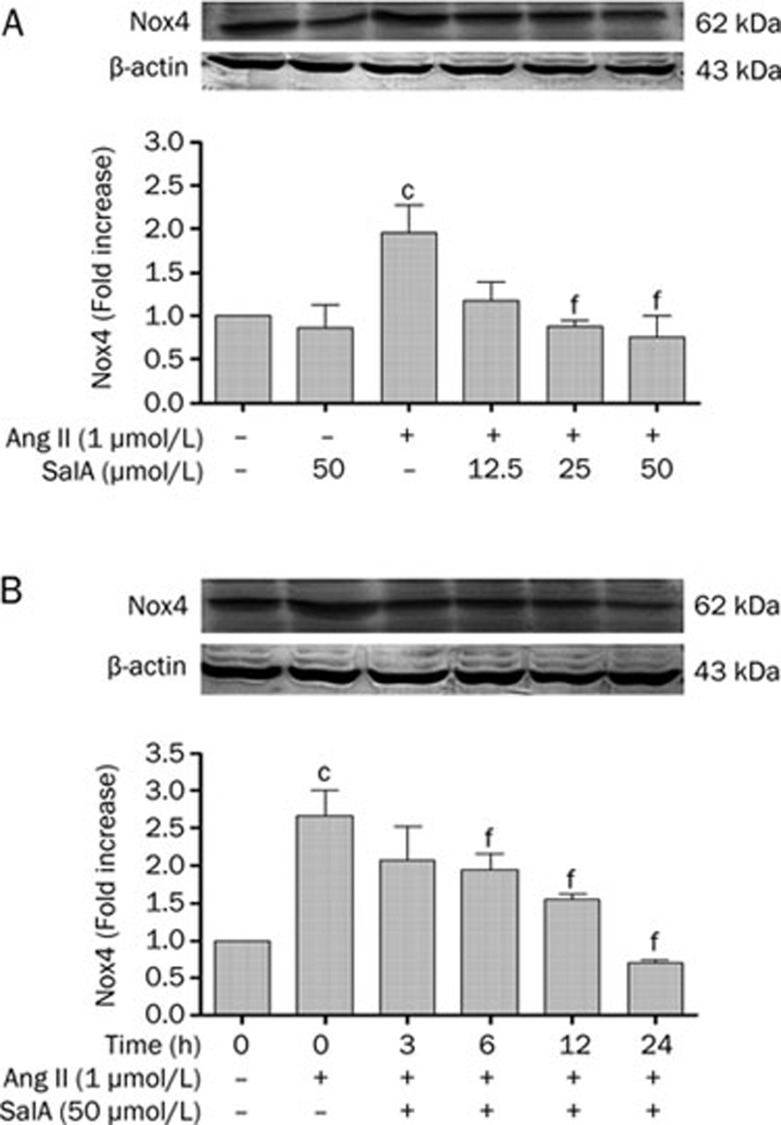
Effects of SalA on the level of Nox4 expression
HUVECs were pretreated with SalA at different concentrations before exposure to Ang II (1 μmol/L) for 24 h. As shown in Figure 5A, Ang II significantly increased the expression level of Nox4, compared with the control (P<0.01). However, the level of Nox4 decreased after pretreatment with SalA (25 and 50 μmol/L) for 12 h, compared with Ang II treatment alone (P<0.01). Pretreatment with SalA (50 μmol/L) for 3, 6, 12, or 24 h led to time-dependent decreases in Nox4 levels. In contrast, Nox4 expression was significantly increased in the Ang II group (P<0.01) (Figure 5B).
Figure 5.
SalA decreased the level of Nox4 induced by Ang II in HUVECs. Pretreated with SalA at different doses for 12 h, HUVECs were incubated with 1 μmol/L Ang II for 24 h. The level of Nox4 was determined by Western blot analysis. SalA (12.5, 25, and 50 μmol/L) downregulated the level of Nox4 in a dose-dependent manner (A). Pretreated with SalA (50 μmol/L) for 3, 6, 12, or 24 h, the level of Nox4 were decreased whereas increased in the Ang II group (B). Data represent means±SEM. n=3. cP<0.01 vs Control. fP<0.01 vs Ang II.
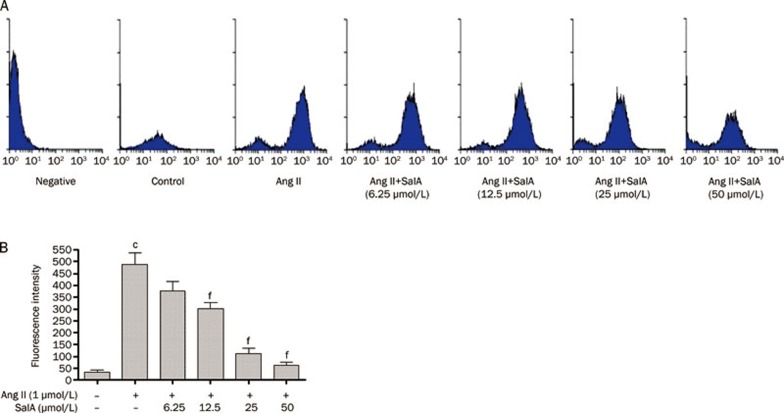
Effects of SalA on ROS production
The DCFH-DA fluorescence method was used to measure ROS production in the HUVECs (Figure 6A). As shown in Figure 6B, the fluorescence intensity of the Ang II-treated group was significantly increased, compared with the control group (P<0.01). However, when the cells were pretreated with SalA (12.5, 25 and 50 μmol/L), the fluorescence intensity significantly decreased (P<0.01), relative to the cells treated with Ang II alone.
Figure 6.
Effects of SalA on ROS production. (A) The level of ROS production measured by FACS. The HUVECs were pretreated with SalA for 12 h prior to incubation of cells with Ang II (1 μmol/L) for 12 h. (B) Fluorescence intensity was determined by statistical analysis. The level of ROS significantly increased in Ang II group, whereas decreased after pretreated by SalA (25 and 50 μmol/L). Data represent means±SEM. n=3. cP<0.01 vs Control. fP<0.01 vs Ang II.
Discussion
Because endothelial cell proliferation is an important variable in the initiation and progression of atherosclerosis, it is of great importance to understand the mechanisms underlying the cardioprotective effects of several drugs. In China, Danshen has been widely used to treat cardiovascular diseases for hundreds of years. Salvianolic acids are the most abundant water-soluble compounds extracted from Danshen. Salvianolic acids, especially SalA and SalB, have been found to have potent anti-inflammatory, antioxidant, antiplatelet, and antifibrotic properties. Previous studies have demonstrated that SalA and SalB are the most abundant components in Danshen and that they share a similar structure. However, SalA produces stronger effects on the above-mentioned functions than SalB does. Some studies have reported that Ang II is an endogenous modulator of endothelium proliferation25. The rate of stent restenosis is high following percutaneous coronary intervention (PCI), mainly due to excessive proliferation of endothelial cells. In the present study, we found that SalA exerted a strong protective effect against the proliferation of HUVECs induced by Ang II, providing evidence for an important potential clinical application of SalA.
To examine whether SalA affects Ang II-induced proliferation of HUVECs, we used the MTT assay to measure cellular proliferation. Treating HUVECs with different concentrations of SalA (6.25–200 μmol/L) for 24 h resulted in no change in cell viability at concentrations of 6.25–50 μmol/L (Figure 2A). Ang II (1 μmol/L) significantly increased cellular proliferation, compared with the control group. Pretreatment with 25 or 50 μmol/L of SalA, compared to treatment with Ang II alone, significantly inhibited cellular proliferation (Figure 2B). This result demonstrated that, in the range of 6.25–50 μmol/L, SalA had no influence on HUVEC viability and that SalA could inhibit the Ang II-induced proliferation of HUVECs in a concentration-dependent manner.
All of the effects ascribed to Ang II coalesce on the high-affinity G-protein coupled receptors localized on the surface of target cells, regulating their physiological and pathophysiological processes. There are two main Ang II receptor subtypes that have been identified thus far, the Ang II type 1 (AT1) and Ang II type 2 (AT2) receptors. In the development and progression of Ang II-mediated high blood pressure, atherosclerosis, and other cardiovascular disease, endothelial cells are some of Ang II's target cells. Ang II, acting through AT1R26, can promote the proliferation of endothelial cells and cause endothelial cell basement membrane egradation, as well as reconstruction migration.
Thomas and Brugge investigated the involvement of Src in cellular proliferation and migration, including the important role this kinase plays in signaling27. They reported that the activation of Src kinase in response to cellular signaling promoted proliferation and survival. The serine/threonine kinase Akt/PKB regulates multiple biological processes, including cell survival, proliferation, and growth. Akt is a member of the protein kinase B group and is a downstream kinase of PI3K. Ser-473 is phosphorylated by S473K thereby activating Akt28. Signaling molecules such as Src and Akt play important roles in many pathophysiological processes, including cell cycle regulation, cell survival, cell growth, glycogen metabolism, and cell migration. Thus, efforts to reduce the proliferation of endothelial cells have recently focused on inhibiting Src and Akt activity. We studied the phosphorylation levels of both Src and Akt to examine the inhibitory effects of SalA on HUVEC proliferation. Ang II has recently been found to cause rapid phosphorylation of Akt, mediated by the PI3K pathway29. As shown in Figure 3A, Ang II increased the level of phospho-Src which reached a peak at 5 min, while simultaneously increasing the level of phospho-Akt (peak at 15 min), as depicted in Figure 4A. However, after pretreatment with 50 μmol/L SalA for 12 h, the phosphorylation levels of both proteins decreased. This result demonstrated that Ang II can activate both Src and Akt phosphorylation, whereas SalA downregulates this modification in a concentration and time-dependent manner.
NADPH oxidase was originally identified and characterized in phagocytes, where it contributes to host defense. All of the components of the NADPH complex have been identified in endothelial cells30, 31, 32, 33. Vascular NADPH oxidase, which plays an important role in both physiology and pathophysiology, is the primary source of ROS in endothelial cells. NADPH oxidase activity varies between vascular cells and between different sites of the vasculature. For example, vascular smooth muscle cells express high levels of Nox1 and Nox4, whereas endothelial cells express high levels of gp91phox/Nox2 and Nox434, 35, 36. ROS have long been implicated in the pathogenesis of cardiovascular diseases, including atherosclerosis, hypertension, and diabetes. There is a growing appreciation for the role of ROS in physiological signaling in many cell types, including vascular endothelial cells. ROS, as a secondary messenger, activates a series of cell protein kinases, including c-Src and Akt/PKB, and this activation plays a decisive role in the process of atherosclerosis. Some evidence indicates that Ang II has a stimulatory effect on ROS production in the endothelium by activating and up-regulating NADPH oxidase37. We sought to clarify whether the inhibitory effect of SalA on the proliferation of HUVECs might be mediated through its antioxidative activity. HUVECs were pretreated with increasing concentrations of SalA for different times prior to incubation with 1 μmol/L Ang II. The results indicated that Ang II treatment increased the level of Nox4 expression, whereas SalA inhibited the Ang II-induced changes in Nox4 expression in a time- and concentration-dependent manner (Figure 5). In addition, the production of ROS was significantly decreased with SalA (25 and 50 μmol/L) treatment compared with treatment with Ang II alone (Figure 6). This result demonstrated that SalA inhibited the induction of Nox4 by Ang II and reduced ROS production accordingly.
Ang II has been proposed to participate in the production of ROS. ROS can activate Src, which can then promote further ROS production38, 39. Increased Src kinase activity was shown to increase Akt phosphorylation. Akt is an important kinase for promoting cellular proliferation and migration. Our data have shown that SalA decreased the level of phosphor-Src and phospho-Akt in a time- and concentration-dependent manner and that SalA also inhibited increases in Nox4 protein expression, ultimately resulting in a significant decrease in ROS production. All of these events contribute in part to the inhibitory effect of SalA on the proliferation of HUVECs.
In summary, we have identified a novel function for SalA by demonstrating the significant role it plays in inhibiting the Ang II-induced proliferation of HUVECs. Further studies are needed to determine the exact mechanism by which SalA acts to protect vascular endothelial cells against Ang II-mediated injury.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Yang LIU.
References
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Hunt BJ. The endothelium in atherogenesis. Lupus. 2000;9:189–93. doi: 10.1191/096120300678828244. [DOI] [PubMed] [Google Scholar]
- Brasen JH, Kivela A, Roser K, Rissanen TT, Niemi M, Luft FC, et al. Angiogenesis, vascular endothelial growth factor and platelet-derived growth factor-BB expression, iron deposition, and oxidation-specific epitopes in stented human coronary arteries. Arterioscler Thromb Vasc Biol. 2001;21:1720–6. doi: 10.1161/hq1101.098230. [DOI] [PubMed] [Google Scholar]
- Alvarez E, Rodino-Janeiro BK, Ucieda-Somoza R, Gonzalez-Juanatey JR. Pravastatin counteracts angiotensin II-induced upregulation and activation of NADPH oxidase at plasma membrane of human endothelial cells. J Cardiovasc Pharmacol. 2010;55:203–12. doi: 10.1097/FJC.0b013e3181ce5f5a. [DOI] [PubMed] [Google Scholar]
- Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, Dikalov S, et al. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther. 2009;331:404–11. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71:226–35. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Chen K, Thomas SR, Keaney JF., Jr Beyond LDL oxidation: ROS in vascular signal transduction. Free Radic Biol Med. 2003;35:117–32. doi: 10.1016/s0891-5849(03)00239-9. [DOI] [PubMed] [Google Scholar]
- Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–86. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- Na AR, Chung YM, Lee SB, Park SH, Lee MS, Yoo YD. A critical role for Romo1-derived ROS in cell proliferation. Biochem Biophys Res Commun. 2008;369:672–8. doi: 10.1016/j.bbrc.2008.02.098. [DOI] [PubMed] [Google Scholar]
- Ali N, Yoshizumi M, Yano S, Sone S, Ohnishi H, Ishizawa K, et al. The novel Src kinase inhibitor M475271 inhibits VEGF-induced vascular endothelial-cadherin and β-catenin phosphorylation but increases their association. J Pharmacol Sci. 2006;102:112–20. doi: 10.1254/jphs.fp0060357. [DOI] [PubMed] [Google Scholar]
- Oda Y, Renaux B, Bjorge J, Saifeddine M, Fujita DJ, Hollenberg MD. c-Src is a major cytosolic tyrosine kinase in vascular tissue. Can J Physiol Pharmacol. 1999;77:606–17. [PubMed] [Google Scholar]
- Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–7. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–42. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham TC, Parikh NU, Siwak DR, Summy JM, McConkey DJ, Kraker AJ, et al. Src activation regulates anoikis in human colon tumor cell lines. Oncogene. 2002;21:7797–807. doi: 10.1038/sj.onc.1205989. [DOI] [PubMed] [Google Scholar]
- Wang SB, Tian S, Yang F, Yang HG, Yang XY, Du GH. Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarction in rats. Eur J Pharmacol. 2009;615:125–32. doi: 10.1016/j.ejphar.2009.04.061. [DOI] [PubMed] [Google Scholar]
- Li HY, Li Y, Yan CH, Li LN, Chen XG. Inhibition of tumor growth by S-3-1, a synthetic intermediate of salvianolic acid A. J Asian Nat Prod Res. 2002;4:271–80. doi: 10.1080/1028602021000049069. [DOI] [PubMed] [Google Scholar]
- Lin TJ, Zhang KJ, Liu GT. Effects of salvianolic acid A on oxygen radicals released by rat neutrophils and on neutrophil function. Biochem Pharmacol. 1996;51:1237–41. doi: 10.1016/0006-2952(96)00067-6. [DOI] [PubMed] [Google Scholar]
- Liu CH, Hu YY, Wang XL, Liu P, Xu LM. Effects of salvianolic acid-A on NIH/3T3 fibroblast proliferation, collagen synthesis and gene expression. World J Gastroenterol. 2000;6:361–4. doi: 10.3748/wjg.v6.i3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MK, Ren DC, Zhang JT, Du GH. Effect of salvianolic acids from Radix Salviae miltiorrhizae on regional cerebral blood flow and platelet aggregation in rats. Phytomedicine. 2002;9:405–9. doi: 10.1078/09447110260571634. [DOI] [PubMed] [Google Scholar]
- Pan HJ, Li DY, Fang F, Chen D, Qi L, Xu TD, et al. Salvianolic acid A demonstrates cardioprotective effects in rat hearts and cardiomyocytes following ischemia/reperfusion injury. J Cardiovasc Pharmacol. PMID:21795988. doi: 10.1097/FJC.0b013e31822de355. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for celluar growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Holmes KL, Otten G, Yokoyama WM. Flow cytometry analysis using the bectondickinson facs calibur. Curr Protocols Immunol. 2001;5:1–22. doi: 10.1002/0471142735.im0504s49. [DOI] [PubMed] [Google Scholar]
- Herr D, Rodewald M, Fraser HM, Hack G, Konrad R, Kreienberg R, et al. Regulation of endothelial proliferation by the rennin-angiotensin system in human umbilicai vein endothelial cells. Reproduction. 2008;136:125–30. doi: 10.1530/REP-07-0374. [DOI] [PubMed] [Google Scholar]
- Mc Ewan PE, Vinson GP, Kenyon CJ. Control of adrenal cell proliferation by AT1 receptors in response to angiotensin II and low-sodium diet. Am J Physiol. 1999;276:E303–309. doi: 10.1152/ajpendo.1999.276.2.E303. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Huang J, Kontos CD. Inhibition of vascular smooth muscle cell proliferation, migration, and survival by the tumor suppressor protein PTEN. Arterioscler Thromb Vasc Biol. 2002;22:745–51. doi: 10.1161/01.atv.0000016358.05294.8d. [DOI] [PubMed] [Google Scholar]
- Das DK, Maulik N, Engelman RM. Redox regulation of angiotensin II signalling in the heart. J Cell Mol Med. 2004;8:144–52. doi: 10.1111/j.1582-4934.2004.tb00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem. 2007;282:35373–85. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- Haurani MJ, Cifuentes ME, Shepard AD, Pagano PJ. Nox4 oxidase overexpression specifically decreases endogenous Nox4 mRNA and inhibits angiotensin II induced adventitial myofibroblast migration. Hypertension. 2008;52:143–9. doi: 10.1161/HYPERTENSIONAHA.107.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–6. doi: 10.1016/s0014-5793(00)02305-x. [DOI] [PubMed] [Google Scholar]
- Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–33. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–17. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- Zhang H, Schmeisser A, Garlichs CD, Plotze K, Damme U, Mugge A, et al. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc Res. 1999;44:215–22. doi: 10.1016/s0008-6363(99)00183-2. [DOI] [PubMed] [Google Scholar]
- Vasant C, Rajaram R, Ramasami T. Apoptosis of lymphocytes induced by chromium (VI/V) is through ROS-mediated activation of Src-family kinases and caspase-3. Free Radic Biol Med. 2003;35:1082–100. doi: 10.1016/s0891-5849(03)00471-4. [DOI] [PubMed] [Google Scholar]
- Gianni D, Taulet N, DerMardirossian C, Bokoch GM. c-Src-mediated phosphorylation of Nox1 and Tks4 induces the reactive oxygen species (ROS)-dependent formation of functional invadopodia in human colon cancer cells. Mol Biol Cell. 2010;21:4287–98. doi: 10.1091/mbc.E10-08-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]