The process of developing novel anticancer agents has become more costly and lengthy than ever1. In addition, agents that target de novo pathway not only put the patients to unpredictable toxicity but also increase the risk in drug development2. Therefore, re-discovering the potential of decade-old drugs for novel anticancer therapeutics provides a new route for drug development. Thalidomide, rapamycin, and azacitidine exemplify the potential of this approach and many more, such as metformin, and valproic acid are in the process of validating their role as new anticancer agents3, 4. Now we have two more members to join the rank. Cardiac glycosides, which have been used to treat cardiac failure for decades, recently emerge as potential anti-cancer agents5. A recent work by TAILLER et al took advantage of high thorough-put screening of 1040 FDA approved agents for antileukemic activity and identified ouabain and zinc pyrithione, which have been used in the past for treating heart failure and dermatologic diseases respectively, as novel antileukemic agents6. They found that these agents induced apoptosis of various leukemic cells through the inhibition of NF-κB pathway. Although these two agents induce apoptosis of leukemic cells through common downstream signal pathway, how they differ in upstream remains elusive. For clarity of discussion, we here focus on the potential of cardiac glycosides.
The notion of using cardiac glycosides for its potential anticancer activity has been around for several decades, due to observation that breast cancer patients with digitalis therapy tends to have lower recurrence7, 8. Some even suggests that the association of high plasma level of digitoxin with reduced risks for cancer9. Using cardiac glycosides for anticancer treatment has a sound mechanistic basis. First, essentially all types of cancer cells have aberrant metabolisms (called Warburg effect) that can be differentiated from normal cells, making cancer metabolism an ideal target for drug development. Second, the toxicity of cardiac glycosides has been well characterized. Thus, the toxicity and cost might be more predictable for the development of this class of drugs. There have been several clinical trials testing the potential of cardiac glycosides, alone or in combination with chemotherapy, for various cancers10, 11.
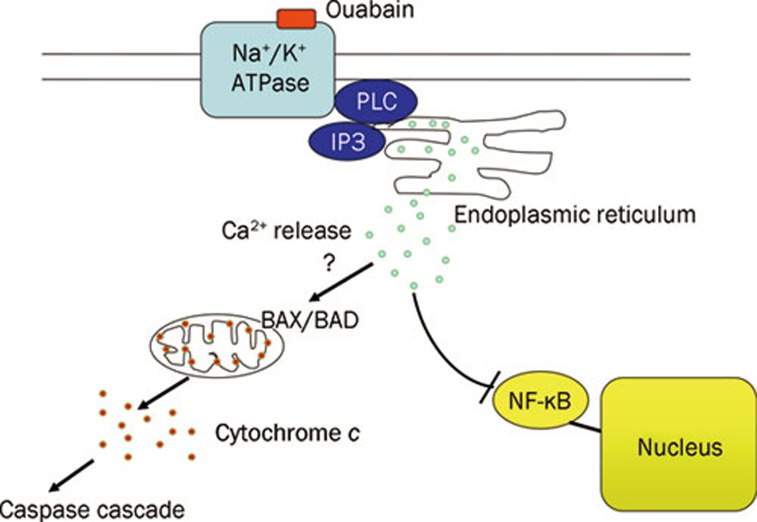
Ouabain binds and suppresses Na+/K+-ATPase, which subsequently leads to apoptosis through depolarization of mitochondria (Figure 1). This mechanism of action is analogous to “mitochondrial priming” of cancer cells by BH3 peptides, which induced permeabilization of mitochondrial outer membrane, prompted release of cytochrome c into cytoplasm, and activated programmed cell death12. The degree of depolarization of mitochondria in cancer cells was correlated with sensitivity to chemotherapeutic agents.
Figure 1.
Proposed mechanisms that induce apoptosis in leukemic cells by cardiac glycosides (ouabain). Binding of ouabain to NA+/K+-ATPase activates phospholipase C (PLC) and inositol-1,4,5-triphosphate (IP3), the latter subsequently binds to IP3 receptor of endoplasmic reticulum, releasing calcium ions into cytoplasm. Increased intracellular calcium ions might induce the apoptosis through inhibition of NF-κB-mediated signal transduction and depolarization of mitochondrial outer membrane.
More relevant to its clinical utility is the finding that primary CD34(+) blasts in acute myeloid leukemia (AML) were more sensitive to ouabain treatment than CD34(–) blasts. In contrast, CD34(+) and CD34(–) cells from healthy donors were insensitive to ouabain. The preferential sensitivity of CD34(+) leukemic blasts is consistent with known differential activation of NF-κB in CD34(+) leukemic cells, but not in unstimulated normal CD34(+) cells13. Therefore, through inhibition of NF-κB, ouabain may selectively eliminate CD34(+) leukemic blasts without detrimental effects to normal hematopoietic progenitors. Another plausible explanation might be that CD34(+) leukemic cells might have higher Na+/K+-ATPase activity, making them sensitive to ouabain. Furthermore, as leukemic blasts are often quiescent and resistant to cytotoxic chemotherapy, ouabain may be used to prime leukemic blasts to enhance chemosensitivity.
However, the application of ouabain to clinical use deserves a note of caution. This drug was dropped out of favor for clinical use for several reasons. First, its bioavailability is poor, compared with other cardiac glycoside, eg digoxin. So it has to be parenterally given, which limits its clinical utility. Second, the activity of ouabain on Na/K ATPase might be dose-dependent. Since endogenous ouabain-like substance is also present in human body at pico-to-nanomolar ranges, suggesting that the level of endogenous ouabain in leukemic patients might be too low to affect NF-κB and that frequent infusion of exogenous ouabain to maintain plasma concentration high enough might be needed to sustain the inhibition of NF-κB14, 15. If so, systemic toxicity might be the limiting factor for its use especially in the elderly patients which constitute the bulk of leukemic patients. Yet, developing semi-synthetic glycosides for cancer therapy has been gaining ground in recent years. A notable agent is UNBS1450 (Unibioscreen), a semi-synthetic cardenolide, which has been tested for various cancers in a phase I trial since 2008. This agent has been shown to deactivate NF-κB in both lung cancer and leukemic cells in vitro at nanomolar levels16. Furthermore, the sensitivity to UNBS1450 appears to correlate with the expression of Na+/K+-ATPase level, implicating that Na/K-ATPase might also be a biomarker for selecting patients for future clinical trials. The findings by Tailler et al highlight the complexity of cancer biology and the potential of using high-throughput screening to discover new application of old drugs for anti-drug discovery, which might some day become a hearty solution for acute myeloid leukemia.
References
- Sukhai MA, Spagnuolo PA, Weir S, Kasper J, Patton L, Schimmer AD. New sources of drugs for hematologic malignancies. Blood. 2011;117:6747–55. doi: 10.1182/blood-2011-02-315283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. New Engl J Med. 2006;355:1018–28. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Sahra IB, Marchand-Brustel YL, Tanti J-F, Bost F. Metformin in cancer therapy: a new perspective for old antidiabetic drug. Mol Cancer Ther. 2010;9:1092–9. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- Stamatopoulos B, Meuleman N, De Bruyn C, Mineur P, Martiat P, Bron D, et al. Antileukemic activity of valproic acid in chronic lymphocytic leukemia B cells defined by microarray analysis. Leukemia. 2009;23:2281–9. doi: 10.1038/leu.2009.176. [DOI] [PubMed] [Google Scholar]
- Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nature Rev Drug Discovery. 2008;7:926–35. doi: 10.1038/nrd2682. [DOI] [PubMed] [Google Scholar]
- Tailler M, Senovilla L, Lainey E, Thepot S, Metivier D, Sebert M, et al. Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia Oncogene 2011. doi: 10.1038/onc.2011.521 [DOI] [PubMed]
- Stenkvist B. Is digitalis a therapy for breast cancer. Oncol Rep. 1999;6:493–6. [PubMed] [Google Scholar]
- Stenkvist B, Pengtsson E, Dahlgvist B, Eriksson O, Jarkrans T, Nordin B. Cardiac glycosides and breast cancer, revisited. New Engl J Med. 1982;306:484. [PubMed] [Google Scholar]
- Haux J, Klepp O, Spigset O, Tretli S. Digitoxin medication and cancer; case control and internal dose-response studies. BMC Cancer. 2001;1:11. doi: 10.1186/1471-2407-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhail T, Kaur H, Ganapathi R, Budd GT, Elson P, Bukowski RM. Phase 1 trial of Anvirzel in patients with refractory solid tumors. Invest New Drugs. 2006;24:423–7. doi: 10.1007/s10637-006-7772-x. [DOI] [PubMed] [Google Scholar]
- Kahn MI, Taft B, Rasku MA, Laber D, Chesney J.A phase 2 trial of biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, interferon, and digoxin in melanoma patientsASCO Chicago2007
- Chonghaile TN, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore VDG, et al. Pretreatment mitochondrial primng correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–33. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman ML, Neering SL, Upchurch D, Grimes B, Howard DS, Rizzieri DA, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia. Blood. 2001;98:2301–7. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- Selden R, Smith TW, Findley W. Ouabain pharmacokinetics in dog and man: determination by radioimmunoassay. Circulation. 1972;45:1176–82. doi: 10.1161/01.cir.45.6.1176. [DOI] [PubMed] [Google Scholar]
- Gottlieb SS, Rogowski AC, Weinberg W, Krichten CM, Hamilton BP, Hamlyn JM. Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation. 1992;86:420–5. doi: 10.1161/01.cir.86.2.420. [DOI] [PubMed] [Google Scholar]
- Juncker T, Cerella C, Teiten M-H, Morceau F, Schumacher M, Ghelfi J, et al. UNBS1450, a steroid cardiac glycoside inducing apoptotic cell death in human leukemia cells. Biochem Pharmacol. 2011;81:13–23. doi: 10.1016/j.bcp.2010.08.025. [DOI] [PubMed] [Google Scholar]