Abstract
A recent study identified a haplotype on a small region of chromosome 12, between markers D12S1725 and D12S1596, shared by all patients with familial neuroblastoma (NB). We previously localized the human MGST1 gene, whose gene product protects against oxidative stress, to this very same chromosomal region (12p112.1–12p13.33). Due to the chromosomal location of MGST1, its roles in tumorigenesis, drug resistance and oxidative stress, and the known sensitivity of NB cell lines to oxidative stress, we considered a role for MGST1 in NB development. Surprisingly there was no detectable MGST1 mRNA or protein in either NB cell lines or NB primary tumor tissue, although all other human tissues, cell lines, and primary tumor tissue examined to date express MGST1 at high levels. The mechanism behind the failure of NB cells and tissue to express MGST1 mRNA is unknown, and involves failure of MGST1 pre-mRNA expression, but does not involve chromosomal rearrangement or nucleotide variation in the promoter, exons, or 3′-untranslated region of MGST1. MGST1 provides significant protection against oxidative stress and constitutes 4 to 6% of all protein in the outer membrane of the mitochondria. As NB cells are extremely sensitive to oxidative stress, and often used as a model system to investigate mitochondrial response to endogenous and exogenous stress, these findings may be due to the lack of expression MGST1 protein in NB. The significance of this finding to the development of neuroblastoma (familial or otherwise), however, is unknown and may even be incidental. While our studies provide a molecular basis for previous work on the sensitivity of NB cells to oxidative stress, and possibly marked variations in NB mitochondrial homeostasis, it also implies that the results of these earlier studies using NB cells are not transferable to other tumor and cell types which express MGST1 at high concentrations.
Keywords: MAPEG, microsomal glutathione transferase, MGST1, MGST2, MGST3, Neuroblastoma, Oxidative stress
Introduction
Neuroblastoma (NB) is the most frequent extracranial solid tumor of childhood and originates from the neural crest cells committed to the adrenal medulla and the sympathetic nervous system. Approximately half of all NB patients are diagnosed with high-risk disease that is characterized by low overall survival rates despite intensive multimodal chemotherapeutic treatment [1]. Molecular studies have identified various somatic alterations that are associated with aggressive disease including amplification of MYCN proto-oncogene [2] or deletions of chromosome 1p and gain of 17q [3]. The subset of NB patients with amplification of the MYCN oncogene, found in approximately 20% of metastasized tumors, have a very poor survival due to defects on pathways that engage or execute apoptosis [4].
Most cases of NB are sporadic and present as either localized or disseminated disease. Familial forms are rare and pedigree structures suggest a dominant mode of inheritance with low penetrance. Linkage studies have yielded various results for different families [5, 6]. A genome-wide linkage analysis was recently performed on a highly informative family including 5 patients affected with NB at different stages of the disease. Two haplotypes co-segregating with the disease, chromosome regions 2p and 12p, were identified with high lod-score values [7]. The haplotype on chromosome 12 and shared by all patients was at 12p112.1–12p13.33 and between markers D12S1725 and D12S1596.
The microsomal glutathione transferase-1 (MGST1) gene is a member of the Membrane-Associated Proteins in Eicosanoid and Glutathione (MAPEG) metabolism family of transmembrane proteins [8]. The MAPEG family is an ancient and diverse family thought to originate prior to the emergence of the cytosolic family of glutathione transferases [9, 10]. The MGST1 protein is ubiquitous in human tissue and cell lines, and is localized to the endoplasmic reticulum and outer mitochondrial membrane where it is thought to protect these membranes from oxidative stress [11]. MGST2 and MGST3, however are not involved in protection against oxidative stress but are involved in leukotriene production, notably the conjugation of LTA4 with glutathione. MAPEG members have also been identified in plants, fungi, and bacteria [12, 13]. The human MGST1 gene displays alternative splicing but all four transcript variants encode the same protein isoform [14]. MGST1 is upregulated in tumor cells and plays a role in development of anti-cancer drug resistance [15]. We previously localized the human MGST1 gene to chromosome 12 [14] in a region with the highest lod-score shared by patients with a familial form of NB [7]. Due to the chromosomal location of MGST1, its roles in tumorigenesis, drug resistance and oxidative stress, coupled with the sensitivity of NB cell lines to oxidative stress, we examined the expression of MGST1 in NB. Despite the ubiquitous nature of MGST1 in human tissue and other cell lines, neither NB cell lines nor NB primary tumor tissue, displayed MGST1 mRNA. These results may explain the sensitivity of NB cell lines, including reports of disruption of mitochondrial potential, to oxidative stress and suggest unique therapeutic opportunities of intervention may exist taking advantage of the fact that the MGST1 pathway is uniquely devoid in NB.
Materials and Methods
Cell lines
The NB5 and NB7 human neuroblastoma cell lines were obtained from Dr. Dwayne Stupack (Department of Pathology, UCSD). The SK-N-AS, SK-N-SH and IMR-32 human neuroblastoma cell lines, as well as all other cell lines, were obtained from the ATCC. All Neuroblastoma cell lines were maintained in RPMI-1640 (Mediatech, Herndon, VA) medium supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) plus 2 mM glutamine in an humidified 5% CO2 incubator at 37°C. The positive control cell line HepG2 obtained from ATCC was maintained in MEM (Mediatech, Herndon, VA) medium supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) plus 2 mM glutamine under the same conditions.
Primary Neuroblastoma Tissue Accrual and RNA preparation
Primary neuroblastoma samples were obtained from the Children’s Oncology Group (COG) Tumor Bank or the Cooperative Human Tissue Network (CHTN). Sample are fully encoded to protect patient confidentiality and are utilized under a UCSD approved IRB protocol (IRB #081062). RNA and protein were extracted from the primary tumors that were crushed over dry ice, lysed in Trizol® (Invitrogen, Carlsbad, CA) and extracted according to manufacturers’ instructions. All work described in this article was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Northern blots
Total RNA was extracted from cells by Trizol LS method (Invitrogen #10296-010) followed by poly A RNA purification with Oligotex mRNA Kit (Qiagen # 70022). Total samples were quantified by spectrophotometry and recovered. The mRNA was loaded onto Nytran SPC Nylon Transfer Membrane (Whatman # 10416285) with BRL Hybri-Slot Manifold apparatus followed by UV cross linking for 3 minutes in Stratalinker (Stratagene). Probes were either generated using PCR amplification or from restriction enzyme digests followed by electrophoresis through GenePure Gold agarose gel (ISC BioExpress # E-3142-125), and purified from the gel by the Qiaquick process (Qiagen). Probes were labeled by Prime-It RmT Random Primer Labeling Kit (Stratagene # 300392) with α32 Phosphorus-labeled dCTP (Perkin Elmer Life Sciences # BLU513Z), hybridized to the desired blot at 65°C overnight in Express Hybridization Buffer (Clontech # 636832) which was supplemented with salmon sperm DNA (Ambion # 9680), followed by washes with hybridization buffer, and imaging on STORM 860 phosphoimager (Molecular Dynamics).
Southern blots
Genomic DNA was extracted from cells by the Promega Wizard Genomic DNA purification method (Promega Lysis Solution #A7943, Protein Precipitation Solution #A7953, and RNase Solution #A7973) and DNA content quantified by spectrophotometry. Genomic DNA (15 – 30 ug) was incubated with an excess of the desired restriction enzymes overnight at 37°C, electrophoresed on a 0.8% GenePure Gold agarose gel (ISC BioExpress # E-31420-125) at 34 volts for 24 hours, then transferred to nylon membrane (Whatman #10416285) by an alkaline transfer method. Probes were radiolabeled as described in the previous section.
Probe descriptions
The exon probes for MGST1 used in Southern Blot analysis corresponded to the previously published report describing the structure of the human MGST1 gene [14]. The MGST1 probe used for the Northern Blot analysis corresponded to the primary full length transcript previously described [14]. The human MGST2 cDNA was 724 bp and derived from human lung tissue (ATCC clone #6141729). The human MGST3 cDNA was 751 bp and derived from human renal tissue (ATCC clone #6189933). The ubiquitin probe was 2.1 kb (Sigma clone #U5007).
Pre-mRNA assay
Total RNA was extracted by Trizol method (Invitrogen), DNase treated with DNase Set Kit (Qiagen), then cleaned-up using RNeasy Kit method (Qiagen). Cellular total RNA was quantified by spectrophotometry. Reverse Transcription was performed using Accuscript High Fidelity 1st Strand Synthesis Kit (Stratagene by Agilent) with upper primer tgagaatgggttgtgtcaagaatatggatataa and lower primer gggcagggagtaagggtggaaggtcggaaagaa using the following conditions: total RNA (198 ng) and primers were heated to 70° C for 30 minutes in cycler and immediately cooled on ice, short spin, then added 1st strand buffer, dNTPs, DTT, and Accuscript. Incubation was at 42° C for 60 min in cycler, followed by 15 min at 70° C, then immediately cooled on ice. Next PCR was performed using the same primers with 2ul of each RT reaction as a template in nuclease free water, 10X Advantage 2 PCR Buffer (Clontech), dNTPs (Clontech), with Advantage 2 Polymerase (Clontech) in a 9700 Gene Amp PCR System (PE Applied Biosystems) with the following conditions: 94° C for 3 minutes (one cycle), 94° C for 45 sec, 56° C for 45 sec, and 72° C for 2 minutes (35 cycles), 72 °C for 10 minutes (one cycle), and then maintaining temperature at 0° C. PCR products were electrophoresed on 1.5% Gene Pure Agarose (ISC BioExpress) gel in TBE. The visualized DNA bands were excised from the gel and del cleaned (Quiagen), cloned into pCR4-TOPO vector (Clontech), and then sequenced at the UCSD CORE facility to confirm nucleotide identity.
Western blot assay
The neuroblastoma cell lines and primary neuroblastoma tissue were assessed for MGST1 protein using the polyclonal antibody developed by Dr. Ralf Morgenstern (Karolinska Institutet, Stockholm, Sweden) using the HepG2 cell line and primary human liver tissue as positive controls. Cellular extracts were prepared using the Active Motif Nuclear Extract Kit (Active Motif # 40010) per manufacturer instructions. The protein extracts were separated on NuPage Bis-Tri SDS Page gels (Invitrogen # NP0302). The separated proteins were transferred to Hybond-P PVDF membrane (Amersham #RPN202F) by gel electrophoresis blotting system (CBS Scientific). Protein detection was accomplished using rabbit polyclonal anti-MGST1 antibody at 1:2000 dilution followed by 1:10,000 dilution of donkey anti-rabbit HRP secondary detection antibody (Amersham ECL Plus detection Kit # RPNZ132) in blocking buffer (TBS Tween 1% BSA). Chemofluorescent detection (estimated sensitivity < 100 pg of enzyme) was then accomplished using a STORM 860 phosphoimager (Molecular Dynamics).
MGST1 Enzymatic quantification using fluorogenic substrate
To determine if very low amounts of MGST1 were present in NB cells and primary tissue, a very sensitive fluorogenic assay was performed using a substrate specific for MGST1 activity [16, 17]. The cells were cultured for four days in a T-shaped 75 cm2 flask. Cells were harvested using trypsin and EDTA, centrifuge 3 minutes at 250 x g, cell pellet suspended in 1ml 0.1 M potassium phosphate, pH6.5, 1% triton X100, and then sonicated on ice 3 times for 10 seconds. The protein concentration was determined using a BCA protein Assay (Thermo Scientific # 23223, 23224). To a black 96 well assay plate (Costar 3915) was add 100 μl phosphate buffer, 5.0 μl 200 mM glutathione, 0.5 μl fluorogenic cresyl violet MGST1 substrate (specific activity 560 nmol/min/mg of MGST1 enzyme protein), followed by cell extract containing 200 ug of protein. Fluorescent assay conditions were excitation at 591 nM and emission at 628 nM. The emissions were quantified every 2 minutes for 20 minutes (sensitivity of 0.02 nanomoles/min per mg of cell extract).
Results
Neuroblastoma cell lines
The expression of MGST1 mRNA was first compared in two common NB cell lines, SK-N-SH and IMR-32, to other commonly used tumor cell lines. There was no detectable MGST1 mRNA in these two NB cell lines but MGST1 expression was detected in other cell lines (Figure 1). Next we examined MGST1 expression in all NB cell lines using full length MGST1 cDNA probe. Human HepG2 hepatoma cells were used as a positive control as these cells express phase II conjugating enzymes, including MGST1, at a level equivalent to cryopreserved human hepatocytes [18]. In contrast to previous reports of upregulation of MGST1 in human tumor cells in vitro and in primary human tumor tissue, there was no detectable MGST1 mRNA expression in all of the NB cell lines (Figure 2). Significant expression was detected with the control ubiquitin as well as for the two other microsomal glutathione transferases (MGST2 and MGST3). A Western blot analysis demonstrated the presence of MGST1 protein in HepG2 cells, but not in NB5 or SK-N-SH cells, as anticipated based on the lack of detectable mRNA presence (data not shown). A sensitive fluorogenic assay using a substrate specific for MGST1 [16, 17] demonstrated significant MGST1 enzymatic assay in HepG2 cells but no detectable activity in NB5 or SK-N-SH cells, as anticipated based on the lack of mRNA or protein presence (data not shown).
Figure 1.

Comparison of MGST1 mRNA expression in neuroblastoma cell lines SK-N-SH and IMR-32 (lanes 22 and 23) to other cell lines. Lanes were loaded with 25 ng of extracted mRNA. Six lanes are blank to allow for orientation and background expression.
Lane Identification: 1 = A549 Lung, 2 = NCI-H460. 3 = NCI-H1299, 4 = blank, 5 = colon HCT116, 6= colon HCT116 p21 null, 7 = colon HCT116 p53 null, 8 = colon HCT-15, 9 = colon HT-29, 10 = blank, 11 = breast MDA-MB-435, 12 = breast MDA-MB-231, 13 = breast MCF7, 14 = ovarian SD-OV-3, 15 = cervical HeLa, 16 = blank, 17 = prostate DU 145, 18 = prostate PC-3, 19 = blank, 20 = melanoma SK-MEL-28, 21 = blank, 22 = neuroblastoma SK-N-SH, 23 = neuroblastoma IMR-32, 24= glioblastoma U-87, 25 = blank, 26 = renal 786-0, 27 = renal ACHN, 28 = Liver HepG2,
Figure 2.
Comparison of mRNA expression in control HepG2 cells versus NB cell lines for microsomal glutathione S-transferase enzymes. The lanes labeled with a “C” correspond to 25 ng of extracted HepG2 mRNA. The mRNA from NB cell lines was loaded at 100 ng (or four times the control samples); Lane 1 contains IMR32 cells, Lane 2 SK-N-SH cells, Lane 3 NB5 cells, Lane 4 NB7 cells, Lane 5 SK-N-AS cells.
Primary human tissue
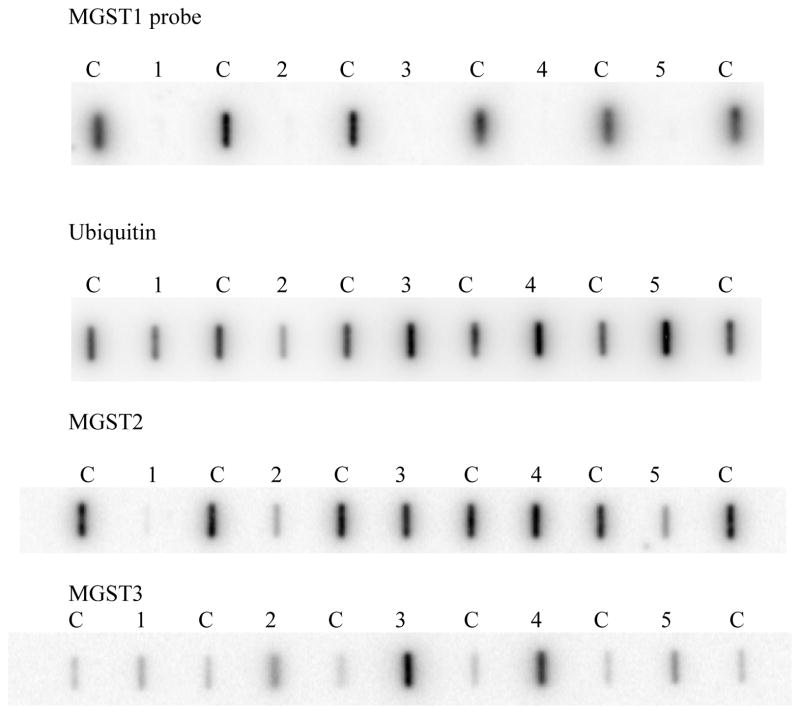
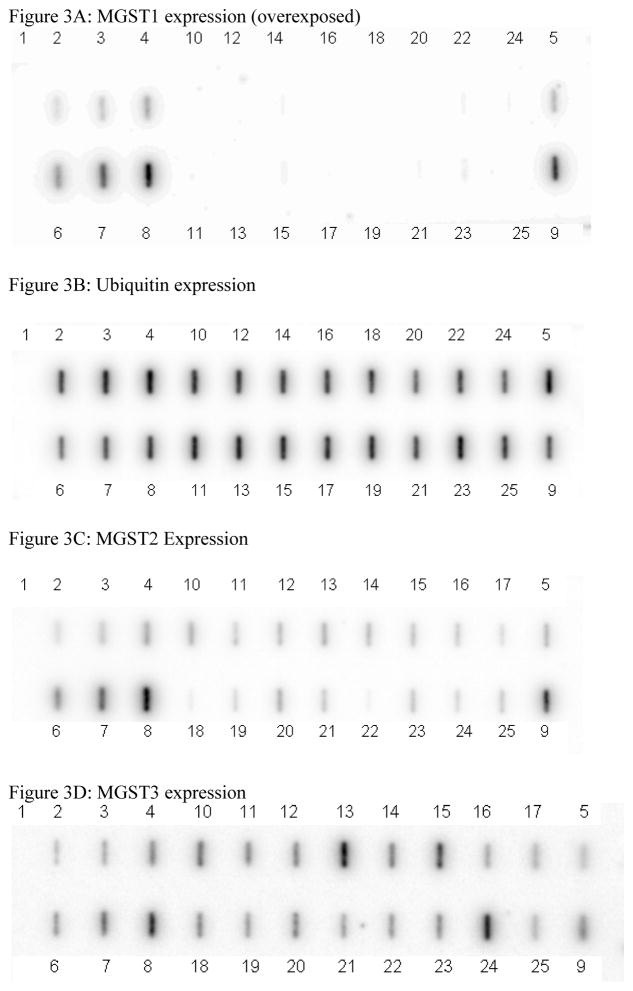
The expression of MGST1 mRNA was examined in primary NB tissue samples from 16 different individuals to determine if the lack of MGST1 mRNA expression was related to the development of NB or simply a “cell culture phenomena”. Human HepG2 hepatoma cells and normal adult human liver tissue were used as positive tissue expressing controls for these studies. There was no detectable MGST1 mRNA expression in the primary tumor tissue, but significant expression was detected with the ubiquitin control probe as well as for two other MAPEG proteins; MGST2 and MGST3 (Figure 3).
Figure 3.
Comparison of MGST1 expression versus ubiquitin, MGST2, and MGST3 expression in primary NB tissue versus HepG2 cells and human liver mRNA.
Lane 1: background; Lanes 2, 3, 4 and 5: HepG2 cells at 75, 150, 300 and 300 ng of mRNA, respectively. Lanes 6, 7, 8, and 9: Primary human liver tissue at 75, 150, 300 and 300 ng of mRNA, respectively. Lanes 10 through 25 contain 150 ng of mRNA extracted from 16 different primary NB tumor tumors.
Pre-mRNA analysis
To determine if the lack of MGST1 mRNA in NB cells and primary tissue was due to a processing or splicing error, the existence of pre-mRNA in NB cell lines and primary tissue was sought using primers that spanned exon 4 and the immediate downstream non-coding region (intron). The presence of MGST1 pre-mRNA could be demonstrated in human primary liver tissue and HepG2 cells, but not in any NB cell line or primary tissue (data not shown).
Southern Blot and Nucleotide Analysis
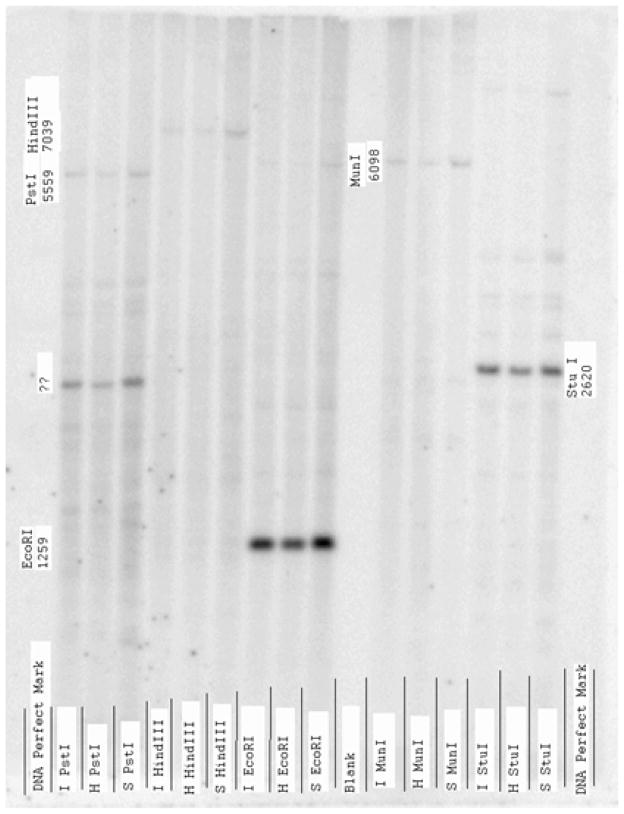
To determine if a chromosomal rearrangement in the MGST1 gene could be detected and explain the lack of pre-mRNA expression in NB cells lines and primary tissue, Southern Blot analysis of the genomic DNA from the NB cell lines was performed. Analysis included probes corresponding to all 4 exons (Exon 1B, 2, 3, 4) of the human MGST1 gene, and eight major restriction enzymes (BamHI, BglII, EcoRI, HindIII, MunI, PstI, StuI, XbaI) were used to digest the genomic DNA. Despite this intensive investigation, there was no obvious difference between the NB cells lines and the HepG2 cells (example of one Southern Analysis provided in Figure 4). The nucleotide sequence of the promoter region for 1 kilobase upstream and all 4 exons for the NB5 and SK-N-SH NB cell lines was completed and compared to the HepG2 GenBank submissions, and also our initial report of the structure of the human MGST1 gene derived from fibroblasts [14] but no obvious explanation for the lack of MGST1 pre-mRNA expression was found (data not shown).
Figure 4.
Representative example of Southern blot analysis of genomic DNA using exon 2 of human MGST1 as a probe. Lanes marked with an I refer to genomic DNA from IMR-32 cell line, S refer to SK-N-SH, and H refer to HepG2.
Summary
The failure to detect any MGST1 mRNA or protein expression in NB cell lines and primary tumor tissue is a completely unanticipated finding. There are numerous reports, including microarray and chip studies, documenting the upregulation of MGST1 in tumor cells. As MGST1 mRNA expression is ubiquitously expressed primarily under the influence of the SP1 transcription factor [19], and SP1 expression is upregulated in NB cell lines, one would have anticipated an increase in MGST1 mRNA similar to that noted with MYCN oncogene expression in NB cells [20, 21].
The mechanism behind the failure of NB cells and tissue to express MGST1 pre-mRNA/mRNA, and the potential role of this finding in the development of NB (if any), will require further investigation. The significance of this finding to the development of neuroblastoma (familial or otherwise) is unknown and may even be incidental. The lack of MGST1 expression in NB, however, does have far reaching implications. NB cells are considered to be extremely sensitive to oxidative stress compared to non-NB derived cell lines but a comprehensive search for the reason did not yield an explanation although the expression of numerous antioxidant enzymes and factors were examined [22–25]. There were even clinical attempts to exploit this sensitivity to oxidative stress by using compounds that auto-oxidize (generate superoxide or hydrogen peroxide) to purge NB cells from bone marrow prior to transplantation [26].
There have been numerous studies investigating the expression of a variety of classical anti-oxidant enzymes and factors in an attempt to explain why NB cells are extremely sensitive to oxidative stress, but no mechanism was identified. Our work now provides a plausible molecular basis for the sensitivity of NB cells to oxidative stress and explains why NB cells are extremely sensitive to GSH depletion [27]. There is also a large compendium of recent work describing significant alterations in mitochondrial permeability and potential upon oxidative stress in NB cell lines that result in apoptosis. Given that MGST1 provides significant protection against oxidative stress [28], and constitutes 4 to 6% of all protein in the outer membrane of the mitochondria, it is plausible that these significant alterations in mitochondrial homeostasis are the result of NB cells being devoid of MGST1 protein. Thus, studies using NB cells to investigate mitochondrial response to endogenous and exogenous stress may not be translatable to other tumor and cell types. We are currently attempting to generate MGST1 expressing clones through use of retroviral and plasmid-based constructs using heterologous promoters to direct the production of MGST1 in NB cell lines. If successful, these cell lines could provide insight into the role of MGST1 in NB development, the sensitivity of NB cells to oxidative stress, and alterations in mitochondrial homeostasis upon oxidative stress.
Conclusions
Human neuroblastoma cell lines and primary tumor tissue fail to express microsomal glutathione S-transferase 1 (MGST1).
Failure to express MGST1 provides an explanation for the inherent sensitivity of neuroblastoma cells to oxidative stress.
Table 1.
Patient characteristics from which primary tumor tissue was obtained.
| UCSD Patient Identifier | Age at diagnosis (years) | Clinical Stage at diagnosis | N-myc Amplification | Event Free Survival (days) |
|---|---|---|---|---|
| 2 | 0.03 | A | negative | 1100 |
| 4 | 0.06 | A | negative | 1017 |
| 6 | 1.38 | A | negative | 1150 |
| 7 | 0.07 | A | negative | 969 |
| 9 | 0.67 | A | negative | 792 |
| 12 | 5.76 | B | negative | 1529 |
| 13 | 0.31 | B | negative | 823 |
| 16 | 0.12 | B | negative | 691 |
| 17 | 2.8 | C | positive | 2433 |
| 21 | 1.5 | C | negative | 1253 |
| 23 | 2.8 | C | positive | 912 |
| 24 | 1.4 | D | positive | 590 |
| 29 | 0.6 | D | positive | 139 |
| 31 | 0.02 | D | positive | 100 |
| 37 | 0.04 | DS | negative | 761 |
| N4152 | 5.23 yrs | D | negative | NA |
Highlights.
Neuoblastoma tumor cells are sensitive to oxidative stress
Neuroblastoma tumor cells and primary human tumor tissue lack MGST1 protein
The lack of MGST1 provides a basis for the sensitivity of neuroblastoma cells to oxidative stress
Acknowledgments
This study was supported by National Institute of Health Award 1RO1ES013687 (MJK), in part by FDA Award FD-R-002319S1 (ALY and MBD), and in part ) by The Swedish Research Council, Swedish Foundation for Strategic Research, VINNOVA and funds from Karolinska Institutet (RM).
Abbreviations
- NB
neuroblastoma
- MAPEG
Membrane-Associated Proteins in Eicosanoid and Glutathione
- MGST1
microsomal glutathione S-transferase-1
Footnotes
Conflict of Interest
None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 4.Hogarty MD. The requirement for evasion of programmed cell death in neuroblastomas with MYCN amplification. Cancer Lett. 2003;197:173–179. doi: 10.1016/s0304-3835(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 5.Maris JM, Weiss MJ, Mosse Y, Hii G, Guo C, White PS, Hogarty MD, Mirensky T, Brodeur GM, Rebbeck TR, Urbanek M, Shusterman S. Evidence for a hereditary neuroblastoma predisposition locus at chromosome 16p12–13. Cancer Res. 2002;62:6651–6658. [PubMed] [Google Scholar]
- 6.Perri P, Longo L, Cusano R, McConville CM, Rees SA, Devoto M, Conte M, Ferrara GB, Seri M, Romeo G, Tonini GP. Weak linkage at 4p16 to predisposition for human neuroblastoma. Oncogene. 2002;21:8356–8360. doi: 10.1038/sj.onc.1206009. [DOI] [PubMed] [Google Scholar]
- 7.Longo L, Panza E, Schena F, Seri M, Devoto M, Romeo G, Bini C, Pappalardo G, Tonini GP, Perri P. Genetic predisposition to familial neuroblastoma: identification of two novel genomic regions at 2p and 12p. Hum Hered. 2007;63:205–211. doi: 10.1159/000099997. [DOI] [PubMed] [Google Scholar]
- 8.Persson B, Jakobsson PJ, Morgenstern R, Mancini J, Ford-Hutchinson A. Common structural features of MAPEG -- a widespread superfamily of membrane associated proteins with highly divergent functions in eicosanoid and glutathione metabolism. Protein Sci. 1999;8:689–692. doi: 10.1110/ps.8.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearson WR. Phylogenies of glutathione transferase families. Methods Enzymol. 2005;401:186–204. doi: 10.1016/S0076-6879(05)01012-8. [DOI] [PubMed] [Google Scholar]
- 10.Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgenstern R, Lundqvist G, Andersson G, Balk L, DePierre JW. The distribution of microsomal glutathione transferase among different organelles, different organs, and different organisms. Biochem Pharmacol. 1984;33:3609–3614. doi: 10.1016/0006-2952(84)90145-x. [DOI] [PubMed] [Google Scholar]
- 12.Bresell A, Weinander R, Lundqvist G, Raza H, Shimoji M, Sun TH, Balk L, Wiklund R, Eriksson J, Jansson C, Persson B, Jakobsson PJ, Morgenstern R. Bioinformatic and enzymatic characterization of the MAPEG superfamily. FEBS J. 2005;272:1688–1703. doi: 10.1111/j.1742-4658.2005.04596.x. [DOI] [PubMed] [Google Scholar]
- 13.Jakobsson PJ, Morgenstern R, Mancini J, Ford-Hutchinson A, Persson B. Membrane-associated Proteins in Eicosanoid and Glutathione Metabolism (MAPEG): A Widespread Protein Superfamily. Am J Respir Crit Care Med. 2000;161:S20–24. doi: 10.1164/ajrccm.161.supplement_1.ltta-5. [DOI] [PubMed] [Google Scholar]
- 14.Kelner MJ, Bagnell RD, Montoya MA, Estes LA, Forsberg L, Morgenstern R. Structural organization of the microsomal glutathione S-transferase gene (MGST1) on chromosome 12p13.1–13.2. Identification of the correct promoter region and demonstration of transcriptional regulation in response to oxidative stress. J Biol Chem. 2000;275:13000–13006. doi: 10.1074/jbc.275.17.13000. [DOI] [PubMed] [Google Scholar]
- 15.Johansson K, Åhlen K, Rinaldi R, Sahlander K, Siritantikorn A, Morgenstern R. Microsomal glutathione transferase 1 in anti-cancer drug resistance. Carcinogenesis. 2007;28:465–470. doi: 10.1093/carcin/bgl148. [DOI] [PubMed] [Google Scholar]
- 16.Alander J, Johansson K, Heuser VD, Farebo H, Järvliden J, Abe H, Shibata A, Ito M, Ito Y, Morgenstern R. Characterization of a new fluorogenic substrate for microsomal glutathione transferase 1. Anal Biochem. 2009;390:52–56. doi: 10.1016/j.ab.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Shibata A, Ito M, Shuto S, Ito Y, Mannervik B, Abe H, Morgenstern R. Synthesis and characterization of a series of highly fluorogenic substrates for glutathione transferases, a general strategy. J Am Chem Soc. 2011;133:14109–14109. doi: 10.1021/ja205500y. [DOI] [PubMed] [Google Scholar]
- 18.Westerink WM, Schoonen WG. Phase II enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol In Vitro. 2007;21:1592–1602. doi: 10.1016/j.tiv.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Forsberg L, Persson L, Kelner MJ, Jakobsson PJ, Morgenstern R. Structure and transcriptional regulation of the human microsomal glutathione transferase 1 (MGST1) and prostaglandin E synthase (PGES) genes. Chem Bio Interact. 2001;133:301–303. [Google Scholar]
- 20.Inge TH, Casson LK, Priebe W, Trent JO, Georgeson KE, Miller DM, Bates PJ. Importance of Sp1 consensus motifs in the MYCN promoter. Surgery. 2002;132:232–238. doi: 10.1067/msy.2002.125387. [DOI] [PubMed] [Google Scholar]
- 21.Tuthill MC, Wada RK, Arimoto JM, Sugino CN, Kanemaru KK, Takeuchi KK, NS N-myc oncogene expression in neuroblastoma is driven by Sp1 and Sp3. Mol Genet Metab. 2003 Sep-Oct;80(1–2):272–80. doi: 10.1016/s1096-7192(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 22.Erlejman AG, Oteiza PI. The oxidant defense system in human neuroblastoma IMR-32 cells predifferentiation and postdifferentiation to neuronal phenotypes. Neurochem Res. 2002;27:1499–1506. doi: 10.1023/a:1021600522299. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Lee J-M, Johnson JA. Microarray Analysis Reveals an Antioxidant Responsive Element driven Gene Set Involved in Conferring Protection from an Oxidative Stress-induced Apoptosis in IMR-32 Cells. J Biol Chem. 2002;277:388–394. doi: 10.1074/jbc.M109380200. [DOI] [PubMed] [Google Scholar]
- 24.Slaughter MR, Thakkar H, O’Brien PJ. Effect of Diquat on the Antioxidant System and Cell Growth in Human Neuroblastoma Cells. Toxicol Appl Pharmacol. 2002;178:63–70. doi: 10.1006/taap.2001.9322. [DOI] [PubMed] [Google Scholar]
- 25.Tiffany-Castiglioni E, Saneto RP, Proctor PH, Perez-Polo JR. Participation of active oxygen species in 6-hydroxydopamine toxicity to a human neuroblastoma cell line. Biochem Pharmacol. 1982;31:181–188. doi: 10.1016/0006-2952(82)90208-8. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds CP, Reynolds DA, Frenkel EP, Smith RG. Selective toxicity of 6-hydroxydopamine and ascorbate for human neuroblastoma in vitro: a model for clearing marrow prior to autologous transplant. Cancer Res. 1982;42:1331–1336. [PubMed] [Google Scholar]
- 27.Anderson CP, Tsai J, Chan W, Park CK, Tian l, Lui RM, Forman HJ, Reynolds CP. Buthione sulphoxime alone and in combination with Melphalan (L-PAM) is highly cytotoxic for human neuroblastoma cell lines. Eur J Cancer. 1997;13:2016–2019. doi: 10.1016/s0959-8049(97)00203-7. [DOI] [PubMed] [Google Scholar]
- 28.Siritantikorn A, Johansson K, Hlen K, Rinaldi R, Suthiphongchai T, Wilairat P, Morgenstern R. Protection of cells from oxidative stress by microsomal glutathione transferase 1. Biochem Biophys Res Commun. 2007;355:592–596. doi: 10.1016/j.bbrc.2007.02.018. [DOI] [PubMed] [Google Scholar]