Abstract
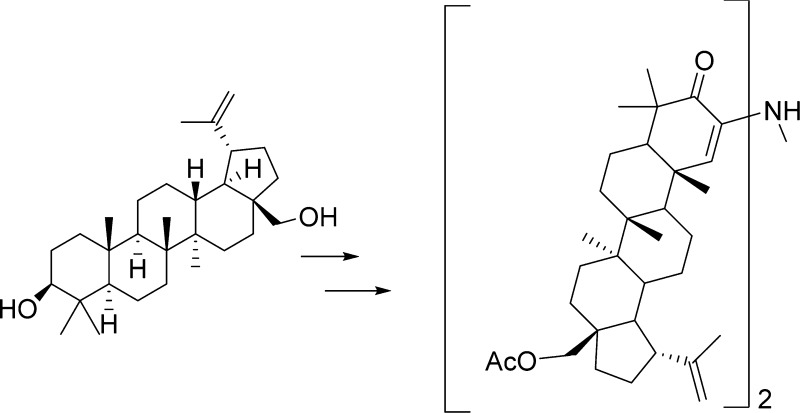
The lupane-type triterpene betulin (1) has been subjected to a series of structural modifications for the purpose of evaluating resultant cancer cell growth inhibitory activity. The reaction sequence 7 → 11 → 12 was especially noteworthy in providing a betulin-derived amine dimer. Other unexpected synthetic results included the 11 and 13/14 → 17 conversions, which yielded an imidazo derivative. X-ray crystal structures of dimer 12 and intermediate 25 are reported. All of the betulin modifications were examined for anticancer activity against the P388 murine and human cell lines. Significant cancer cell growth inhibition was found for 4, 8, 9, 15/16, 19, 20, 24, and 26, which further defines the utility of the betulin scaffold.
Extracts of birch bark (Betula species) usually contain a mixture of betulin (1) as a major component accompanied by related pentacyclic
triterpenoids and have been used in traditional medicine in local
areas through the ages.2a Although betulin
(1) has only moderate anticancer, antibacterial, antifungal,
and antiviral activity and other biological properties, structural
modifications are readily accessed to provide derivatives both known
and new that have improved potency in these areas.2−4 Betulin (1) is a relatively accessible starting molecule for such medicinal
chemistry research.2 Among other avenues,
we have employed betulin in SAR synthesis efforts focused on cephalostatin
1 (2), a powerful marine organism anticancer constituent.5a−5c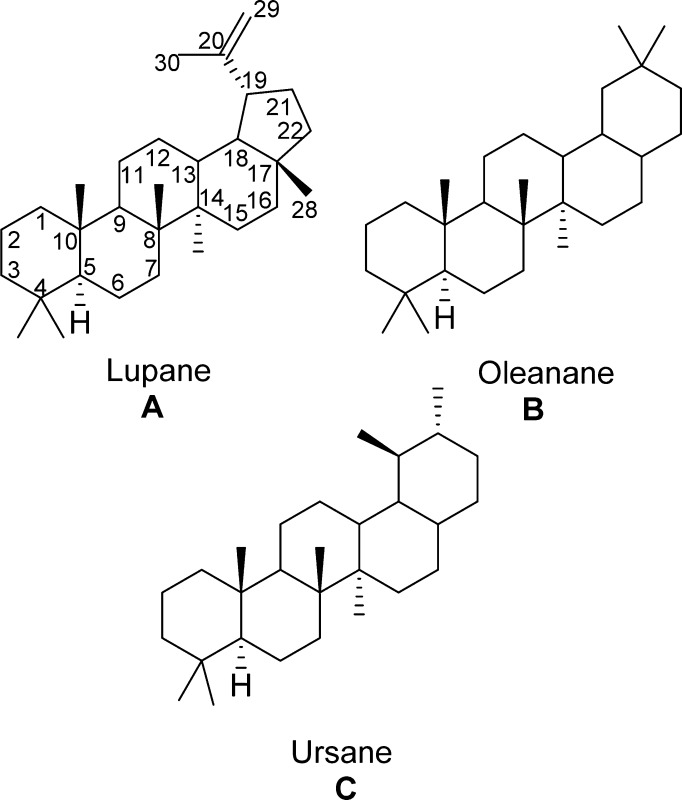
Nature’s infinite ability in biosynthesis productivity and the promise of the discovery of new anticancer drugs inspired the NCI in September 1957 to advance a carefully targeted research program (CCNSC) for the discovery and development of important, new naturally occurring and synthetic drugs for improving human cancer treatments. That same month was the start of our group’s immediate commitment to the discovery of new anticancer drugs derived from natural products and the beginning of our continuing collaboration with the NCI. The first such collaborative research was aimed at evaluating species of the Labiatae family, one of several known at the time to be most frequently used in traditional cancer treatments.6
In that early period we were also evaluating other plant constituents such as those from the white birch (Betula papyrifera) bark. We pursued the lupanes (A), the betulin/betulinic acid series7 from birch bark, and the 11-ene oleanane (B)/oleanolic acid and the 11-ene ursole (C)/ursolic acid from salvia species.6 However significant anticancer activity was not detected using the then current NCI evaluation system (generally murine in vivo Walker 256 sarcoma and/or leukemia L1210). Subsequently a large number of advances in both triterpenoid chemistry2 and cancer cell growth inhibition evaluations have shown the above early triterpene leads to have cancer cell growth inhibitory activity. Indeed triterpenes representing type A2,3 (including betulinic acid) are now in human cancer clinical trials,4 and B8 and C9 have generated considerable scientific and medicinal interest due to the increasing diverse types of biological activities, which now include antiviral.10
Cephalostatin 1 (2) was first
isolated by our group in 1988 from the Indian Ocean marine worm Cephalodiscus gilchristi.5a−5c The natural product
is a unique bis-steroid, biosynthetically constructed by the organism
in an unsymmetrical coupling of two C27 steroidal units
by way of the C-2/C-3 pyrazine ring. The first total synthesis of
this molecule was achieved in 1998.11 In
order to advance our ongoing structure–activity relationship
investigations of this promising anticancer lead, we attempted the
synthesis of pyrazino-bis-lupane 3 in order to further
explore structure–activity boundaries.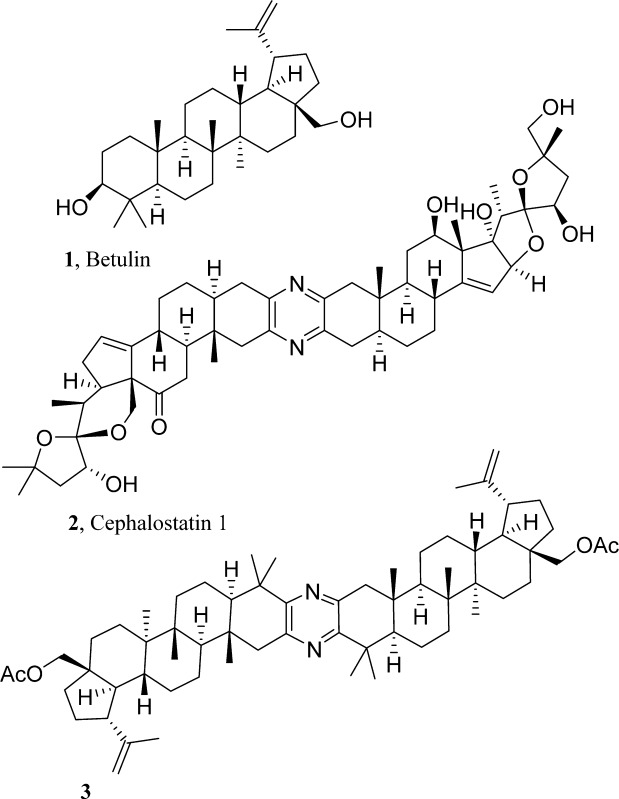
Results and Discussion
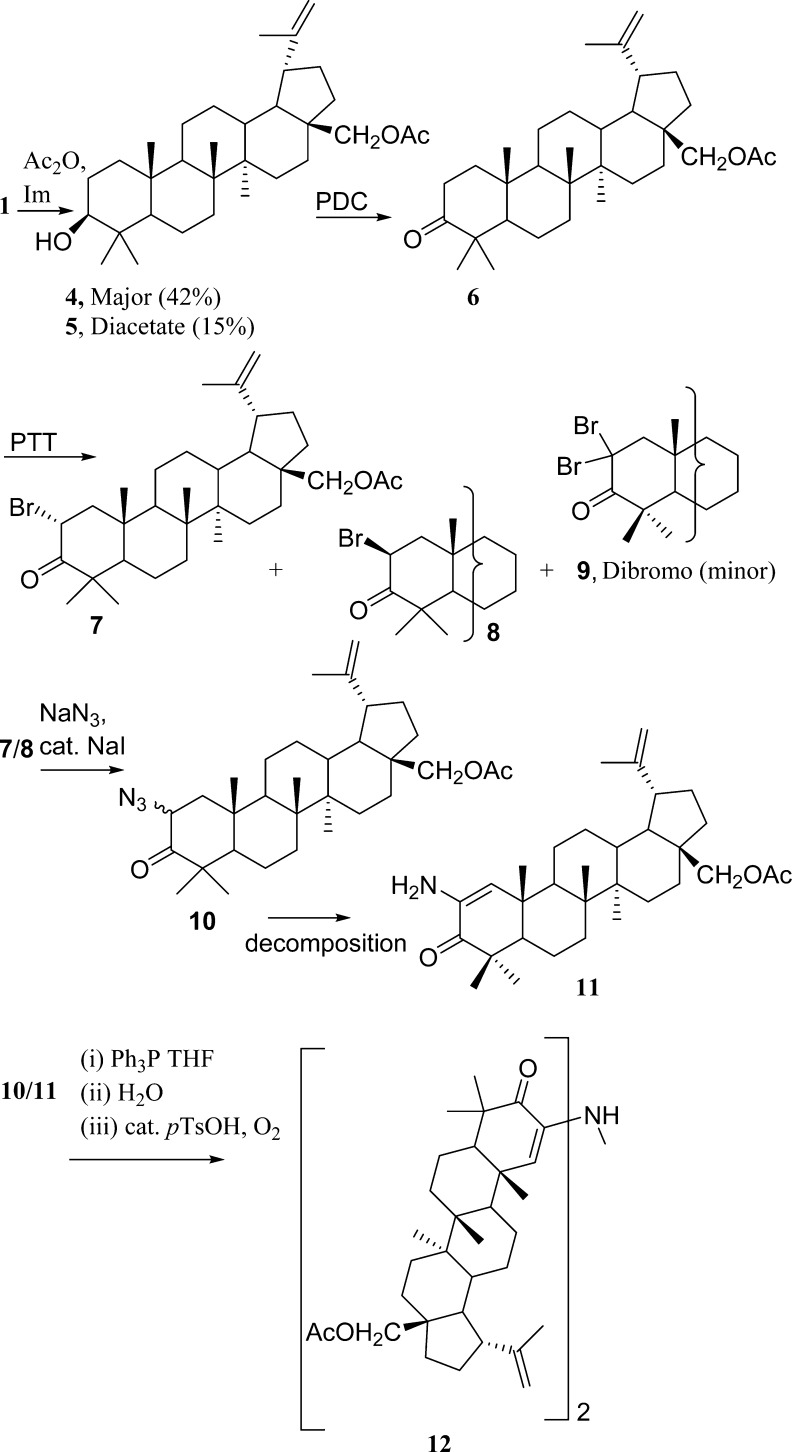
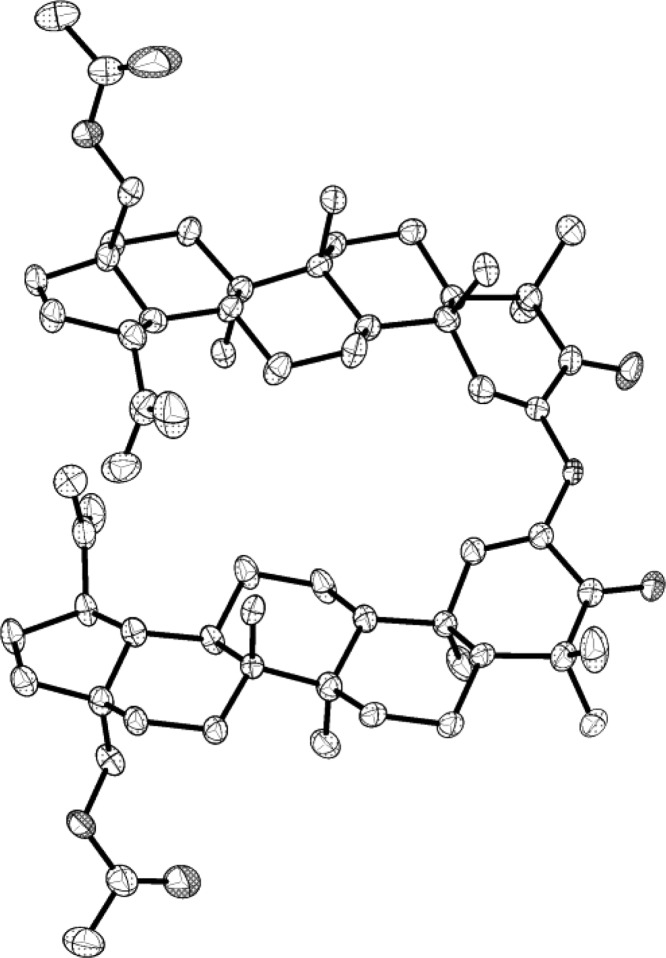
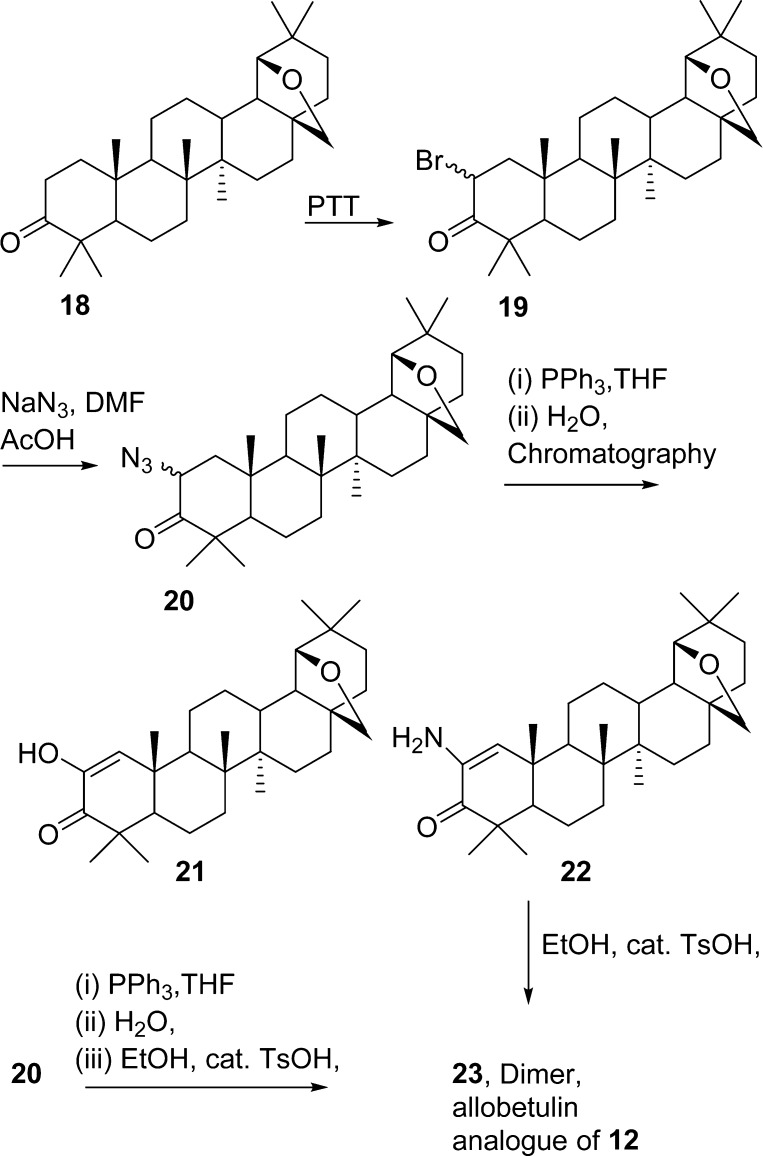
The first approach pictured a reductive dimerization of 2-azido ketone 10. However the synthesis and isolation of azide 10 proved difficult, as it decomposed rapidly to the enamine 11, which, in turn, provided the novel betulin dimer 12 (Scheme 1). The dimer was crystallized, and the structure elucidated using X-ray crystallography (Figure 1). Both the dimer and its precursors were evaluated against murine and human cancer cell lines (Table 1). The absence of significant cancer cell growth inhibition with 12 caused by the presumed rotation around the secondary amine bridge of dimer 12 suggests a critical need for a strictly rigid pyrazine skeleton. However, our and others’ experience probing for a route to structurally simple steroid pyrazine dimers based on the cephalostatins indicates a need for more closely approximating the cephalostatin E and F ring complexity.12
Scheme 1.
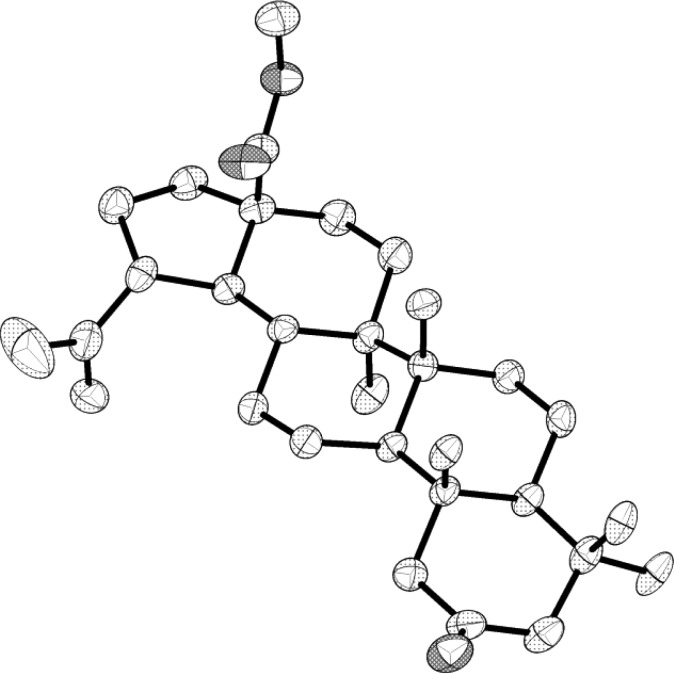
Figure 1.
X-ray crystal structure of dimer 12.18 Hydrogen atoms and labels have been omitted for clarity. Thermal ellipsoids are shown at the 50% probability level.
Table 1. Murine and Human Cancer Cell Line Data [ED50 and GI50, μg/mL].
| cell linea |
|||||||
|---|---|---|---|---|---|---|---|
| compound no. | P388 | BXPC-3 | MCF-7 | SF-268 | NCI-H460 | KM20L2 | DU-145 |
| 1 | 9.3 | >10 | >10 | 7.4 | >10 | >10 | |
| 4 | 0.805 | 4.1 | 3.0 | 4.0 | 2.5 | 5.0 | 7.6 |
| 5 | 0.806 | >10 | >10 | >10 | >10 | >10 | >10 |
| 6 | 0.12 | >10 | 8.0 | 10.6 | 5.2 | 12.7 | >10 |
| 7 | 0.725 | 3.1 | 4.1 | 10.1 | 12.8 | 9.6 | 9.0 |
| 8 | >10 | 3.4 | 3.2 | 3.4 | 2.8 | 4.4 | 6.1 |
| 9 | 0.391 | 1.0 | 2.5 | 4.4 | 4.4 | 4.2 | 3.8 |
| 10 | 6.6 | 5.0 | >10 | 4.4 | 4.2 | 5.7 | |
| 11 | >10 | 7.9 | >10 | >10 | >10 | >10 | >10 |
| 12 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| 13/14 | >10 | 5.6 | 4.8 | 7.0 | 6.2 | 5.1 | 8.8 |
| 14 | >10 | 9.1 | 5.8 | 6.1 | 8.0 | 5.9 | >10 |
| 15/16 | >10 | 3.4 | 3.8 | 4.2 | 3.2 | 4.0 | 9.4 |
| 17 | 14.5 | 11.2 | >10 | >10 | >10 | >10 | |
| 17a | 1.9 | 2.0 | 6.9 | 2.8 | 4.9 | 5.8 | |
| 18 | >10 | >10 | >10 | >10 | >10 | >10 | |
| 19 | 3.1 | 5.0 | 3.4 | 2.9 | 2.7 | 6.7 | |
| 20 | 3.1 | 4.7 | 8.7 | 3.8 | 7.9 | 4.5 | |
| 21 | >10 | 7.1 | >10 | 8.8 | 8.0 | 9.3 | |
| 22 | 10.8 | 11.2 | >10 | >10 | >10 | >10 | |
| 23 | >10 | >10 | >10 | >10 | >10 | >10 | |
| 24 | >10 | 2.4 | 2.7 | 2.7 | 2.3 | 2.2 | 2.3 |
| 25 | >10 | 8.2 | 11.6 | >10 | 13.1 | >10 | >10 |
| 26 | 3.9 | 6.1 | >10 | 4.4 | >10 | >10 | |
Cancer cell lines in order: murine lymphocytic leukemia (P388); lung (NCI-H460); colon (KM20L2); prostate (DU-145); pancreas (BXPC-3); breast (MCF-7); CNS (SF-268).
Acetylation of the primary hydroxy group of 1 yielded the monoacetate 28-O-acetylbetulin 4(13) as the major product (42%) and the diacetate 5 as a minor component (15%). The compounds were separated using silica gel column chromatography (SGC, DCM). Oxidation of 4(14) using pyridinium dichromate gave the ketone 6 in good yield (92%). Bromination of 6(14) using phenyltrimethylammonium tribromide yielded a mixture of 2α- and 2β-bromo ketones 7/8 (57/31%), which was separated by SGC. Upon scale-up, the dibromo derivative 9 was also isolated (15%). The dibromo derivative was unstable upon standing and decomposed over time.
Treatment of the bromo ketone (7/8) mixture with excess NaN3 in DMF and a catalytic amount of NaI afforded the azide 10, which rapidly decomposed to the enamine 11.14 The enamine was also unstable and decomposed over some time to an orange resin, which contained several dimers, detected by mass spectroscopic analysis. The 2-azido ketone 10 was not isolated. If the reaction was carried out at room temperature (rt) with 1.3 equivalents of NaN3, then a 2α/β-azido ketone mixture was isolated but decomposed in air to a red solid mixture. When the 2-bromo ketone was treated with NaN3 on a small scale (30 mg) in either DMF or N-methyl-2-pyrrolidine (NMP) using a catalytic amount of HOAc,15a−15c the 2α/β-azido ketone mixture 10 was obtained along with a minor amount of enamine 11. Upon scale-up, 11 was again the major product and decomposition of the material to a red solid mixture was observed.
When tetramethylguanidinium azide (TMGA)11 in acetonitrile was used instead of NaN3, the result was a mixture of the α-azido ketone (10), the enamine (11), and the starting bromide.
Owing to the rapid decomposition of the azido ketone 10 to the enamine 11, reaction products (obtained from the reaction of 7 with TMGA/DMF/CH3CN or with NaN3/DMF) were immediately allowed to react without further purification using the Staudinger reaction16 with Ph3P in the hope of forming the iminophosphorane from any azide remaining in the product mixtures. The phosphorane would then be hydrolyzed in aqueous THF with the prospect of obtaining the 2-amino ketone, which was expected to spontaneously dimerize and undergo autoxidation to give pyrazine 3, as is routinely observed for α-amino ketones under these conditions.15c,17
No pyrazines were isolated using this method from either reaction mixture. When the experiment was carried out with epimers 7/8 using the NaN3/DMF/NaI method, again the reaction products were subjected directly to the Staudinger reaction. After air oxidation in EtOH with a catalytic amount of p-toluenesulfonic acid, a bright yellow, amorphous powder precipitated following several days of stirring at rt. The precipitate was recrystallized from DCM/MeOH to yield crystals of 12, whose structure was confirmed by X-ray crystallography. This product was not obtained when enamine 11 was stirred in EtOH and a catalytic amount of p-toluenesulfonic acid for several days at room temperature.
Hydrogenation of the azido/enamine mixtures was also performed using 10% Pd/C in a mixture of MeOH and EtOAc containing a catalytic amount of glacial HOAc.19 Again, the reaction did not yield pyrazine 3. In the event that amine products were present in this unresolved mixture, a catalytic amount of p-toluenesulfonic acid in EtOH was employed to promote dimerization, but with no effect.
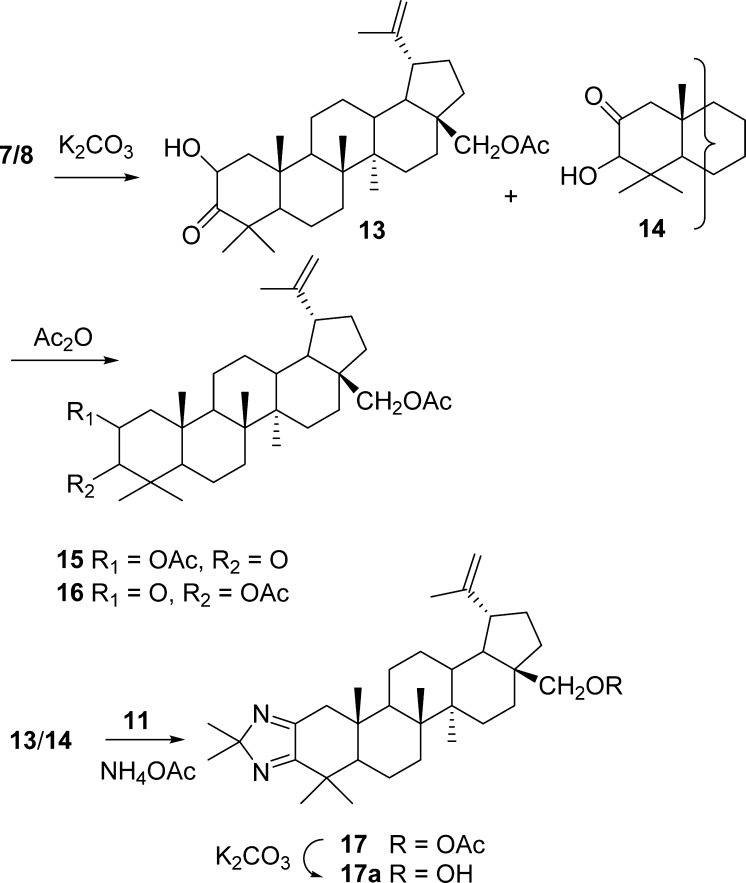
The route to the pyrazine via the unstable azide 10 was unsuccessful, and a further attempt at obtaining 3 using a different approach involved condensation of 11 with 2-hydroxy ketone 13 was pursued as outlined in Scheme 2.20a,20b The preparation and purification of 2α-hydroxy ketone 13 again presented difficulties and was obtained as the major isomer accompanied by the 2-oxo-3-hydroxy derivative 14. The α-orientation of the C-2 hydroxy group of 13 was assigned based on the coupling constants J1α,2β = 13 Hz and J1β,2β = 6 Hz. SGC [DCM/acetone (1.5%)] of the 13/14 regiomeric mixture gave an inseparable mixture in a ratio of 50:50. Further attempts at purification of this mixture by chromatography on silica gel eluting with hexanes/EtOAc (4:1) gave a compound that was slow to elute and was found to be the 2-oxo-3-hydroxy-28-O-acetylbetulin derivative 14. The 2-hydroxy ketone 13 was not isolated and may have decomposed on the column. Acetylation of the hydroxy ketone (13/14) mixture in order to simplify separation gave a mixture of acetylated products 15/16. That served to confirm the 2-hydroxy/3-hydroxy ketone mixture as the starting material and was further established by deshielded H-2 and H-3 resonances due to the C-2 and C-3 acetoxy groups, with the coupling constants for the H-2β resonance [J1α,2β = 13 Hz and J1β,2β = 6 Hz] of 15 augmented by the H-3 singlet in compound 16. The expected absence of the two hydroxy resonances in the 1H NMR spectrum and the presence of two O-acetyl resonances at δ 2.18 and 2.14 were observed.
Scheme 2.
The enamine (11) was then allowed to react with the hydroxy ketone mixture 13/14 in MeOH/DCM in the presence of NH4OAc employing controlled addition of the hydroxy ketone mixture to prevent its homodimerization. The products were separated by SGC to yield another mixture of dimers (by MS analysis) that resisted separation, along with compound 17, whose structure was proposed based on 1D NMR (APT) and 2D NMR (HSQC, COSY, HMBC) analysis. Deprotection of the C-28 acetate group with K2CO3 gave the alcohol with MS and NMR data supporting structure 17a. Clearly, the considerable steric constraints caused by the C-4 gem-dimethyl group in a 1,3 relationship with the C-10 methyl group imposed a series of synthesis challenges.
Finally, in order to eliminate any unpredicted steric and/or unwanted chemical interactions from the betulin E-ring olefin and 28-OAc functionalities, preparation of 2-azido-3-oxoallobetulin 20 was undertaken from 2α-bromo-3-oxoallobetulin 19 using published methods21 (Scheme 3). However this method gave inconsistent results. A mixture containing only enamino ketones was obtained in a repeat experiment and used in the azide reduction procedure with triphenylphosphine. Chromatographic separation of the products did not yield dimers, and the products were identified as the enolic ketone 21 and the enamine 22.
Scheme 3.
When the products from the preparation of 20 were not separated by column chromatography but taken directly to the reduction step followed by air oxidation in EtOH with a catalytic amount of p-toluenesulfonic acid, an amorphous powder precipitated in low yield following a six-day stirring period. The solid precipitate showed an MS ion at m/z = 890 corresponding to dimer 23, presumed to be the allobetulin analogue of 12, and was submitted for biological evaluation. The dimer 23 was also obtained in low yield when the enamine 22 was stirred for 2 days in EtOH with a catalytic amount of p-toluenesulfonic acid. Hydrogenation of the enamine (22) over 10% Pd/C gave a polar mixture of products that resisted separation.
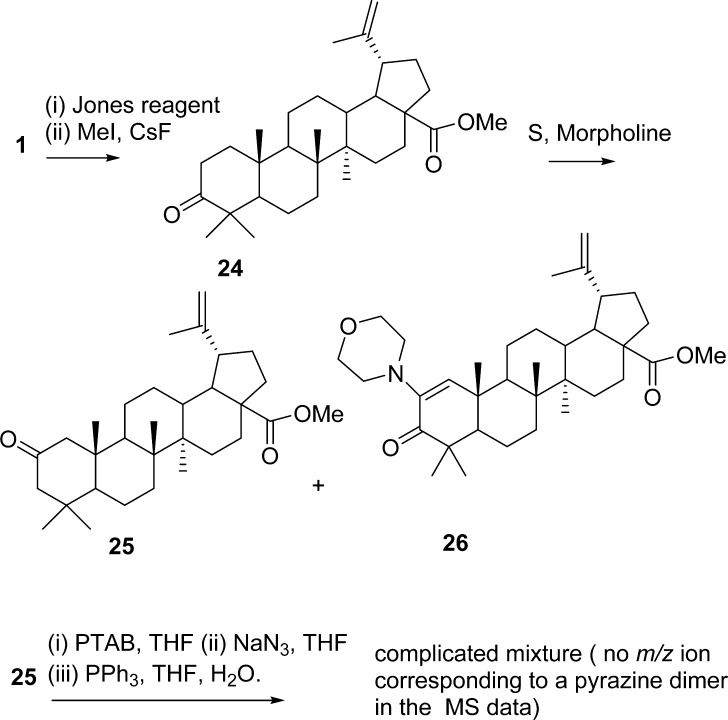
In a continued effort to moderate possible steric effects presented by the C-4 dimethyl group, the synthesis of the 2-oxo-methyl ester 25 was performed (Scheme 4) using the Willgerodt–Kindler reaction22 and was isolated along with the 2-morpholino-3-oxo derivative 26 from ketone 24.23 The structure of 25 was elucidated using X-ray crystallography on crystals obtained by recrystallization from MeOH (Figure 2). Bromination of 25 yielded a mixture of brominated products, and treatment with NaN3 followed by azide reduction with PPh3 in THF gave complex mixtures. Preliminary evaluation of such mixtures using MS data and NMR analysis indicated that this route did not yield any pyrazines and the synthetic methods utilized lacked the necessary practicality to be refined.
Scheme 4.
Figure 2.
X-ray crystal structure of ketone 25.18 Hydrogen atoms and labels have been omitted for clarity. Thermal ellipsoids are shown at the 50% probability level.
All compounds prepared in the study were examined for cancer cell growth inhibition (Table 1) against a series of murine and human cancer cell lines. The cancer cell growth inhibition of betulin improves upon acetylation at C-28 (4). However diacetate 5 was considered inactive. When the monoacetate 4 was oxidized to the C-3 oxo derivative (6), a decrease in activity against some cell lines resulted. Bromination at C-2 (7/8) caused a return to the modest cancer cell growth activity seen for the parent molecule. The β-bromo isomer gave slightly higher activity when compared to the α-isomer. Bromination at C-2 was previously shown to improve the activity of certain triterpenoid derivatives.24 The enamine 10 and the dimer 12 proved to be inactive.
In summary, attempts at the synthesis of a bis-pyrazine-lupane using betulin, instead, led to a new lupane dimer (12). The 2-azido ketone 10 was difficult to prepare and was obtained as part of a mixture containing the slightly more stable enamino ketone 11 or 22. A series of methods aimed at dimerization and hence pyrazine formation of a 10/11 mixture failed to yield the target pyrazines. The dimer 12 was the only pure compound isolated from the reaction product mixtures when 10/11 was allowed to react in an aza-Wittig-type reaction followed by air oxidation. That suggests the bulky C-4 gem-dimethyl group and the 1,3 steric relationship with the axial C-10 methyl group interfered with the formation of a pyrazine dimer. Similar compounds lacking the C-4 gem-dimethyl group readily dimerize under these conditions to give pyrazines. However, the specialized chemical considerations learned here will be useful to our future research in this field.
Experimental Section
General Experimental Procedures
Reagents and anhydrous solvents were purchased from Acros Organics (Fisher Scientific) and Sigma-Aldrich Chemical Co. and were used as received. For thin-layer chromatography, Analtech silica gel GHLF Uniplates were used and visualized with short-wave UV irradiation and use of an iodine chamber or a 10% H2SO4/EtOH dip followed by heating. Solvent extracts of aqueous solutions were dried over MgSO4. For column chromatography, silica gel (230–400 mesh ASTM) from E. Merck (Darmstadt, Germany) was used. Melting points are uncorrected and were determined with a Fisher–Johns melting point apparatus. Optical rotations were measured by use of a Perkin-Elmer 241 polarimeter, and the [α]D values are given in 10–1 deg cm2 g–1. 1H and 13 C NMR spectra were recorded on Varian Unity INOVA 400 and 500 instruments with deuterated solvents. HRMS were obtained with a Jeol JMS-LCmate mass spectrometer. Elemental analyses were determined by Galbraith Laboratories, Inc. The X-ray crystal structure data were obtained on a Bruker APEX2 CCD diffractometer using Mo Kα (0.71073 Å) radiation.
Betulin (1)
Small quantities of betulin (1) were purchased from Sigma-Aldrich, and larger quantities were isolated25 from the bark of birch trees (Betula papyrifera), collected in the state of Maine by extraction with hot toluene (80 °C) for 3 h. The crude extract was washed with aqueous 5% K2CO3, dried (MgSO4), and recrystallized from hot toluene to yield 1 as a colorless solid: Rf = 0.11 (DCM); mp 252–255 °C; lit.13 mp 250–252 °C; 1H and 13C NMR data were consistent with those reported.26a,26b
28-O-Acetylbetulin (4) and 3,28-Di-O-acetylbetulin (5)
A solution of betulin (1) (1.0 g, 2.3 mmol), Ac2O (12 mL, 0.124 mol, excess), and imidazole (0.35 g, 4.6 mmol) in CHCl3 (40 mL) was heated under reflux (61–62 °C) until TLC (DCM) showed product development was almost complete (∼2 h). The reaction mixture was cooled to room temperature and washed with 10% HCl, (10 mL), H2O (10 mL), and brine (10 mL) and dried (Na2SO4), and the solvent was removed under reduced pressure. TLC indicated the presence of three compounds. Separation was achieved using SGC with DCM as the eluent to give 3,28-di-O-acetylbetulin 5 (0.16 g, 15%): Rf 0.5 (DCM); mp 228–229 °C, lit.13 mp 216–218 °C; [α]20D +15 (c 0.4, CHCl3), lit.13 [α]20D +20 (c 1.67, CHCl3); 1H and 13 C NMR spectroscopic data of 5 were in agreement with published data;26a,26c HREIMS m/z 526.3999 (calcd for C34H54O4, 526.4022); 28-O-acetylbetulin (4) (0.454g, 42%); Rf 0.2 (DCM); mp 210–212 °C, lit.13 mp 210–212 °C; [α]20D +6 (c 0.4, CHCl3), lit.13 [α]D20 +8.5 (c 1.58, CHCl3); 1H and 13C NMR spectroscopic data of 4 were in agreement with published data;26c13C NMR (CDCl3, 100 MHz) δ 171.6, 150.1, 109.8, 78.9, 62.8, 55.3, 50.3, 48.7, 47.7, 46.3, 42.7, 40.8, 38.8, 38.7, 37.5, 37.1, 34.5, 34.2, 29.7, 29.5, 27.9, 27.4, 27.0, 25.2, 21.0, 20.8, 19.1, 18.3, 16.1, 16.0, 15.4, 14.7, and betulin (1) (0.117 g, 11%).
3-Oxo-28-O-acetylbetulin (6)
The monoacetate 4 (0.75 g, 1.55 mmol) was dissolved in dry DCM (40 mL) and stirred under N2 at room temperature. Pyridinium dichromate (0.77g, 1.99 mmol) was added. The mixture was a bright orange color initially, which darkened over time. The reaction was allowed to proceed for a total of 20 h, and at this point starting material was present as a faint spot by TLC (DCM). The mixture was filtered through silica gel and eluted with Et2O (500 mL). The Et2O layer was concentrated to a colorless foam solid (0.73 g, 98%). Further purification by SGC (eluent: DCM) gave the ketone 6 as a colorless solid: mp 81–82 °C (0.69 g, 92%); Rf 0.41 (DCM); lit.27a mp 117–119 °C; [α]20D +29 (c 0.2, CHCl3), lit.27a [α]20D +38 (c 1.35, CHCl3); 1H and 13C NMR spectroscopic data of 6 were in agreement with published data;27b HREIMS m/z 482.3779 (calcd for C32H50O3, 482.3760); anal. C 74.04, H 10.32%, calcd for C32H50O3, C 79.62, H 10.44%.
2α/β-Bromo-3-oxo-28-O-acetylbetulin 7/8 and 2,2-Dibromo-3-oxo-28-O-acetylbetulin 9
A solution of 6 (0.10g, 0.2 mmol) in anhydrous THF (5 mL) was stirred at 0 °C under N2. Phenyltrimethylammonium tribromide (0.085 g, 0.25 mmol, 1.3 equiv) was dissolved in THF (5 mL) and cooled to 0 °C before adding to the solution of the starting material in THF. The mixture was stirred for 2 h, quenched with brine, and diluted with EtOAc. The organic layer was separated and washed with brine followed by H2O, dried (Na2SO4), filtered, and concentrated to a colorless oil. TLC showed two products when compared to the starting material in DCM, and these were separated by flash SGC (50 g, column size 40 × 1 1/2 cm, elution rate: 1 mL/0.5 min). 2α-Bromo-3-oxo-28-O-acetylbetulin (7) (0.066g, 57%): mp 114 °C; Rf (DCM) 0.38; 1H NMR (CDCl3, 400 MHz) δ 5.05 (1H, dd, J = 13.4, 6.2 Hz, H-2), 4.67, 4.58 (each 1H, nm, H-29), 4.24, 3.82 (each 1H, d, J = 12 Hz, H-28), 2.62 (1H, dd, J = 12.8, 6.0 Hz, H-2 e), 2.44 (1H, ddd, J = 11, 5.5 Hz, H-19), 2.07 (3H, s, OCOCH3), 1.66, 1.17, 1.11, 1.07, 1.06, 0.95 (each 3H, s, CH3), 1.97–1.00 (CH, CH2); 13C NMR (CDCl3, 100 MHz) δ 207.0, 171.6, 149.9, 110.1, 62.7, 56.6, 52.7, 52.6, 49.7, 49.3, 48.6, 47.6, 46.2, 42.8, 41.0, 39.9, 37.5, 34.5, 33.7, 29.6, 29.5, 27.0, 26.2, 24.9, 21.7, 21.0, 19.2, 19.1, 16.0, 15.9, 14.6; HREIMS m/z 560.2889 (calcd for C32H49BrO3, 560.2865); anal. C 67.48, H 8.70%, calcd for C32H49BrO3, C 68.43, H 8.79 %.
2β-Bromo-3-oxo-28-O-acetylbetulin (8) (0.036 g, 31%): Rf (DCM) 0.28; 1H NMR (400 MHz, CDCl3) δ 5.02 (1H, t, J = 10.2 Hz, H-2), 4.61, 4.52 (each 1H, s, H-29), 4.17, 3.77 (each 1H, d, J = 11 Hz, H-28), 2.34–2.42 (2H, m, 2H, H-1e, H-19), 2.00(3H, s,OCOCH3), 1.61 (3H, s, CH3), 1.05 (6H, s, 2 × CH3), 0.95 (6H, s, 2 × CH3), 0.89–1.99 (CH, CH2), 0.72 (3H, s, CH3); 13C NMR (CDCl3, 100 MHz) δ 209.0, 171.5, 149.8, 110.00, 62.6, 54.0, 52.0, 51.4, 49.6, 48.6, 47.5, 47.4, 46.2, 42.7, 40.8, 39.2, 37.8, 34.5, 32.6, 29.6, 29.5, 29.2, 26.9, 25.1, 21.9, 21.0, 20.0, 19.8, 19.1, 18.8, 15.3, 14.6. On scale-up the 2,2-dibromo derivative 9 was also isolated (19% yield): mp 112–115 °C; Rf (DCM) 0.43; HREIMS m/z 638.1945 (calcd for C32H48Br2O3, 638.1971); 1H NMR (CDCl3, 400 MHz) δ 4.69, 4.60 (each 1H, s, H-29), 4.25, 3.85 (each 1H, d, J = 8 Hz, H-28), 3.62, 3.11 (each 1H, d, J = 16 Hz, H-1), 2.47 (1H, m, H-19), 2.08 (3H, s, OCOCH3), 1.69 (3H, s, CH3), 1.23 (6H, s, 2 × CH3), 1.05, 1.01, 0.92 (each 3H, s, CH3); HREIMS m/z 638.1945 (calcd for C32H48Br2O3, 638.1971).
2α/β-Azido-3-oxo-28-O-acetylbetulin, 10
To a solution of the 2α/β-bromo ketone (7/8) mixture (0.03 g, 0.05 mmol) in DMF (1 mL) were added HOAc (0.01 mL) and NaN3 (0.02 g, 0.31 mmol, 6.2 equiv). The reaction mixture was stirred at rt for 1 h. Iced water was added, and the precipitate was collected by filtration and dried under vacuum to afford 20 mg (74% yield) of the α/β isomers with a minor amount of 11 as an impurity. Column chromatography was carried out using a gradient elution of hexanes/EtOAc (6:1), affording the 2α/β-azido-3-oxo-28-O-acetylbetulin mixture (10) as a colorless oil, which solidified on standing (8 mg, 28% yield): 1H NMR (CDCl3, 400 Hz) δ 4.67, 4.57 (each 2H, nm, H-29), 4.19–4.26 (4H, m, H-28a, H-2α, H-2β), 3.82 (2H, m, H-28b), 2.42(1H, m, H-19), 2.27 (1H, dd, J = 12, 6 Hz,), 2.06 (6H, s, OCOCH3), 1.67, 166, 1.124, 1.12, 1.11, 1.08, 1.07, 1.01, 1.00, 0.95 (s, 12 CH3); HRMS (APCI) + m/z 524.3856 [M + H]+ (calcd for C32H50N3O3, 524.3852).
The bulk of the product mixture decomposed on the column as a red band appearing upon loading the material. The remaining fractions contained mixtures (7 mg) and 2,3-dioxo-28-O-acetylbetulin (5 mg, 6%). Some of the baseline material was removed with DCM/MeOH 1% to yield a yellow residue, 15 mg.
2-Enamino-3-oxo-28-O-acetylbetulin (11)
To a solution of the α/β isomers bromo-3-oxo-28-O-acetylbetulin (7/8) (0.6 g, 1.18 mmol) in anhydrous DMF (60 mL) under N2 were added NaN3 (0.84g, 13 mmol, 11 equiv) and a catalytic amount of NaI. The suspension was heated to 50 °C for 1 h. The reaction was terminated by the addition of H2O (100 mL). The aqueous fraction was extracted with EtOAc/Et2O (1:2, 2 × 200 mL). The combined organic extract was washed with H2O and brine (Na2SO4) and concentrated under vacuum to yield a yellow solid (0.44 g, 88%): mp decomposes at 115 °C; 1H NMR (CDCl3, 400 MHz) δ 6.15 (1H, s, H-2), 4.70, 4.61 (each 1H, s, H-29), 4.25, 3.85 (each 1H, d, J = 12 Hz, H-28), 3.38 (2H, bs, NH2), 2.45 (1H, ddd, J = 11, 6 Hz, H-19), 2.07 (3H, s, OCOCH3), 1.69, 1.15, 1.09, 1.08, 1.06, 0.97 (each 3H, s, 6 × CH3), 1.0–2.0 (CH, CH2); 13C NMR (CDCl3, 100 MHz) δ 201.2, 171.6, 150.0, 135.8, 129.0, 110.0, 62.8, 53.6, 48.7, 47.6, 46.3, 45.6, 44.2, 43.0, 41.6, 38.0, 37.7, 34.5, 33.8, 29.7, 29.5, 27.9, 27.0, 25.2, 21.74, 21.1, 21.1, 20.4, 19.1, 19.0, 16.4, 14.7; HREIMS m/z 495.3721 (calcd for C32H49NO3, 495.3712).
Further purification using gravity SGC, eluting with hexanes/EtOAc (8:2), caused decomposition of the enamine 11, and the enolic ketone (NMR and MS data) was obtained in some of the fractions. Attempts at recrystallization resulted in decomposition of 11, and when stored for longer periods NMR analysis indicated decomposition to complex mixtures.
1,2, 1′,2′-Ene, 2,2′-Bisamino-3,3′-oxo-28,28′-O-acetylbetulin 12
To a solution of 2α/β-bromo-3-oxo-28-O-acetylbetulin (7/8) (0.68 g, 1.2 mmol) in anhydrous DMF (40 mL) under N2 were added NaN3 (1.0 g, 15.6 mmol, 13 equiv) and a catalytic amount of NaI. The mixture was stirred at 50 °C under nitrogen for 2 h. The solvent was evaporated under reduced pressure, keeping the solution at 43–45 °C using an oil bath. When the mixture was completely dry, CHCl3 (50 mL) was added and the organic solution was washed with brine (2 × 20 mL), dried (Na2SO4), and concentrated to yield 11, which was stored under vacuum and taken directly to the next step. To a solution of enamine 11 in anhydrous THF (20 mL) was added Ph3P (1.0 g, 3.81 mmol, 3 equiv). The yellow-colored solution was stirred under N2 at rt for 24 h, then heated at 40 °C for 22 h. Water (0.5 mL) was added, and the reaction mixture was stirred at rt for 24 h, then concentrated and dried on a vacuum pump to remove H2O. The yellow oil was taken up in EtOH (20 mL), and an immediate precipitate was observed upon stirring at rt. p-Toluenesulfonic acid was added (cat. amount), and the yellow mixture was stirred at rt for 3 days and monitored by TLC. The reaction mixture was concentrated to minimum volume, and a fine precipitate developed, which was filtered through Celite and washed with CH3Cl and EtOAc. The combined washings and mother liquor were concentrated and purified (gradient elution; hexanes/EtOAc, 9:1 → EtOAc 100%) to give the main fraction as a yellow powder (0.153 g, 27%). Two successive recrystallizations from DCM/MeOH afforded crystals of 12 suitable for X-ray crystallography: mp 205 °C, [α]20D −39 (c 0.2, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 6.37 (3H, m, H-1, H-1′, N–H), 4.70, 4.62 (each 2H, s, H-29, H-29′), 4.27, 3.83 (each 2H, d, J = 12 Hz, H-28, H-28′), 2.43 (2H, ddd, J = 10, 6 Hz, H-19, H-19′), 2.06 (6H, s, 2 × OCOCH3), 2.0–1.03 (CH, CH2), 1.69 (6H, s, 2 × CH3), 1.14 (6H, s, 2 × CH3), 1.09 (18 H, s, 6 × CH3), 0.96 (6H, s, 2 × CH3). 13C NMR (CDCl3, 100 MHz) δ 200.8, 171.7, 149.9, 133.6, 132.4, 110.0, 62.8, 53.0, 48.7, 47.5, 46.38, 46.0, 44.4, 43.1, 41.7, 38.3, 37.8, 34.6, 33.9, 29.8, 29.7, 27.9, 27.0, 25.3, 21.8, 21.3, 21.1, 20.6, 19.4, 18.9, 16.5, 14.8; HRMS (APCI+) m/z 974.7530 [M + H+] (calcd for C64H96NO6, 974.7232); anal. C 72.66, H 9.23, N 1.42%, calcd for C64H95NO6·CH2Cl2, C 73.69; H 9.23, N, 1.32%. This compound was stored in the dark, as it is light sensitive and goes from bright yellow to dark orange with prolonged exposure to light.
X-ray Crystal Structure of 12
a. Data Collection
A clear, pale yellow, plate-like specimen (0.08 mm × 0.32 mm × 0.36 mm) of 12 grown from a DCM/MeOH solution was mounted on the end of a thin glass fiber using Apiezon type N grease and optically centered. Cell parameter measurements and data collection were performed at 123 ± 1 K with a Bruker APEX2 CCD diffractometer using Mo Kα (0.71073 Å) radiation. A sphere of reciprocal space was collected using the Multi-Run scheme available in the Bruker APEX228a data collection package. Three sets of frames were obtained, with each set using 0.50°/frame steps to cover 182° in ω at fixed φ values of 0°, 120°, and 240°, respectively, for a total of 1092 frames. Data were integrated and reduced using Bruker SAINT,28b resulting in 99.9% coverage of all unique reflections to a resolution of 0.830 Å with an average redundancy of 2.27.
b. Crystal Data
C64H97NO6, fw = 976.43, monoclinic, P21, a = 13.5315(6) Å, b = 16.3954(7) Å, c = 14.1737(6) Å, β = 106.5240(10)°, V = 3014.6(2) Å3, Z = 2, ρc = 1.076 Mg m–3, μ(Mo Kα) = 0.067 mm–1, λ = 0.710 73 Å, F(000) = 1072.
c. Structure Solution and Refinement
A total of 25 001 reflections were measured, of which 11 004 were independent (Rint = 0.0209) and 10 215 were considered observed (Iobs > 2σ(I)). Final unit cell parameters were determined by least-squares fit of 9928 reflections covering the resolution range of 8.280 to 0.800 Å. Data were corrected for absorption effects using the multiscan method (SADABS).28c The calculated minimum and maximum transmission coefficients (based on crystal size) are 0.9763 and 0.9947. Statistical analysis with the XPREP program in SHELXTL28d indicated the space group was P21. Routine direct methods structure solution was performed with SHELXTL, which produced the majority of non-hydrogen atom coordinates on the single 12 molecule in the asymmetric unit. Upon initial refinement with SHELXTL, the solvent in the voids of the asymmetric unit became discernible in the Fourier difference map. However, subsequent refinement steps were unable to resolve the electron density into chemically reasonable solvent molecules. Thus, application of the solvent removal utility in PLATON SQUEEZE28e to the integrated data produced the solvent-free data set that was used to finalize the refinement. After full isotropic refinement was completed, the thermal parameters for all non-hydrogen atoms were then allowed to refine anisotropically. Subsequently, hydrogen atoms were added at idealized positions, and bond distances with their isotropic thermal parameters were fixed at 1.5 times the Uiso values of their bonding partners for the methyl hydrogens and 1.2 times the Uiso values of their bonding partners for all others. The model was allowed to refine with hydrogens constrained as atoms riding on their bonding partners. This resulted in a final standard residual R1 value of 0.0521 for observed data and 0.0552 for all data. Goodness of fit on F2 was 1.041, and the weighted residual on F2, wR2, was 0.1399 for observed data and 0.1421 for all data. The final Fourier difference map showed minimal electron density, with the largest difference peak and hole having values of 0.320 and −0.236 electron Å–3, respectively. Final bond distances and angles were all within expected and acceptable limits for the 12 molecule.
2-Hydroxy-3-oxo-28-O-acetylbetulin (13) and 2-Oxo-3-hydroxy-28-O-acetylbetulin (14)
To a mixture of 2α/β-bromo-3-oxo-28-O-acetylbetulin (7/8 1.0 g, 1.78 mmol) in 83% aqueous acetone (249 mL) was added a solution of K2CO3 (0.25 g, 1.81 mmol, ∼1 equiv) in H2O (51 mL).20b The reaction mixture was heated under reflux for 24 h. The initially cloudy mixture became clear at high temperatures, and TLC (DCM/acetone 2%) showed all starting material had reacted after 24 h. The cooled solution was concentrated to half volume, and a 1% aqueous HCL solution was added until the pH was neutral (52 mL). The mixture was extracted with Et2O (3 × 80 mL), washed with 5% NaHCO3 (2 × 50 mL) and H2O (1 × 50 mL), dried (Na2SO4), concentrated, and redried under vacuum (1.0 g). NMR analysis of the crude sample showed the major product as 2-α-hydroxy-3-oxo-28-O-acetylbetulin compound 13, with 14 as a minor component. However once chromatography was completed, the NMR indicated a 50:50 mixture of the alcohols 13/14. The crude product was therefore taken forward in the reaction with the enamino ketone to miminize the number of potential products formed.
SGC: column size (23 × 4 cm); eluent DCM/acetone 1.5%; collected at 20 mL × 1.5 min. Material eluted in fractions 28–42 was concentrated to a colorless solid of ∼1 g. Analysis by NMR indicated a 50:50 mixture of 13/14. Further attempts at purification of this mixture by chromatography on silica gel eluting with hexanes/EtOAc (4:1) gave a compound that was slow to elute and was found to be the 2-oxo-3-hydroxy-28-O-acetylbetulin derivative 14, as the only compound isolated. NMR assignments were made by comparing the data with the crude material isolated initially, which was enriched with the 2-hydroxy-3-ketone and the pure 2-oxo-3-hydroxy derivative.
2-α-Hydroxy-3-oxo-28-O-acetylbetulin (13):
1H NMR (CDCl3, 400 MHz) δ 4.68, 4.59 (each 1H, s, H-29), 4.52 (1H, ddd, J = 13, 6, 4 Hz, H-2), 4.23 (1H, m, H-28), 3.84 (m, 1H, H-28), 3.55 (1H, d, J = 4 Hz, OH-2, D2O exchange experiment), 2.39–2.46 (m, 2H, H-19, H-1e), 2.07 (s, 3H, OCOCH3,), 2.0–0.9 (CH, CH2), 1.66 (s, 3H, H-30) 1.16, 1.13, 1.09, 1.08, 0.94 (each 3H, s, CH3); 13C NMR (CDCl3, 100 MHz) 216.7, 171.7, 149.9, 110.1, 69.6, 62.7, 57.8, 53.9 (2C), 50.0, 49.9, 48.7, 47.7, 46.3, 42.8, 42.4, 41.0, 37.9, 37.5, 34.0, 31.7, 27.0, 24.9, 24.5, 21.3, 21.1, 20.99, 19.1, 16.6, 16.2, 14.6.
2-Oxo-3-hydroxy-28-O-acetylbetulin (14):
1H NMR (CDCl3, 400 MHz) δ 4.68, 4.59 (each 1H, s, H-29), 4.23 (1H, d, J = 11 Hz, H-28), 3.84 (3H, m, H-28, H-3), 3.42 (1H, d, J = 4.8 Hz, OH-3, D2O exchange experiment), 2.50 (d, 1H, J = 12.4 Hz, H-1e), 2.39–2.46 (1H, m, H-19), 2.07 (s, 3H, OAc), 2.0–0.9 (CH, CH2), 1.67 (s, 3H, H-30), 1.25, 1.12, 0.96, 0.79, 0.65 (each 3H, s, CH3); 13C NMR (CDCl3, 100 MHz) 211.4, 171.7, 149.9, 110.0, 83.0, 62.7, 54.5, 53.5, 51.0, 50.3, 48.6, 47.6, 46.3, 45.5, 43.9, 42.8, 41.3, 37.4, 34.5, 34.0, 29.6, 29.5, 29.2, 27.0, 24.9, 21.0, 20.9, 18.4, 16.9, 16.3, 15.6, 14.7; HRMS (APCI+) m/z 499.3751 (M + H)+ (calcd for C32H51O4, 499.3787).
The mixture of alcohols was acetylated for further characterization.
2-O-Acetyl/3-O-acetyl-3/2-oxo- (15/16)
To a solution of the alcohols 13/14 (0.21 g, 0.42 mmol) in pyridine was added Ac2O (2 mL), and the colorless solution stirred at rt overnight under a drying tube. TLC (hexanes/EtOAc, 4:1) showed complete conversion to product, which was one spot by TLC. Iced H2O (50 mL) was added, and a white gelatinous precipitate was observed. A mixture of petroleum ether/EtOAc/Et2O (2:2:1, 50 mL) was added. The mixture was stirred until clear. The organic fraction was extracted from the aqueous layer and dried (MgSO4), filtered, and concentrated to a colorless glass (0.1 g), which was found to be a 50:50 mixture of acetylated products 15/16: 1H NMR (CDCl3, 400 MHz) δ 5.60 (1H, dd, J = 10, 6 Hz, H-2, 15), 4.93 (1H, s, H-3 16), 4.70 (s, 2H, H-29 15/16), 4.60 (m, 2H, H-29 15/16), 4.26 (2H, d, J = 11 Hz, H-28 15/16), 3.85 (1H, d, J = 11 Hz 15/16), 2.48–2.42 (3H, m, H-1e (16), H-19, 15/16), 2.22 (1H, dd, J = 13, 6 Hz, H-1e 15), 2.18, 2.14 (each 3H, s, C-2/3-OCOCH3, 15/16), 2.08 (6H, s, C-28-OCOCH3, 15/16), 2.3–1.0 (m, CH, CH2), 1.69, 1.68, 1.21, 1.13, 1.11, 1.10, 1.09, 1.04, 1.02, 0.96, 0.84, 0.83 (each 3H, s, CH3, 15/16) ppm; 13C NMR (CDCl3, 100 MHz) δ 209.3, 204.5, 171.6 (171.5), 170.5, 170.1, 149.9 (149.8), 110.0 (109.9), 84.0 (C-3, 16), 71.8 (C-2, 15), 62.7 (62.6), 57.2, 55.4, 57.2, 50.1 (50.04), 48.64 (48.62), 48.60, 47.6, 46.3 (46.2), 46.0, 43.5 (43.3), 42.78 (42.74), 41.2, 40.9, 38.1, 37.4 (37.39), 34.5, 33.8, 33.7, 30.9, 29.6, 29.5, 29.46 (29.46), 28.8, 27.1, 26.9, 24.9 (24.87), 24.7, 21.0 (3C), 20.9, 20.7, 20.6, 19.1, 19.0, 18.97, 18.4, 17.3, 16.8, 16.5, 16.2, 15.7, 14.7, 14.6; HRMS (APCI+) m/z 541.3898 [M + H]+ (calcd for C34H53O5 541.3893).
Condensation of the Enamine 11 and the Hydroxy Ketones 13 and 14 (ref (20a))
To a two-necked round-bottom flask fitted with a dropping funnel and a reflux condenser was added enamine 11 (0.33 g, 0.66 mmol) followed by NH4OAc (0.2 g, 2.6 mmol, 4 equiv) and MeOH (20 mL). The mixture was heated at reflux for 1 h to ensure dissolution, and the 2/3-hydroxy ketone 13/14 mixture (0.33 g, 0.66 mmol) in MeOH (0.5 mL) was added dropwise to the reaction mixture. The solution was heated at reflux for 48 h under N2 and monitored by TLC. The reaction mixture was cooled, quenched with H2O, and extracted with DCM. The organic layer was washed with brine, followed by H2O, and dried (MgSO4). Concentration led to a yellow solid mixture (0.41g, ∼60% yield) separated by flash SGC gradient elution (hexanes/EtOAc, 7:3 → 5:5, column size 25 × 4 cm). Examination of the fractions by NMR and MS showed the first fraction contained a dimer with a molecular ion at m/z 974 (0.059 g), which was not purified further, the second was enamine 11 (0.10 g), and a third fraction was concentrated to a colorless solid, imidazole derivative 17: 0.08 g; mp 185–190 °C; Rf = 0.24 (hexanes/EtOAc, 1:1); [α]20D +24 (c 0.1, CHCl3); [α]20D +33 (c 0.34, CH3Cl); 1H NMR (CDCl3, 400 MHz) δ 4.67, 4.58 (each 1 H, s, H-29), 4.22, 3.82 (1H, d, J = 11 Hz, H-28), 2.96 (1H, d, J = 16 Hz, H-1e), 2.42 (1H, ddd, J = 11, 6 Hz, H-19), 2.07 (s, 3H, OCOCH3), 1.99 (1H, d, J = 16 Hz, H-1a), 1.9–1.0 (CH, CH2), 1.66, 1.39, 1.36, 1.24, 1.19, 1.05, 0.97, 0.77 (each 3H, s, CH3); 13C NMR (CDCl3, 100 MHz) δ 173.5 (C-3), 171.6 (C-31, OCOCH3), 164.8 (C-2), 150.0 (C-20), 110.1(C-29) 102.0 (C-33, (CH3)2C), 62.8 (C-28), 53.3 (C-5), 48.7 (C-18), 48.4 (C-9), 47.7 (C-19), 46.4 (C-17), 42.83 (C-14), 42.81 (C-1), 40.9 (C-8), 38.7 (C-10), 37.7 (C-13), 36.3 (C-4), 34.6 (C-22), 33.1 (C-7), 30.7 (C-24), 29.7 (C-21), 29.68 (C-16), 27.2 (C-15), 25.3 (C-12), 24.6 (C-23), 24.1 (C-35, CH3C), 23.8 (C-34, CH3C), 21.4 (C-11), 21.1 (OCOCH3), 19.8 (C-6), 19.3 (C-30), 16.5 (C-25), 15.6 (C-26), 14.7 (C-27); HRMS (APCI+) m/z 535.4262 [M + H]+ (calcd for C35H55N2O2, 535.4264).
Deacetylation of Compound 17
To a solution of 17 (0.07g, 0.13 mmol) in aqueous MeOH 88% (30 mL) and DCM (3 mL) was added K2CO3 (0.056 g, 4.05 mmol), and the mixture stirred for 2 days at rt and monitored by TLC (DCM/MeOH 2%, starting material Rf = 0.38 product, Rf = 0.28). After 48 h, H2O/DCM, 50:50 (40 mL), was added, and the organic layer was separated, dried (MgSO4), and concentrated in vacuo. The resulting yellow glass (36 mg) was purified by flash column chromatography on silica gel to provide 17a as a colorless solid, which was recrystallized from MeOH (16 mg, 44% yield): mp 288 °C; [α]20D +35 (c 0.2, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 4.70, 4.61 (each 1H, s, H-29), 3.80, 3.35 (each 1H, d, J = 8 Hz, H-28), 3.0 (1H, d, J = 16 Hz, H-1e), 2.39 (m, H-19), 1.99 (1H, d, J = 16 Hz, H-1a), 2.01–1.00 (CH, CH2), 1.69, 1.42, 1.39, 1.28, 1.23, 1.07, 1.01, 0.77 (each 3H, s, CH3); HRMS (APCI+) m/z 493.4107 [M + H]+ (calcd for C33H53N2O, 493.4158).
2-Bromoallobetulone 19.
(29) The solid acid montmorillonite clay K10 was used to carry out the rearrangement of betulin to allobetulin according to a procedure reported previously:30 mp 271 °C (colorless crystals from DCM/MeOH 95%); [α]20D +40 (c 0.2, CHCl3) (lit.30 mp 266–268 °C); [α]20D +65 (c 0.3, CHCl3). Allobetulin was oxidized with pyridinium dichromate to yield the keto derivative 18 (allobetulone) with mp 210–212 °C; [α]20D +68 (c 0.3, CHCl3) (lit.27a 229–231 °C; [α]22D +86 (c 1, CHCl3). A solution of the ketone 18 (0.5 g, 1.14 mmol) in dry THF was cooled to 0 °C in an ice bath, and an ice cold solution of PTAB (0.45 g, 0.119 mmol, 1.05 equiv) in THF (15 mL) was added in one batch. The mixture became colorless in approximately 5 min, and after 10 min stirring brine (30 mL) was added. The mixture was extracted with DCM (20 mL × 2) and washed with H2O (20 mL × 2), and the organic phase was dried (MgSO4) and concentrated to a white foam solid (0.566 g). Examination of the solid by 1D and 2D NMR showed the major product in the mixture of epimers 19 was the 2α-bromo ketone. Recrystallization from CH3Cl/MeOH gave colorless needles of 2α-bromoallobetulone: mp 231–233 °C; [α]20D +49 (c 0.2, CHCl3) (lit.29 mp 234–235 °C, [α]20D +35 (c 3, CHCl3); 1H NMR data for the crystalline mixture of 2α/β-bromo ketones (CDCl3, 400 MHz) δ 5.15–5.06 (2H, m, H-2α/β), 3.72, 3.43 (each 2H, d, H-28), 3.51 (2H, s, H-19), 2.66 (1H, dd, J = 13, 6 Hz, H-1e (2α-Br)), 2.46 (1H, m, H-1e (2β-Br)), 2.06 (1H, m, H-1a, (2β-Br)), 1.7 (1H, t, J = 13 Hz, H-1a (2α-Br)), 1.7–0.7 (CH, CH2), 1.18, 1.12, 1.07, 1.00, 0.92, 0.89, 0.78 (each 6H, CH3).
2-Azido-3-oxoallobetulone 20 (ref (21))
Sodium azide (0.375 g, 5.85 mmol, 6 equiv) was added to a solution of 2-bromo-3-oxoallobetulone (19) (0.5 g, 0.96 mmol) in NMP (10 mL) and Ac2O (0.5 mL). After stirring at rt under N2 for 22 h, the reaction was quenched by the addition of iced H2O, and the resulting yellow precipitate was collected by filtration and dried under vacuum. Column chromatography (SGC) afforded 20 (150 mg, 54%); 1H NMR (CDCl3, 400 MHz) δ 4.30–4.22 (2H, m, H-2α, H-2β), 3.75, 3.43 (each 2H, d, H-28 α/β), 3.51 (s, 2H, H-19 α/β), 2.34 (1H, dd, H-1), 2.17 (1H, t, J = 12 Hz, H-1), 1.7–0.8 (CH2), 1.14, 1.13, 1.12, 1.09 1.07, 1.01, 0.95, 0.93, 0.92 (2 × CH3), 0.89, 0.79, 0.78, 0.77 (each 3H, α/β CH3); 13C NMR corresponded with reported data;21 HRMS (APCI+) m/z 482.3755 (M + H)+ (calcd for C30H48N3O2, 482.3747).
Reaction of 20 with PPh3/H2O
To a stirred solution of the azide 20 (0.150 g, 0.31 mmol) in dry THF (4 mL) was added a THF (1 mL) solution of PPh3 (0.175 g, 0.66 mmol). The light yellow solution was stirred for 17 h, with no evolution of N2 observed. TLC (successive development used, hexanes 100% followed by hexanes/EtOAc, 9:1) showed all the azide had reacted and the presence of more polar products. H2O (0.125 mL) was added, and the reaction was stirred for a further 24 h. The reaction mixture was concentrated, and the crude yellow solid was purified using column chromatography (gradient elution; hexanes 100% → hexanes/EtOAc, 8:2) to afford the 2,3-diketone 21 (36 mg, 25%) [Rf = 0.58 (PhCH3/EtOAc 20%); 1H NMR data for 21 were in agreement with reported data]27a and the enamine 22 (34 mg, 24%), which was recrystallized from DCM and MeOH: mp 153 °C; [α]20D +21 (c 0.11, CHCl3); 1H NMR (CHCl3, 400 MHz) δ 6.19 (1H, s, H-1), 3.75 (1H, d, J = 8 Hz, H-28), 3.51 (1H, s, H-19), 3.43 (1H, d, J = 8 Hz, H-28), 1.72–1.0.7 (ring CH2), 1.14, 1.08, 1.06, 1.00, 0.91, 0.89, 0.78 (each 3H, s, 7 × CH3).; HRMS (APCI+) m/z 454.3690 (M + H)+ (calcd for C30H48NO2 454.3685).
Conversion of 20 into Dimer 23
2-Azido-3-oxoallobetulone (20) (0.05 g, 0.104 mmol) was dissolved in dry THF (2 mL), and PPh3 (0.05 g, 0.19 mmol, 1.8 equiv) was added. The reaction was stirred at rt for 1 h before H2O (0.05 mL) was added, and stirring was continued overnight. The mixture was concentrated and dried under high vacuum. EtOH (2 mL) and pTsOH (3.5 mg) were added, and the reaction was stirred at rt for 6 days. The precipitate was filtered and dried to yield dimer 23 as a yellow solid in 7% yield. Compound 23: mp chars >300 °C; [α]20D +47 (c 0.1, CHCl3); 1H NMR (CDCl3, 400 MHz) δ 7.13 (1H, s), 6.47 (1H, s), 3.76 (2H, d, J = 8.8 Hz, H-28), 3.48 (4H, m, H-19, H-28), 1.8–0.78 (CH,CH2), 1.16, 1.12, 1.10, 1.04, 0.94 (2 × CH3), 0.82 (s, CH3); HSMS (APCI+) m/z 890.7042 (M + H)+ (100% of base) (calcd for C60H92NO4 890.7026).
Methyl 2-Oxo-28-betulonate 25
Betulonic acid was prepared from betulin (1) and had the following physical properties: mp 235–239 °C (MeOH); [α]20D +25 (c 0.4, MeOH) (lit.31 mp 247–249 °C, lit.32 mp 250–254 °C, [α]20D +32 (c 0.4)). Methylation afforded methyl betulonate (24): mp 140–142 °C, [α]20D +21 (c 0.5, MeOH) (lit.32 mp 161–165 °C, [α]20D +28 (c 0.4) according to the literature methods.31,33 To a solution of 24 (1 g, 2.14 mmol) in morpholine (20 mL) was added sulfur (0.75 g, 23.4 mmol, 11 equiv). The reaction mixture was heated at reflux for 3 h. The morpholine was removed by fractional distillation into a collection flask immersed in a (dry ice/ispropyl alcohol) cooling bath. The resulting dark brown residue was dissolved in DCM (80 mL) and washed with aqueous 10% HCl (5 × 20 mL). The organic layer was washed with H2O (30 mL), and the mixture was stirred overnight (∼20 h). The phases were separated, and the organic fraction was dried (MgSO4) and concentrated to a crude solid (2 g). Purification using SGC (hexanes/Et2O, 6:1) gave 25 as a foam (0.27 g, 25% yield, crystallized from EtOH): mp 147–148 °C; [α]20D +15 (c 1.25, MeOH); 1H NMR δ 4.71, 4.57 (each 1H, s, H-29), 3.63 (3H, s, −COOCH3), 2.95 (1H, ddd, J = 10.5, 4 Hz, H-19), 2.35 (1H, dd, J = 12, 2 Hz, H-1e), 2.24–2.15 (3H, m), 2.10 (1H, dd, J = 12, 2, Hz, H-3e), (CH, CH2), 1.65, 1.00, 0.97, 0.88, 0.83, 0.80 (each 3H, s, CH3); 13C NMR (CDCl3, 400 MHz) δ 212.3, 176.6, 150.3, 109.7, 56.5, 56.45, 56.1, 55.7, 51.3, 50.2, 49.3, 46.9, 43.0, 42.5, 41.1, 39.0, 38.1, 36.9, 33.8, 33.3, 32.1, 30.6, 29.7, 25.3, 23.1, 21.0, 19.4, 18.9, 17.2, 15.6, 14.7; HRMS (APCI+) m/z 469.3678 (M + H)+ (calcd for C31H48O3, 469.3682).
X-ray Crystal Structure of 25
a. Data Collection
A clear, colorless, plate-like crystal (0.07 mm × 0.22 mm × 0.34 mm) of 25 grown from a MeOH solution was mounted on the end of a thin glass fiber using Apiezon type N grease and optically centered. Cell parameter measurements and data collection were performed at 123 ± 1 K with a Bruker APEX2 CCD diffractometer using Mo Kα (0.71073 Å) radiation. A sphere of reciprocal space was collected using the Multi-Run scheme available in the Bruker APEX228a data collection package. Three sets of frames were obtained, with each set using 0.50°/frame steps to cover 182° in ω at fixed φ values of 0°, 120°, and 240°, respectively, for a total of 1092 frames. Data were integrated and reduced using Bruker SAINT,28b resulting in 99.9% coverage of all unique reflections to a resolution of 0.830 Å with an average redundancy of 4.485.
b. Crystal Data
C31H48O3, fw = 468.69, orthorhombic, P212121, a = 9.3498(8) Å, b = 15.3827(14) Å, c = 18.6860(17) Å, V = 2687.5(4) Å3, Z = 4, ρc = 1.158 Mg m–3, μ(Mo Kα) = 0.072 mm–1, λ = 0.71073 Å, F(000) = 1032.
c. Structure Solution and Refinement
A total of 22 151 reflections were measured, of which 4939 were independent (Rint = 0.0501) and 4239 were considered observed (Iobs > 2σ(I)). Final unit cell parameters were determined by least-squares fit of 5455 reflections covering the resolution range of 9.343 to 0.897 Å. Data were corrected for absorption effects using the multiscan method (SADABS).28c The calculated minimum and maximum transmission coefficients (based on crystal size) are 0.9953 and 0.9756. Statistical analysis with the XPREP program in SHELXTL28d indicated the space group was P212121. Routine direct methods structure solution was performed with SHELXTL, which produced the majority of non-hydrogen atom coordinates on the single molecule of 25 in the asymmetric unit. The remaining non-hydrogen atoms were found in subsequent difference maps. After full isotropic refinement was completed, the thermal parameters for all non-hydrogen atoms were then allowed to refine anisotropically. Hydrogen atoms were added at idealized positions, and bond distances with their isotropic thermal parameters were fixed at 1.5 times the Uiso values of their bonding partners for the methyl hydrogens and 1.2 times the Uiso values of their bonding partners for all others. The model was then allowed to refine with hydrogens constrained as atoms riding on their bonding partners. This resulted in a final standard residual R1 value of 0.0526 for observed data and 0.0631 for all data. Goodness of fit on F2 was 1.055, and the weighted residual on F2, wR2, was 0.1338 for observed data and 0.1400 for all data. The final Fourier difference map showed minimal electron density, with the largest difference peak and hole having values of 0.328 and −0.201 electron Å–3, respectively. Final bond distances and angles were all within expected and acceptable limits for the 25 molecule.
Methyl 2-morpholino-3-oxo-28-betulonate 26
When morpholine was not removed by distillation and the reaction was stopped with the addition of water followed by extraction with DCM (20 mL), the reaction products differed. The organic layer was washed with water (10 mL), 10% HCl (10 mL), and saturated aqueous NaHCO3 (10 mL), dried, and concentrated to a crude solid. The reaction mixture was separated using SGC to give, along with 25, the morpholino derivative 26 as a colorless solid: mp 125 °C; [α]20D +76 (c 0.5, CHCl3); 1H NMR (400 MHz, CDCl3) δ 5.96 (1H, s, H-1), 4.74, 4.61 (each 1H, s, H-29), 3.77 (4H, m, 2 × CH2 morpholine), 3.65 (3H, s, OCH3), 2.99 (1H, m, H-19), 2.85 (2H, m, CH2 morpholine), 2.50 (2H, m, CH2 morpholine), 2.3–0.8 (CH, CH2), 1.69, 1.12, 1.02, 0.99, 0.96, 0.93 (each 3H, s, 6 × CH3); 13C NMR (CDCl3, 100 MHz) δ 201.6, 176.5,150.5, 144.6, 134.8, 109.7, 66.7(2C), 56.5, 52.2, 51.3, 49.4 (2C), 49.3, 46.9, 45.4, 45.2, 42.6, 41.3, 38.5, 38.1, 36.9, 33.5, 32.1, 30.5, 29.6, 29.1, 25.7, 21.7, 21.0, 20.5, 19.5, 19.4, 16.1, 14.6; HSMS (APCI +) m/z 552.4059 (M + H)+ (calcd for C35H54NO4 552.4053).
The remaining material from the column was recrystallized from EtOH/DCM to yield large yellow needle crystals, mp 274 °C, which when analyzed by NMR were found to be the side product dithioxalodimorpholide.33a,33b
Cancer Cell Line Procedures
Inhibition of human cancer cell growth was assessed using the NCI’s standard sulforhodamine B assay as previously described.34 Briefly, cells in a 5% fetal bovine serum/RPMI1640 medium were inoculated in 96-well plates and incubated for 24 h. Serial dilutions of the compounds were then added. After 48 h, the plates were fixed with trichloroacetic acid, stained with sulforhodamine B, and read with an automated microplate reader. A growth inhibition of 50% (GI50, or the drug concentration causing a 50% reduction in the net protein increase) was calculated from optical density data with Immunosoft software. In order to determine growth characteristics of cells being tested in the SRB assay and to ensure that the cells maintain a confluency of approximately 80%, in situ experiments were conducted for each of these lines. Also during the inoculation phase of the screening assays, cells were trypsinized, centrifuged, resuspended, and counted by either hemacytometer (human) or Coulter counter (mouse). The cells were then adjusted to the appropriate concentration.
Mouse leukemia P388 cells35 were incubated for 24 h in a 10% horse serum/Fisher medium followed by a 48 h incubation with serial dilutions of the compounds. Cell growth inhibition (ED50) was then calculated using a Z1 Beckman/Coulter particle counter.
Acknowledgments
We acknowledge the financial support from grants R01CA90441-01-05, 2R56CA090441-06A1, and 5-RO1CA90441-07-08 from the Division of Cancer Treatment and Diagnosis, NCI, DHHS; the Arizona Disease Control Research Commission; J. W. Kieckhefer Foundation; Margaret T. Morris Foundation; the Robert B. Dalton Endowment Fund; as well as Dr. William Crisp and Mrs. Anita Crisp. In addition we are very pleased to thank Prof. R. Dunlap and Mr. B. Deane as well as Hardwood Products Company LLC, Guilford, Maine, for providing scale-up amounts of state of Maine birch bark.
Supporting Information Available
X-ray crystallographic data for compounds 12 and 25 as well as copies of the 1H and 13C NMR spectra of compounds 11, 12, 17, 25, and 26. This material is available free of charge via the Internet at http://pubs.acs.org. Crystallographic data have been deposited with Cambridge Crystallographic Data Center as supplementary publication nos. CCDC 971646 (12) and CCDC 971647 (25). This can be obtained free of charge on application to Cambridge Crystallographic Data Center, 2 Union Rd, Cambridge CBZ 1EZ, UK [fax: (+44) 1223-336 033; e-mail: deposit@ccdc.cam.ac.uk].
The authors declare no competing financial interest.
Author Status
‡ In memoriam of Lee Williams, deceased September 3, 2013.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- For Antineoplastic Agents Part 594 refer to Hadimani M. B.; MacDonough M. T.; Strecker T. E.; Lopez R.; Sriram M.; Nguyen B. L.; Kessler R. J.; Ghatak A.; Shirali A. R.; Liu L.; Garner C. M.; Pettit G. R.; Hamel E.; Chaplin D. J.; Mason R. P.; Trawick M. L.; Pinney K. G. J. Nat. Prod. 2013, 7691668–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Krasutsky P. A. Nat. Prod. Rep. 2006, 23, 919–942. [DOI] [PubMed] [Google Scholar]; b Gautier G.; Legault J.; Piochon-Gauthier M.; Pichette A. Phytochem. Rev. 2011, 10, 521–544. [Google Scholar]; c Dahaen W.; Mashentseva A. A.; Seitembetov T. S. Molecules 2011, 16, 2443–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Cancer Etiology, Diagnosis and Treatments, Series 2010; Salvador J. A. R., Ed.; Nova Science Publishers, Inc.: New York, 2010. [Google Scholar]; e Connolly J. D.; Hill R. A. Nat. Prod. Rep. 2007, 24, 465–486. [DOI] [PubMed] [Google Scholar]
- a Csuk R.; Stark S.; Nitsche C.; Barthel A.; Siewert B. Eur. J. Med. Chem. 2012, 337–335. [DOI] [PubMed] [Google Scholar]; b Urban M.; Vlk M.; Dzubak P.; Hajduch M.; Sarek J. Bioorg. Med. Chem. 2012, 20, 3666–3674. [DOI] [PubMed] [Google Scholar]; c Qian K.; Kim S.-Y.; Hung H.-Y.; Huang L.; Chen C.-H.; Lee K.-H. Biol. Org. Med. Chem. Lett. 2011, 21, 5944–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Csuk R.; Barthel A.; Kluge R.; Ströhl D.; Kommera H.; Paschke R. Bioorg. Med. Chem. 2010, 18, 1344–1355. [DOI] [PubMed] [Google Scholar]; e Gauthier C.; Legault J.; Rondeau S.; Pichette A. Tetrahedron Lett. 2009, 50, 988–991. [Google Scholar]; f Vasilevsky S. F.; Govdi A. I.; Shults E. E.; Shakirov M. M.; Sorokina I. V.; Tolstikova T. G.; Baev D. S.; Tolstikov G. A.; Alabugin I. V. Biorg. Med. Chem. 2009, 17, 5164–5169. [DOI] [PubMed] [Google Scholar]; g Goff R. D.; Thorson J. S. Org. Lett. 2009, 11, 461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Koohang A.; Majewski N. D.; Szotek E. L.; Mar A. A.; Eiznhamer D. A.; Flavin M. T.; Xu Z.-Q. Bioorg. Med. Chem. Lett. 2009, 19, 2168–2171. [DOI] [PubMed] [Google Scholar]; i Gauthier C.; Legault J.; Piochon M.; Lavoie S.; Tremblay S.; Pichette A. Bioorg. Med. Chem. Lett. 2009, 19, 2310–2314. [DOI] [PubMed] [Google Scholar]; j Kumar V.; Rani N.; Aggarwal P.; Sanna V. K.; Singh A. T.; Jaggi M.; Joshi N.; Sharma P. K.; Irchhaiya R.; Burman A. C. Bioorg. Med. Chem. Lett. 2008, 18, 5058–5062. [DOI] [PubMed] [Google Scholar]
- Mullaer F. B.; Kessler J. H.; Medema J. P. Anti-Cancer Drugs 2010, 21, 215–227. [DOI] [PubMed] [Google Scholar]
- a Pettit G. R.; Inoue M.; Kamono Y.; Herald D.; Arm C.; Dufresne C.; Christie N.; Schmidtm J. M.; Doubek D. L.; Krupa T. S. J. Am. Chem. Soc. 1988, 110, 2006–2007. [Google Scholar]; b Pettit G. R.; Kamano Y.; Inoue M.; Dufresne C.; Boyd M. R.; Herald C. L.; Schmidt J. M.; Doubek D. L.; Christie N. L. J. Org. Chem. 1992, 57, 429–431. [Google Scholar]; c Pettit G. R.; Xu J.-P.; Schmidt J. M.; Boyd M. R. Bioorg. Med. Chem. Lett. 1995, 5172027–2032. [Google Scholar]
- Pettit G. R.; Klinger H.; Jorgensen N.-O. N. Phytochemistry 1966, 5, 301–309. [Google Scholar]
- Pettit G. R.; Green B.; Bowyer W. J. J. Org. Chem. 1961, 26, 2879. [Google Scholar]
- a Yuan W.; Wang P.; Deng G.; Li S. Phytochemistry 2012, 75, 67–77. [DOI] [PubMed] [Google Scholar]; b Yang H.; Cho H.-J.; Sim S. H.; Chung Y. K.; Kim D.-D.; Sung S. H.; Kim J.; Kim Y. C. Bioorg. Med. Chem. Lett. 2012, 22, 2079–2083. [DOI] [PubMed] [Google Scholar]; c Zhang H.; Samadi A. K.; Karumanchi V. R.; Cohen M. S.; Timmermann B. N. J. Nat. Prod. 2011, 74, 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Nhiem N. X.; Lim H. Y.; Kiem P. V.; Minh C. V.; Thu V. K.; Tai B. H.; Quang T. H.; Song S. B.; Kim Y. H. Bioorg. Med. Chem. Lett. 2011, 21, 6143–6147. [DOI] [PubMed] [Google Scholar]; e Wang X.-Y.; Chen X.-L.; Tang H.-F.; Gau H.; Tian X.-R.; Zhang P.-H. Planta Med. 2011, 77, 1550–1554. [DOI] [PubMed] [Google Scholar]; f Barros F. W. A.; Bandeira P. N.; Lima D. J. B.; Meira A. S.; de Farias S. S.; Albuquerque M. R. J. R.; dos Santos H. S.; Lemos T. L. G.; de Morais M. O.; Costa-Lotufo L. V.; do Ó Pessoa C. Bioorg. Med. Chem. 2011, 19, 1268–1276. [DOI] [PubMed] [Google Scholar]; g Oramos-Royo S. M.; Chávez H.; Martín-Rodríguez P.; Fernández-Pérez L.; Ravelo Á. G.; Estévez-Braun A. J. Nat. Prod. 2010, 73, 2029–2034. [DOI] [PubMed] [Google Scholar]; h Cusk R.; Schwarz S.; Kluge R.; Ströhl D. Eur. J. Med. Chem. 2010, 45, 5718–5723. [DOI] [PubMed] [Google Scholar]; i Huang X.-a.; Liang Y.-j.; Cai X.-l.; Feng X.-q.; Zhang C.-h.; Fu L.-w.; Deng W.-d. Bioorg. Med. Chem. Lett. 2009, 19, 6515–6518. [DOI] [PubMed] [Google Scholar]; j Cheng K.; Liu J.; Liu X.; Li H.; Sun H.; Xie J. Carbohydr. Res. 2009, 344, 841–850. [DOI] [PubMed] [Google Scholar]; k Li J.-F.; Zhao Y.; Cai M.-M.; Fei X.-F; Li J.-X. Eur. J. Med. Chem. 2009, 44, 2796–2806. [DOI] [PubMed] [Google Scholar]
- a Nhiem N. X.; Tai B. H.; Quang T. H.; Kiem P. V.; Minh C. V.; Nam N. H.; Kim J. H.; Im L.-R.; Lee Y.-M.; Kim Y. H. Bioorg. Med. Chem. Lett. 2011, 21, 1777–1781. [DOI] [PubMed] [Google Scholar]; b Csuk R.; Siewert B. Tetrahedron Lett. 2011, 52, 6616–6618. [Google Scholar]; c Shyu M.-H.; Kao T.-C.; Yen G.-C. J. Agric. Food Chem. 2010, 58, 6110–6118. [DOI] [PubMed] [Google Scholar]; d Kassi E.; Sourlingas T. G.; Spiliotaki M.; Papoutsi Z.; Pratsinis H.; Aligiannis N.; Moutsatsou P. Cancer Invest. 2009, 27, 723–733. [DOI] [PubMed] [Google Scholar]; e Meng Y.-Q.; Liu D.; Cai L.-L.; Chen H.; Cao B.; Wang Y.-Z. Bioorg. Med. Chem. 2009, 17, 848–854. [DOI] [PubMed] [Google Scholar]
- a Bori I. D.; Hung H.-Y.; Qian K.; Chen C. H.; Morris-Natschke S. L. Tetrahedron Lett. 2012, 53, 1987–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Xiong J.; Kashiwada Y.; Chen C.-C.; Qian K.; Morris-Natschke S. L.; Lee K.-H.; Takaishi Y. Bioorg. Med. Chem. 2010, 18, 6451–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Qian K.; Kuo R.-Y.; Chen C.-H.; Huang L.; Morris-Natschke S. L.; Lee K. H. J. Med. Chem. 2010, 53, 3133–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Kazakova O. B.; Giniyatullina G. V.; Yamansarov E. Y.; Tolstikov G. A. Bioorg. Med. Chem. Lett. 2010, 20, 4088–4090. [DOI] [PubMed] [Google Scholar]
- La Cour T. G.; Guo C.; Bhandaru S.; Boyd M. R.; Fuchs P. L. J. Am. Chem. Soc. 1998, 120, 692–707. [Google Scholar]
- a Pettit G. R.; Moser B. R.; Mendonca R.; Knight J. C.; Hogan F. J. Nat. Prod. 2012, 75, 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pettit G. R.; Mendonca R. F.; Knight J. C.; Pettit R. K. J. Nat. Prod. 2011, 74, 1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier C.; Legault J.; Bouchard Lebrun M. J.; Dufour P.; Pichette A. Bioorg. Med. Chem. 2006, 14, 6713–6725. [DOI] [PubMed] [Google Scholar]
- Drogemuller M.; Flessner T.; Jautelat R.; Scholz U.; Winterfeldt E. Eur. J. Org. Chem. 1998, 2811–2831. [Google Scholar]
- a Schonecker B.; Ponsold K. J. Prakt. Chem. 1971, 313, 817–824. [Google Scholar]; b Smith S. C.; Heathcock C. H. J. Org. Chem. 1992, 57, 6379–6380. [Google Scholar]; c Cerny I.; Pouzar V.; Budensinsky M.; Brasar P. Collect. Czech. Chem. Commun. 2000, 65, 1597–1608. [Google Scholar]
- Staudinger H.; Meyer J. Helv. Chim. Acta 1919, 2, 635–646. [Google Scholar]
- Lotowski Z.; Morzycki J. W.; Niewczas I. S.; Zdanowicz M. Collect. Czech. Chem. Commun. 2002, 67, 47–54. [Google Scholar]
- CCDC 971646 (12) and CCDC 971647 (25) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- Kramer A.; Ullmann U.; Winterfeldt E. J. Chem. Soc., Perkin Trans. 1 1993, 23, 2865–2867. [Google Scholar]
- a Haak E.; Winterfeldt E. Synlett. 2004, 8, 1414–1418. [Google Scholar]; b Numazawa M.; Nagaoka M. Steroids 1982, 393345–355. [DOI] [PubMed] [Google Scholar]
- Hajduch M.; Sarak J. Patent WO 2001090096 A2, 20011129.
- Sejbal J.; Klinot J.; Protiva J.; Vystrcil A. Collect. Czech. Chem. Commun. 1986, 51, 118–127. [Google Scholar]
- Deng Y.; Snyder J. K. J. Org. Chem. 2002, 67, 2864–2873. [DOI] [PubMed] [Google Scholar]
- Urban M.; Vlk M.; Dzubak P.; Hajduch M.; Sarek J. Bioorg. Med. Chem. 2012, 20, 3666–3674. [DOI] [PubMed] [Google Scholar]
- Bender C.; Sauter M. Ger. Patentschrift, DE 10204278 C1, 20030807, 2003.
- a Patra A.; Chaudhuri S. K.; Panda S. K. J. Nat. Prod. 1988, 51, 217–220. [Google Scholar]; b Tinto W.; Blair L. C.; Alli A. J. Nat. Prod. 1992, 55, 395–398. [Google Scholar]; c Hiroya K.; Takahashi T.; Miura N.; Naganuma A.; Sakamoto T. Bioorg. Med. Chem. 2002, 10, 3229–3236. [DOI] [PubMed] [Google Scholar]
- a Korovin A. V.; Tkachev A. V. Russ. Chem. Bull., Int. Ed. 2001, 50, 304–310. [Google Scholar]; b Ashavina O.; Flekhter O. B.; Galin F. Z.; Kabalnova N. N.; Baltina L. A.; Tolshkov G. A. Chem. Nat. Compd. 2003, 39, 207–211. [Google Scholar]
- a APEX2; Bruker AXS Inc.: Madison, WI, USA, 2010.; b SAINT-Plus; Bruker AXS Inc.: Madison, WI, USA, 2009.; c SADABS; Bruker AXS Inc.: Madison, WI, USA, 2008.; d SHELXTL: Sheldrick G. M. Acta Crystallogr. 2008, A64, 112–122. [DOI] [PubMed] [Google Scholar]; e PLATON SQUEEZE: Spek A. L. Acta Crystallogr. 2009, D65, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinot J.; Vystrcil A. Collect. Czech. Chem. Commun. 1966, 31, 1079–1092. [Google Scholar]
- Li T.-S.; Wang J.-X.; Zheng X.-J. J. Chem. Soc., Perkin Trans. 1 1998, 3957–3965. [Google Scholar]
- Kim D. S. H. L; Chen Z.; Nguyen V.-T; Pezzuto S. Q.; Lu Z.-Z. Synth. Commun. 1997, 2791607–1612. [Google Scholar]
- Urban M.; Sarek J.; Klinot J.; Korinkova K.; Hajduch M. J. Nat. Prod. 2004, 67, 1100–1105. [DOI] [PubMed] [Google Scholar]
- a Yu Y. P., Lin Y. Y.; Liu B. X.. Acta Crystallogr. Sect. E 2009, 65 (3), 0511. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Horton W. J.; Berghe J. V. D. J. Am. Chem. Soc. 1948, 7072425–2427. [Google Scholar]
- Monks A.; Scudiero D.; Skehan P.; Shoemaker R.; Paull K.; Vistica D.; Hose C.; Langley J.; Cronise P.; Viagro-Wolff A.; Gray-Goodrich M.; Campbell H.; Mayo J.; Boyd M. J. Natl. Cancer Inst. 1991, 83, 757–766. [DOI] [PubMed] [Google Scholar]
- Suffness M.; Douros J.. Methods in Cancer Research, Vol. XVIA; DeVita V. T. Jr.; Busch H., Eds.; Academic Press: New York, 1979; Vol. 16, pp 73–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.