Abstract
Aim:
AVE8134 is a structurally novel potent PPARα agonist. The aim of this study is to investigate the efficacy of AVE8134 on lipid profile and glucose metabolism in dyslipidemic mice and type 2 diabetic rats.
Methods:
A cell based PPAR Gal4 transactivation assay was constructed for testing the activities of AVE8134 at 3 different PPAR isoforms in vitro. Transgenic human Apo A1 (hApo A1) mice and insulin-resistant ZDF rats were used to evaluate the effects of AVE8134 in vivo.
Results:
AVE8134 was a full PPARα dominated PPAR agonist (the values of EC50 for human and rodent PPARα receptor were 0.01 and 0.3 μmol/L, respectively). AVE8134 was not active at PPARδ receptor. In female hApo A1 mice, AVE8134 (1–30 mg·kg−1·d−1, po for 12 d) dose-dependently lowered the plasma triglycerides, and increased the serum HDL-cholesterol, hApo A1 and mouse Apo E levels. In female ZDF rats, AVE8134 (3–30 mg·kg−1·d−1 for 2 weeks) improved insulin-sensitivity index. In pre-diabetic male ZDF rats (at the age of 7 weeks), AVE8134 (10 mg·kg−1·d−1 for 8 weeks) produced an anti-diabetic action comparable to rosiglitazone, without the PPARγ mediated adverse effects on body weight and heart weight. In male ZDF rats (at the age of 6 weeks), AVE8134 (20 mg·kg−1·d−1 for 12 weeks) increased mRNA levels of the target genes LPL and PDK4 about 20 fold in the liver, and there was no relevant effect with rosiglitazone.
Conclusion:
AVE8134 improves lipid profile and glucose metabolism in dyslipidemic mice and type 2 diabetic rats.
Keywords: AVE8134, peroxisome proliferator-activated receptor, type 2 diabetes, glucose, serum lipids, rosiglitazone, transgenic human Apo A1 (hApo A1) mouse, insulin-resistant ZDF rat
Introduction
Type 2 diabetes (T2D) is an increasing health problem in all western and developing countries1, 2. The disease is slow progressing and is characterized by disregulated glucose and lipid metabolism. Insulin resistance is a hallmark of early stage type 2 diabetes and at a more progressed stage partial or absolute β-cell failure leads to a relative or absolute lack of insulin1, 3, 4, with the metabolic consequences of overt type 2 diabetes.
The morbidity and mortality of type 2 diabetes is caused by micro- as well as by macrovascular damage as consequences of the complex metabolic dysfunction. Whereas the micro-vascular complications are mainly correlated with high plasma glucose levels, the macrovascular damage and related cardiovascular mortality are associated with an atherogenic lipid profile1, 5. Lipid profiles in type 2 diabetes are usually characterized by high triglycerides and low HDL-C6, 7. An ideal treatment for T2D would need to improve both, glucose and lipid control6, 8.
Glitazones (rosiglitazone, or pioglitazone) are insulin-sensitizing agents in clinical use for improving glucose control by activation of PPARγ receptors. They have little or no positive effect on the lipid profile9. Their clinical efficacy is limited by the mechanism-based side effects, mainly the risk of edema, heart failure and weight gain but probably also by other complications10, 11, 12.
Fibrates (eg, fenofibrate) are weak PPARα agonists in clinical use for the treatment of mixed dyslipidemia. They decrease high plasma triglycerides and elevate low HDL-levels13, 14. The increase of serum Apo A1 and Apo A2 are less pronounced in clinical trials15, but could be more distinctly shown in several transgenic animal models16, 17, 18. The effect of fenofibrate and other fibrates on insulin sensitivity has not been clearly demonstrated in humans19, 20 and only moderate efficacy on glucose control has been demonstrated in animal models of T2D at high doses18. In contrast to the results reported with the weak PPARα activator fenofibrate, a few studies with more potent PPARα compounds have demonstrated stronger effects in animal models of T2D21.
In this paper we report the efficacy of AVE8134, a potent structurally novel PPARα agonist in established pharmacological models for dyslipidemia, insulin resistance and T2D. The efficacy and markers of side effects were compared to the clinically used PPARγ agonist rosiglitazone and to the PPARα agonist fenofibrate.
Materials and methods
Test compounds
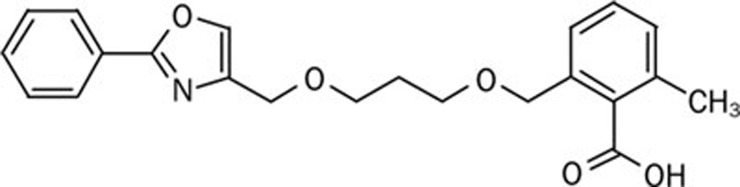
AVE8134 (Figure 1) was synthesized by Sanofi-Aventis Deutschland GmbH. Rosiglitazone was used from commercially available tablets (8 mg/tablet). Fenofibrate was purchased from Sigma-Aldrich (Steinheim, Germany).
Figure 1.
Chemical structure of AVE8134.
Compounds were pre-dissolved in ethanol and then transferred into 50 °C heated Solutol® and finally suspended or dissolved in 0.5% methylcellulose (w/v) to the final volume of 5 mL for rats and 10 mL for mice to achieve doses as indicated in the Results section. Drugs were administered once daily by oral gavage between 07:00 and 08:00 AM.
Gal4 assay
To test the PPAR activities of AVE8134 on the 3 different PPAR isoforms, human embryo kidney cells (HEK 293) were transfected with two genetic elements: 1) a luciferase reporter gene plasmid, containing five GAL4 binding sites upstream of the firefly luciferase reporter gene and 2) a PPAR expression plasmid, encoding a fusion protein of the N-terminus of the glucocorticoid receptor (GR), the DNA binding domain of the yeast GAL4 protein (GAL4) and the respective PPAR ligand binding domain (PPAR-LBD). HEK 293 cells transfected with both, the reporter plasmid and the PPAR expression plasmid produce a GR-GAL4-PPAR-LBD fusion protein which binds to the GAL4 binding sites located upstream of the firefly luciferase reporter gene. If a cell permeable PPAR agonist such as AVE8134 binds to and activates the respective PPAR-LBD, the expression of the firefly luciferase reporter gene is induced. This induction is dose-dependent and is measured as a chemiluminescence signal after addition of an appropriate firefly luciferase substrate.
Animals
Female C57BL/6-Tg(APO A1)1Rub/J mice were purchased from Charles River Germany (Sulzfeld, Germany) and treatment started at the age of 11–13 weeks. Male lean (ZDF/Crl-Fa/?) and obese (ZDF/Crl-fa/fa) Zucker Diabetic fatty (ZDF) rats were obtained from Charles River Belgium (Brussels, Belgium) and treatment started at the age of 7 and 10 weeks for male and female rats, respectively. They were housed in a temperature-controlled room at 23±2 °C with controlled humidity at 55%±5%. They were kept on a 12:12-h light-dark cycle (light on 06:00). All animals had free access to water and to a standard pellet rodent chow (mice and female ZDF rats: ssniff® Soest, Germany; male ZDF rats: Purina rat chow 5008), unless otherwise indicated. Mice and rats were acclimatized at minimal for 1 week before start of the treatment period and randomly allocated into study groups using a stratification method based on body weight. Animal studies were performed according to the German animal protection law as well as according to international animal welfare legislation and rules.
Experimental procedure
For analysis of blood glucose and HbA1c blood was drawn from tail tip from conscious animals using glass capillaries. For all other parameters blood was drawn from the retroorbital vein plexus during short-term isoflurane anesthesia or at the end of the study after laparotomy from the abdominal aorta during deep isofluran/nitric oxide anesthesia. Serum was obtained after centrifugation using Sarstedt® Serum-Gel tubes (Sarstedt, Nümbrecht, Germany).
Mice were treated for 12 d. At the end of the study blood was collected terminally from abdominal aorta during terminal anaesthesia from non-fasted animals.
Female ZDF rats were treated for 14 d. On the last day of the study after an overnight fast blood was collected retroorbitally in short-term isoflurane anesthesia for measuring of fasting blood glucose and fasting insulin levels and subsequent calculation of the insulin resistance index (HOMAIR=fasted-glucose [mmol/L] × fasted-insulin [mU/L]/22.5).
Male ZDF rats were treated for 8 weeks. Food consumption and body weight were measured twice a week. The amount of diet for an additional control group was restricted to levels below the estimated consumption of the AVE8134 group: the starting value was 7 g chow/100 g body weight for the first week and than adapted to 8 g/100 g body weight. The chow for that control group was divided into two portions and was offered twice daily when light was switched on and off, all other groups had free access to food, except before measurements of fasted blood glucose, insulin and before oral glucose tolerance test. After two weeks of treatment an oral glucose tolerance test (oGTT) was performed. Briefly, after an overnight fast, the animals were treated with the drugs or vehicle between 06:30 and 07:00, glucose (2 g/kg) was administered orally 2 h later in a volume of 5 mL/kg, blood was drawn from the tip of the tail from using glass capillaries at 0, 30, 60, 90, 120, and 180 min after glucose administration. The glycemic index (GI) was calculated as area under the curve (AUC) of glucose response during oGTT. Blood glucose concentration before oral glucose load (0 min) was defined as baseline of AUC calculation.
Hepatic gene expression
Male 6-week old ZDF (n=5) rats were treated for 12 weeks, at the end of the study livers were dissected. Liver tissue samples (40–50 mg) were homogenized and lysed in RLT buffer (Qiagen, Hilden, Germany) with an UltraTurrax homogenizer (IKA Labortechnik, Staufen, Germany). Total RNA was isolated from the tissue lysate and cleaned up with the Qiagen Rneasy kit according to the manufacturers protocol. The isolated total RNA from 5 rats were pooled and subsequently analysed in a microarray experiment. Synthesis of labeled cRNA, hybridization, washing and staining of Affymetrix GeneChip arrays (Affymetrix, Santa Clara, CA, USA) were carried out as described22. Briefly, 10 μg of total RNA was utilized to generate cDNA, using the Superscript Choice System for cDNA synthesis (Life Technologies, Karlsruhe, Germany). After purification and subsequent precipitation, the cDNA was used as template for an in vitro transcription reaction (Enzo BioArray High Yield RNA Transcript Labeling Kit, distributed by Affymetrix, Santa Clara, CA, USA) according to the manufacturers protocol. At each case 15 μg of the labeled cRNAs was then fragmented and hybridized overnight onto RG U34A GeneChip arrays (Affymetrix, Santa Clara, CA, USA). The RG U34A GeneChip array contains probes for ∼8000 rat mRNA transcripts and EST clusters. After hybridization and washing, the GeneChip arrays were stained using the antibody amplification protocol provided by Affymetrix (Affymetrix, Santa Clara, CA, USA). Finally, the GeneChip arrays were scanned with a confocal laser scanner (Hewlett Packard). GeneChip 4.0 software (Affymetrix, Santa Clara, CA, USA) was used for quantitative analysis of the scanned images. Further data analysis including estimation of regulation (comparison vehicle treated versus compound treated) and weighting of the statistical significance of those regulations was performed using proprietary software GECKO2 developed within Aventis23.
Clinical chemistry
Serum levels of cholesterol, triglycerides, and safety variables: aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (AP), as well as blood glucose and HbA1c were determined on a Hitachi 912 analyzer, using the respective Roche clinical chemistry kits for human diagnostics. Serum insulin concentrations were determined using a commercial rat and mouse ELISA kit (MERCODIA, Upsala, Sweden). Serum adiponectin concentrations were measured using a commercial mouse RIA kit (LINCO, USA). Assays were performed according to the instructions from the suppliers.
Statistical analysis
Data are presented as mean±SEM. Depending on the homogeneity of variances (Levene test), significant differences were calculated by a one-way ANOVA followed by a post hoc Dunnett́s test. Kruskal-Wallis test was used, if variances were not homogeneous. When testing for differences between repeated measured parameters, a two-way ANOVA (for repeated measures) followed by a post hoc Dunnett́s test was used. For repeated measured parameters, a rank transformation was performed if variances were not homogeneous. For all statistical calculations software Everstat V5 [(Sanofi-Synthelabo) based on SAS 8]) was used. A P<0.05 was considered to be statistically significant.
Results
In vitro activity
AVE8134 is a full PPARα dominated PPAR agonist, more potent on human than on rodent PPARα receptor. AVE8134 is not active on PPARδ (Table 1) and other nuclear receptors (data not shown) and has only partial activity on PPARγ (≅40%). In humans AVE8134 is PPARα selective, in rodents the split between PPARα and PPARγ is more than 20 fold lower (Table 1).
Table 1. EC50 values of AVE8134 in cell based PPAR Gal4 transactivation assays, relative to fenofibrate, rosiglitazone, or GW501516 for PPARα, PPARγ, or PPARδ, respectively.
| Species | PPARα EC50 | PPARγ EC50 | PPARδ EC50 | PPARγ/α EC50 ratio |
|---|---|---|---|---|
| Human | 0.01 (μmol/L) full activation | 4 (μmol/L) partial activation (≅40%) | >10 (μmol/L) | 400 |
| Rat | 0.3 (μmol/L) full activation | 5 (μmol/L) partial activation (≅40%) | >10 (μmol/L) | 17 |
Effects on lipid metabolism in vivo: AVE8134 lowers serum triglycerides and increases HDL-cholesterol and Apo A1
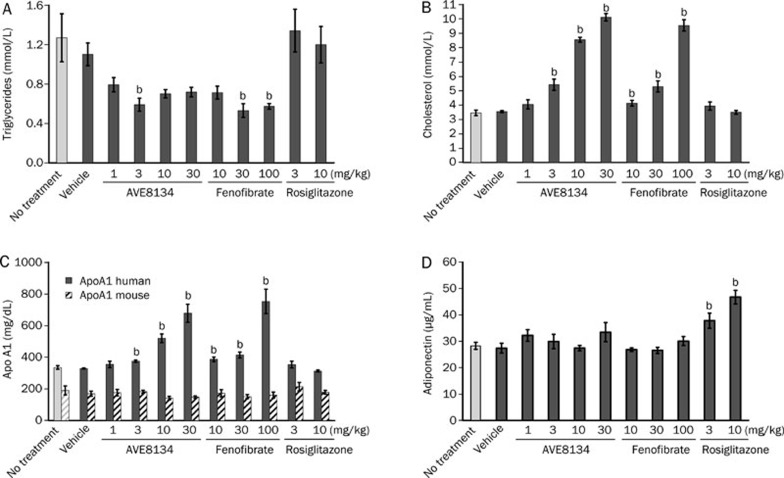
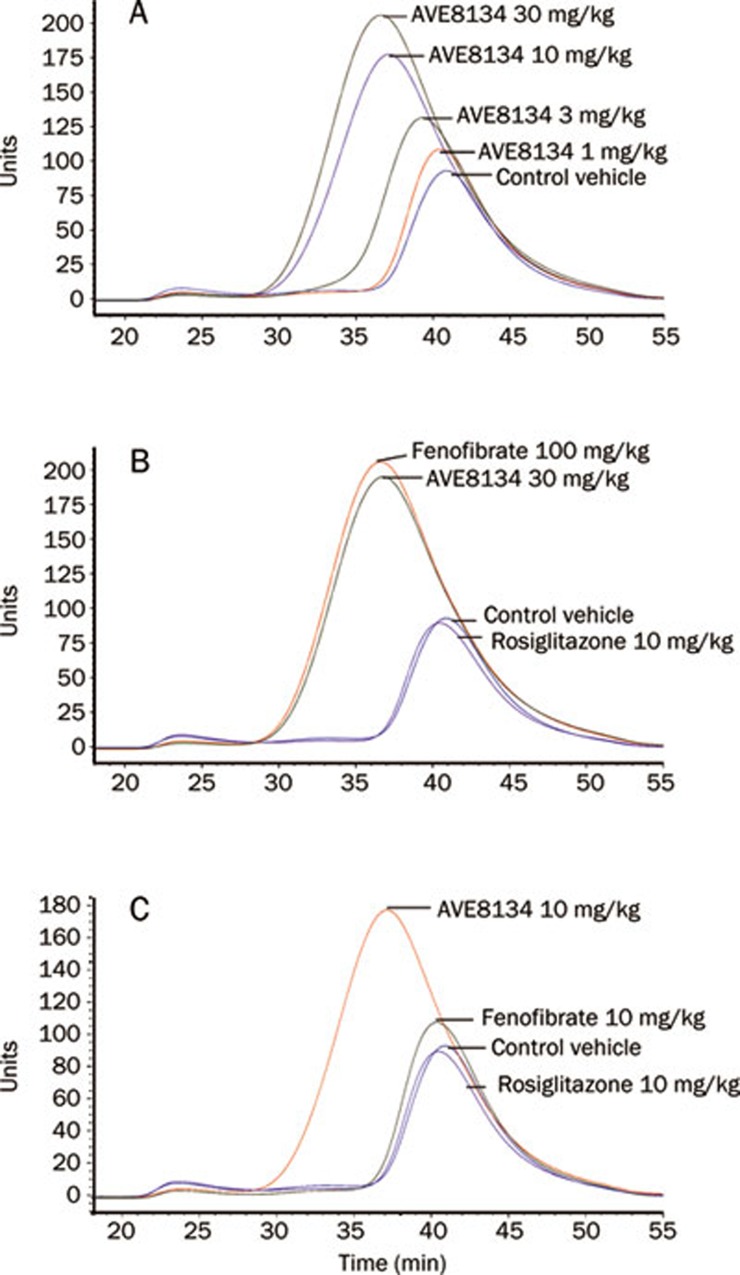
In hApo A1 transgenic mice 12 d of treatment with AVE8134 decreased serum triglycerides, increased dose-dependently serum total cholesterol, as well as human Apo A1 (hApo A1) and mouse Apo E. Mouse Apo A1 was unchanged (Figure 2 and Table 2). Lipoprotein pattern showed that more than 90% of serum cholesterol was in the HDL fraction and the measured total cholesterol reflected predominantly HDL cholesterol. Treatment with AVE8134 further decreased the VLDL and LDL fraction and increased the HDL fraction (Figure 3). AVE8134 was already maximal effective on serum triglycerides at the medium dose (3 mg/kg). Higher doses were needed to increase hApo A1. Fenofibrate had comparable effects on serum lipids and hApo A1 but at 10 fold higher doses. Both PPARα agonists, AVE8134 as well as fenofibrate had no effect on serum adiponectin and heart weight (Figure 2 and Table 2). In contrast rosiglitazone did not change serum lipids, but increased serum adiponectin and heart weight dose-dependently.
Figure 2.
Effects of AVE8134, fenofibrate and rosiglitazone on serum triglycerides (A), serum cholesterol (B), serum Apo A1 (C), and adiponectin (D) in female Apo A1 mice after multiple administrations for 12 d. Mean±SEM (n=6). bP<0.05 vs vehicle control.
Table 2. Effects of AVE8134, fenofibrate, and rosiglitazone in female hApo A1 mice on body weight (bw), relative liver weight, relative heart weight, and Apo E. bP<0.05 vs vehicle control.
| Treatment | Dose (mg/kg) | Weight gain (g) | Food consumption (g/d) | Liver weight (% bw) | Heart weight (% bw) | Apo E (mg/L) |
|---|---|---|---|---|---|---|
| Control | No treatment | 1.28±0.25 | 3.52±0.08 | 4.34±0.07 | 0.45±0.01 | 0.95±0.07 |
| Vehicle | 0.57±0.18 | 3.19±0.14 | 4.58±0.12 | 0.45±0.01 | 1.04±0.09 | |
| AVE8134 | 1 | 0.89±0.43 | 3.24±0.23 | 4.69±0.06 | 0.44±0.01 | 1.25±0.10 |
| 3 | 0.96±0.55 | 3.29±0.03 | 5.40±0.11 | 0.44±0.01b | 2.00±2.20b | |
| 10 | 0.96±0.13 | 3.38±0.15 | 6.95±0.13 | 0.45±0.01b | 3.22±0.17b | |
| 30 | 1.48±0.31 | 3.23±0.05 | 7.73±0.13 | 0.45±0.01b | 3.53±0.08b | |
| Fenofibrate | 10 | 0.76±0.16 | 3.40±0.10 | 5.09±0.06 | 0.47±0.01b | 1.49±0.07 |
| 30 | −0.14±0.43 | 3.15±0.09 | 6.12±0.16 | 0.46±0.01b | 2.32±0.29b | |
| 100 | 1.49±0.23 | 3.25±0.05 | 7.46±0.20 | 0.43±0.01b | 3.18±0.14b | |
| Rosiglitazone | 3 | 0.81±0.26 | 3.49±0.17 | 4.60±0.09 | 0.47±0.02 | 1.29±0.09 |
| 10 | 0.57±0.23 | 3.25±0.15 | 4.68±0.11 | 0.49±0.02 | 1.31±0.16 |
Figure 3.
Effects of AVE8134, fenofibrate and rosiglitazone on serum FPLC lipoprotein pattern in female Apo A1 mice after multiple administrations for 12 d. (A) AVE8134 dose response: 1, 3, 10, and 30 mg·kg−1·d−1 vs vehicle control. (B) Fenofibrate 100 mg·kg−1·d−1 and AVE8134 30 mg·kg−1·d−1 vs vehicle control. (C) Fenofibrate 10 mg·kg−1·d−1, rosiglitazone 10 mg·kg−1·d−1 and AVE8134 10 mg·kg−1·d−1 vs vehicle control.
AVE8134 improves insulin resistance in female ZDF rats
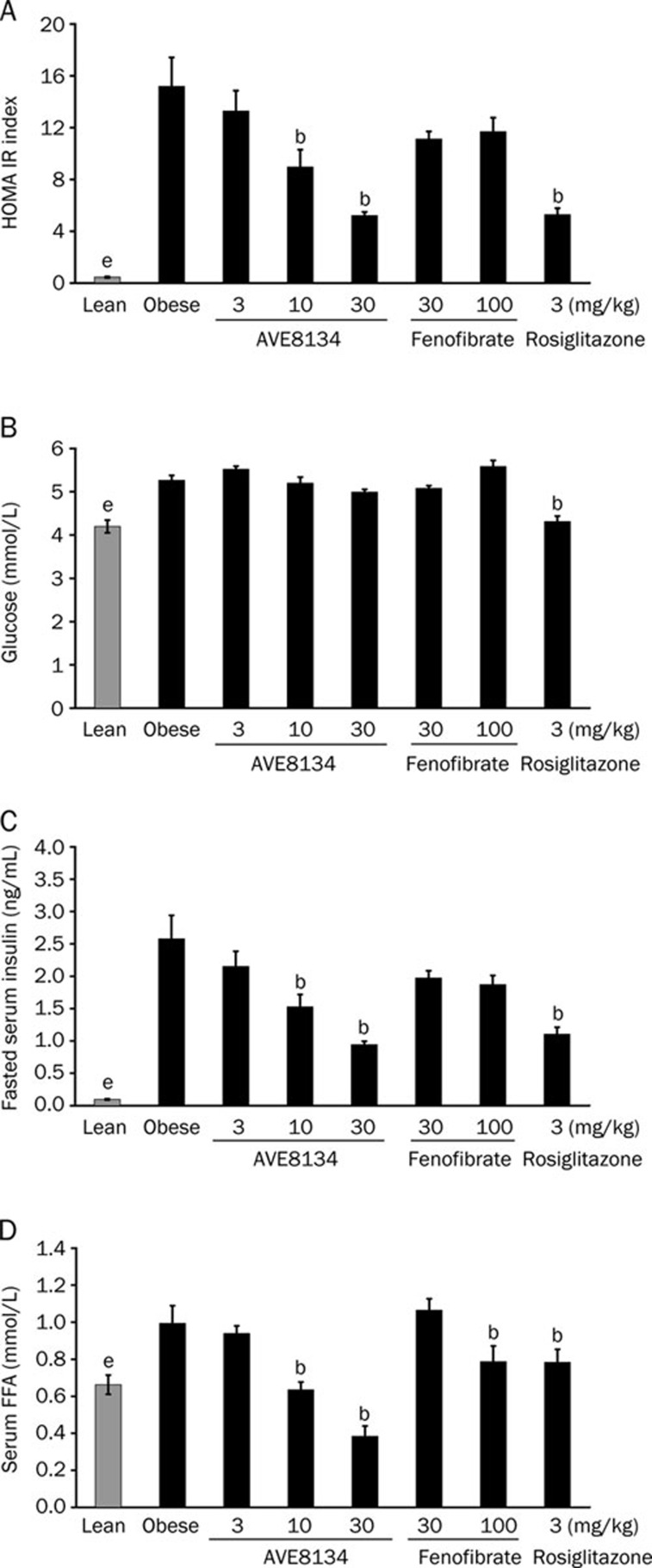
The effect of AVE8134 on glucose control was first investigated in insulin-resistant female, obese ZDF rats after 2 weeks of treatment and the effects were compared to fenofibrate and rosiglitazone. Compared to lean control animals, the fasted blood glucose and non-fasted free fatty acids (FFA) levels in 10–12 weeks old female obese ZDF rats were slightly elevated. However, the fasted insulin levels in the obese animals were several-fold above the level of the lean control animals.
Treatment with AVE8134 dose-dependently decreased fasted serum insulin levels resulting in an improved HOMA IR (homeostasis model assessment test). AVE8134 decreased dose-dependently serum FFA but has no additional effect on fasted blood glucose in female ZDF rats. Rosiglitazone decreased insulin and FFA levels and additionally slightly decreased fasted blood glucose resulting in an improved HOMA IR. The effect of AVE8134 at 30 mg/kg was comparable to the effect with an optimal efficacious dose of rosiglitazone. In contrast to AVE8134, the weak PPARα agonist fenofibrate had no significant effect on fasted insulin, fasted blood glucose and HOMA IR (Figure 4), but was still effective on serum FFA levels. AVE8134 and rosiglitazone (only) slightly increased the food consumption, while fenofibrate had no influence on food consumption in the female ZDF rat (data not shown), because altered food consumption could be a cause for improved glucose control.
Figure 4.
Effects of AVE8134, fenofibrate and rosiglitazone treatment on HOMA IR (A), fasted blood glucose (B), fasted Insulin (C), and non-fasted free fatty acids (FFA) (D) in female ZDF rats after multiple administration for 2 weeks. Mean±SEM (n=8). bP<0.05 vs obese control. eP<0.05 lean control vs obese control.
AVE8134 has anti-diabetic effects comparable to rosiglitazone without PPARγ side effects in male ZDF rats
Treatment of male ZDF rats was started at the age of 7 weeks when animals were in a pre-diabetic state. Blood glucose was already significantly elevated and further increased in untreated animals during the 8 weeks of the study.
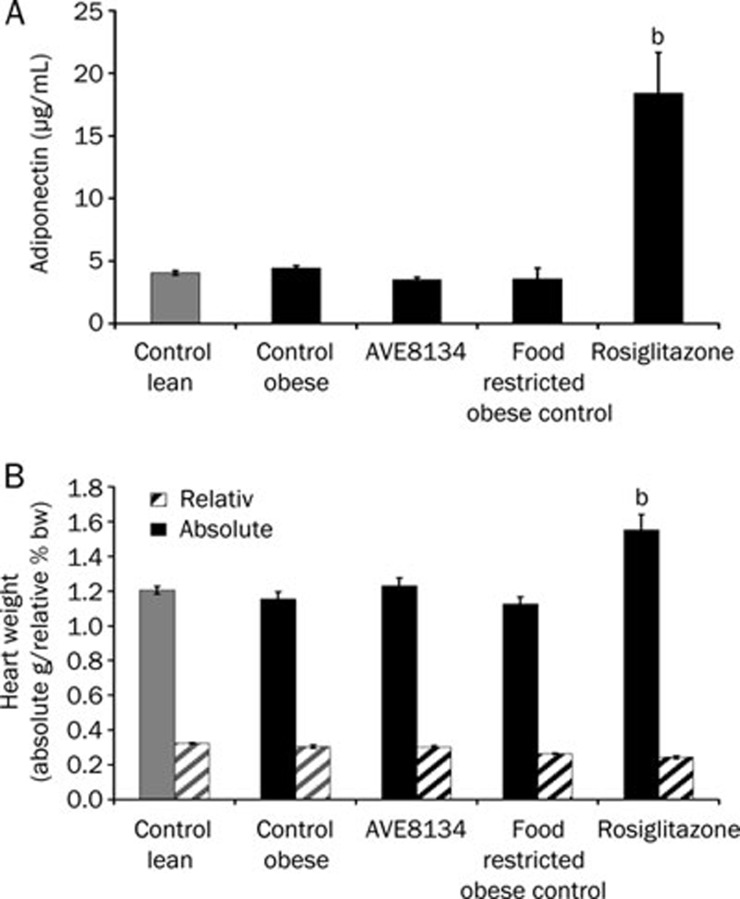
AVE8134 lowered blood glucose, serum triglycerides and cholesterol, improved HbA1c and glucose tolerance. From results of a pilot study as well as from published reports with PPARα agonists21, it was known that potent PPARα agonists decreased food intake in male ZDF rats. Therefore, a second control group received same amount of food as calculated for AVE8134 treated animals. AVE8134 was more effective in glucose control than the effect achieved with partial food restriction. Rosiglitazone showed the expected anti-diabetic effect in male ZDF rats, lowered serum triglycerides, increased weight gain and prevented further increase of serum cholesterol. The efficacy of AVE8134 on glucose control was comparable to rosiglitazone, but unlike rosiglitazone, AVE8134 did not significantly increase the body weight gain or heart weight (Figure 5 and 6). Serum adiponectin, a biomarker for PPARγ activation was 5-fold increased by rosiglitazone after 8 weeks of treatment, while it was not influenced by AVE8134.
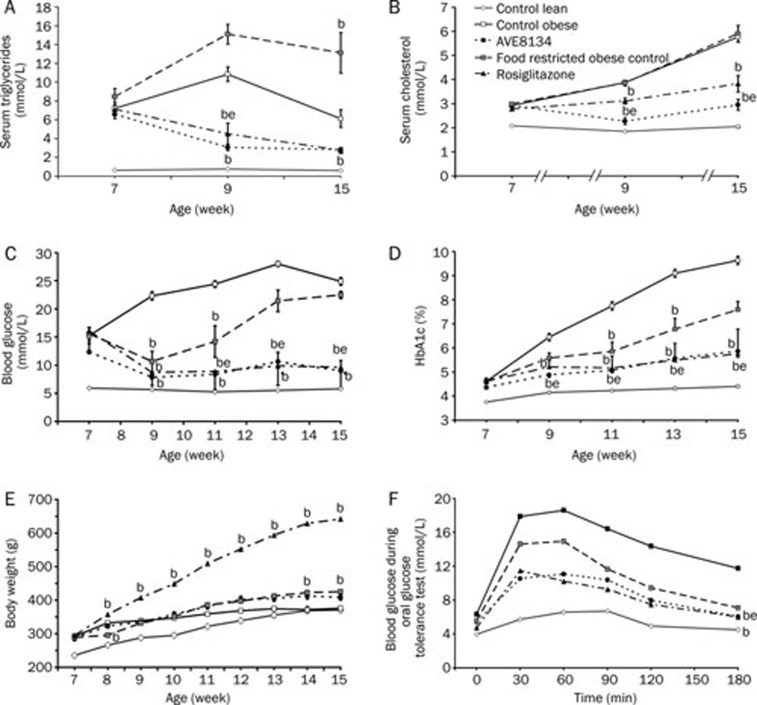
Figure 5.
Effects of AVE8134 (10 mg·kg−1·d−1), and rosiglitazone (3 mg·kg−1·d−1) on serum triglycerides (A), serum cholesterol (B), blood glucose (C), HbA1c (D), body weight (E), and oral glucose tolerance test (F) in male ZDF rats after 8 weeks treatment. Mean±SEM (n=6). bP<0.05 vs obese control. eP<0.05 vs food restricted obese control.
Figure 6.
Effects of AVE8134 (10 mg·kg−1·d−1) and rosiglitazone (3 mg·kg−1·d−1) on serum adiponectin (A), relative and absolute heart weight (B), in male ZDF rats after 8 weeks treatment. Mean±SEM (n=6). bP<0.05 vs obese control.
Food restriction alone (to amounts consumed by the AVE8134 group) decreased blood glucose and improved glucose tolerance during the first 2 weeks of the study, however, during the following 6 weeks on restricted food blood glucose, and HbA1c increased, at the end of the study blood glucose reached the level of the ad libitum fed control group. Food restriction increased the serum triglycerides (Table 3, Figure 5 and 6).
Table 3. Effect of AVE8134 and rosiglitazone in male ZDF rats on food consumption during 8 weeks treatment. Glycemic index (GI) after 2 weeks treatment, calculated as AUC of blood glucose during oGTT. Non-fasted insulin before and after treatment. Safety parameter: serum activity of transaminases (AST and ALT) and alkaline phosphatase (AP) after 8 weeks treatment. bP<0.05 vs obese control.
| Lean control | Obese control | AVE8134 10 mg/kg | Food restricted | Rosiglitazone 3 mg/kg | ||
|---|---|---|---|---|---|---|
| Food consumption (g·animal−1·d−1) | 21±0.3 | 41±1.3 | 29±0.8b | 30±1.4b | 44±1.3 | |
| Age/treatment (week) | ||||||
| Insulin (μg/L) | 7/0 | 0.41±0.01 | 11.46±1.89 | 13.44±3.29 | 10.30±1.24 | 9.17±1.28 |
| 9/2 | 0.56±0.07 | 3.87±0.33 | 3.84±0.36 | 10.30±1.31 | 4.79±0.54 | |
| 13/6 | 0.76±0.12 | 2.68±0.24 | 5.17±0.39 | 11.32±3.07 | 7.29±1.24 | |
| 15/8 | <0.40 | 1.08±0.37 | 0.44±0.11 | 1.93±0.51 | 2.64±0.68 | |
| AUC oGTT (mmol·L−1·min) | 272±24 | 1541±50 | 601±30 | 954±137 | 663±100 | |
| AST (U/L) | 135±4 | 109±8 | 125±9 | 151±17 | 105±4 | |
| ALT (U/L) | 52±2 | 102±10 | 42±4 | 149±30 | 81±3 | |
| AP (U/L) | 87±2 | 253±13 | 990±52 | 223±11 | 156±33 |
AVE8134 was well tolerated and no relevant changes in serum safety parameters were seen, no increase in serum transaminases activity, only increase in serum alkaline phosphate activity was observed (Table 3). As in mice the rodent specific PPARα effect on liver weight was also seen in male and female ZDF rats, the liver weight was 2 fold increased with AVE8134 (data not shown).
Hepatic gene induction of lipoprotein lipase (LPL) and PDK4
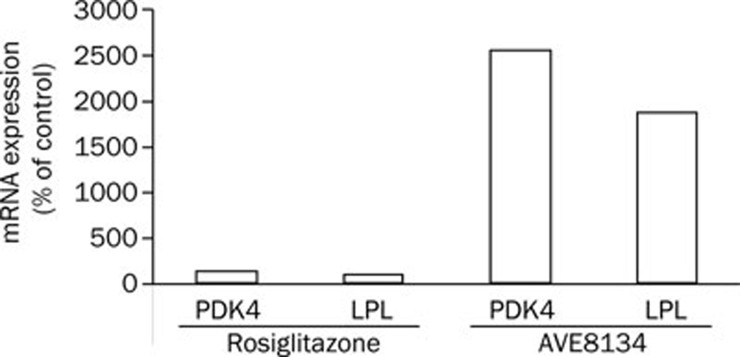
In a pilot study in male ZDF rats the in vivo effect on PPARα target genes was investigated. Treatment was started at the age of 6 weeks, animals were sacrificed after 12 weeks treatment. AVE8134 increased mRNA levels for LPL and PDK4 about 20 fold in the liver, there was no relevant effect with rosiglitazone (Figure 7).
Figure 7.
Relative mRNA levels for PDK4 or LPL in liver after treatment of male ZDF rats with rosiglitazone (3 mg·kg−1·d−1) or AVE8134 (20 mg·kg−1·d−1). Data are obtained in a microarray experiment described in Materials and methods and expressed as relative expression values in comparison to the vehicle treated control group.
Discussion
In humans, treatment with PPARα agonists like fenofibrate increases HDL and Apo A1 and decreases serum triglycerides. The effect on HDL and Apo A1 in rats and mice is not present, because the human – but not the mouse – Apo A1 promoter has a positively regulated element that responds to the PPARα receptor16, 17. To investigate the effects of PPARα agonists on Apo A1 and HDL in rodents a transgenic mouse model that expresses the human Apo A1 gene was used16. As expected in that mouse model, AVE8134 and fenofibrate increased only the human Apo A1 and had no relevant effect on mouse Apo A1. Before treatment and in vehicle treated control mice, HDL cholesterol accounted for more than 90% of total cholesterol. Treatment with the PPARα agonists AVE8134 and fenofibrate decreased the Apo E carrying fractions low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) below the limit of detection. In mice and humans, Apo E is present in the HDL and also in non-HDL lipoprotein particles24. The increase of Apo E in the hApo A1 mouse indicates that the activation of the human Apo A1 promoter and subsequent increase in hApo A1 resulted in an increase in HDL particle number. The qualitative effects of a PPARα agonist on serum triglycerides, HDL and Apo A1 in the hApo A1 transgenic mice can be expected in humans, however the effect size can not be translated in humans, therefore the in vivo effect of AVE8134 on serum lipids was investigated by direct comparison to fenofibrate to estimate the relative in vivo potency of AVE8134. Data from the study in hApo A1 mice demonstrated that in vivo, AVE8134 is a potent PPARα agonist with 10-fold higher potency than fenofibrate. AVE8134 and fenofibrate were effective on triglycerides in the lowest tested dose, whereas effects on HDL and Apo A1 were seen with both drugs only with 10-fold higher doses. That corresponds with clinical observations with fenofibrate. The drug is very effective on triglycerides but has in clinical doses only moderate effects on HDL and Apo A1. PPARγ agonists improve insulin-sensitivity in insulin-resistant models and type 2 diabetic patients. An effect in an insulin-sensitive hApo A1 mouse can not be expected, nevertheless the known effect on serum adiponectin and side effects of rosiglitazone were already seen in that model, even after a short treatment period. Rosiglitazone increased serum adiponectin and heart weight. It is still under discussion whether or not a potent PPARα agonist will improve glycaemic control by improving insulin sensitivity, the effect of PPARγ agonists like rosiglitazone on glycaemic control and insulin sensitivity in humans is proven, but linked to the side effects edema, weight gain, and potential cardiovascular risk. In type 2 animal models, fenofibrate has moderate effects, but it is much less effective than rosiglitazone. These findings with fenofibrate and rosiglitazone could be confirmed in an insulin-resistance model, the female ZDF rat. On normal chow, female ZDF rats are not diabetic, but insulin resistant. The female animals have massive but stable hyper-insulinemia starting from age of 8 weeks for further several weeks. So the HOMA IR index is a predictive parameter for insulin sensitivity in that model. AVE8134 dose dependently improved the HOMA IR index and was as effective as rosiglitazone, whereas fenofibrate has no significant effect, only a trend without correlation to dose was observed, which fits to clinical observations. Based on results in female ZDF rats, the anti-diabetic effect of AVE8134 was investigated in a diabetes model, the male ZDF rat. In male ZDF rats, fenofibrate and other PPARα agonists have only a minor effect and part of the reported effects of PPARα agonists on glucose control in male ZDF rats could be related to decreased food intake, which is typical for these drugs in male ZDF rats21. PPARγ agonist rosiglitazone could prevent the onset of diabetes, despite the increased food intake under rosiglitazone treatment. In the male ZDF rat, non-fasted blood glucose together with HbA1c value are predictive and robust parameters to monitor the diabetes progression. With rosiglitazone, a daily dose of 3 mg/kg is established as the optimal dose in male ZDF rats. It could be demonstrated that not only did AVE8134 have an anti-diabetic effect comparable to rosiglitazone but also that the anti-diabetic effect of AVE8134 was not solely a result of decreased food intake. At start of treatment, the male ZDF rats were in an early diabetic state with already elevated blood glucose and both AVE8134 and rosiglitazone decreased blood glucose and prevented the onset of overt diabetes for the following weeks of treatment. Food restriction delayed only the onset of diabetes, at the end of the study blood glucose and HbA1c of food restricted control were at the same level than ad libitum fed obese control. Adiponectin is a validated in vivo biomarker for PPARγ activation25 and increased body weight gain and elevated heart weight are the PPARγ mediated side effects in ZDF rats. In vitro results demonstrate that AVE8134 is a PPARα dominated PPAR agonist but never the less AVE8134 is also a weak partial PPARγ agonist.
In a pilot study in male ZDF rats it was observed that AVE8134 is in vivo a very potent PPARα agonist. Treatment with AVE8134 increased the PPARα target genes LPL and PDK4 about 20 fold in the liver. With the investigated dose of 10 mg/kg in male ZDF rats no PPARγ activation was detectable, no increase in serum adiponectin or PPARγ-mediated side effects on weight gain or absolute heart weight were seen, food restriction has also no influence on heart weight nor serum adiponectin. The absolute heart weight is more predictive than relative heart weight as PPARγ mediated side effect. We observed in several studies with male ZDF that there is no difference in absolute heart weight between lean and obese ZDF rats, the heart increased in growing lean and obese animals in parallel with age and reached a plateau of about 1.2 g in adult animal. Effects on relative heart weight are masked by PPARγ mediated increase in visceral body fat pad mass. In contrast to rosiglitazone, AVE8134 was shown to attenuate the progression of heart failure and to increase survival in rats26.
The increase in serum alkaline phosphatise activity is a typical biomarker of PPARα activation, seen with several PPARα agonists in rats and the dose related increase of alkaline phospahtase was observed with fenofibrate27. This is not relevant in humans since in humans fibrates decrease serum alkaline activity28 and the decrease in serum alkaline phosphatase activity has been used to monitor compliance of fibrates in clinical trials29.
Conclusion
With the observed lipid lowering and anti-diabetic effects in animal models it can be expected that AVE8134 is a potent PPARα agonist which will improve an atherogenic lipid profile with the potential to optimize glucose homeostasis without PPARγ side effects in clinical trials.
Author contribution
Hans-Ludwig SCHÄEFER and Wolfgang LINZ designed and performed the studies and wrote the paper. Eugen FALK designed and performed the serum lipoproteine and lipid analysis. Marcus KORN designed and performed gene expression experiments. Maike GLIEN designed and performed the in vivo glucose tolerance tests. Heiner GLOMBIK designed and synthesized the test compound. Wolfgang WENDLER designed and performed the in vitro assays. Andreas W HERLING and Hartmut RÜTTEN designed research.
Acknowledgments
We thank Thomas HENNIG, Carolin JOERG, Kerstin KLEINSCHMIDT, MANFRED SCHMALZ, PIERRE WENSKI and Gabriele BIEMER-DAUB for helpful support in conducting animal studies, animal care and serum clinical chemistry. We thank Marion SCHNEIDER for performance of the cellular assays and Gertraud JERABEK-SANDOW for performance of the gene expression studies.
We thank David CROWTHER for reviewing the manuscript.
References
- Kiess W, Bottner A, Bluher S, Raile K, Galler A, Kapellen TM. Type 2 diabetes mellitus in children and adolescents — the beginning of a renal catastrophe. Nephrol Dial Transplant. 2004;19:2693–6. doi: 10.1093/ndt/gfh455. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ. Type 2 diabetes–a matter of beta-cell life and death. Science. 2005;307:380–4. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ, White MF. Molecular insights into insulin action and secretion. Eur J Clin Invest. 2002;32:3–13. doi: 10.1046/j.1365-2362.32.s3.2.x. [DOI] [PubMed] [Google Scholar]
- Agrawal RP, Sharma P, Pal M, Kochar A, Kochar DK. Magnitude of dyslipedemia and its association with micro and macro vascular complications in type 2 diabetes: a hospital based study from Bikaner (Northwest India) Diabetes Res Clin Pract. 2006;73:211–4. doi: 10.1016/j.diabres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120:S12–8. doi: 10.1016/j.amjmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Valabhji J, Watson M, Cox J, Poulter C, Elwig C, Elkeles RS. Type 2 diabetes presenting as diabetic ketoacidosis in adolescence. Diabet Med. 2003;20:416–7. doi: 10.1046/j.1464-5491.2003.00942.x. [DOI] [PubMed] [Google Scholar]
- Cziraky MJ. Management of dyslipidemia in patients with metabolic syndrome. J Am Pharm Assoc. 2004;44:478–88. doi: 10.1331/1544345041475643. [DOI] [PubMed] [Google Scholar]
- Buse JB, Tan MH, Prince MJ, Erickson PP. The effects of oral anti-hyperglycaemic medications on serum lipid profiles in patients with type 2 diabetes. Diabetes Obes Metab. 2004;6:133–56. doi: 10.1111/j.1462-8902.2004.00325.x. [DOI] [PubMed] [Google Scholar]
- Berlie HD, Kalus JS, Jaber LA. Thiazolidinediones and the risk of edema: a meta-analysis. Diabetes Res Clin Pract. 2007;76:279–89. doi: 10.1016/j.diabres.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Patel C, Wyne KL, McGuire DK. Thiazolidinediones, peripheral oedema and congestive heart failure: what is the evidence. Diab Vasc Dis Res. 2005;2:61–6. doi: 10.3132/dvdr.2005.010. [DOI] [PubMed] [Google Scholar]
- Fruchart JC, Duriez P. Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today (Barc) 2006;42:39–64. doi: 10.1358/dot.2006.42.1.963528. [DOI] [PubMed] [Google Scholar]
- Sharpe M, Ormrod D, Jarvis B. Micronized fenofibrate in dyslipidemia: a focus on plasma high-density lipoprotein cholesterol (HDL-C) levels. Am J Cardiovasc Drugs. 2002;2:125–32. doi: 10.2165/00129784-200202020-00006. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, Howey DC, McErlean E, Wang MD, et al. Effects of a potent and selective PPAR-alpha agonist in patients with atherogenic dyslipidemia or hypercholesterolemia: two randomized controlled trials. JAMA. 2007;297:1362–73. doi: 10.1001/jama.297.12.1362. [DOI] [PubMed] [Google Scholar]
- Berthou L, Duverger N, Emmanuel F, Langouet S, Auwerx J, Guillouzo A, et al. Opposite regulation of human versus mouse apolipoprotein A-I by fibrates in human apolipoprotein A-I transgenic mice. J Clin Invest. 1996;97:2408–16. doi: 10.1172/JCI118687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennuyer N, Poulain P, Madsen L, Berge RK, Houdebine LM, Branellec D, et al. Beneficial effects of fibrates on apolipoprotein A-I metabolism occur independently of any peroxisome proliferative response. Circulation. 1999;99:2445–51. doi: 10.1161/01.cir.99.18.2445. [DOI] [PubMed] [Google Scholar]
- Nadeau KJ, Ehlers LB, Aguirre LE, Reusch JE, Draznin B. Discordance between intramuscular triglyceride and insulin sensitivity in skeletal muscle of Zucker diabetic rats after treatment with fenofibrate and rosiglitazone. Diabetes Obes Metab. 2007;9:714–23. doi: 10.1111/j.1463-1326.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- Rosenson RS, Wolff DA, Huskin AL, Helenowski IB, Rademaker AW. Fenofibrate therapy ameliorates fasting and postprandial lipoproteinemia, oxidative stress, and the inflammatory response in subjects with hypertriglyceridemia and the metabolic syndrome. Diabetes Care. 2007;30:1945–51. doi: 10.2337/dc07-0015. [DOI] [PubMed] [Google Scholar]
- Rosenson RS. Effects of peroxisome proliferator-activated receptors on lipoprotein metabolism and glucose control in type 2 diabetes mellitus. Am J Cardiol. 2007;99:96B–104B. doi: 10.1016/j.amjcard.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Yao J, Woods JW, Zycband EI, Liu C, Li Z, et al. Peroxisome proliferator-activated receptor (PPAR)-alpha agonism prevents the onset of type 2 diabetes in Zucker diabetic fatty rats: A comparison with PPAR gamma agonism. Endocrinology. 2006;147:4252–62. doi: 10.1210/en.2005-1535. [DOI] [PubMed] [Google Scholar]
- Voss MD, Beha A, Tennagels N, Tschank G, Herling AW, Quint M, et al. Gene expression profiling in skeletal muscle of Zucker diabetic fatty rats: implications for a role of stearoyl-CoA desaturase 1 in insulin resistance. Diabetologia. 2005;48:2622–30. doi: 10.1007/s00125-005-0025-2. [DOI] [PubMed] [Google Scholar]
- Theilhaber J, Ulyanov A, Malanthara A, Cole J, Xu D, Nahf R, et al. GECKO: a complete large-scale gene expression analysis platform. BMC Bioinformatics. 2004;5:195. doi: 10.1186/1471-2105-5-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida BY, Blanche PJ, Nichols AV, Yashar M, Paigen B. Effects of atherogenic diet consumption on lipoproteins in mouse strains C57BL/6 and C3H. J Lipid Res. 1991;32:559–68. [PubMed] [Google Scholar]
- Yang B, Brown KK, Chen L, Carrick KM, Clifton LG, McNulty JA, et al. Serum adiponectin as a biomarker for in vivo PPARgamma activation and PPARgamma agonist-induced efficacy on insulin sensitization/lipid lowering in rats. BMC Pharmacol. 2004;4:23. doi: 10.1186/1471-2210-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz W, Wohlfart P, Baader M, Breitschofpf K, Falk E, Schäfer HL, et al. The peroxisome proliferator-activated receptor-α (PPAR- α) agonist, AVE8134, attenuates the progression of heart failure and increases survival in rats. Acta Pharmacol Sin. 2009;30:935–46. doi: 10.1038/aps.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J, Dewa Y, Muguruma M, Kuroiwa Y, Yasuno H, Shima T, et al. Effect of fenofibrate on oxidative DNA damage and on gene expression related to cell proliferation and apoptosis in rats. Toxicol Sci. 2007;97:44–54. doi: 10.1093/toxsci/kfm011. [DOI] [PubMed] [Google Scholar]
- Day AP, Feher MD, Chopra R, Mayne PD. The effect of bezafibrate treatment on serum alkaline phosphatase isoenzyme activities. Metabolism. 1993;42:839–42. doi: 10.1016/0026-0495(93)90056-t. [DOI] [PubMed] [Google Scholar]
- Elisaf M. Effects of fibrates on serum metabolic parameters. Curr Med Res Opin. 2002;18:269–76. doi: 10.1185/030079902125000516. [DOI] [PubMed] [Google Scholar]