Abstract
The purpose of this research was to set up a sensitive and consistent UPLC-UV and UPLCMS/MS method to analyze emodin and its glucuronidated metabolite, and to determine how gender differences affect its pharmacokinetic behaviors. In addition, a breast cancer resistance protein inhibitor dipyridamole was used to test how significant the absolute oral biovailabilty of emodin or its glucuronide is increased. A sensitive and fast UPLC-MS/MS method was successfully applied to determine emodin and its metabolite in male and female SD rat plasma. The absolute oral bioavailability of emodin was extremely low whether in male rats (7.5%) and female rats (5%). Following a single intravenous injection of 4 mg/kg emodin, the emodin plasma concentration-time data fit for a good two-compartment model either in male or female SD rats. The t1/2α were 13.26±6.28min (male rats) and 13.52±7.28min (female rats). The t1/2β were 187.38±0.16min (male rats) and 118.50±83.09min (female rats). Emodin showed significant gender differences in i.v. PK profiles with higher AUC values in male (422.71 ± 163.40 mg*μg/ml) than female (282.52 ± 98.42 mg*μg/ml) SD rats (n=6). Emodin glucuronide was suggested a good fit for single compartmental model for the plasma emodin metabolite concentrations. The t1/2Ke were 167.40±50.91min(male rats) and 251.31±114.20min (female rats), the area under the curve (AUC0-∞, i.v.) were 2210.02 ± 950.09 mg*μg/ml and 1054.42 ± 290.31 mg*μg/ml (female rats)(n=6). There was no good fit for any PK compartmental model for the plasma concentration-time data for single dose oral administration of emodin (8mg/kg) and its metabolite. Analyzing the oral PK data using non-compartmental model, Cmax, Tmax and AUC0-∞, p.o. of emodin in male rats were: 0.31±0.094 were μg/ml, 18.00±6.71min and 65.76±34.77 mg*μg/ml respectively; whereas Cmax, Tmax and AUC0-∞, p.o. of emodin in female rats were: 0.039±0.011 μg/ml, 18.75±7.51min and 33.82±4.09 mg*μg/ml respectively. The parameters of emodin glucuronide were significant different with emodin, the Cmax, Tmax and AUC0-∞, p.o of emodin glucuronide in male rats were 6.69±1.06 μg/ml, 240min and 2261.89±655.87 mg*μg/ml respectively, in female rats, the Cmax, Tmax and AUC0-∞, p.o. were 1.81±0.58 μg/ml, 60min and 458.50±373.29 mg*μg/ml respectively. The absolute bioavailability of emodin glucuronide was 60% (male rats) and 15% (female rats). The absolute bioavailability of emodin was no significant changed (7.3%) in male rats by using dipyridamole, the bioavailability of metabolite of emodin was significant declined to 14.6%.
Keywords: emodin, absolute oral bioavailability, pharmacokinetics, UPLC-MS/MS, emodin
1. Introduction
Emodin (1, 3, 8-trihydroxy-6-methylanthraquinone) is one of the major active ingredients of rhubarb (rheum officinale B.), aloe (aloe barbadensis M.), and leaf of senna (cassia angustifolia) plants. Decades ago, emodin was extensively studied for its traditional pharmacological activities. However, recent studies have put emodin back into limelight with its anti-cancer activities in several types of cancer cells with apoptosis as a possible mechanism of action [1, 2]. Emodin also has an inhibitory effect on cancer cell migration [3] and invasion [4] in in vitro studies and that is why emodin has good prospects[5] and promising future in the anticancer treatment market [6].
In our previous studies, emodin showed extremely fast metabolism in rat intestine and liver microsomes which were derived from 5 species of animals such as mouse, dog and human [7]. Moreover, intestinal absorption and disposition of emodin was investigated in rats by using the in situ intestinal perfusion model. After conducting in vitro and in situ studies, we confirmed that Phase II metabolism (glucuronidation) was major route of metabolism of emodin in rats. Even though emodin absorption and disposition studies have been conducted in vitro and in situ, little is known about its pharmacokinetics and metabolism in vivo. Shia [8] et al also found that emodin glucuronide is the main metabolite of emodin after performed emodin pharmacokinetics in rats by using HPLC analysis method. The only study showing oral pharmacokinetics of emodin showed emodin glucuronide as a major metabolite present in rat plasma. In the study, the plasma samples were analyzed using HPLC and therefore the sensitivity of the sample analysis was significantly low. Furthermore, no information regarding the absolute oral bioavailability of emodin in rats is available. There is also lack of information of gender effect on absolute oral bioavailability of emodin in rats.
In modern era, more and more people, no matter men or women aspire to be fit and healthy. Therefore, in addition to the above mentioned effects, emodin’s health food and slimming product [9] intake has increased due to its weight loss property through its traditionally known stimulant laxative activity. As described previously, metabolism of emodin in vitro was gender different in rats and other four species animal [10]. However, again, no study has been done to show the gender differences in oral bioavailability of emodin in rodents as well as humans. Hence, we propose to study gender-dependent difference in absorption and metabolism of emodin as the main aim of our study.
Therefore, the purpose of this study is to 1) develop a sensitive and reliable UPLC-MS/MS method to determine emodin concentration using in vivo single dose (8mg/kg) oral and intravenous (4mg/kg) PK model; 2) study pharmacokinetic behavior of emodin and its metabolite in rats.
2. Experimental
2.1. Chemicals and reagents
Emodin (≥98%, HPLC grade) was purchased from Chengdu Mansite Pharmaceutical Company (China). Daidzein (≥98%, HPLC grade) was purchased from Sigma (St. Louis, MO,USA). PEG300 and oral suspensions were purchased from PCCA laboratories (TX, Houston, USA). The extraction experimental procedures of emodin 3-O-β-D-glucuronide were essentially the same as published previously [7]. The 3D structure of emodin glucuronide was confirmed by mass spectrometer and 1H-NMR [7]. All other materials (typically analytical grade or better) were used as received.
2.2. Instruments and conditions
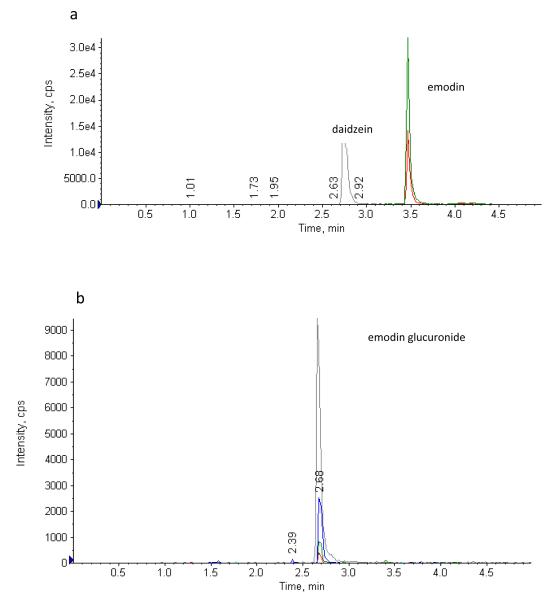
An API 3200 Qtrap triple quadrupole mass spectrometer (Applied Biosystem/MDS SCIEX, Foster City, CA, USA) was used to determine the plasma concentrations of emodin and emodin glucuronide. The quantitative analysis of emodin in aqueous and plasma medium were done by using MRM (Multiple Reaction Monitoring) method in positive ion mode. The main working parameters for mass spectrum were set as follows: ionspray voltage, 4.5 kV; ion source temperature, 350°C; gas1, 40 psi; gas2, 20 psi; curtain gas, 10 psi. Daidzein was used as an internal standard in quantification of emodin and emodin glucuronide in plasma. Emodin and emodin 3-O-β-D-glucuronide were determined by MRM method in negative mode (Fig. 1 a, b). The quantification was performed using MRM method with the transitions of m/z 269 → m/z 243 for emodin, m/z 253 → m/z 132 for daidzein (IS), m/z 445 → m/z 268.6 for emodin 3-O-β-D-glucuronide. The compound dependent parameters in MRM mode for emodin and other compounds were summarized in Table 1.
Fig. 1.

The structure of emodin
Table 1.
Compound dependent parameters for emodin and its glucuronide and IS in MRM mode for UPLC-MS/MS analysis.
| Analyte | Q1 Mass | Q3 Mass |
Dwell time | DP | CEP | CE | CXP |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| u | u | Ms | V | V | V | V | |
| emodin | 269 | 243 | 150 | 65 | 24 | 40 | 2 |
|
| |||||||
| emodin 3-O-β-D-glucuronide | 445 | 268.6 | 150 | 29 | 20 | 30 | 2 |
|
| |||||||
| Daidzein | 253 | 132 | 150 | 75 | 21 | 52 | 2 |
UPLC conditions for analyzing emodin in plasma were: system, Waters Acquity™ with diode array detector (DAD); column, Acquity UPLC BEH C18 column (50 ×2.1 mm I.D., 1.7 mm, Waters, Milford, MA, USA); mobile phase B, 100%acetonitrile, mobile phase A, 100% aqueous buffer (2.5 mM NH4Ac, pH 7.4); flow rate 0.45 ml/min; gradient, 0 to 0.1 min, 85%A, 0.1 to 1.8 min, 85-60%A, 1.8 to 2.2 min, 60-40%A, 2.2 to 2.8 min, 40-85%A, 2.8 to 3.2 min, 85%A, wavelength, 254 nm for emodin and its glucuronide and daidzein; and injection volume, 10 μL; column temperature, 45°C; and injection volume, 10 μl.
2.3. Pharmacokinetic study in vivo
2.3.1 Animals
Male Sprague-Dawley rats (250-300g, 8-10 weeks old) and female Sprague-Dawley rats (200-230g, 8-10 weeks old) were purchased from Harlan Laboratory (Indianapolis, IN) and kept in an environmentally controlled room (temperature: 25 ±2°C, humidity: 50±5%, 12 h dark-light cycle) for at least 1 week before the experiments.
2.3.2 Pharmacokinetic Experiments
Male and female Sprague-Dawley rats were assigned to two groups, and the animals were fasted overnight before drug administration. Emodin was freshly prepared just before i.v. administration by dissolving it in 80% pH9.0 NaHCO3 solution and 20% PEG300 at dose of 4mg/kg and after the resulting solution was filtered through 0.22 μm membrane, it was injected into the tail vein. In case of oral administration, emodin was dispersed in oral suspending vehicle and was given to rats at dose of 8mg/kg orally. Blood samples were withdrawn from tail vein at 5, 10, 15, 30, 60, 120, 240, 480, 720, 1440 minutes after injection administration and at 5, 15, 30, 60, 120, 240, 480, 720, 1440 minutes after oral administration. Each blood sample was centrifuged at 5000 rpm for 5 minutes. Afterwards, 100μl of serum was mixed with 320 μl of methanol containing 50 μM internal standard (daidzein), and the mixture was centrifuged at 13,000rpm for 20 minutes. The supernatant was then removed and dried under nitrogen gas, and the residue was reconstituted in 100 μl of methanol, vortexed and then injected into UPLC/MS/MS for analysis.
2.3.3 Preparation of standard and quality control samples
Stock solution of emodin was prepared in methanol at a concentration of 2mg/ml. The stock solution of daidzein (IS) was prepared in methanol at a concentration of 100μg/ml. Calibration standard samples for emodin were prepared in blank rat plasma at concentrations of 39, 78, 156,312.5, 625, 1250, 2500, 5000, 10000ng/ml. Three quality control (QC) samples were prepared as low (39ng/ml), medium (625ng/ml), and high (10000ng/ml) concentrations in the same way as the plasma sample preparation for calibration. QC samples were stored at −20°C until analysis. Calibration standard samples for emodin glucuronide were prepared in blank rat plasma at concentrations of 78, 156, 312.5, 625, 1250, 2500, 5000, 10000ng/ml. Three quality control (QC) samples for emodin glucuronide were prepared as low (78ng/ml), medium (625ng/ml), and high (10000ng/ml) concentrations in the same way as the plasma sample preparation for calibration. QC samples were stored at −20°C until analysis.
2.3.4 Method validation
2.3.4.1 Calibration curve and LLOQ
Calibration curves were prepared according to section 2.3.3. The linearity of each calibration curve was confirmed by plotting the peak area ratio of emodin to IS in rat plasma. 1/x least-squares linear regression method was used to determine the slope, intercept and correlation coefficient of linear regression equation. The lower limit of quantification (LLOQ) was determined based on the signal-to noise ratio of 10:1.
2.3.4.2 Precision and accuracy
The “Intra-day” and “Inter-day” precision and accuracy of emodin were determined with three different concentrations of six QC samples in the same day or on three different days.
2.3.4.3 Extraction recovery
The extraction recovery of emodin was determined by comparing (a) the peak areas obtained from blank plasma spiked with analytes before the extraction with (b) those from samples to which analytes were added after the extraction.
2.3.4.4 Stability
Short-term (25°C for 24h), long-term (−20°C for 90 days) and three freeze-thaw cycle stabilities of emodin were determined.
2.3.5 Pharmacokinetic data analysis
In oral and intravenous PK studies, emodin was rapidly metabolized to emodin-3-O-β-D-glucuronide, the only metabolite of emodin that was observed by UPLC-MS/MS.
The pharmacokinetic parameters for 8mg/kg oral and intravenous emodin PK studies in male and female rats were calculated using WinNonlin 3.3 software. The area under the concentration-time curve (AUC) was calculated using the trapezoidal rule with extrapolation to time infinity. The oral absolute oral bioavailability was calculated as F= [(AUC0-∞, p.o.) / (AUC0-∞, i.v.)] × (Dose i.v./Dose p.o. ) ×100%, where AUC0-∞, p.o. and AUC0-∞, i.v. were corresponding to area under the curve from zero to infinity after oral and i.v. administration respectively.
2.4 Statistical analysis
The data in this paper were presented as mean±RSD, if not specified otherwise. Significance differences were assessed by using unpaired Student’s t-test with P value of <0.05 to be considered as statistically significant.
Results and Discussion
3.1 Chromatography and mass spectrometry
A specific, reliable and sensitive method to determine emodin concentration was established. The LLOQ (lower limit of quantitation) of emodin was 20ng/ml by using the UPLC-MS/MS method, which was more than 100 times lower than the published method by Shia et al [8] which used HPLC to quantify emodin. In our study, methanol was used to precipitate rat plasma protein, which improved recovery. This newly established method enabled us to determine the concentration of emodin in vivo after administration of a low dose of emodin. Typical chromatograms of spiked emodin and emodin glucuronide in rat plasma sample were shown in Fig.2a and Fig.2b, respectively.
Fig.2.
Sample chromatograms of different analytes in current UPLC system with MS detection.
a A blank plasma sample spiked with emodin (10000ng/ml) and daidzein (5000ng/ml).
b A blank plasma sample spiked with emodin glucuronide (10μM).
3.2 Linearity, precision and accuracy, recovery, matrix effect, stability
The standard curves for emodin in plasma was linear in the concentration range of 39ng/ml to 10000ng/ml with correlation coefficient values>0.998 (the weight is 1/×). The lower limit of quantification (LLOQ) was 20ng/ml, defined as a signal-to-noise ratio of ≥10. The standard curves for emodin glucuronide in plasma was linear in the concentration range of 78ng/ml to 10000ng/ml with correlation coefficient values>0.999 (the weight is 1/×). The lower limit of quantification (LLOQ) was 40ng/ml, defined as a signal-to-noise ratio of ≥10. Intra-day and inter-day precision and accuracy were determined by measuring six replicates of QC samples (emodin and its glucuronide) at three concentration levels in rat plasma. The precision and accuracy were shown in Table 2. The results showed that the precision and accuracy values were well within the 10% acceptance range.
Table 2.
Intra-day and Inter-day precision and accuracy for emodin and its glucuronide in MRM mode of UPLC-MS/MS analysis
| Intra-day (n=6) | Inter-day (n=6) | ||||
|---|---|---|---|---|---|
| Analyte | Concentration (ng/ml) |
Accuracy (Bias, %) |
Precision (CV, %) |
Accuracy (Bias, %) |
Precision (CV, %) |
| Emodin | 39 | 90.46 | 6.42 | 89.23 | 7.93 |
| 625 | 98.34 | 4.98 | 96.32 | 5.44 | |
| 10000 | 104.75 | 2.83 | 102.09 | 4.72 | |
| Emodin glucuronide | 78 | 92.45 | 3.89 | 95.98 | 7.52 |
| 625 | 101.13 | 4.62 | 99.68 | 9.13 | |
| 10000 | 105.85 | 4.83 | 102.43 | 6.74 | |
The mean extraction recoveries determined using three replicates of QC samples of emodin at three concentration levels in rat plasma were found 78.6.2±2.85%, 73.2±1.77%, and 77.9±1.45% for 39ng/ml, 625ng/ml and 10000ng/ml, respectively. The mean extraction recoveries determined using three replicates of QC samples of emodin glucuronide at three concentrations were 77.2±3.2%, 80.3±1.75% and 74.4±2.0% for 78ng/ml, 625ng/ml and 10000ng/ml, respectively.
The stability of emodin in plasma and was evaluated by analyzing three replicates of quality control samples containing 39, 625 and 10000ng/ml emodin after short-term storage (25°C, 4h), long-term cold storage (−20°C, 90days) and within three freeze-thaw cycles. All the samples displayed 95-105% recoveries after various stability tests, which were shown in Table 3. In earlier studies, Shia et al [8] developed a HPLC method to analyze emodin and emodin glucuronide in plasma samples. The method was not sensitive enough to detect the plasma concentrations of emodin and emodin glucuronide upto 24 hours. Therefore, a new highly sensitive UPLC-MS/MS method was developed and validated. The method showed significantly lower limit of quantification of 20ng/ml with signal to noise ratio of 10:1. The inter and intra day precision for emodin and emodin glucuronide analysis was always within the acceptable range of 10%. The validated method was successfully used to analyze the rat plasma samples after single dose oral and i.v. administration of emodin 8mg/kg in male and female rats.
Table 3.
Short-term stability, long-term stability and freeze-thaw cycles of emodin in rat plasma (n=3)
| Recovery% (Mean±SD) | |||
|---|---|---|---|
| Concentration (ng/mL) | Short-term stability |
Long-term stability |
Freeze-thaw cycle |
| 39 | 102.74±3.70 | 100.32±9.15 | 91.34±13.29 |
| 625 | 104.64±6.93 | 92.45±8.33 | 102.43±10.93 |
| 10000 | 99.83±4.78 | 94.65±6.89 | 102.53±9.63 |
3.3 Pharmacokinetic results
Plasma male rats showed 4 folds higher plasma concentrations (AUC values) of total emodin (emodin+glucuronide) than female rats after single dose oral 8mg/kg emodin administration suggesting significant gender-dependent differences (Figure 3 and table 4). The observed gender difference in total emodin oral PK is due to higher AUC values of only emodin glucuronide (more than 5 folds) and not emodin aglycone in male than female rats (Figure 4 and table 5). Similar to AUC, the Cmax values for emodin and emodin glucuronide were also higher in male (7 and 3.5 folds respectively) than female rats.
Fig. 3.
a Plasma concentration –time curves of total emodin after i.v. administration of emodin in SD male and female rats(4 mg/kg). Each point represents an average of six determinations and the error bars are standard deviations of the mean.
b Plasma concentration –time curves of total emodin after orally dose of 8mg/kg emodin in SD male and female rats (8mg/kg)(n=6). Each point represents an average of six determinations and the error bars are standard deviations of the mean.
Table 4.
Pharmacokinetics parameters of total emodin (emodin+glucuronide) after oral administration (n=6)
| Parameters | I.V. | Oral | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| t1/2Ke (min) | 228.94±28.74 | 380.66±230.87 | - | - |
| Vc (mL/kg) | 290.21±76.13 | 350.32±134.23 | - | - |
| AUC (min*μg /mL) |
1839.08±446.09 | 2146.47±497.26 | 3034.59±968.99 | 762.07±321.89 |
| Cl (mL/min/kg) | 2.30±0.51 | 1.91±0.57 | - | - |
| Cmax (μg/ml) | - | - | 4.10±0.98 | 1.59±0.59 |
| Tmax (min) | - | - | 226.56±51.82 | 132.08±70.07 |
Fig. 4.

a Plasma concentration-time curves of emodin after oral administration of emodin in SD male and female rats(8 mg/kg). (n=6). Each point represents an average of six determinations and the error bars are standard deviations of the mean.
b Plasma concentration-time curves of emodin glucuronide after oral administration of emodin in SD male and female rats(8 mg/kg). (n=6). Each point represents an average of six determinations and the error bars are standard deviations of the mean.
Table 5.
Pharmacokinetics parameters of emodin and emodun glucuronide after oral administration (n=6)
| Emodin | Emodin glucuronide | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Cmax (μg/ml) | 0.31±0.094 | 0.039±0.011 | 6.69±1.06 | 1.81±2.58 |
| Tmax (min) | 18.00±6.71 | 18.75±7.51 | 240 | 60 |
| AUC (min*μg/ml) |
65.70±34.77 | 33.82±4.09 | 2661.89±635.87 | 458.50±373.29 |
Plasma concentrations profile for emodin and emodin glucuronide after 4mg/kg emodin intravenous administration in male and female SD rats showed no gender-dependent difference (Fig 5). Emodin aglycone plasma concentrations declined biexponentially and could be detected only up to 4 hours in both male and female rats. Furthermore, emodin glucuronide was detected in plasma only 5 minutes after the i.v. administration of emodin in both male and female rats. The pharmacokinetic parameters for the emodin and emodin glucuronide after emodin 4mg/kg intravenous administration in male and female rats were listed in table 6.
Fig. 5.

a Plasma concentration-time curves of emodin after i.v. administration of emodin in SD male and female rats(4 mg/kg). (n=6). Each point represents an average of six determinations and the error bars are standard deviations of the mean.
b Plasma concentration-time curves of emodin glucuronide after i.v. administration of emodin in SD male and female rats(4 mg/kg). (n=6). Each point represents an average of six determinations and the error bars are standard deviations of the mean
Table 6.
Pharmacokinetics parameters of emodin and emodun glucuronide after intravenous injection (n=6)
| Emodin | Emodin glucuronide | |||
|---|---|---|---|---|
|
| ||||
| Parameters | Male | Female | Male | Female |
| A (μg/mL) | 11.52±16.40 | 7.38±5.67 | - | - |
| B (μg/mL) | 1.59±1.55 | 3.28±4.62 | - | - |
| α (1/mL) | 0.066±0.036 | 0.063±0.03 | - | - |
| β(1/mL) | 0.014±0.021 | 0.011±0.011 | - | - |
| t1/2Ke (min) | - | - | 167.40±50.91 | 251.31±114.20 |
| t1/2α(min) | 13.26±6.28 | 13.52±7.28 | - | - |
| t1/2β(min) | 187.38±174.52 | 118.50±83.09 | - | - |
| Vd /Vc(mL/kg) | 213.49±129.84 | 156.64±85.21 | 122.62±40.63 | 230.81±87.34 |
| AUC (min*μg /mL) | 422.71±163.40 | 282.52±98.42 | 2210.02±950.09 | 1054.42±290.31 |
| Cl (mL/min/kg) | 2.64±0.86 | 3.98±1.56 | 0.56±0.28 | 0.67±0.11 |
| K12 (1/min) | 0.030±0.015 | 0.023±0.017 | - | - |
| K21 (1/min) | 0.038±0.040 | 0.078±0.15 | - | - |
| K10 (1/min) | 0.022±0.020 | 0.032±0.018 | - | - |
A gender dependent significantly higher oral systemic bioavailability of total emodin in male than female SD rats has been shown for the first time.
Pharmacokinetic Studies
Significantly higher oral systemic bioavailablity of total emodin in male than female rats (Fig 3 and 4) may be explained by higher intestinal emodin absorption and lower intestinal excretion of emodin glucuronide in male than female SD rats [7]. Furthermore, the male rat intestinal and liver microsome studies published previously by our lab has also shown that the rate of glucuronidation of emodin was significantly higher in male than female SD rats at lower as well as higher concentrations in liver and intestine [7]. This higher rate of metabolism if excreted faster may also cause apparent increase in the intestinal absorption in male rats thereby causing increased oral bioavailability of total emodin (especially emodin glucuronide) in male than female rats.
Interestingly in another study, i.v. administration of emodin did not show any gender-dependent difference in total systemic bioavailability of emodin as well as individual systemic bioavailability of emodin and emodin glucuronide in male and female rats. This suggested that there was no difference in the elimination pathway of emodin and emodin glucuronide in male and female rats.
3. Conclusion
A rapid, sensitive and specific UPLC-MS/MS method was developed, validated and successfully applied for quantifying emodin in aqueous and rat plasma samples. Significant gender dependent difference of 4 folds was observed in absolute oral bioavailability of total emodin in male (1.6%) and female (0.4%) SD rats.
Acknowledgements
This work was mainly supported by National Basic Research Program of China (No. 2009CB522808), and the Key Project of National Natural Science Foundation of China (No. U0832002) both to ZL. MH was also supported by NIH GM070737.
References
- 1.Srinivas G, Anto RJ, Srinivas P, Vidhyalakshmi S, Senan VP, Karunagaran D. Emodin induces apoptosis of human cervical cancer cells through poly(ADP-ribose) polymerase cleavage and activation of caspase-9. Eur J Pharmacol. 2003;473:117–125. doi: 10.1016/s0014-2999(03)01976-9. [DOI] [PubMed] [Google Scholar]
- 2.Yi JY, J He, Gao R, Sang F, Tang H, Ye X, D. R. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108–116. doi: 10.1158/0008-5472.can-2820-2. [DOI] [PubMed] [Google Scholar]
- 3.Huang Q, Shen HM, Ong CN. Emodin inhibits tumor cell migration through suppression of the phosphatidylinositol 3-kinase-Cdc42/Rac1 pathway. Cell Mol Life Sci. 2005;62:1167–1175. doi: 10.1007/s00018-005-5050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Q, Shen HM, Ong CN. Inhibitory effect of emodin on tumor invasion through suppression of activator protein-1 and nuclear factor-kappaB. Biochem Pharmacol. 2004;68:361–371. doi: 10.1016/j.bcp.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Xiao W, Deng HZ, Ma Y. [Summarization of the clinical and laboratory study on the rhubarb in treating chronic renal failure] Zhongguo Zhong Yao Za Zhi. 2002;27:241–244. 262. [PubMed] [Google Scholar]
- 6.Wang GH, Nie QX, Li H, Zang C, Zhang BX, Zhao XM. [Comparative study on in vitro drug-release between Tuizhang ophthalmic gel and Tuizhang oculentum] Zhongguo Zhong Yao Za Zhi. 2007;32:683–687. [PubMed] [Google Scholar]
- 7.Liu W, Tang L, Ye L, Cai Z, Xia B, Zhang J, Hu M, Liu Z. Species and gender differences affect the metabolism of emodin via glucuronidation. AAPS J. 12:424–436. doi: 10.1208/s12248-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shia CS, Hou YC, Tsai SY, Huieh PH, Leu YL, Chao PD. Differences in pharmacokinetics and ex vivo antioxidant activity following intravenous and oral administrations of emodin to rats. J Pharm Sci. 99:2185–2195. doi: 10.1002/jps.21978. [DOI] [PubMed] [Google Scholar]
- 9.Kwan TH, Tong MK, Leung KT, Lai CK, Poon WT, Chan YW, Lo WH, Au TC. Acute renal failure associated with prolonged intake of slimming pills containing anthraquinones. Hong Kong Med J. 2006;12:394–397. [PubMed] [Google Scholar]
- 10.Srinivas G, Babykutty S, Sathiadevan PP, Srinivas P. Molecular mechanism of emodin action: transition from laxative ingredient to an antitumor agent. Med Res Rev. 2007;27:591–608. doi: 10.1002/med.20095. [DOI] [PubMed] [Google Scholar]