Abstract
Aim:
To investigate the effect of genistein on bone homeostasis in mandibular subchondral bone of rats.
Methods:
Female SD rats were administered with genistein (10 and 50 mg/kg) or placebo by oral gavage for 6 weeks. Then the animals were sacrificed, and histomorphology and micro-structure of mandibular condyle were examined using HE staining and micro-CT analysis, respectively. The expression levels of alkaline phosphatase (ALP), osteocalcin (OC), osteoprotegerin (OPG), the receptor activator of nuclear factor κB ligand (RANKL) and estrogen receptors (ERs) in mandibular condyle were detected using real-time PCR. Cultured osteoblasts were prepared from rat mandibular condyle for in in vitro study. The cells were treated with genistein (10−7 or 10−4 mol/L) for 48 h. The expression of the bone homeostasis-associated factors and estrogen receptors (ERs) was detected using real-time PCR, and ER silencing was performed.
Results:
At both the low- and high-doses, genistein significantly increased the bone mineral density (BMD) and bone volume, and resulted in thicker subchondral trabecular bone in vivo. In both in vivo and in vitro study, the low-dose genistein significantly increased the expression of ALP, OC and OPG, but decreased the expression of RANKL and the RANKL/OPG ratio. The high-dose genistein decreased the expression of all these bone homeostasis-associated factors. Both the low and high doses of genistein significantly increased the expression of ERβ, while ERα expression was increased by the low dose genistein and decreased by the high dose genistein. ERβ silencing abrogated most of the effects of genistein treatment.
Conclusion:
In rat mandibular condylar subchondral bone, low-dose genistein increases bone formation and inhibit bone resorption, while excess genistein inhibits both bone formation and resorption. The effects of genistein were predominantly mediated through ERβ.
Keywords: genistein, estrogen receptor, mandibular subchondral bone, osteoblast, alkaline phosphatase, osteocalcin, osteoprotegerin, the receptor activator of nuclear factor κB ligand (RANKL)
Introduction
Osteoporosis is a systemic disease characterized by reduced bone mass and structural deterioration of bone tissue. Osteoporosis has become a public health issue threatening a large portion of the population over 50 years of age1, 2. Estrogen replacement therapy (ERT) is effective in reducing or reversing postmenopausal bone loss3. In addition to its use for the treatment of postmenopausal symptoms, potential benefits of ERT include a reduction in the risk of osteoarthritis4, 5, 6 and prevention of cardiovascular disease and dementia7. However, recent studies have shown that ERT is associated with a higher risk for breast, endometrial and ovarian cancer as well as cardiovascular disease and stroke8, 9. Due to the side-effects of long-term ERT use, the number of ERT users has fallen dramatically.
Observational studies suggest that Asian women who consume traditional diets high in isoflavones from soybean have a lower rate of postmenopausal fractures than Caucasian women10, 11. Phytoestrogen, especially genistein, which structurally resembles 17β-estradiol, is abundant in soybean products. Previous animal studies have shown that genistein treatment increased bone mineral density (BMD) and improved bone biomechanical performance in ovariectomized (OVX) rodents12, 13. Clinical studies have also demonstrated that phytoestrogen can effectively increase the BMD of vertebrae and hip bones in postmenopausal women without side effects, which suggested that genistein plays an important role in the regulation of bone formation and bone resorption14, 15, 16, 17. phosphatase (ALP) and osteocalcin (OC) are widely used markers for bone formation. Genistein treatment (45 mg/kg by oral gavage) was observed to significantly increase the serum ALP and OC levels in OVX rats18. In the process of bone resorption, osteoprotegerin (OPG) and the receptor activator of nuclear factor κB ligand (RANKL) constitute a complex mediator system. The RANKL to OPG ratio is the key regulatory determinant of bone resorption19. It is suggested that genistein (10 mg/kg, subcutaneous administration) significantly increased serum OPG level as well as decreased serum RANKL level and the RANKL/OPG ratio in OVX rats20. Additionally, the most abundant protein in bone is type I collagen. Type I collagen carboxy terminal telopeptide (CTX) is formed during bone collagen breakdown and is liberated into the circulation. Serum concentration of CTX reflects the degree of bone resorption21.
In the literature, the effects of genistein on bone were almost exclusively focused on changes in long bones or lumbar spines in OVX animals or postmenopausal women with estrogen deficiency. It has been suggested that patients with a history of osteoporotic fractures tend to have increased mandibular bone loss and relevant trabecular micro-structural changes22. Additionally, a dense trabecular pattern in the mandibular alveolar process has been proven to be a reliable sign of normal BMD in dentate middle-aged subjects whereas a sparse trabecular pattern indicates osteopenia23. In developed countries, a large proportion of the population visits dentists annually, and dental radiographs are performed routinely. Thus, researchers worldwide have recently tried to develop methods for using the jawbones, especially the mandible, to predict skeletal BMD23. The mandibular condyle, one important section of the mandible, is one of the most common sites of OA24. Furthermore, the articular surfaces of the mandibular condyle are composed of fibrocartilage that is distinct from the hyaline cartilage of the appendicular skeleton25. Unlike appendicular joints whose cartilage and subchondral bone are separated by tidemark and calcified cartilage, mandibular condylar cartilage and its subchondral bone are not obviously separated by an osteochondral interface26. However, in the literature few reports focused on the effect of phytoestrogens on mandibular bone, particularly in intact animals with normal estrogen levels. How genistein affects bone homeostasis in mandibular subchondral bone and whether different doses of genistein play different roles in this process remain unclear. The aim of the present study was to investigate the effect of different doses of genistein on bone homeostasis in the mandibular condylar subchondral bone in intact female rats.
Materials and methods
Genistein treatment in vivo
All experimental procedures administered to the animals were approved by the Animal Research Committee of the Fourth Military Medical University. Thirty female 7-week-old SD rats, weighing 180–190 g, were provided by the animal center of the Fourth Military Medical University. All animals were housed under conditions of 22 °C and 30%–60% relative humidity with a normal day-night rhythm, consisting of a 12 h:12 h light-dark cycle. Animals were randomly assigned into the control, low- or high-dose genistein-treated groups, 10 animals in each group. Rats had free access to tap water and a phytoestrogen-free diet where corn oil replaced soybean oil. Based on a series of safety studies with genistein, it is estimated that the no observed adverse effect level (NOAEL) of genistein is 50 mg·kg−1·d−1 for rats, and the no observed effect level (NOEL) is 10 mg·kg−1·d−127. Thus, in the present study, 50 mg·kg−1·d−1 genistein was chosen as the high dose, and 10 mg·kg−1·d−1 as the low dose. The animals were treated once daily with genistein (10 or 50 mg/kg body weight, 99.5% pure, Winherb Med Sci Co Ltd, China) or placebo by oral gavage. Genistein was dissolved in a placebo solution (0.9% NaCl, 2% Tween 80, and 0.5% methyl cellulose in water). Application volume was 5 mL/kg body weight. Changes in body weight during the experimental period were taken into account in calculating the genistein dosage. All animals were sacrificed following 6 weeks of genistein administration.
Histological analysis
Under deep anesthesia, 12 rats (4 in each group) were sacrificed for histological analysis. The right temporomandibular joints (TMJs), including the mandibular ramus, were dissected and fixed in 4% paraformaldehyde (pH 7.4) overnight at 4 °C and then decalcified for 1 week in Kristensen's fluid (sodium formate 52.2 g, formic acid 174.2 mL, 1000 mL distilled water). Samples were then dehydrated and embedded in paraffin, followed by cutting into 5 μm middle-sagittal sections. HE staining was carried out for histological analysis. As we previously reported26, two square areas (0.5 mm×0.5 mm) were selected under the interface of the cartilage and subchondral bone. They were located in the center of the middle and posterior third sections of the condylar cartilage that appeared consistent among animals. Using a computer-assisted image analyzing system (Leica Qwin Plus, Leica Microsystem Imaging Solutions Ltd, Cambridge, United Kingdom), the 2D measurements within the selected squares were performed by directed measurement of the trabecular bone area (B.Ar in mm2) and perimeter (B.Pm in mm). Then, the histomorphometric parameters of the subchondral trabecular bone were calculated according to Parfitt's formula as follows: bone volume fraction (BV/TV)=B.Ar/T.Ar; trabecular thickness (Tb.Th)=B.Ar/B.Pm; trabecular number (Tb.N)=B.Pm/T.Ar; trabecular separation (Tb.Sp)=(T.Ar–B.Ar)/B.Pm. The means of the data from two squares were used for statistical analysis.
Micro-computed tomography (micro-CT) analysis
As reported previously26, the left mandibular condyles from animals for histological analysis were scanned in a micro-CT system (GE eXplore Locus SP, London, UK) at an isotropic spatial resolution of 8 μm and a peak voltage of 80 kV. Twelve condyles, 4 in each group, were inserted in parallel into a home-made, round synthetic foam and mounted in the cylindrical specimen holders. Four projections were performed for each scan angle in each slice. These projections had an exposure time of 3000 ms each and were averaged in order to improve the signal-to-noise ratio. The time of each scanning was over 270 min. General Electric Health Care MicroView ABA 2.1.2 Software was used to analyze reconstructive images of condyles and to calculate the parameters of architecture and mineralization. Similar to histomorphometric analysis, the interface of the cartilage and subchondral bone was divided into anterior, middle and posterior regions. As shown in Figure 1, two cubic regions of interest (ROI) (0.5 mm×0.5 mm×0.5 mm) were selected in the center of the middle and posterior regions of the condyle for the model-independent, three-dimensional morphometric analysis (Figure 1). The following micro-structural parameters were obtained: 1) BMD; 2) BMC; 3) trabecular bone volume fraction (BV/TV); 4) bone surface-to-volume ratio (BS/BV); 5) mean trabecular thickness (Tb.Th); 6) trabecular number (Tb.N), 7) mean trabecular separation (Tb.Sp), and 8) bone volume (BV). The means of the data from two ROIs were used for statistical analysis.
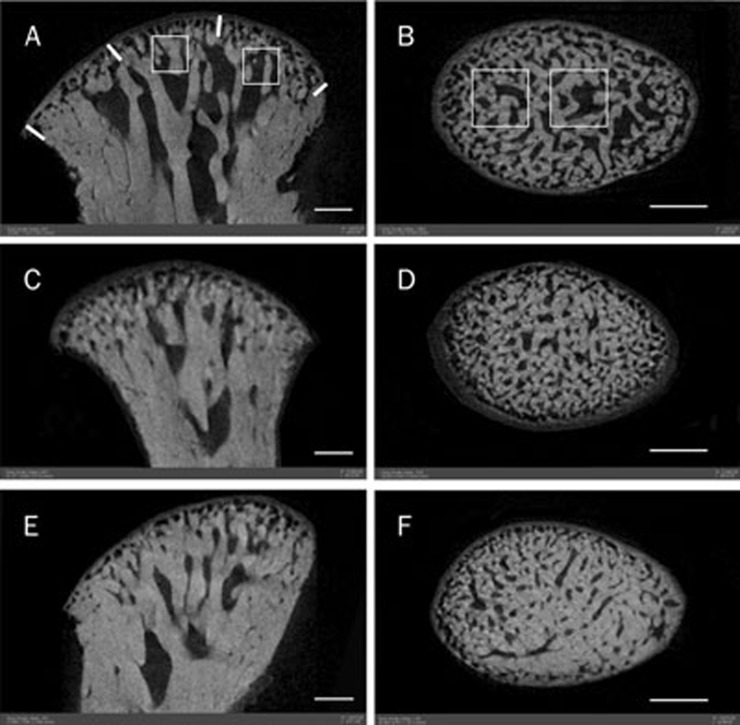
Figure 1.
Sagittal central and coronal section of mandibular condyle scanned by micro-CT. A and B from control group, C and D from low dose genistein-treatment group, and E and F from high dose genistein-treatment group. The area of trabecular bone in both genistein-treatment groups was obviously higher than that in control group. White cubes in A and B represented the selected regions of interest for three-dimensional morphometric analysis. Scale bar is 500 μm.
Quantitative real-time PCR in vivo
Under deep anesthesia, the remaining 18 rats (6 in each group) were sacrificed for real-time PCR. Twelve mandibular condyles in each group were randomly assigned into 3 samples. Mandibular condylar subchondral bone samples were pulverized in liquid nitrogen. The total RNA was isolated using a standard TRIzol® protocol (Invitrogen, Carlsbad, CA, USA), followed by first-strand cDNA synthesis with the RevertAidTM First Strand cDNA Synthesis Kit (Fermentas). Real-time PCR was performed in an ABI 7500 Fast thermal cycler. The protocol comprised 40 cycles of 94 °C for 5 s, 62 °C for 34 s, and 72 °C for 1 min each. The detected cytokines were ALP, OC, OPG, RANKL, ERα, and ERβ. Table 1 shows the sequences of the primers used in this study. Expression levels of all detected cytokines were normalized according to ribosomal protein 18S RNA levels to account for differences arising from reverse transcription efficiency and quality of the total RNA28.
Table 1. Primer sequences for ALP, OC, OPG, RANKL, ERα, ERβ, and S18.
| Gene | Sequence | Fragment length (bp) | Accession number |
|---|---|---|---|
| ALP | F: 5′-CGAGCAGGAACAGAAGTTTGC-3′ R: 5′-TGGCCAAAAGGCAGTGAATAG-3′ | 105 bp | NM_013059 |
| OC | F: 5′-ATGCCACTGCGTATTGGTTGA-3′ R: 5′-TCCGCTAGCTCGTCACAATTG-3′ | 122 bp | M25490 |
| OPG | F: 5′-AGCTGGCACACGAGTGATGAA-3′ R: 5′-CACATTCGCACACTCGGTTGT-3′ | 106 bp | U94330 |
| RANKL | F: 5′-GGAGGATTTTTCAAGCTCCGG-3′ R: 5′-TGAAAGCCCCAAAGTACGTCG-3′ | 103 bp | NM_057149 |
| ERα | F: 5′-TGCGCAAGTGTTACGAAGTGG-3′ R: 5′-TTCGGCCTTCCAAGTCATCTC-3′ | 108 bp | NM_012689 |
| ERβ | F: 5′-AAAAACTCACCGTCGAGCCTT-3′ R: 5′-GCTGAATACTCATGGCGGTTG-3′ | 124 bp | NM_012754 |
| S18 | F: 5′-CGGCTACCACATCCAAGGAA-3′ R: 5′-GCTGGAATTACCGCGGCT-3′ | 187 bp | M11188 |
ALP, alkaline phosphatase; OC, osteocalcin; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor κB ligand; ERα, estrogen receptor α ERβ, estrogen receptor β S18, ribosomal protein S18, a housekeeping gene.
Serum chemistry
Before animals were sacrificed, blood was taken from the abdominal aorta by puncture exsanguination. Serum was collected by centrifugation and stored at -80 °C for further biochemical analysis. Serum bone-ALP, OC, OPG, RANKL, and CTX levels were measured by enzyme-linked immunosorbent assay (ELISA) using commercial kits (CSB-E11865r, CSB-E05129r, CSB-E07404r, and CSB-E05126r from Cusabio Biotech Co, Ltd, and QRCT-301330013301EIA\UTL from Adlitteram Diagnostic Laboratories) according to the manufacturer's instructions. The absorbance was read on an Elx800TM microplate spectrometer (Bio-tec).
Culture of primary osteoblasts and genistein treatment in vitro
Primary osteoblasts were obtained from the mandibular subchondral bone of 8-week-old SD rats. In brief, the mandibular condyles were dissected with aseptic technique. The soft tissues and cartilage were removed to obtain mandibular subchondral bone, which was reduced to small fragments and gently digested at 37 °C with 0.125% trypsin for 8 min. The digestion process was repeated 6 times. Fractionated cells were collected from the final four digestions and combined together as the primary osteoblasts. Cells were plated at a density of 1×104/cm2 into appropriate dishes and then cultured under 37 °C and 5% CO2 conditions. Culture medium was DMEM containing 10% FBS, 100 U/mL penicillin and 100 U/mL streptomycin. The cell medium was changed every three days.
For experiments, osteoblasts from the second generation were seeded in 12-well plates at a concentration of 3×104/cm2. It has previously been identified that genistein increases OPG levels with a maximum effect at 10−7 mol/L29. Thus, in the present study, 10−7 mol/L genistein was regarded as physiological or low dose, and 10−4 mol/L as the high dose in vitro. At confluence, cells were treated with 0.1% DMSO (Sigma D-2650) or genistein (10−7 and 10−4 mol/L, Sigma G6649) for an additional 48 h. Cells were collected for total RNA isolation. The mRNA expression levels of ALP, OC, OPG, RANKL, ERα, and ERβ were evaluated by real-time PCR.
RNAi of ERs and genistein treatment
Small interfering RNA (siRNA) duplexes targeted against rat ERα and ERβ genes were designed and synthesized by Gene-Pharma (Shanghai, China). The siRNA sequences for ERα (GeneBank Accession No NM 012689) were as follows: sense, 5′-GGGAGCUGGUUCAUAUGAUTT-3′ and anti-sense, 5′-AUCAUAUGAACCAGCUCCCTT-3′. The siRNA sequences for ERβ (GeneBank Accession No NM 012754) were as follows: sense, 5′-GAGCACACCUUACCUGUAATT-3′ and anti-sense, 5′-UUACAGGUAAGGUGUGCUCTT-3′. The nonsense siRNA sequences were as follows: sense, 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and anti-sense, 5′-ACG UGA CAC GUU CGG AGA ATT-3′. Briefly, cells were grown in 6-well plates for 36 h and transfected at 70%–80% confluency. ERα and ERβ siRNAs were transfected with Lipofectamine 2000 (Invitrogen), respectively. Transfection medium was replaced after 6 h. At confluence, cells were treated with 0.1% DMSO (Sigma D-2650), 10−7 mol/L or 10−4 mol/L genistein for another 48 h. Cells were collected for total RNA isolation. The mRNA expression levels of ALP, OC, OPG, and RANKL mRNA were evaluated by real-time PCR.
Statistical analysis
SPSS 13.0 (SPSS Inc, Chicago, IL, USA) was used to analyze and describe the data. Single-factor analysis of variance was adopted. When significant main effects were found, specific comparisons between groups were made by Student's t test. P-values were considered to be statistically significant when less than 0.05.
Results
Morphology and micro-architectural properties of mandibular condylar subchondral bone
In the mandibular condyles of control, low-, and high-dose genistein-treated groups, condylar cartilage covering subchondral bone was arranged regularly with good continuity in each layer (Figure 2). As identified by histomorphological analysis, the mandibular subchondral trabecular bone became larger and thicker after both high- and low-dose genistein treatment (Table 2), which was also identified by micro-CT (Figure 1). As shown in Table 3, genistein induced significant increase in BMD, BMC, and bone volume in both the low-dose (P<0.05, P<0.05, P<0.01, respectively) and high-dose genistein-treated groups (all P<0.01). The BMD in the high-dose genistein-treated group was higher than that in the low-dose group (P<0.05). With respect to the micro-architecture of subchondral bone, the BV/TV and Tb.Th increased in both low-dose (P<0.05, P<0.01) and high-dose genistein-treated groups (both P<0.01), as well as obvious decrease in BS/BV, Tb.N and Tb.Sp in both genistein-treated groups (P<0.05, P<0.01). The BV/TV in the high-dose genistein-treated group was higher than that in low-dose group (P<0.01), while the Tb.Sp was lower in the high-dose group than that in the low-dose group (P<0.05).
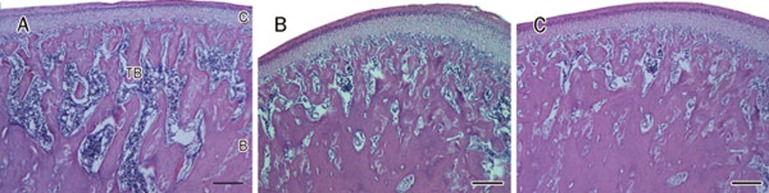
Figure 2.
The histological morphology of mandibular condyle. (A−C) showed the morphology of middle-posterior region of mandibular condyle from control, low dose and high dose genistein-treatment groups. The area of trabecular bone in genistein-treatment group was obviously higher than that in control group. The space between trabecular bones in genistein-treatment group was obviously less than that in control group. C, cartilage; B, subchondral bone; TB, trabecular bone. Scale bar is 200 μm.
Table 2. Values of the histomorphological parameters after genistein treatment. Mean±SEM. bP<0.05, cP<0.01 vs control group. eP<0.05, fP<0.01 low dose (10 mg/kg) genistein-treatment groups.
| Parameters | Control group | Genistein (10 mg/kg) | Genistein (50 mg/kg) |
|---|---|---|---|
| BV/TV (%) | 57.76±2.35 | 63.15±2.92b | 72.4±3.37cf |
| Tb.Th (μm) | 58.02±4.66 | 75.45±5.02c | 83.18±3.45ce |
| Tb.N (#/mm3) | 114.73±2.31 | 9.34±0.88c | 9.03±0.81c |
| Tb.Sp (μm) | 45.64±4.35 | 29.52±2.88c | 26.48±3.35c |
BV/TV, trabecular bone volume fraction; Tb.Th, mean trabecular thickness; Tb.N, mean trabecular number; Tb.Sp, mean trabecular separation.
Table 3. Values of BMD and microstructural parameters after genistein treatment. Means±SEM. bP<0.05, cP<0.01 vs control group. eP<0.05, fP<0.01 vs low dose (10 mg/kg) genistein-treatment groups.
| Parameters | Control group | Genistein (10 mg/kg) | Genistein (50 mg/kg) |
|---|---|---|---|
| BMD (mg/mm3) | 761.273±38.076 | 887.893±31.714b | 993.276±47.386ce |
| BMC (mg) | 0.097±0.004 | 0.111±0.007b | 0.124±0.008c |
| Bone volume (mm3) | 0.079±0.002 | 0.089±0.005c | 0.093±0.004c |
| BV/TV (%) | 61.297±1.903 | 67.228±2.578b | 73.568±1.782cf |
| Tb.Th (μm) | 61.748±1.444 | 72.794±1.999c | 77.593±7.111c |
| BS/BV (%) | 31.993±1.184 | 27.806±1.325c | 25.923±2.112c |
| Tb.N (#/mm3) | 10.170±0.296 | 9.182±0.611b | 9.088±0.525b |
| Tb.Sp (μm) | 0.102±3.654 | 31.204±1.504c | 28.176±1.850ce |
BMD, bone mineral density; BMC, bone mineral content; BV/TV, trabecular bone volume fraction; BS/BV, bone surface-to-volume ratio; Tb.Th, mean trabecular thickness; Tb.N, trabecular number; Tb.Sp, mean trabecular separation; BV, bone volume.
Effect of genistein-treatment on the mRNA expression of ALP, OC, OPG, RANKL, ERα, and ERβ in vivo
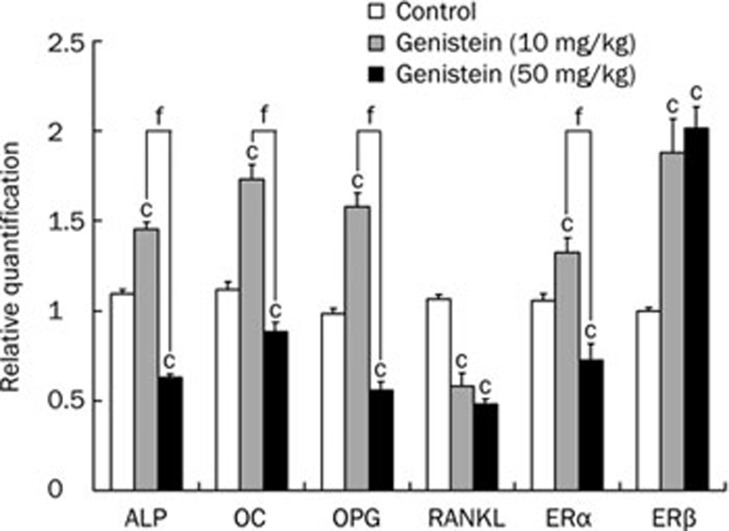
As shown in Figure 3, gene expression of ALP, OC, OPG, ERα, and ERβ increased in the low-dose genistein-treated group (all P<0.01) compared with the control group, but that of RANKL decreased (P<0.01). In the high-dose genistein-treated group, however, the gene expression of ALP, OC, OPG, RANKL, and ERα decreased significantly (all P<0.01), but that of ERβ still increased compared with the control group (P<0.01). Gene expression of ALP, OC, OPG, and ERα in the low-dose genistein-treated group was higher than that in the high-dose group (all P<0.01). Additionally, the RANKL/OPG ratio decreased in both genistein-treated groups.
Figure 3.
Effect of genistein treatment on the mRNA expression of ALP, OC, OPG, RANKL, ERα, and ERβ. Values were normalized to the expression of S18. Data shown are mean±SD (n=3). cP<0.01 indicates significant difference between genistein-treatment and control groups. fP<0.01 indicates significant difference between high and low dose genistein-treatment groups.
Effect of genistein-treatment on serum levels of bone-ALP, OC, OPG, RANKL, and CTX
Compared with the control group, the serum b-ALP, OC, and OPG levels increased significantly (all P<0.01), but the sRANKL level decreased (P<0.01) in the low-dose genistein-treated group (Table 3). No significant difference was found in the CTX level between control and low-dose genistein-treated groups. However, compared with the control group, not only the serum sRANKL and CTX (both P<0.01), but also b-ALP, OC, and OPG levels decreased (P<0.05, P<0.05, and P<0.01, respectively) in the high-dose genistein-treated group. The serum b-ALP, OC, OPG, and CTX levels in the low-dose genistein-treated group were higher than those in the high-dose group (P<0.01, P<0.01, P<0.01, and P<0.05, respectively). The RANKL/OPG ratio decreased in both genistein-treated groups (Table 4).
Table 4. Values of serum bone markers after genistein treatment. Mean±SEM. bP<0.05, cP<0.01 vs control group. eP<0.05, fP<0.01 vs low dose (10 mg/kg) genistein-treatment groups.
| Parameters | Control group | Genistein (10 mg/kg) | Genistein (50 mg/kg) |
|---|---|---|---|
| b-ALP (U/L) | 118.00±9.90 | 151.33±9.01c | 98.93±13.02bf |
| OC (ng/mL) | 9.95±1.27 | 13.93±2.55c | 7.76±1.73bf |
| OPG (pg/mL) | 335.24±18.50 | 403.67±19.36c | 255.64±23.14cf |
| sRANKL (pg/mL) | 22.53±3.18 | 13.15±2.17c | 11.64±2.56c |
| CTX (ng/mL) | 53.93±7.49 | 49.40±8.07 | 38.46±6.62ce |
b-ALP, bone alkaline phosphatase; sRANKL, soluble receptor activator of nuclear factor κB ligand.
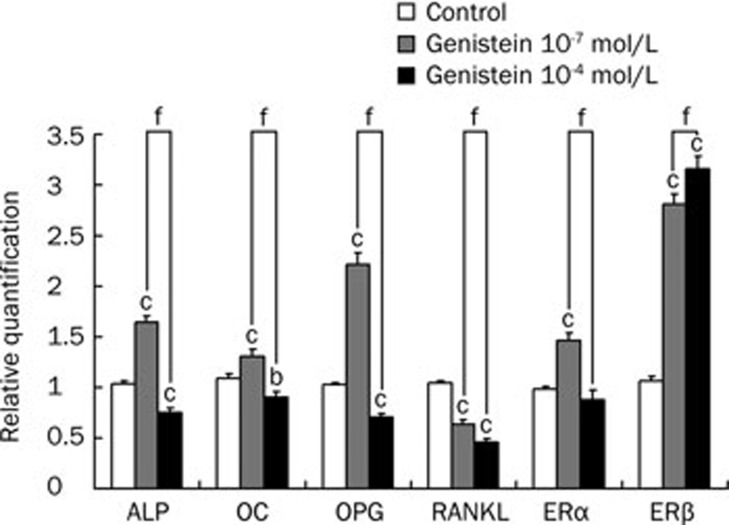
Effect of genistein-treatment on the mRNA expression of ALP, OC, OPG, RANKL, ERα, and ERβ in vitro
As shown in Figure 4, genistein affected osteoblasts from mandibular condylar subchondral bone in a concentration-dependent manner. The presence of 10−7 mol/L genistein in the culture medium induced a significant decrease in the gene expression of RANKL (P<0.01), as well as increase in ALP, OC, OPG, ERα, and ERβ (all P<0.01). However, the presence of 10−4 mol/L genistein reduced the expression of ALP, OC, OPG, RANKL (P<0.01, P<0.05, P<0.01, and P<0.01, respectively), as well as increase in ERβ expression (P<0.01). The expression of ALP, OC, OPG, RANKL and ERα in the 10−7 mol/L genistein-treated group were higher than those in the 10−4 mol/L group (all P<0.01). Alternatively, expression of ERβ was lower in the low-dose group than in the high-dose group (both P<0.01). The RANKL/OPG ratio was decreased by both 10−4 and 10−7 mol/L genistein treatment, respectively.
Figure 4.
Effect of genistein treatment on the mRNA expression of ALP, OC, OPG, RANKL in osteoblasts. Values were normalized to the expression of 18S. Data shown are mean±SD (n=3). bP<0.05, cP<0.01 indicate significant difference between genistein treatment and control group. fP<0.01 indicates significant difference between high and low dose genistein-treatment groups.
Effect of ER silencing on the effect of genistein treatment
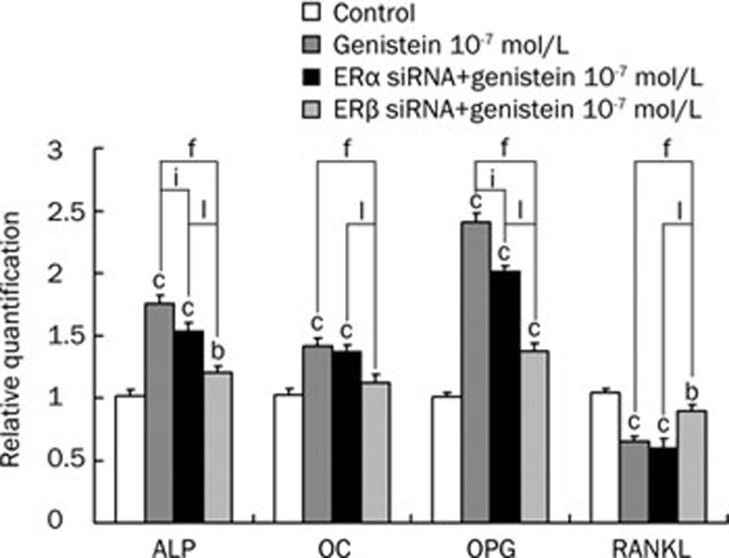
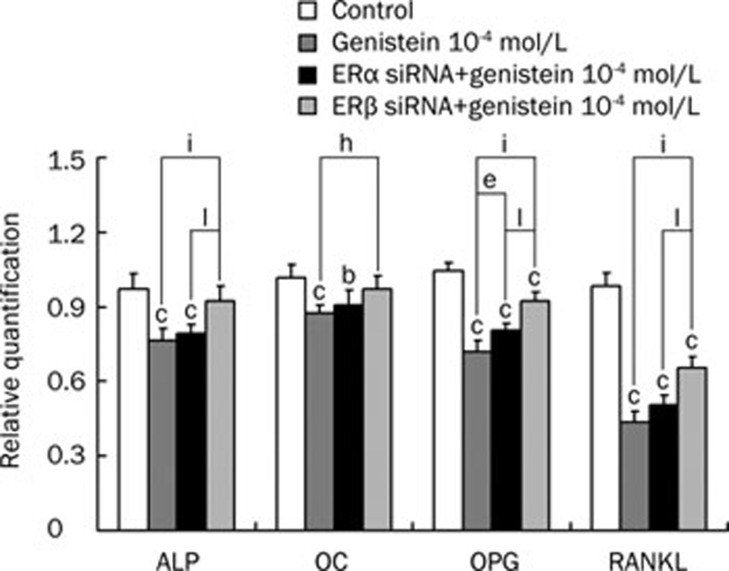
In order to explore the function of ERα and ERβ, we performed gene silencing experiments in osteoblasts. The mRNA levels of ERα and ERβ were decreased in cells as detected by real-time PCR (Figure 5). ERα and ERβ siRNA were able to knockdown 72% and 80% of ER expression, respectively. As shown in Figure 6 and Figure 7, ERβ silencing reversed the effect of both 10−7 and 10−4 mol/L genistein treatment in the expression of ALP, OC, OPG, and RANKL. ERα silencing only reversed the expression of ALP and OPG (both P<0.01) in 10−7 mol/L genistein-treatment group, and that of OPG in the 10−4 mol/L genistein-treated group (P<0.05). Importantly, the effect of ERβ silencing was greater than that of ERα silencing in nearly all of the groups with the exception of OC expression in the 10−4 mol/L genistein-treated group (all P<0.01).
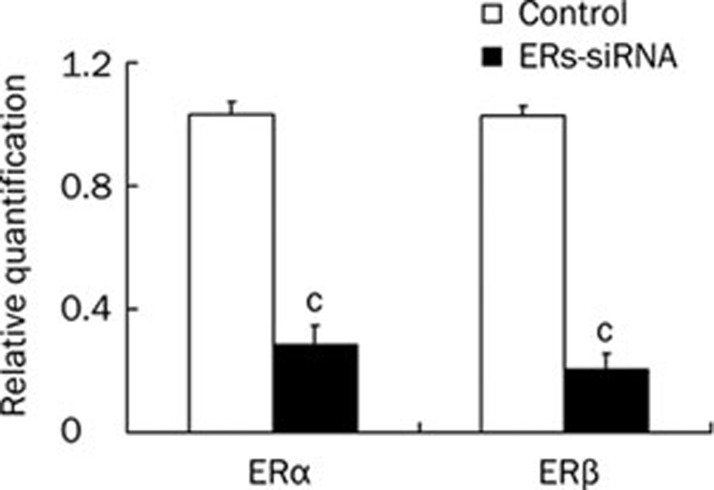
Figure 5.
Effect of ERα and ERβ siRNA on the mRNA expression of ERα and ERβ in osteoblasts. Values were normalized to the expression of 18S. Data shown are mean±SD (n=3). cP<0.01 vs control.
Figure 6.
Effect of ERα and ERβ silencing on the expression of ALP, OC, OPG, RANKL in osteoblasts treated by 10−7 mol/L genistein. Values were normalized to the expression of 18S. Data shown are mean±SD (n=3). bP<0.05, cP<0.01 indicate significant difference between genistein treatment and control group. fP<0.01 indicates significant difference between 10−7 mol/L genistein-treatment group and ERα siRNA+10−7 mol/L genistein-treatment group. iP<0.01 indicates significant difference between 10−7 mol/L genistein-treatment group and ERβ siRNA+10−7 mol/L genistein-treatment group. lP<0.01 indicates significant difference between ERα siRNA+10−7 mol/L genistein-treatment group and ERβ siRNA+10−7 mol/L genistein-treatment group.
Figure 7.
Effect of ERα and ERβ silencing on the expression of ALP, OC, OPG, RANKL in osteoblasts treated by 10−4 mol/L genistein. Values were normalized to the expression of 18S. Data shown are mean±SD (n=3). bP<0.05, cP<0.01 indicate significant difference between genistein treatment and control group. eP<0.05 indicates significant difference between 10−4 mol/L genistein-treatment group and ERα siRNA+10−4 mol/L genistein-treatment group. hP<0.05, iP<0.01 indicate significant difference between 10−4 mol/L genistein-treatment group and ERβ siRNA+10−4 mol/L genistein-treatment group. lP<0.01 indicates significant difference between ERα siRNA+10−4 mol/L genistein-treatment group and ERβ siRNA+10−4 mol/L genistein-treatment group.
Discussion
In the literature, the protective effects of genistein in OVX rats and mice preventing OVX-induced bone loss have been reported in appendicular bone or vertebrae for various treatment periods (2–15 weeks), application routes (oral gavage, intraperitoneal administration, subcutaneous administration) and doses of genistein (3–50 mg·kg−1·d−1)18, 20, 30, 31, 32, 33, 34, 35, 36. Similarly, oral administration of genistein (54 mg·kg−1·d−1) increased BMD of the spine and hip in osteopenic postmenopausal Caucasian women with an observation period of 24 months17. In the present study on female rats with normal estrogen levels, both low and high doses of genistein treatment were observed to induce significant increase in BMD, BMC and bone volume, as well as inducing thicker and larger trabecular bone in the mandibular subchondral bone. These results were consistent with the above-mentioned studies on appendicular bone or vertebrae17, 18, 20, 30, 31, 32, 33, 34, 35, 36, indicating that the effect of genistein on mandibular subchondral bone is similar to that on appendicular bone or vertebrae.
Similar to the biphasic effects of estrogen depending on dosage37, genistein was also observed to have a biphasic cell proliferative response, stimulation at low concentrations and inhibition at high concentrations38, 39, 40. In the present study low dose genistein treatment increased the expression of ALP, OC, and OPG at the serum level and mRNA level both in vivo and in vitro. Low-dose genistein treatment also decreased the expression of RANKL and the RANKL/OPG ratio in agreement with previous studies20, 41, 42, 43, 44. These data suggest that normal doses of genistein improve bone formation and inhibit bone resorption. However, high-dose genistein treatment decreased not only the expression of RANKL and the RANKL/OPG ratio, but also that of ALP, OC, and OPG. It indicates that excess genistein inhibits both bone resorption and bone formation in rat mandibular subchondral bone. This phenomenon is consistent with the fact that the number of both active osteoclasts and active osteoblasts is increased in postmenopausal osteoporosis induced by estrogen deficiency45. Although bone formation was also inhibited by excess genistein, the RANKL/OPG ratio was still lower in the high-dose genistein-treated group than in the control group, which is in line with the increased BMD and bone volume in the high-dose genistein-treated group. Interestingly, though bone formation was inhibited by excess genistein to some extent, high doses of genistein induced more mandibular subchondral trabecular bone compared to low-dose genistein. This discrepancy might be due to the sampling time-point, or the asynchronism between microstructural parameters and the expression of bone homeostasis-associated factors. Allowing for these considerations, further studies with additional time-points are needed.
A mechanism has been proposed in which genistein affects bone homeostasis directly via ER activation in bone34. The two subtypes of ER, ERα, and ERβ, are distinct proteins encoded by separate genes located on different chromosomes. Genistein is almost exclusively ERβ selective and binds with a 7- to 30-fold greater affinity to ERβ than to ERα40. Furthermore, genistein activates transcriptional activation of ERβ at a 10-fold lower concentration than for ERα40. In the present study, the expression of ERβ was increased significantly by both low and high doses of genistein, while the expression of ERα was increased by low dose of genistein, but decreased by high dose of genistein. Furthermore, ERβ silencing reversed the effect of genistein treatment on the expression of ALP, OC, OPG, and RANKL. The effect of ERβ silencing was greater than that of ERα silencing. It seems that the effect of genistein on rat mandibular subchondral bone was preferentially mediated via ERβ activation, which is in keeping with the previous report that the effect of genistein on skeletal muscle myosin heavy chain expression was predominantly mediated through ERβ46.
In the literature, a few studies support ERα's involvement in the adaptive response of osteoblasts to mechanical strain47, 48, 49, 50. ERα−/− mice produced three times less new cortical bone in response to the same mechanical stimulus as their ERα+/+ littermates47, 48. Similarly, osteoblasts derived from ERα−/− mice failed to increase in number in response to mechanical stimulation, which can be rescued by transfection with fully functional ERα49, 50. The decreased expression of ERα in the high-dose genistein-treated group may imply a decreased adaptive capacity of mandibular subchondral bone to mechanical loads. Considering the difference and fluctuation of estrogen levels in individual people, the proper dose of genistein should be studied in more depth prior to wide application of genistein in the treatment of osteoporosis or OA.
In summary, low-dose genistein can increase bone formation and inhibit bone resorption, while excess genistein can inhibit both bone resorption and bone formation in rat mandibular condylar subchondral bone, which was predominantly mediated through ERβ.
Author contribution
Shi-bin YU and Guang-ying DONG designed the present study and wrote the manuscript. Yong-qi LI performed the animal treatments, sample preparation and micro-CT analysis. Xiang-hui XING performed the ELISA assay, PCR and revised the manuscript. Hui WANG performed the osteoblast culture, ER silencing and PCR in vivo. Xi-li WENG performed the HE staining, data analysis and revised the manuscript.
Acknowledgments
The authors thank Mr Jun WANG (Xijing Hospital, Fourth Military Medical University) for technical assistance in Micro-CT. This work was financially supported by the National Natural Science Foundation of China (No 30901699 and No 30928028).
References
- Hohenhaus MH, McGarry KA, Col NF. Hormone therapy for the prevention of bone loss in menopausal women with osteopenia: is it a viable option. Drugs. 2007;67:2311–21. doi: 10.2165/00003495-200767160-00002. [DOI] [PubMed] [Google Scholar]
- Levine JP. Effective strategies to identify postmenopausal women at risk for osteoporosis. Geriatrics. 2007;62:22–30. [PubMed] [Google Scholar]
- Fitzpatrick LA. Estrogen therapy for postmenopausal osteoporosis. Arq Bras Endocrinol Metabol. 2006;50:705–19. doi: 10.1590/s0004-27302006000400016. [DOI] [PubMed] [Google Scholar]
- Nevitt MC, Cummings SR, Lane NE, Hochberg MC, Scott JC, Pressman AR, et al. Association of estrogen replacement therapy with the risk of osteoarthritis of the hip in elderly white women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1996;156:2073–80. [PubMed] [Google Scholar]
- Zhang Y, McAlindon TE, Hannan MT, Chaisson CE, Klein R, Wilson PW, et al. Estrogen replacement therapy and worsening of radiographic knee osteoarthritis: the Framingham Study. Arthritis Rheum. 1998;41:1867–73. doi: 10.1002/1529-0131(199810)41:10<1867::AID-ART20>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Spector TD, Nandra D, Hart DJ, Doyle DV. Is hormone replacement therapy protective for hand and knee osteoarthritis in women? The Chingford Study. Ann Rheum Dis. 1997;56:432–4. doi: 10.1136/ard.56.7.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JC. Hormone replacement therapy: review, update, and remaining questions after the Women's Health Initiative Study. Curr Osteoporos Rep. 2004;2:12–6. doi: 10.1007/s11914-004-0009-z. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer Lancet 19973501047–59. [PubMed] [Google Scholar]
- Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study Lancet 2003362419–27. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr. 2003;78:593S–609S. doi: 10.1093/ajcn/78.3.593S. [DOI] [PubMed] [Google Scholar]
- Messina M, Ho S, Alekel DL. Skeletal benefits of soy isoflavones: a review of the clinical trial and epidemiologic data. Curr Opin Clin Nutr Metab Care. 2004;7:649–58. doi: 10.1097/00075197-200411000-00010. [DOI] [PubMed] [Google Scholar]
- Chanawirat A, Khemapech S, Patumraj S, Siriviriyakul P. Genistein replacement therapy on endothelial dysfunction and bone loss in bilateral ovariectomized rats. Clin Hemorheol Microcirc. 2006;34:309–14. [PubMed] [Google Scholar]
- Erlandsson MC, Islander U, Moverare S, Ohlsson C, Carlsten H. Estrogenic agonism and antagonism of the soy isoflavone genistein in uterus, bone and lymphopoiesis in mice. APMIS. 2005;113:317–23. doi: 10.1111/j.1600-0463.2005.apm_113502.x. [DOI] [PubMed] [Google Scholar]
- Chen YM, Ho SC, Lam SS, Ho SS, Woo JL. Beneficial effect of soy isoflavones on bone mineral content was modified by years since menopause, body weight, and calcium intake: a double-blind, randomized, controlled trial. Menopause. 2004;11:246–54. doi: 10.1097/01.gme.0000094394.59028.46. [DOI] [PubMed] [Google Scholar]
- Wu J, Oka J, Higuchi M, Tabata I, Toda T, Fujioka M, et al. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial. Metabolism. 2006;55:423–33. doi: 10.1016/j.metabol.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Morabito N, Crisafulli A, Vergara C, Gaudio A, Lasco A, Frisina N, et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res. 2002;17:1904–12. doi: 10.1359/jbmr.2002.17.10.1904. [DOI] [PubMed] [Google Scholar]
- Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. 2007;146:839–47. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
- Li B, Yu S. Genistein prevents bone resorption diseases by inhibiting bone resorption and stimulating bone formation. Biol Pharm Bull. 2003;26:780–6. doi: 10.1248/bpb.26.780. [DOI] [PubMed] [Google Scholar]
- Sliwiński L, Folwarczna J, Nowińska B, Cegieła U, Pytlik M, Kaczmarczyk-Sedlak I, et al. A comparative study of the effects of genistein, estradiol and raloxifene on the murine skeletal system. Acta Biochim Pol. 2009;56:261–70. [PubMed] [Google Scholar]
- Bitto A, Burnett BP, Polito F, Marini H, Levy RM, Armbruster MA, et al. Effects of genistein aglycone in osteoporotic, ovariectomized rats: a comparison with alendronate, raloxifene and oestradiol. Br J Pharmacol. 2008;155:896–905. doi: 10.1038/bjp.2008.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tähtelä R, Thölix E. Serum concentrations of type I collagen carboxyterminal telopeptide (ICTP) and type I procollagen carboxy-and aminoterminal propeptides (PICP, PINP) as markers of metastatic bone disease in breast cancer. Anticancer Res. 1996;16:2289–93. [PubMed] [Google Scholar]
- Shoji K, Elsubeihi ES, Heersche JN. Effects of ovariectomy on turnover of alveolar bone in the healed extraction socket in rat edentulous mandible. Arch Oral Biol. 2011;56:114–20. doi: 10.1016/j.archoralbio.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Jonasson G, Alstad T, Vahedi F, Bosaeus I, Lissner L, Hakeberg M. Trabecular pattern in the mandible as bone fracture predictor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e42–51. doi: 10.1016/j.tripleo.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Griffin CJ, Powers R, Kruszynski R. The incidence of osteoarthritis of the temporomandibular joint in various cultures. Aust Dent J. 1979;24:94–106. doi: 10.1111/j.1834-7819.1979.tb03615.x. [DOI] [PubMed] [Google Scholar]
- Milam SB. Pathophysiology and epidemiology of TMJ. J Musculoskelet Neuronal Interact. 2003;3:382–90. [PubMed] [Google Scholar]
- Jiao K, Dai J, Wang MQ, Niu LN, Yu SB, Liu XD. Age- and sex-related changes of mandibular condylar cartilage and subchondral bone: a histomorphometric and micro-CT study in rats. Arch Oral Biol. 2010;55:155–63. doi: 10.1016/j.archoralbio.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Michael MR, Wolz E, Davidovich A, Pfannkuch F, Edwards JA, Bausch J. Acute, subchronic and chronic safety studies with genistein in rats. Food Chem Toxicol. 2006;44:56–80. doi: 10.1016/j.fct.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Diel P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr. 2009;48:213–20. doi: 10.1007/s00394-009-0004-3. [DOI] [PubMed] [Google Scholar]
- Viereck V, Gründker C, Blaschke S, Siggelkow H, Emons G, Hofbauer LC. Phytoestrogen genistein stimulates the production of osteoprotegerin by human trabecular osteoblasts. J Cell Biochem. 2002;84:725–35. doi: 10.1002/jcb.10087. [DOI] [PubMed] [Google Scholar]
- Ishida H, Uesugi T, Hirai K, Toda T, Nukaya H, Yokotsuka K, et al. Preventive effects of the plant isoflavones, daidzin and genistin, on bone loss in ovariectomized rats fed a calcium-deficient diet. Biol Pharm Bull. 1998;21:62–6. doi: 10.1248/bpb.21.62. [DOI] [PubMed] [Google Scholar]
- Nakajima D, Kim CS, Oh TW, Yang CY, Naka T, Igawa S, et al. Suppressive effects of genistein dosage and resistance exercise on bone loss in ovariectomized rats. J Physiol Anthropol Appl Human Sci. 2001;20:285–91. doi: 10.2114/jpa.20.285. [DOI] [PubMed] [Google Scholar]
- Ye SF, Saga I, Ichimura K, Nagai T, Shinoda M, Matsuzaki S. Coumestrol as well as isoflavones in soybean extract prevent bone resorption in ovariectomized rats. Endocr Regul. 2003;37:145–52. [PubMed] [Google Scholar]
- Pie JE, Park JH, Park YH, Ryu YM, Kim KN, Suh SW, et al. Effect of genistein on the expression of bone metabolism genes in ovariectomized mice using a cDNA microarray. J Nutr Biochem. 2006;17:157–64. doi: 10.1016/j.jnutbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Hertrampf T, Gruca MJ, Seibel J, Laudenbach U, Fritzemeier KH, Diel P. The bone-protective effect of the phytoestrogen Genistein is mediated via ERα-dependent mechanisms and strongly enhanced by physical activity. Bone. 2007;40:1529–35. doi: 10.1016/j.bone.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Hertrampf T, Schleipen B, Offermanns C, Velders M, Laudenbach U, Diel P. Comparison of the bone protective effects of an isoflavone-rich diet with dietary and subcutaneous administrations of genistein in ovariectomized rats. Toxicol Lett. 2009;184:198–203. doi: 10.1016/j.toxlet.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Dai R, Ma Y, Sheng Z, Jin Y, Zhang Y, Fang L, et al. Effects of genistein on vertebral trabecular bone microstructure, bone mineral density, microcracks, osteocyte density, and bone strength in ovariectomized rats. J Bone Miner Metab. 2008;26:342–9. doi: 10.1007/s00774-007-0830-4. [DOI] [PubMed] [Google Scholar]
- Cheng P, Ma X, Xue Y, Li S, Zhang Z. Effects of estradiol on proliferation and metabolism of rabbit mandibular condylar cartilage cells in vitro. Chin Med J (Engl) 2003;116:1413–7. [PubMed] [Google Scholar]
- Maggiolini M, Bonofiglio D, Marsico S, Panno ML, Cenni B, Picard D, Ando S. Estrogen receptor α mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Mol Pharmacol. 2001;60:595–602. [PubMed] [Google Scholar]
- Power KA, Thompson LU. Ligand-induced regulation of ERα and ERβ is indicative of human breast cancer cell proliferation. Breast Cancer Res Treat. 2003;82:209–21. doi: 10.1023/A:1026114501364. [DOI] [PubMed] [Google Scholar]
- Rajah TT, Du N, Drews N, Cohn R. Genistein in the presence of 17beta-estradiol inhibits proliferation of ERbeta breast cancer cells. Pharmacology. 2009;84:68–73. doi: 10.1159/000226123. [DOI] [PubMed] [Google Scholar]
- Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J Bone Miner Res. 2008;23:715–20. doi: 10.1359/jbmr.080201. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Altavilla D, Squadrito G, Romeo A, Adamo EB, Marini R, et al. Effects of the phytoestrogen genistein on the circulating soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin system in early postmenopausal women. J Clin Endocrinol Metab. 2004;89:188–92. doi: 10.1210/jc.2003-030891. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li Q, Wan HY, Helferich WG, Wong MS. Genistein and a soy extract differentially affect three-dimensional bone parameters and bone-specific gene expression in ovariectomized mice. J Nutr. 2009;139:2230–6. doi: 10.3945/jn.109.108399. [DOI] [PubMed] [Google Scholar]
- Ha H, Lee HY, Lee JH, Jung D, Choi J, Song KY, et al. Formononetin prevents ovariectomy-induced bone loss in rats. Arch Pharm Res. 2010;33:625–32. doi: 10.1007/s12272-010-0418-8. [DOI] [PubMed] [Google Scholar]
- Lerner UH. Bone remodeling in post-menopausal osteoporosis. J Dent Res. 2006;85:584–95. doi: 10.1177/154405910608500703. [DOI] [PubMed] [Google Scholar]
- Velders M, Solzbacher M, Schleipen B, Laudenbach U, Fritzemeier KH, Diel P. Estradiol and genistein antagonize the ovariectomy effects on skeletal muscle myosin heavy chain expression via ER-beta mediated pathways. J Steroid Biochem Mol Biol. 2010;120:53–9. doi: 10.1016/j.jsbmb.2010.03.059. [DOI] [PubMed] [Google Scholar]
- Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature. 2003;424:389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- Lee KC, Jessop H, Suswillo R, Zaman G, Lanyon LE. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-alpha and -beta. J Endocrinol. 2004;182:193–201. doi: 10.1677/joe.0.1820193. [DOI] [PubMed] [Google Scholar]
- Jessop HL, Suswillo RF, Rawlinson SC, Zaman G, Lee K, Das-Gupta V, et al. Osteoblast-like cells from estrogen receptor alpha knockout mice have deficient responses to mechanical strain. J Bone Miner Res. 2004;19:938–46. doi: 10.1359/jbmr.2004.19.6.938. [DOI] [PubMed] [Google Scholar]
- Zaman G, Cheng MZ, Jessop HL, White R, Lanyon LE. Mechanical strain activates estrogen response elements in bone cells. Bone. 2000;27:233–9. doi: 10.1016/s8756-3282(00)00324-0. [DOI] [PubMed] [Google Scholar]