Abstract
Genistein, one of the most active natural flavonoids, exerts various biological effects including chemoprevention, antioxidation, antiproliferation and anticancer. More than 30 clinical trials of genistein with various disease indications have been conducted to evaluate its clinical efficacy. Based on many animals and human pharmacokinetic studies, it is well known that the most challenge issue for developing genistein as a chemoprevention agent is the low oral bioavailability, which may be the major reason relating to its ambiguous therapeutic effects and large interindividual variations in clinical trials. In order to better correlate pharmacokinetic to pharmacodynamics results in animals and clinical studies, an in-depth understanding of pharmacokinetic behavior of genistein and its ADME properties are needed. Numerous in vitro/in vivo ADME studies had been conducted to reveal the main factors contributing to the low oral bioavailability of genistein. Therefore, this review focuses on summarizing the most recent progress on mechanistic studies of genistein ADME and provides a systemic view of these processes to explain genistein pharmacokinetic behaviors in vivo. The better understanding of genistein ADME property may lead to development of proper strategy to improve genistein oral bioavailability via mechanism-based approaches.
Keywords: genistein, flavonoids, bioavailability, pharmacokinetics, ADME, metabolism, transporter, BCRP
Introduction
Genistein (4', 5, 7-trihydroxyisoflavone), one of the major soy isoflavones, has attracted major interests in the last few decades since several epidemiological studies indicated that intake of soy rich diet may be one of the important factors contributing to the lower incidence of breast and prostate cancer in Asian countries [1–5]. The beneficial effects did not appear to be race-dependent since immigrants from Asia to western countries who take less soybean enriched food have the similar cancer incidence as their western counterparts [2, 6, 7].
Among many isoflavones, genistein is the most studied and has demonstrated a wide variety of biological activities that make it a good candidate as a chemopreventive agent [8, 9]. Its chemoprevention mechanism includes down-regulation of genes related to cell proliferation and cell cycle, induction of apoptosis, inhibition of NF-κB activation and reduction of Akt protein level, down-regulation of androgen-mediated carcinogenesis, and antioxidation effects [10–16]. These molecular targets have been succinctly summarized by Banerjee et al and Sarkar et al in their previous review articles [17, 18].
Several completed clinical trials indicated that genistein has favorable beneficial effects on reducing the serum PSA (prostate specific antigen), blood cholesterol and displayed an inverse correlation between genistein intake and breast cancer risk [19, 20]. Although there were some concerns based on in vitro and rodent data which suggested that genistein stimulated proliferation of mammary cancer cells and tumor growth in mice [21, 22], a Meta-analysis of 18 studies including over 9000 breast cancer cases concluded that high soy intake was modestly associated with reduced breast cancer risk (odds ratio=0.86 and 95% confidence interval= 0.75–0.99) [23]. A recent published nested case-control study including 140,420 subjects during 10.6 years of follow up showed 66% reduction in breast cancer risk with higher concentration of plasma genistein (>354ng/ml) in Japanese women [24]. Strikingly, a randomized, double-blinded, placebo-controlled study of 383 women indicated that the magnitude of the protective association was similar in western populations despite their markedly different levels of intake compared to Asian women [25]. Intake of genistein may protect against the development of breast and prostate cancer and did not cause the development of new, estrogen-dependent breast or reproductive tissue cancers [20, 26]. In addition to its chemoprevention effect on breast and prostate cancer, genistein can be selectively cytotoxic to neoplastic and preneoplastic cells as well as lung and pancreatic cancer cell lines in vitro [27–29].
However, numerous pharmacokinetic studies showed that genistein has low oral bioavailability and its plasma or tissue concentrations were much lower than its in vitro IC50 [30–32] which could affect its in vivo efficacy and may also be the major factor contributing to the large variations or somewhat ambiguous pharmacological results in clinical trials [32, 33]. The cause of the poor absolute oral bioavailability of genistein have been investigated for two decades [20, 34], and significant progresses had been made to improve our understanding of genistein in vivo pharmacokinetics. But the consensus regarding pharmacokinetic behaviors of genistein remained unclear due to the inconsistent and complicated results observed by various biopharmaceutical investigations. Recently, several review articles had summarized the key factors that affecting the bioavailability of genistein in human from age, gender, food matrices, dose frequency, and ADME properties [35, 36]. This review focused on the updates of the recent progress on revealing the mechanisms of genistein absorption, metabolism, distribution, excretion and other factors responsible for the overall bioavailability. This review should strengthen our understanding on genistein poor intrinsic bioavailability and shed lights on the possible strategy to improve its oral bioavailability in human clinical trials.
It is noted that genistein and daidzein are two predominant isoflavones consumed as daily diet. Daidzein can also mitigate the adverse effects caused by genistein [37]. Many previous studies indicated that daidzein has similar ADME properties as genistein because of their similar structures [38, 39]. In order to reduce redundancy, only genistein was chosen as lead compound in this review paper, and the mechanism study of genistein's ADME can be also applied to daidzein.
Definition of Bioavailability
There are two kinds of calculations on the oral bioavailability of genistein discussed in previous publications. One refers to the absolute bioavailability which is calculated by comparing the plasma/urine AUCoral to AUCiv after dose correction (classic pharmacokinetics definition). It is the most accurate measurement and commonly used in animal studies when AUC is available following intravenous administration [40, 41]. The other definition is to calculate plasma or urine AUCoral and obtain the % recovery based on the administrated dose. It is usually applied in clinical pharmacokinetics or nutrition studies as the intravenous administration is not available due to practical or ethical issues [31, 32]. Because various sample process and analytical assays for genistein quantification have been used among numerous publications, i.e., radioactive label [32, 42], enzymatic hydrolysis treatment [43, 44] and direct measurement [40, 45], either free genistein (aglycone only) or total genistein (including metabolites) concentrations were reported as genistein. Since enzymatic hydrolysis (containing glucuronidases/sulfatases and glucosidase) treatment was used to process samples in most of clinical studies, the bioavailability in this paper is referred to the total genistein to avoid confusion and ambiguous comparison. However, considering most of the genistein presented in vivo is conjugates [40, 46], the bioavailability of genistein aglycone will also be reviewed when the data is available because aglycone is generally considered to be more biological active compared to genistein conjugates [47–49].
Genistein pharmacokinetic studies in animals
Our studies showed that more than 80% of genistein was converted to glucuronides and sulfates in FVB mice following both intravenous and oral administrations of genistein at 20 mg/kg and the absolute bioavailability of genistein aglycone was 23.4% [40]. Genistein showed very long half-life (46 hr) in vivo after oral administration suggesting recycling of genistein is substantial or an unknown mechanism of elimination in vivo [40]. Pharmacokinetic studies using different dose (2, 20 and 80 kg/mg) of genistein did not show proportional increase in term of AUC, which indicated non-linear pharmacokinetics as reported by Zhou et al. [46].
Some other pharmacokinetic studies showed that genistein had poor oral bioavailability as genistein aglycone but decent bioavailability as total genistein in rodents [41, 42, 50]. The plasma level of free genistein is usually 10 fold less than the value of total genistein, represented by much lower AUC in plasma, indicating the extensive metabolism in vivo. Andrade et al. determined that absolute bioavailability of genistein aglycone was <15% while the bioavailability of total genistein was close to 90% after oral administration of soy protein isolate in female Balb/c mice [41]. The similar results were reported by Coldham et al [42] that absolute bioavailability of free genistein was 6.8% while the total genistein bioavailability was >55% after oral administration of 4 mg/kg genistein in Wistar rats using radioactive labeled genistein. The higher bioavailability of total genistein indicated that genistein has a good absorption in GI tract and its extensive metabolism is one of the major reasons causing low oral bioavailability for genistein aglycone.
Most pharmacokinetic studies including ours showed that genistein has a short Tmax (<2 hr) indicating that genistein is absorbed fast after oral administration, but Tmax will extend significantly if soy protein was administrated. The terminal half life (t1/2) of total genistein is usually longer in oral administration than in i.v. administration and double peaks displayed in plasma concentration vs time profile indicated that enterohepatic recycling is substantial, which may partially explained why genistein has low oral bioavailability but long half-life in vivo [40, 42, 46]. Genistein pharmacokinetics in animals were summarized in Table 1 and it listed pharmacokinetic parameters of genistein from available animal data. Clearance and volume of distribution were not reported here because they need to be normalized by the absolute bioavailability, which were not available in most pharmacokinetics studies.
Table 1.
Summary of genistein pharmacokinetics in animals
| Species | Dose (mg/kg) | Analyte | Tmax (hr) | Cmax (μM) | AUC inf/AUC0-t (μM*h) | Elimination half-life (hr) | Bioavailability (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Wistar rat | 4 | Genistein | 0.5 | 0.08 ± 0 | 0.57 | 16.8 | 6.8 | [42] |
| 4 (i.v.) | Genistein | 25.63 ± 4.41 | 8.33 | 0.9 | [42] | |||
| 4 | Gen-Glu | 0.5 | 0.68 ± 0.45 | 4.57 | 5.8 | [42] | ||
| 4 (i.v.) | Gen-Glu | 0.1 | 5.93 ± 0.53 | 3.78 | 31.9 | [42] | ||
| 4 | Total | 0.5 | 10.15 ± 1.25 | 53.85 | 9.1 | 55.2 | [42] | |
| 4 (i.v.) | Total | 0.1 | 71.98 ± 25.07 | 97.64 | 14.4 | [42] | ||
| Wistar rat | 20a | Total | 11.0 ± 2.3 | [117] | ||||
| SD rat | 13b | Total | ~0.4 | [54] | ||||
| SD rat | 4 | Total | 0.5 ± 0.1 | 4.1 ±0.9 | 14.51 ± 5.66 | 4.5 ± 1.4 | [59] | |
| 20 | Total | 0.5 ± 0.1 | 12.3 ±2.4 | 45.85 ± 11.77 | 4.41± 1.2 | [59] | ||
| 40 | Total | 2 ± 0.3 | 18.1 ± 0.4 | 115.81± 20.55 | 5.3± 0.9 | [59] | ||
| 1 (i.v.) | Total | 9.41± 0.74 | 0.20± 0 | [59] | ||||
| 1.6 (i.v.)c | Total | 12.6 ± 3.3 | 9.74 ± 1.44 | 0.19 ± 0 | [59] | |||
| 64c | Total | 8.0 ± 1.0 | 13.7 ± 3.7 | 189.70 ± 45.77 | 7.5 ± 0.7 | 48.66 | [59] | |
| SD rat | 12.5 (i.v.) | Genistein | 15.27 | 10.3 | [46] | |||
| 6.25 | Genistein | 0.41 | 1.67 | 3.2 | 21.9 | [46] | ||
| 12.5 | Genistein | 1.0 | 5.11 | 8.5 | 33.5 | [46] | ||
| 50 | Genistein | 2.77 | 11.35 | 8.4 | 19.0 | [46] | ||
| SD rat | 20d | Total | 6.5 ± 3 | 3.53 ± 1.03 | 19.05 ± 6.69 | 7.0 ± 1.8 | 29.14 ± 12.35 | [144] |
| 20e | Total | 5.0 ± 3.5 | 3.93 ± 1.87 | 30.38 ± 21.08 | 3.6 ± 1.8 | 57.78 ± 17.40 | [144] | |
| 20f | Total | 4.8 ± 3.8 | 7.68 ± 1.67 | 49.54 ± 10.35 | 5.8 ± 2.1 | 62.34 ± 8.07 | [144] | |
| SD rat | 40 | Genistein | 4.8 ± 5.0 | 0.1 0± 0.02 | 0.54 ± 0.1 | 3.6 ± 0.9 | [92] | |
| 40 | Gen-Sult | 1.5 ± 1.7 | 0.5 5± 0.2 | 3.6 ± 2.1 | 4.3 ± 1.1 | [92] | ||
| 40 | Gen-Glu | 1.9 ± 1.9 | 8.6 ± 1.3 | 42.9 ± 34.5 | 4.3 ± 0.8 | [92] | ||
| CD2F1 mice | 9.2 (i.v.) | Total | 110.74 | 9.77 | [34] | |||
| 25.7 (i.v.) | Total | 241.48 | 21.96 | [34] | ||||
| 52 (i.v.) | Total | 286.6 | 46.67 | 0.7 ± 0.3 | [34] | |||
| 180 | Total | 4.1 | 12 | [34] | ||||
| 45 | Total | 2.6 | 20.5 | [34] | ||||
| Balb/c mice | 1.2 (i.v.) | Total | 0.65 | 1.4 | [41] | |||
| 1.2 (i.v.) | Genistein | 0.18 | 0.16 | [41] | ||||
| 1.2 | Total | 0.57 | 1.4 | 89 | [41] | |||
| 1.2. | Genistein | 0.02 | 0.23 | 9.4 | [41] | |||
| 1.2.g | Total | 0.55 | 1.23 | 86 | [41] | |||
| 1.2.g | Genistein | 0.03 | 1.0 | 14 | [41] | |||
| FVB mice | 20 | Genistein | 1.3 ± 0.3 | 0.71 ± 0.22 | 6.1 ± 0.5 | 46.37 ± 30.56 | 23.4 | [40] |
| 20 (i.v.) | Genistein | 25.85 ± 13.8 | 14.2 ± 9.87 | [40] | ||||
| 20 | G-7-G | 2.8 ± 2.3 | 0.98 ± 0.94 | 2.79 ± 2.26 | [40] | |||
| 20 (i.v.) | G-7-G | 0 | 57.93 ± 17.98 | 26.19 ± 4.67 | ||||
| 20 | G-4'-G | 1.5 ± 1.1 | 0.53 ± 0.38 | 2.55 ± 0.79 | [40] | |||
| 20 (i.v.) | G-4'-G | 0.1 ± 0.1 | 14.26 ± 4.86 | 11.40 ± 2.55 | ||||
| 20 | G-4'-S | 3.0 ± 2.2 | 0.25 ± 0.22 | 1.08 ± 0.72 | [40] | |||
| 20 (i.v.) | G-4'-S | 0.1 ± 0.1 | 3.97 ± 0.57 | 2.24 ± 0.32 | ||||
| 20 | G-7-S | 3.0 ± 2.2 | 0.65 ± 0.56 | 2.85 ± 1.58 | [40] | |||
| 20 (i.v.) | G-7-S | 0.1 ± 0.1 | 10.74 ± 1.64 | 6.86 ± 1.23 | ||||
| Monkey | 4.8 | Total | ~0.1 μM | [54] |
After administration of isoflavone-rich extract from soy flour by stomach tube and the dose is equivalent to 20 mg genistein/kg body weight
After daily administration of diets containing soy protein isolate and dose is the equivalent genistein amount in the diet
After administration of genistin (genistein glucoside) and genistin doses of 64 and 1.6 mg/kg are equivalent to genistein doses of 40 and 1 mg/kg, respectively.
After administration of aglycone mixture (genistein, daidzein and glycitein at the ratio of 1:0.5:0.2) and genistein dose is 11.76 mg/kg.
after administration of glycoside mixture (genistin, daidzein and glycitin) and genistin dose is 19.09 mg/kg
After administration of NOVASOY™ containing isoflavone
After administration of SPI (soy protein isolate) with the same equivalent dose
Genistein pharmacokinetic studies in humans
Consistent to genistein pharmacokinetics in animals, genistein was extensively metabolized in human, and the unconjugated genistein concentration in plasma was very low [51–53]. The genistein aglycone was accounted for only 3.7% in the first 2h and 1.6 % at steady state in women after single oral dose of 50 mg genistein [32]. Gu et al. also found that genistein aglycone only accounts to <1% in human plasma after soy beverage consumption [54]. Similarly, the genistein aglycone was found to be less than 1% in human urine [55]. The major metabolites identified in human are phase II metabolites including glucuronides and sulfates, but not phase I metabolites. Adlercreutz et al. showed that 62.5 % of genistein was presented as monoglucuronides, followed by 12.5 % of diglucuronides, 11.5 % sulfoglucuronides, 3.5 % disulfates, 2.8 % monosulfates, and then 0.9 % free genistein in men urine samples after soy diet consumption [56]. Gu et al. showed that 78% of genistein presented as glucuronides and 20% presented as sulfates in human plasma while 86% of genistein appeared as glucuronides and 13% appeared as sulfates in urine sample [54]. Although large interindividual variations were observed in human clinical pharmacokinetic studies, it is clear that the plasma level of total genistein is in micro molar range while the genistein aglycone is in hundred nano molar range in vivo [53].
The half-life (t1/2) reported in most human pharmacokinetic study is the half-life of total genistein, and it is actually pseudo values because it is the combination of metabolism and excretion rates of multiple metabolites. Therefore, we usually observe that t1/2 of genistein aglycone is much shorter than the t1/2 of total genistein [51]. Genistein pharmacokinetics results in human were summarized in Table 2.
Table 2.
Summary of genistein pharmacokinetics in humans
| Source | Dose (mg/kg) | No. of subjects | Analyte | Tmax (hr) | Plasma concentration (μM) | AUC inf(μM*h) | Urinary excretion % of intake | Elimination half-life (hr) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Pure genistein | 50 | 6 | Total | 5.2 | 1.26 ± 0.27 | 16.8 | 6.8 | [32] | |
| Soy beverage | 13 | 10 | Total | 1.1 | [54] | ||||
| Clover phytoestrogen (Trinovin) | 240 | 40 | Total | 0.656 | [115] | ||||
| Soy beverage | 1 | 12 | Total | 4.4 ± 0.4 | 0.65 ± 0.04 | 8.7 ± 0.9 | 7.9 ± 0.7 | [44] | |
| Gen_Glu | 6.0 | 0.50 | 8.0 | 8.4 | [44] | ||||
| Gen_Sult | 4.5 | 0.14 | 1.6 | 5.7 | [44] | ||||
| Soymilk | 0.2 μmol | 12 | Total | 5.9 ± 0.4 | 0.41 ± 0.06 | 4.51 ± 0.69 | 20.2 | [31] | |
| Β-glucosidase treated soymilk | 43.1 μmol | 12 | Total | 1.1 ± 0.1 | 0.87 ± 0.05 | 8.55 ± 0.90 | 30.3 | [31] | |
| Fermented soymilk | 43.3 μmol | 12 | Total | 1.0 ± 0 | 1.02 ± 0.06 | 10.13 ± 1.18 | 28.0 | [31] | |
| Soymilk | 0.59 | 14 | Total | 1.78 ± 0.83 at 6h | 27.6 | [143] | |||
| soygerm | 0.59 | 14 | Total | 0.47 ± 0.29 at 6h | 29.7 | [143] | |||
| Soy food PTI G-2535 | 2 | 4 | Total | 6.0 | 5.12 | 91.1 | [166] | ||
| 4 | 3 | Total | 4.5 | 14.12 | 301.4 | [166] | |||
| 8 | 3 | Total | 8.0 | 16.34 | 221.2 | [166] | |||
| Soy food PTI G-4660 | 2 | 4 | Total | 6.0 | 5.02 | 92.2 | [166] | ||
| 4 | 3 | Total | 4.5 | 12.18 | 205.7 | [166] | |||
| 8 | 4 | Total | 3.0 | 4.34 | 112.0 | [166] | |||
| 13C-labled genistein | 0.4 | 16 | Total | 0.55 ± 0.09 | 6.75 ± 1.29 | 8.9 ± 1.4 | [39] | ||
| 0.8 | 16 | Total | 0.87± 0.14 | 9.77 ± 1.32 | 8.3 ± 1.2 | [39] | |||
| Soy food | 1–16 | 30 | Total | 3.5–8.0 | 0.9–27 | 7.7–18 | 9.2 | [51] | |
| Aglycone | 3.2 | [51] | |||||||
| Soymilk powder | 0.92 | 7 | Total | 1.43 ± 0.41 at 6h | 10.31 ±4.08 | [137] | |||
| 1.83 | Total | 2.78 ± 0.75 at 6h | 9.96 ±4.14 | [137] | |||||
| 2.78 | Total | 4.59 ± 1.35 at 6h | 10.49 ±4.03 | [137] | |||||
| Soybean flour | 0.97 | 6 | Total | 8.0 ± 0.7 | 4.09 ± 0.94 | 22 ± 4 | 5.7 ± 1.3 | [167] | |
| Soy beverage | 1 | 12 | Total | 10.0 ± 3.5 | [55] | ||||
| Gen-Glu | 6.0 ± 0.04 | [55] | |||||||
| Gen-Sult | 6.0 ± 0.04 | [55] | |||||||
| Soy nuts | 9.8 | 10 | Total | 4.9 ± 0.8 | 0.59 ± 0.11 | 10.1 ± 2.1 | 25.2 ± 5.3 | 10.8 ± 0.7 | [82] |
| 19.6 | Total | 4.0 ± 0.7 | 1.22 ± 0.30 | 17.3 ± 3.9 | 13.4 ± 2.1 | 10.0 ± 0.5 | [82] | ||
| 39.2 | Total | 6.0 ± 0.6 | 2.21 ± 0.36 | 31.2 ± 6.7 | 15.8 ± 2.7 | 9.6 ± 0.4 | [82] | ||
| Soy isoflavone formulation | 3.3 | 20 | Aglycone | 3.5 | 0.03 | 0.191 | 0.1 | 4.0 | [53] |
| Total | 5.5 | 2.0 | 32.4 | 13.9 | 10.3 | [53] | |||
| Non-fermented soybeans | 2.85 μmol | 11 | Total | 5.0 ± 0.7 | 0.73 ± 0.08 | 9.20 ± 1.39 | [168] | ||
| Fermented soybeans | 29.32 μmol | Total | 2.1 ± 0.6 | 1.04 ± 0.07 | 11.05 ± 0.84 | [168] | |||
| Baked soybean powder | 30.2 | 7 | Total | 8.0 | 2.44 | 17.6 | 8.4 | [169] |
Mechanistic Studies on genistein ADME
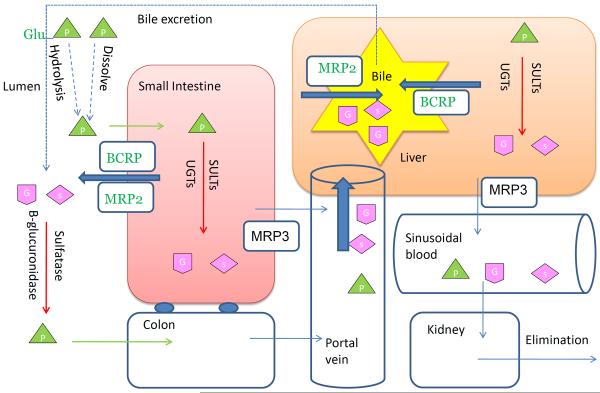
The profile of plasma or urine concentration against time represents the combined effects of how body affects the disposition of genistein. In order to systemically investigate the pharmacokinetics of genistein and its low oral bioavailability, a detailed understanding of ADME property is necessary and pivotal. We proposed a schematic diagram showing genistein in vivo disposition after oral administration (Fig. 2) and it revealed the mechanism of genistein ADME from the following perspectives.
Figure 2.
Schematic representation showing the disposition of genistein in systemic circulation after oral administration of genistin or genistein (vertical view).  : genistin;
: genistin;  : genistein;
: genistein;  : genistein glucuronides;
: genistein glucuronides;  : genistein sulfates;
: genistein sulfates;
Physical and chemical property
Gensitein (4', 5, 7-trihydroxyisoflavone) is a heterocyclic diphenol with three hydroxyl groups and its chemical name is 4',5,7-trihydroxy-isofavonethe or 5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one [57]. Genistein, containing in soy-derived foods, is present and ingested mainly as genistin, a glycoside form of genistein [58]. Because of low permeability of genistin, its absorption depends on the permeation of genistein after bacteria hydrolysis of genistin [59]. Genistein is a lipophilic compound (Log P=3.04) and has low molecular weight (Mw= 270) [60]. It showed low aqueous solubility in water (5.3 μM), and the highest solubility in methanol (32.5 mM) at 25°C [61]. The poor aqueous solubility may be one of the reasons for non-linear pharmacokinetic behaviors of genistein and may explain why simply increasing the dose cannot improve its bioavailability through saturation of metabolic enzymes [46]. Genistein is thermodynamically and chemically stable and its antioxidant activity sustains for more than 20 days at pH= 7 at 70 °C [62].
Absorption
Genistein is absorbed rapidly and nearly completely in vivo due to small molecular weight and favorable lipophilic property. It showed high permeability (>3×10−5 cm/s) in the human Caco-2 cell model at 37°C [63, 64] where any compound with permeability more than 1×10−5 cm/s is considered as high permeable in humans [65, 66]. Bidirectional transport in BCRP or MDR overexpressed MDCKII cells indicated that genistein is the weak substrate of BCRP with the efflux ratio close to 2, but not a substrate of P-glycoprotein [67]. Considering the high permeability of genistein in Caco-2 and MDCKII cells, passive diffusion is the major transport mechanism but BCRP may play a role in limiting its intestinal absorption [67, 68].
In situ intestinal perfusion studies showed that genistein has moderate to high absorption in rat/mouse intestine with 77 % absorption at 10 μM and 46.4 % at 12 μM [69, 70], while the lowest absorption percentage (28%) was reported when concentration of genistein was 35 μM in perfusate [63]. Genistein showed region dependent absorption in intestine and the amounts absorbed in duodenum (44%), colon (35%) were higher (p<0.05) than that in terminal ileum (18%) and jejunum (16%) [63]. It is understandable since duodenum is a more absorbing intestinal segment than ileum and jejunum, and colon has less expression level of BCRP which may increase its absorption, but the real mechanism remained unclear.
The Pharmacokinetic studies confirmed the in vitro and in situ absorption studies by showing that 56% of genistein was absorbed in male rats and completely absorbed (~100%) in female rats [42, 71]. Tmax and absorption rate constant indicated that genistein is rapidly absorbed in vivo, which is consistent with the transport results shown in Caco-2 cell model.
Inconsistent results were reported comparing oral bioavailability of genistin and genistein after their oral administrations. Steensma et al found that genistein (aglycone) showed higher bioavailability based on the portal vein plasma concentration at the first point of detection at 15 min after dosing [72] which was in agreement with what Liu et al. reported that permeability of genistein was 5 times higher than genistin in Caco-2 cells and genistin was quickly hydrolyzed to genistein in the upper intestine of rat to be absorbed [64]. Kwon et al showed the opposite results that genistin (glycoside form) had higher bioavailability (48.66 %) than aglycone (30.75 %) [59] after 40 mg/kg oral administration in rat. In any means, oral administration of genistin showed comparable plasma level compared to genistein indicating the hydrolysis of genistin to genistein is rapidly and completely in intestine and did not affect its absorption.
Human clinical studies showed that genistein had moderate absorption after oral administration of soy supplements with high contents of genistein or genistin [45, 73]. Overall, the fast and extensive absorption of genistein was not the obstacle responsible for its low oral bioavailability. The absorption results in different models were summarized in Table 3.
Table 3.
Summary of genistein absorption at in vitro, in situ and in vivo models in rodents and humans
| Human Caco-2 cells | |
|
| |
| Permeability | 3×10−5 cm/sa [63], 3.6×10−5 cm/sa [64], 5×10−5 cm/sa [128] |
|
| |
| Kinetic constants | 5.02 h−1b [170] |
|
| |
| Rat IEC-18 cells Kinetic constants | 4.02 h−1b [170] |
|
| |
| Human HCEC cells Kinetic constants | 3.79 h−1b [170] |
|
| |
| Rat intestine perfusion | |
|
| |
| P*eff | >1.5b [64] |
|
| |
| P*w | >7b [64] |
|
| |
| %absorbed | 77% @ one-site rat intestine perfusion using 10 μM genisteinb [70], |
| 28% (≈ 450 nmol/30 min) @ four-site rate intestine perfusion using 35 μM genisteinb [63] | |
| 44% in duodenum, 35% in colon, 16% in jejunum and 18% in terminal ileumb [63] | |
| 46.4% @ one-site rat intestine perfusion using 12 μM genisteinb [69] | |
|
| |
| uptake | 5.5% uptake in the intestinal tissueb [69] |
|
| |
| Mouse intestine perfusion | 4.5 nmol/30 min/10 cm using 12 μM genistein (small intestine) [133] |
|
| |
| Rat pharmacokinetics | |
|
| |
| Recovery in urine | 66.4–67.3%b [71], 20%a [117] |
|
| |
| Tmax | 0.5 hr at 4 and 20 mg/kg, 2 hr at 40 mg/kgb [59] |
|
| |
| Recovery in bile after genistein infusion into the duodenum | 70–75% at 0–4 hr periodb [89] |
|
| |
| Absorption | 56% in male and 111% in female ratsb [42] |
|
| |
| Mice pharmacokinetics | |
|
| |
| Absolute bioavailability | 89%b [41] |
|
| |
| Tmax | 1.25 hr at 20 mg/kga [40] |
|
| |
| Human pharmacokinetics | |
|
| |
| Absorption rate constants | 0.24–0.5 h−1 after soymilk ingestiona [73] |
Measurement of free genistein
Measurement of total genistein
% represented the recovery of genistein
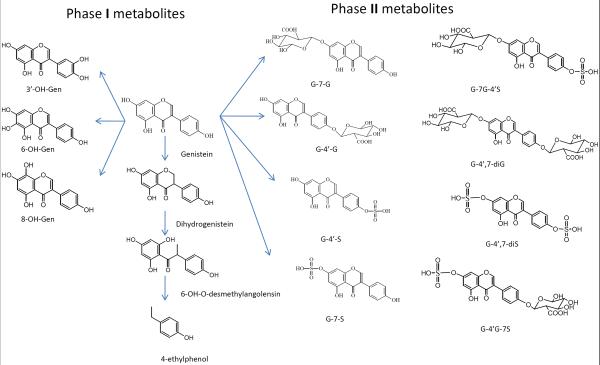
Metabolism
Genistein goes through diverse and extensive metabolism in vivo and its metabolism reaction includes oxidation, reduction and conjugation [74–76]. The structures of major metabolites of genistein in vivo were showed in Fig. 1. The major metabolic pathways of genistein are glucuronidation and sulfation [40, 44, 45] with limited CYP reaction and the levels of glucuronidations were much greater than the corresponding sulfates or aglycones in humans [43]. Genistein was fast metabolized with plasma Tmax less than 30 min after i.v., p.o. and i.p. administration of genistein, indicating the extremely high metabolism rate in intestine and liver [40]. Because of high activity of UGTs and SULTs in intestine [77, 78], genistein was extensively metabolized in the enterocytes. After entering into portal vein, the remaining genistein aglycone can be further sequestered by liver and go through hepatic metabolism [79, 80]. Several other tissues, like kidney, heart and lung were also reported to have high expression level of UGTs and SULTs, which should be enable to metabolize genistein in the organs [78, 81]. Metabolisms of genistein in different models were summarized in Table 4.
Figure 1.
Genistein metabolism pathway and its major phase I and phase II metabolites in vivo.
Table 4.
Summary of genistein metabolism in various models
| Human liver microsome | Six hydroxylation products, the major ones: 5,6,7,4'-tetrahydroxyisoflavone, 5,7,8,4'-tetrahydroxyisoflavone, 5,6,3',4'-tetrahydroxyisoflavone [75, 99], monoglucuronides, diglucuronides [87, 171] |
|
| |
| Wistar rat liver microsome | 5,6,7,4'-tetrahydroxyisoflavone, 5,7,8,4'-tetrahydroxyisoflavone [96] |
|
| |
| Human breast cancer cells ZR-75-1, BT-20, T47D | G-7-S [94] |
|
| |
| MCF-7 | G-7-S, hydroxylated and methylated form of genistein sulfate [102] |
|
| |
| Human mammary epithelial (HME) cells | No significant metabolites [102] |
|
| |
| Rat small intestine | 31.3% glucuronides [69] |
|
| |
| Rat anaerobic caecal cultures | 4-hydroxyphenyl-2-propionic acid, 6'-hydroxy-O-desmethylangolensin, dihydrogenistein glucuronide [172] |
|
| |
| Mice | |
|
| |
| Intestine, liver S9 fraction | G-7-G, G-4'-G, G-7-S, G-4'-S [93] |
|
| |
| plasma | G-7-G, G-4'-G, G-7-S, G-4'-S [40] |
|
| |
| Human metabolism | |
|
| |
| In breast | G-7-G, G-4'-G and G-7-G is the major one [101] |
|
| |
| In prostate | Monoglucuronide is the major one with trace amounts of diglucuronide and sulfate [113] |
|
| |
| In plasma | G-4',7-diG, G-4',7-diS, G-7G-4'S, G-7-G, G-4'-G, G-7-S, G-4'-S were found and the major conjugates are G-7G-4'S, G-4',7-DiG [45, 52] |
| 48% glucuronide, 8% sulfate, 30% mixed conjugates (one glucuronide and one sulfate) [44] | |
|
| |
| In urine | Mono and di-glucuronide, mono and di-sulfate, mix glucuronides and sulfate [52] |
| 53–76% in monoglucuronide, 12–26% diglucuronide, 2–15% sulfoglucuronide and 1–4% disulfate fractions [56] | |
| 3'-OH, 8-OH, 6-OH, 3',6-diOH, 3',8-diOH genistein [98] | |
| Five hydroxylation products, 5,6,7,4'-tetrahydroxyisoflavone, 5,7,8,4'-tetrahydroxyisoflavone, 5,6,3',4'-tetrahydroxyisoflavone, 5,7,8,3',4'-pentahydroxyisoflavone, 5,6,7,3',4'-pentahydroxyisoflavone, [75] | |
| 6'-hydroxy-O-desmethylangolensin [173] | |
| Dihydrogenistein [74, 173] | |
% represented the recovery of genistein
UGT-mediated metabolism
In human clinical studies, genistein was mainly presented as glucuronide in plasma and urine [44, 54, 56, 82]. Recent studies found that G-7G-4'S and G-4',7-diG were the major metabolites in human plasma, and G-7-G and G-4'-G were the major metabolites in human urine [45, 52, 83, 84]. In rats and mouse pharmacokinetic studies, monoglucuronide was the major metabolites in plasma after oral administration of genistein [40, 46]. G-7-G and G-4'-G were found in human hepatocytes and Caco-2 cells, and G-7-G was the major glucuronidation product [85].
Human UGT studies showed that UGTs (1A1, 1A4, 1A6, 1A7, 1A9 and 1A10) catalyzed 7-and 4'-glucuronidation of genistein [43, 44, 55, 86]. Tang et al., reported that the rank order of metabolism capability for genistein glucuronide was UGT1A8 > 1A9 > 1A10 > 1A1 in term of clearance (Table 5) [87] and Doerge et al., found the consistent results that the rank decreased in the order of 1A9 > 1A10 > 1A1 > 1A6 > 1A7 except UGT1A8 was not measured [43]. The ability of human tissue microsomes to catalyze the glucuronidation of genistein decreased in the order of kidney > colon > liver [43]. It is not surprised since human kidney and colon has been shown to have higher expression level of UGT1A9 and 1A10 than liver [77, 88], and the higher glucuronidation activity in colon was consistent with a previous study that showed genistein infused into rat duodenum was rapidly converted to glucuronides in the intestine [89]. When genistein concentration increased, glucuronidation rate always increased in human liver microsome but the rate first went up and then came down in human intestinal microsome [87]. Furthermore, Tang et al., found that the prediction of metabolic “fingerprinting” to genistein (systematic metabolic profiling studies utilizing expressed human UGT isoforms in determining major metabolites and predicting the major organ of metabolism) was rather successful in the liver microsome, but not successful in the intestinal microsomes. The results showed that genistein has UGT isoform-specific metabolic patterns that are concentration-dependent [87]. UGT 1A9 and 1A10 followed classic Michaelis-Mentern kinetics for genistein glucuronidation, whereas UGT1A8 followed autoactivation kinetics and UGT1A1 showed substrate inhibition kinetics when genistein concentration increased from 2.5 to 35 μM [87].
Table 5.
Summary of apparent kinetic parameters of genistein glucuronidation in different rat microsomes and human UGT isoforms
| Kinetic parameters | Sprague-Dawley rats | Human UGT isoforms | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | Colon | Liver | UGT1A1 | UGT1A8 | UGT1A9 | UGT1A10 | |
| Vmax1 (nmol/min/mg) | 1.445 | 3.171 | 1.423 | 0.435 | 3.713 | NA | 1.24 | 2.09 | 12.34 |
| Km1 (μM) | 4.04 | 6.628 | 8.239 | 3.186 | 23.6 | NA | 2.24 | 1.29 | 1.04 |
| Vmax1 /Km1 (ml/min/mg) | 0.357 | 0.478 | 0.172 | 0.136 | 0.157 | NA | 1.81 | 0.62 | 0.08 |
| Vmax2 (biphasic) (nmol/min/mg) | 0.041 | 1.59E3 | 0.826 | ||||||
| Km2 (μM) | 7.994 | 2.74E3 | 0.71E-7 | ||||||
| Vmax-0 (autoactivation) (nmol/min/mg) | NA | 0.01 | |||||||
| Vmax-d (autoactivation) (nmol/min/mg) | NA | 1.23 | |||||||
| R2 | 0.998 | 0.995 | 0.963 | 0.995 | 0.989 | NA | 0.96 | 0.98 | 0.99 |
| AIC | −55.67 | −19.13 | −21.85 | −53.61 | −17.9 | NA | −7.98 | −26.91 | −25.23 |
In rat UGTs, the rank order of metabolism ability for genistein glucuronide was UGT1A7 > 2B1 > 1A6 > 1A1 [90]. Genistein glucuronidation at different intestine region and liver were also determined before [70]. Apparent kinetic parameters of genistein glucuronidation in different rat microsomes and human UGT isoforms were [70, 87, 91] summarized in Table 5. The results showed that intestinal microsome has larger clearance than liver microsome indicating the significant role of intestine in term of genistein metabolism.
When UGT1A isoforms were knockout, UGT2Bs contributed and compensated genistein glucuronidation at some extent based on the similar excretion of genistein glucuronides found in jejunum and ileum between Gunn (UGT 1A deficient) and Wistar rats [90]. However, the excretion of genistein glucuronides found in duodenum, colon and bile were significantly decreased in Gunn rats compared to wild-type Wistar rats [90]. In pharmacokinetic studies conducted by our group, we found that plasma genistein glucuronides level did not change significantly in Gunn rats compared to Wistar rats, indicating that UGT2B may compensate for glucuronidation of genistein by UGT 1A. To our surprise, we found that genistein sulfate concentrations significantly increased by more than 100-fold in Gunn rats after oral administration indicating besides UGT2B compensation, sulfotransferase (SULTs) may have higher activity and make major contribution to genistein metabolism in the absence of UGT1A (unpublished data, Kaustubh Kulkarni and Ming Hu).
SULT-mediated metabolism
Sulfotransferase is the second most important metabolism pathway for genistein in humans after consumption of soy food [52, 54, 56]. Genistein sulfates were also found to have antioxidant activity similar to genistein in plasma [48]. Hosoda et al. found monosulfate (G-7-S, G-4'-S), disulfate (G-4'S-7S), sulfoglucuronide (G-7G-4'S and G-7S-4'G) in human plasma and G-7G-4'S took account of 54% of the total genistein metabolites. The levels of genistein sulfates were much lower in urine compared to the values in plasma [84]. Similar results were found in rodents in that genistein sulfates levels were slightly lower than its corresponding glucuronides in mice [40] but were significantly lower or below the detection limit in rats plasma, intestine and bile after oral administration of genistein [46, 70, 90, 92].
Human SULTs (1A1, 1A2, 1E and 2A1) were found to catalyze genistein sulfation and the activity decreased in the order of 1A1*2>2A1>1E>1A2=1A3 [43]. Genistein can be converted into G-4'-S and G-7-S in Caco-2 cells [93] and 95% genistein was converted to G-7-S in breast cancer cell lines, such as ZR-75-1, BT-20 and T-47D cells [94].
CYP-mediated metabolism
Although oxidative metabolites are not the major biotransformation products of genistein in human [74, 75], several phase I metabolites of genistein, mainly hydroxylated metabolites, have been identified in vitro and in vivo [75, 85, 95–97]. 3'-OH-Gen, 6-OH-Gen, 8-OH-Gen are the major hydroxylated metabolites found in human liver microsomes [75] while 3'-OH-Gen and 8-OH-Gen were the major oxidative metabolites found in human urine (no NMR confirmation) [98]. Four monohydroxylated and two dihydroxylated metabolites were found in rat liver microsomes [96]. As for enzymatic research, Hu et al. identified that CYP1A2 was the predominantly isoform responsible for 3'-OH-genistein (Orobol) by using inhibitory monoclonal antibodies of various CYP isoforms [99] and the same results were confirmed by Breinholt et al. that Orobol was the only metabolite formed after incubation with CYP1A2 [95]. Other than CYP1A2, CYP 2E1, CYP2D6 and CYP3A4 also catalyzed genistein oxidation reaction but to a lesser extent [95, 99]. Bacteria flora in colon catalyzed reductive biotransformation of genistein to dihydrogenistein, which were found in rat urine [74, 97], however it may not occur in rat small intestine [69]. Unlike daidzein, genistein has less intestinal bacteria product indicating bacteria degradation is not the major metabolism pathway of genistein [74]. Genistein metabolism experimental results were summarized in Table 2.
Metabolism in breast and prostate tissues
Aside from the metabolism in intestine and liver, whether genistein goes through extensive metabolism in breast and prostate tissues is important to evaluate the composition and efficacy of genistein in the active site [86, 100]. Bolca et al. [101] found that 98% of genistein was presented as glucuronides in breast tissue after 5 days soy milk/supplement consumption in healthy women. Both G-7-G and G-4'-G were determined, but glucuronidation in C7-position was preferred. No monosulfates or sulfoglucuronides of genistein were observed in breast tissue.
In vitro studies performed in human breast cancer and mammary epithelial cells showed different metabolic pathway of genistein. Peterson showed that G-7-S was the major metabolites in several breast cancer cell lines including ZR-75-1, BT-20 and T47D cells [94]. IC50 values of these cell lines did not correlate well with the production of G-7-S indicating that sulfate was less active on inhibition of cell growth compared to aglycone. Similar results were observed in mammary epithelial cells that genistein was converted to genistein-7-sulfate and its hydroxylated or methylated form of genistein sulfate [102]. The discrepancy of genistein metabolism pathway between in vitro cell lines and in vivo human breast tissue is unclear, and the possible explanation may be the high expression level of sulfotransferase in breast cancer cell line [94].
Similarly, Guy et al determined that genistein was mainly presented as glucuronides in human prostate after taking soy supplement for 3 days at 112.5 mg isoflavone aglycone/day. Genistein diglucuronide and sulfate were also found but in a trace amount, the metabolism of genistein in prostate was consistent with its liver and intestine metabolism [45, 103].
Distribution
Uptake and distribution of genistein in tissues and plasma may hold the key to the potent chemoprevention effects in these active sites. Coldham et al., found that genistein has the highest concentrations in gut (18.5 μg/g), and followed by liver (0.98 μg/g), plasma (0.79 μg/g) and then other reproductive tissues (uterus, ovary, vagina and prostate, ranged from 0.12–0.28 μg/g) in rats [71]. Comparison of tissue residues would suggest different mechanisms of uptake, possibly by interaction with estrogen receptors. The high concentration in GI tract was sufficient to have direct antiproliferative effects while the concentrations in other tissues also exceed its EC50 to compete with estradiol for activation of estrogen receptor [71, 104]. Consistent results were reported by Zhou et al who showed that most of genistein was accumulated in stomach (1.83 μg/g), followed by intestine (1.50 μg/g), liver (1.13 μg/g), kidney (0.41 μg/g), lung (0.27 μg/g), heart (0.23 μg/g), brain (0.1 μg/g), reproductive organs (0.09–0.22 μg/g), and then muscle (0.07 μg/g) at 6 hr after oral dose of 12.5 mg/kg genistein in rats [46]. Chang et al showed different results in that genistein accumulation was higher in reproductive tissues than in liver and brain of male rat, but much higher genistein concentrations were found in female liver [105]. Gilani et al recently showed slightly different results in that genistein is the highest in plasma (6.18 μM), followed by liver (0.45 nmol/g) and mammary gland (0.11 nmol/g) in rats [106]. Overall, genistein showed higher tissue distribution in GI tract and liver but did not show concentrated accumulation in other tissues as compared to plasma, the possible reason was that most of genistein was sequestered by intestine and liver to get metabolized and enterohepatic recycling keeps genistein in these organs. And high hydrophilic genistein conjugates prevents their permeation and accumulation in other tissues, except for urine excretion.
Breast tissue distribution
According to the epidemiological studies, intake of isoflavone can potentially decrease the incidence of breast and prostate cancer. The major proposed pharmacological mechanism of genistein is to competitively bind to estrogen receptors (ER) α and β, with a higher affinity to ERβ than ERα to block estrogen signaling in breast cancer cells [107–109]. Recently, some studies showed genistein may promote carcinogenesis in mammary tissues [20, 110]. Therefore, it is crucial to understand the distribution of genistein in breast tissue in order to correlate exposure with its protective or adverse response to breast cancer. Genistein showed relatively low concentration in the breast tissue compared to plasma after intake of high dose of soy isoflavones in human which indicated weak estrogenic response on the breast [111]. Bolca et al. reported that total genistein ranged from 92 to 494 pmol/g while the total genistein concentration in plasma ranged from 135 to 2831 nmol/L. Further analytic measurement showed that the genistein aglycone only accounted for 2 % of total genistein in breast, and the major metabolites identified was G-7-G [101].
Maubach et al also reported similar results in that intake of soy-based food supplements for 5 consecutive days did not result in significantly higher genistein concentrations in human breast tissue when compared to the placebo group. The genistein concentration were in the low nanomolar range in breast, whereas concentrations were a hundred-fold higher in plasma [112]. These results suggested that genistein may not accumulate in breast and the biological effect may be correlated to the tissue concentration.
Prostate tissue distribution
The genistein concentration in human prostate tissue is highly variable among different publications. Guy et al., reported that the genistein concentration recovered after β-glucuronidase/sulfatase hydrolysis in human prostate was 0.58 nmol/g, lower than the plasma concentration (0.78 μM) after receiving 112.5 mg isoflavone/day for 3 days (genistein content=65.7%) [113]. Genistein aglycone only represented less than 10% of the total genistein concentrations in human prostate [113]. Hong et al., published similar results that genistein concentration in prostate was about half the plasma concentration (87 ng/ml vs 155 ng/ml) in human with normal dietary intake [114]. However, Rannikko et al found that genistein concentration in prostate was over two-fold higher than their plasma after receiving 240 mg of clover phytoestrogen daily for 2 week period [115]. Interestingly, even though the placebo group also showed two-fold prostate genistein concentration compared to the plasma value, suggesting that genistein can be accumulated in prostate. Farhan et al., reported that concentration of genistein in prostatic tissue were about 10-fold higher compared to plasma in mice at 24 hr after administration of 250 μg genistein per mouse via a gastric tube [116].
Plasma protein binding
Plasma protein binding is the important parameter to measure unbound drug in vivo. Because of its lipophilicity, genistein showed high plasma protein binding (>80%) after 0.5, 2 and 24 hr in rats [71] indicating genistein has good protein binding affinity and it may explain why genistein stays in vivo for long time as bound portion may act as a reservoir or depot from which genistein is slowly released as the unbound form.
Excretion
As genistein goes through extensive phase II metabolism in vivo, it is mainly excreted as its conjugation forms. In vivo/in vitro ADME studies revealed that intestinal, biliary and renal excretions are the excretion pathways for genistein metabolites [46, 55, 91]. The role of intestine in disposition of genistein is not limited to the metabolism, and also greatly extended to the excretion of genistein conjugates [63, 91]. The highly active efflux transporters play important roles in excreting genistein metabolites back to lumen to decrease bioavailability of total genistein. After oral administration of genistein, only a small portion of genistein aglycone was excreted through bile and a high level of genistein glucuronides was detected in bile indicating that genistein is mainly excreted in the form of glucuronides. The cumulative biliary excretion of genistein glucuronides accounted for 61%, 82% and 85% of total genistein at dose of 6.25, 12.5 and 50 mg/kg genistein, respectively [46]. Genistein glucuronides and sulfates were moderately recovered in urine indicated that urinary excretion is one of the major pathways for eliminating genistein conjugates [54, 117].
Glucuronide and sulfate conjugates are much more hydrophilic than the parent compound and they cannot traverse through the cellular membrane in enterocytes and hepatocytes by passive diffusion, and require the action of efflux transporters for their exit from the cells [118]. Because of the coupling of metabolic enzymes and efflux transporters, excretion of genistein phase II metabolites is not only dependent on the activity of conjugating enzymes, but also rely on the capacity of efflux transporters [119]. Therefore, metabolic parameters obtained in vitro (Km, Vmax, Clint) cannot always predict or correlate with in vivo metabolic properties of genistein, which complicated scenarios used to explain its in vivo excretion [91].
Role of BCRP in total genistein disposition
Breast cancer resistant protein (BCRP), a member of the ATP-binding cassette transporter family, is highly expressed in the apical membrane of enterocytes and hepatocytes facing bile canaliculus [120, 121]. It is responsible to facilitate intestinal and biliary excretion of several compounds and their metabolites [122–124]. Genistein and its glucuronide and sulfate conjugation are the substrates of BCRP [67, 125], which has been demonstrated using both in vitro cell culture models and in vivo pharmacokinetic studies [67, 68]. Enokizono reported that genistein showed weak efflux (efflux ratio=2) in BCRP overexpressed MDCKII cells and the AUC0–4 of genistein aglycone in BCRP −/− mice was 2-fold greater than wild-type mice after oral administration of genistein [67]. The Kp (tissue to plasma concentration ratio) of brain and testis of genistein were significantly increased in BCRP knockout mice and the genistein concentrations in epididymis and ovary were also higher in the BCRP knockout mice compared to the wild-type mice [67].
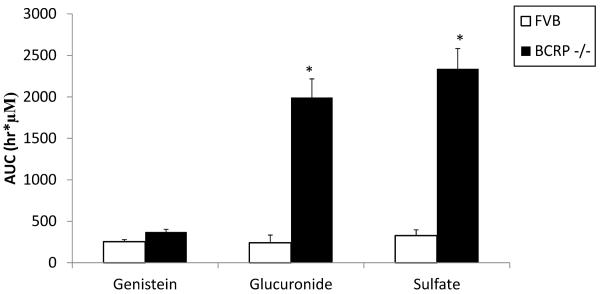
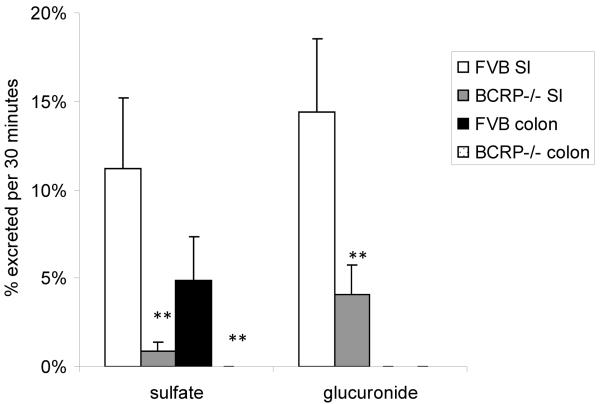
A mice intragastric pharmacokinetic study performed by Alvarez et al. suggested significant exposure increase of genistein conjugates in the BCRP knockout mice than in the wild-type FVB mice, as signified by 7–10 fold increase in phase II conjugates levels (Fig. 3a) [68]. Zhu et al., previously found that BCRP played a dominant role in genistein sulfate excretion and a significant role in genistein glucuronide excretion in the mouse intestine where the excretion of genistein sulfates and glucuronides decreased substantially (>90% for sulfates and >70% for glucuronides decrease, respectively) in small intestine and colon of BCRP knockout mice, when compared to the wild-type FVB mice (Fig. 3b) [93]. Our own group measured biliary excretion of genistein, its glucuronides and sulfates after genistein intestinal perfusion and found that biliary excretion of genistein sulfate was significantly decreased in BCRP knockout mice while genistein and its glucuronides did not change significantly (Fig. 3c). In Caco-2 cells, genistein metabolites excretion was delayed or decreased with the addition of dipyridamole and fumitremorgin C (BCRP inhibitors). Recently, Caco-2 transport and biliary excretion studies conducted by our own group showed BCRP can determine the biodistribution of genistein glucuronides and sulfate in enterocytes and bile, which greatly decreased intestinal efflux and biliary excretion of genistein conjugates while increased basolateral efflux towards systemic circulation in BCRP knockout mice. It subsequently increased the total bioavailability of genistein in vivo (Fig. 2) (unpublished, Zhen Yang and Ming Hu), which is consistent with what Alvarez et al reported recently [68].
Figure 3a.
Plasma AUC of genistein and its major glucuronide, sulfate in wild-type and BCRP knockout mice [68]. * indicated p<0.05.
Figure 3b.
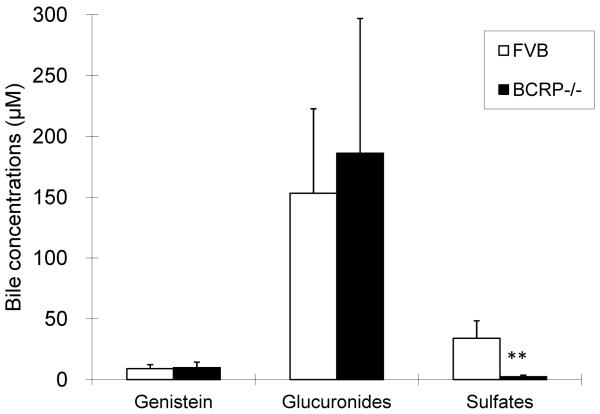
The intestinal excretion of genistein sulfate and glucuronide in wild-type and BCRP knockout mice. Reproduced from reference [93] with permission from the author. ** indicated p<0.01.
Figure 3c.
The biliary excretion of genistein and its sulfate and glucuronide in wild-type and BCRP knockout mice (unpublished data, Zhen Yang and Ming Hu).** indicated p<0.01.
Role of MRP in total genistein disposition
Multidrug resistance protein (MRP) family including MRP2 and MRP3 has been shown to be the major transporters involved in the excretion of conjugates of genistein [124, 126–129]. There are nine MRP transporters identified so far and MRP2 is located at apical membrane of enterocytes and hepatocytes facing bile canaliculus while MRP3 is located at basolateral side of enterocytes and hepatocytes [130–132].
Chen et al. reported that 0.1 μM leukotriene C4 (MRP inhibitor) inhibited excretion of genistein glucuronides and sulfates at both apical and basolateral sides of Caco-2 cells indicating MRP2 and MRP3 may mediate genistein conjugates efflux in cells since they (MRP2 and MRP3) are highly expressed in Caco-2 cells [128]. Zhu et al. also showed that genistein metabolites (glucuronides and sulfates) excretion was delayed or decreased with the addition of MK-571 (an MRP2 inhibitor) in Caco-2 cells [133]. Wang et al. found that biliary efflux of genistein glucuronides was significantly lower in TR negative (MRP2 deficient) rats as compared to wild type Wistar rats in intestinal perfusion model [127]. This probably was due to the fact that in TR negative rats, the lack of hepatic Mrp2 protein expression was also accompanied by decreased expression levels of hepatic Bcrp efflux transporters [134]. This dual decrease in the hepatic efflux transporter levels reduced the biliary efflux and thereby increased plasma concentrations of genistein and its metabolites in TR negative rats as compared to wild type rats. However, using MRP2 inhibitors in mouse perfusion model did not decrease genistein sulfates excretion, which indicated that sulfates may not be the good substrate of MRP2 [133]. Genistein (20 mg/kg) pharmacokinetic studies in TR negative rats conducted by our group further showed that bioavailability of genistein glucuronides did not increase significantly compared to wild-type Wistar rats, and the reason could possibly be attributed to the compensation effect of BCRP to transport genistein glucuronides in the absence of MRP (unpublished, Kaustubh Kulkarni and Ming Hu).
Genistein glucuronides had much lower abundance in urine samples of MRP3 −/− than of wild-type mice. MRP3-mediated transport was tested in vesicular transport experiments, and both human and mouse MRP3 were found to transport genistein glucuronides in a time- and ATP-dependent manner [126]. It indicated that MRP3 was the important transporter that transports genistein or its glucuronides across the basolateral membrane of enterocytes and hepatocytes into the blood circulation for subsequent urinary excretion.
Overall, these experimental results revealed that genistein glucuronides were good substrates for MRP and BCRP, and genistein sulfates were strong substrates for BCRP in rodents. Inhibition or deletions of BCRP significantly increased the plasma levels of both genistein aglycone and metabolites, thereby increased the bioavailability of total genistein.
Enterohepatic Recycling
Genistein is extensively absorbed in enterocytes, converted to conjugates and then enter the portal vein. When the blood circulation passes through liver, the remaining genistein aglycone are sequestered, converted and the metabolites are excreted into the bile. Because of large amounts of bacteria in terminal small intestine and colon, these conjugates can be hydrolyzed and reabsorbed in intestine, thereby enable the reabsorption of genistein to complete the enterohepatic recycling (Fig. 2) [135]. Enterohepatic recycling of genistein is the key reason for prolonged residence time of genistein in vivo and the double peaks in the profile of plasma concentration vs. time after oral administration of genistein [40, 46, 89].
Yasuda et al. found that ~16% of administrated dose was excreted in bile within 36 h in rats [136]. Chen et al. determined that 6.4% of absorbed genistein was biliary excreted in rats after 2.5 hr intestinal perfusion of 10 μM genistein [70]. Wang et al. also found large amounts of genistein metabolites (~150 nmol/30 min) were excreted in the bile of Wistar rats in the intestinal perfusion model [90]. Sfakianos et al. showed more convincing results that infusion of genistein into the duodenum resulted in 80% of administrated genistein presented as G-7-G in bile. If it is introduced to portal vein, ~100% of dose was transported into the bile. Furthermore, infusion of genistein glucuronide (G-7-G) into the duodenum also led to its appearance in bile, which explained the efficient recirculation process [89]. All these data are in agreement that genistein undergoes substantial enterohepatic recycling.
Enterohepatic recycling of genistein in human, like other isoflavone, has been proposed and genistein metabolites go through enterohepatic recycling can be eventually excreted via the kidney [82, 137, 138].
Enteric Recycling
Large amounts of genistein metabolites generated in enterocytes can be excreted back into lumen by efflux transporters expressed at the apical side of enterocytes, i.e. BCRP and MRP2 (Fig. 2) [63, 64]. Similarly to the enterohepatic recycling, bacteria in small intestine and colon deconjugate these metabolites and enable them to be reabsorbed in intestine. Therefore, intestinal conjugation, efflux back to lumen, hydrolysis by bacteria, further reabsorption and conjugation constitutes the enteric recycling [119]. Genistein was extensively metabolized in intestine after oral administration and several studies showed that intestine is more important than liver on disposition of genistein [30, 70]. The coupling of metabolic enzymes and efflux transporters in intestine enables enteric recycling and it represents one of the main pathways for genistein elimination. The detailed information describing coupling of conjugating enzymes and efflux transporters has been reviewed earlier by our group [139, 140]. The duo recycling scheme involving both enteric and enterohepatic is the reason for extensive metabolism and prolonged systemic exposure of genistein in vivo.
Role of gender in pharmacokinetics of genistein
Gender difference was often reported in genistein clinical studies and this is expected considering the major mechanism of genistein is to interact with estrogen receptors [71, 141]. Coldham and coworkers have shown that 2 fold higher oral bioavailability of both genistein aglycone (14.6 vs 6.8 %) and total genistein (110.8 vs 55.2%) in female than in male SD rats [71]. The results suggested significantly higher concentrations of 14C genistein in liver in female SD rats than male one, thereby suggesting high enterohepatic recycling as a possible mechanism. Furthermore, the radioactive genistein concentrations were significantly higher in reproductive organs in female than in male SD rats. Besides the similar gender difference in 2-fold exposure level of genistein, Chang et al also found that significantly shorter elimination half-life in male rats (2.97 ± 0.14 hr) compared to female rats (4.26 ± 0.29 hr) which may explain why female rats has higher bioavailability of genistein than male rats [105]. However, gender difference in pharmacokinetics of genistein appears not obvious in humans. Cassidy et al observed significant gender difference in the bioavailability of daidzein, but not genistein [142]. Zhang et al. and Sepehr et al. reported the similar results that genistein did not show significant difference in exposure level between men and women [143, 144], but whether gender difference exists on tissue distribution of genistein remains unknown.
Our own group found out that plasma genistein glucuronide concentrations were significantly higher (>2 fold) in female than in male Sprague Dawley rats after genistein oral administration at 20mg/kg. Also, the plasma genistein glucuronide concentrations were almost 3 folds higher than genistein aglycone in female whereas no significant difference in plasma genistein aglycone and genistein glucuronide concentrations was observed in male SD rats. To our surprise, we discovered significantly higher plasma genistein sulfate concentrations in male rats suggesting possible compensation for genistein glucuronidation, which appeared to have been saturated in male rats (unpublished, Kaustubh Kulkarni and Ming Hu).
Role of female sex hormones in pharmacokinetics of genistein
Since significant gender difference was observed in genistein pharmacokinetics, the obvious thought came to mind was if sex hormones play any role in genistein pharmacokinetics. Genistein had been shown to regulate both male and female sex hormones in vivo but whether sex hormone alters genistein pharmacokinetics is unclear [145, 146]. We chose premenstrual female rats as a model and grouped the rats into two groups based on varying levels of estrogen in the estrus cycle: elevated estrogen levels (female rats in proestrus and oestrus phase) and basal estrogen levels (female rats in metoestrus and diestrus phase). In pharmacokinetic studies, total genistein (parent + metabolite) concentrations in plasma were significantly higher in basal levels of estrogen as compared to elevated levels of estrogen group of female rats after oral administration of 20 mg/kg genistein. Furthermore, both genistein aglycone and genistein glucuronide concentrations were significantly higher in basal estrogen level group than elevated estrogen level group of female rats (unpublished, Kaustubh Kulkarni and Ming Hu).
To delineate the possible mechanism of action for this observed genistein oral pharmacokinetics, we also performed rat intestinal perfusion with bile duct cannulation using female rats in two different groups. In the study, female rats in both group showed no significant differences in genistein absorption and intestinal metabolite excretion. However, biliary excretion of genistein glucuronide was significantly higher in female rats in basal level estrogen group than in elevated level estrogen group suggesting efficient enterohepatic recycling as a possible mechanism of action for the observed higher bioavailability of genistein aglycone in basal over elevated estrogen level group (unpublished, Kaustubh Kulkarni and Ming Hu).
In order to conclusively study the role of estrogen in oral genistein bioavailability, we used female ovariectomized rats as a model for the lack of plasma estrogen levels in these rats. In pharmacokinetic studies, female ovariectomized rats showed similar plasma concentrations of genistein aglycone and genistein glucuronide as that of basal estrogen level group of female rats after oral administration of genistein at 20 mg/kg. This meant, in comparison to elevated estrogen level group of female rats, the female ovariectomized rats showed significantly higher genistein aglycone and genistein glucuronide plasma concentrations. Taken together, this data suggested that genistein oral pharmacokinetics was female sex hormone, estrogen-dependent (unpublished, Kaustubh Kulkarni and Ming Hu).
The possible reasons for altered oral pharmacokinetics of genistein may be derived from regulations of enzymes and transporters by sex hormones. Sex hormones can directly regulate gene expression of drug-metabolizing enzymes, especially on mRNA expression of UGT in liver and kidney in mice [147]. Bcrp1 expression in mouse tissues can be altered by sex hormones and possibly medicated by certain nuclear receptors via a transcriptional mechanism [148].
Effects of genistein on enzymes and transporters
Since genistein is the substrate of multiple metabolic enzymes and efflux transporters, their interaction was suggested as an important mechanism for genistein chemoprevention effects [149, 150]. Coadministration of genistein with chemotherapeutic drugs (i.e., docetaxel, cisplatin and gemcitabine) also enhanced their efficacy in animal models and cells [28, 151–153]. The mechanism of cancer protective effects of genistein and its interaction with drugs should be considered further to improve their efficacy and reduce their side effects. The herb-drug interaction usually includes two perspectives containing enzyme-based and transporter based interactions [154, 155].
Genistein showed various inhibitory and induction effects on the activities of cytochrome P450 and phase II enzymes which resulted in the detoxification of carcinogens or prevention of degradation for active compounds. Chen et al. found that genistein inhibited the formation of α-hydroxytamoxifen via inhibition of CYP1A2 to decrease the side effect of tamoxifen [156]. Farhan demonstrated that genistein reduced the activity of CYP24 in DU-145, a human prostate cancer cell line and subsequently prevented degradation of active metabolite of vitamin D [116]. Laurenzana et al reported that genistein decreased both CYP2C and CYP3A expression in liver microsomes in male rats after feeding genistein at 1250 ppm for 14 days [157]. Genistein has been shown to induce UDP-glucuronosyltransferase activity by ~4 fold at 5 μM after 5 days incubation in LNCap cells [158] and inhibit the activity of sulfotransferase 1A1 and 1E1 in vitro [159, 160].
Isoflavone is often reported as a modulator on the activity of ABC transporter [161]. Genistein was found as the inhibitor of BCRP with EC50 at 14.9 μM for increasing accumulation of mitoxantrone in overexpressing BCRP MCF-7 MX100 cells [162]. It subsequently increased cytotoxicity of mitoxantrone (IC50) in MCF-7 MX100 cells after combination with genistein. Coadministration of 100 mg/kg of genistein and daidzein significantly increased the exposure level and tissue distribution of nitrofurantoin in mice [163]. Genistein showed similar inhibitory effects of increasing methotrexate transport in BCRP-expressing membrane vesicle [164], and of elevated topotecan accumulation in K562/BCRP cells [165]. All these results indicated that genistein may improve potency of chemotherapeutic drugs by increasing their uptake and exposure levels.
Conclusion
ADME studies revealed that genistein has favorable absorption property in intestine but its poor solubility may prevent absorption of larger doses without proper formulations. Extensive metabolism is definitely one of the major reasons contributing to the low oral bioavailability of genistein, but high expression level of efflux transporters, especially BCRP, may be the most pivotal factor responsible for low oral bioavailability of total genistein. The coupling of metabolic enzymes and efflux transporters plays an important role on genistein distribution and elimination. It enables enteric and enterohepatic recycling and significantly decreases exposure level of genistein and prolongs its residence time in vivo. Efflux transporter, like BCRP can determine the biodistribution of genistein conjugates, and subsequently affect their pharmacokinetics and bioavailability. Genistein and its conjugates mainly accumulated in GI tract and liver because of dual recycling mechanisms and their distributions in reproductive organs are not concentrated compared to plasma levels. Gender and sex hormone did change genistein in vivo pharmacokinetics and bioavailability in animals which may guide clinical trials (e.g. design of dose regimen. Genistein has been shown to change the activity of both enzyme and efflux transporter in vivo, indicating the potential herb-drug interaction in clinical trials. The ADME results suggested that genistein has intrinsically low oral bioavailability because of two major obstacles, metabolic enzyme and efflux transporter. By manipulating enzyme activity only may not achieve the goal to improve genistein oral bioavailability due to compensation mechanism, which has been proved in Gunn rats. But recent progress on transporter studies showed promising results that total genistein bioavailability can be substantially increased by knockout of BCRP and the effect can be extended to other isoflavones. Further studies should focus on developing the practical approaches to increase genistein bioavailability and its efficacy in clinical trials.
Acknowledgments
This work was supported by grants from the National Institute of Health [GM070737] to Ming Hu at University of Houston.
Abbreviations
- G-7-G
genistein-7-glucuronide
- G-4'-G
genistein-4'-glucuronide G-4'-S, genistein-4'-sulfate
- G-7-S
genistein-7- sulfate
- G-4',7-diG
genistein-4',7-diglucuronide
- G-4',7-diS
genistein-4',7-disulfate
- G-7G-4'S
genistein-7-glucuronide-4'-sulfate
- G-4'G-7S
genistein-4'-glucuronide-7-sulfate
- UGTs
UDP-Glucuronosyltransferase
- SULT
sulfotransferase
- AUCinf
area under the plasma concentration-time curve from time zero to infinity
- Cmax
maximum plasma concentration
- t1/2
terminal half-life
- I.V.
intravenous administration
- P.O.
oral gavage
- I.P.
intraperitoneal injection
- BCRP
breast cancer resistance protein
- MRP
multidrug resistance associated protein.
Reference
- 1.Ziegler RG, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85(22):1819–27. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 2.Yatani R, et al. Geographic pathology of latent prostatic carcinoma. Int J Cancer. 1982;29(6):611–6. doi: 10.1002/ijc.2910290602. [DOI] [PubMed] [Google Scholar]
- 3.Severson RK, et al. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989;49(7):1857–60. [PubMed] [Google Scholar]
- 4.Lee HP, et al. Dietary effects on breast-cancer risk in Singapore. Lancet. 1991;337(8751):1197–200. doi: 10.1016/0140-6736(91)92867-2. [DOI] [PubMed] [Google Scholar]
- 5.Adlercreutz H, et al. Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone binding globulin (SHBG) J Steroid Biochem. 1987;27(4–6):1135–44. doi: 10.1016/0022-4731(87)90200-7. [DOI] [PubMed] [Google Scholar]
- 6.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55(1):1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 7.Tham DM, Gardner CD, Haskell WL. Clinical review 97: Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab. 1998;83(7):2223–35. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- 8.Steele VE, et al. Cancer chemoprevention agent development strategies for genistein. J Nutr. 1995;125(3 Suppl):713S–716S. doi: 10.1093/jn/125.3_Suppl.713S. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar FH, Li Y. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer Metastasis Rev. 2002;21(3–4):265–80. doi: 10.1023/a:1021210910821. [DOI] [PubMed] [Google Scholar]
- 10.Bitto A, et al. Genistein administration & climacteric symptoms: from plasma levels to biological activity. Indian J Med Res. 2007;125(4):508–9. [PubMed] [Google Scholar]
- 11.Park SS, et al. Genistein-induced apoptosis via Akt signaling pathway in anaplastic large-cell lymphoma. Cancer Chemother Pharmacol. 2005;56(3):271–8. doi: 10.1007/s00280-004-0974-z. [DOI] [PubMed] [Google Scholar]
- 12.Rabiau N, et al. Genistein and daidzein act on a panel of genes implicated in cell cycle and angiogenesis by polymerase chain reaction arrays in human prostate cancer cell lines. Cancer Epidemiol. 2010;34(2):200–6. doi: 10.1016/j.canep.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Pugalendhi P, Manoharan S. Chemopreventive potential of genistein and daidzein in combination during 7,12-dimethylbenz[a]anthracene (DMBA) induced mammary carcinogenesis in Sprague-Dawley rats. Pak J Biol Sci. 2010;13(6):279–86. doi: 10.3923/pjbs.2010.279.286. [DOI] [PubMed] [Google Scholar]
- 14.He H, et al. Genistein down-regulates the constitutive activation of nuclear factor-kappaB in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Phytother Res. 2009;23(6):868–73. doi: 10.1002/ptr.2715. [DOI] [PubMed] [Google Scholar]
- 15.Chodon D, Ramamurty N, Sakthisekaran D. Preliminary studies on induction of apoptosis by genistein on HepG2 cell line. Toxicol In Vitro. 2007;21(5):887–91. doi: 10.1016/j.tiv.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Polkowski K, Mazurek AP. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol Pharm. 2000;57(2):135–55. [PubMed] [Google Scholar]
- 17.Banerjee S, et al. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269(2):226–42. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar FH, et al. The role of genistein and synthetic derivatives of isoflavone in cancer prevention and therapy. Mini Rev Med Chem. 2006;6(4):401–7. doi: 10.2174/138955706776361439. [DOI] [PubMed] [Google Scholar]
- 19.Lazarevic B, et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: a randomized, placebo-controlled, double-blind phase 2 clinical trial. Nutr Cancer. 2011;63(6):889–98. doi: 10.1080/01635581.2011.582221. [DOI] [PubMed] [Google Scholar]
- 20.Taylor CK, et al. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr Rev. 2009;67(7):398–415. doi: 10.1111/j.1753-4887.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 21.Wietrzyk J, et al. Genistein alone or combined with cyclophosphamide may stimulate 16/C transplantable mouse mammary cancer growth. Med Sci Monit. 2004;10(11):BR414–9. [PubMed] [Google Scholar]
- 22.Allred CD, et al. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61(13):5045–50. [PubMed] [Google Scholar]
- 23.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98(7):459–71. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki M, et al. Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: a nested case-control study from the Japan Public Health Center-based prospective study group. J Clin Oncol. 2008;26(10):1677–83. doi: 10.1200/JCO.2007.13.9964. [DOI] [PubMed] [Google Scholar]
- 25.Jerome-Morais A, Diamond AM, Wright ME. Dietary supplements and human health: for better or for worse? Mol Nutr Food Res. 2011;55(1):122–35. doi: 10.1002/mnfr.201000415. [DOI] [PubMed] [Google Scholar]
- 26.Lampe JW, et al. Plasma isoflavones and fibrocystic breast conditions and breast cancer among women in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2579–86. doi: 10.1158/1055-9965.EPI-07-0368. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, et al. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65(15):6934–42. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 28.Mohammad RM, et al. Cisplatin-induced antitumor activity is potentiated by the soy isoflavone genistein in BxPC-3 pancreatic tumor xenografts. Cancer. 2006;106(6):1260–8. doi: 10.1002/cncr.21731. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, et al. Apoptosis-inducing effect of chemotherapeutic agents is potentiated by soy isoflavone genistein, a natural inhibitor of NF-kappaB in BxPC-3 pancreatic cancer cell line. Pancreas. 2004;28(4):e90–5. doi: 10.1097/00006676-200405000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Manach C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 31.Kano M, et al. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006;136(9):2291–6. doi: 10.1093/jn/136.9.2291. [DOI] [PubMed] [Google Scholar]
- 32.Setchell KD, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131(4 Suppl):1362S–75S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 33.Larkin T, Price WE, Astheimer L. The key importance of soy isoflavone bioavailability to understanding health benefits. Crit Rev Food Sci Nutr. 2008;48(6):538–52. doi: 10.1080/10408390701542716. [DOI] [PubMed] [Google Scholar]
- 34.Supko J, Malspeis L. Plasma pharmacokinetics of genistein in mice. Int J Oncol. 1995;7(4):847–54. doi: 10.3892/ijo.7.4.847. [DOI] [PubMed] [Google Scholar]
- 35.Cassidy A. Factors affecting the bioavailability of soy isoflavones in humans. J AOAC Int. 2006;89(4):1182–8. [PubMed] [Google Scholar]
- 36.Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer. 2007;57(1):1–10. doi: 10.1080/01635580701267677. [DOI] [PubMed] [Google Scholar]
- 37.Snyder RD, Gillies PJ. Reduction of genistein clastogenicity in Chinese hamster V79 cells by daidzein and other flavonoids. Food Chem Toxicol. 2003;41(10):1291–8. doi: 10.1016/s0278-6915(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 38.Rowland I, et al. Bioavailability of phyto-oestrogens. Br J Nutr. 2003;89(Suppl 1):S45–58. doi: 10.1079/BJN2002796. [DOI] [PubMed] [Google Scholar]
- 39.Setchell KD, et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr. 2003;77(2):411–9. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- 40.Yang Z, et al. Simultaneous determination of genistein and its four phase II metabolites in blood by a sensitive and robust UPLC-MS/MS method: Application to an oral bioavailability study of genistein in mice. J Pharm Biomed Anal. 2010;53(1):81–9. doi: 10.1016/j.jpba.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrade JE, et al. Absolute bioavailability of isoflavones from soy protein isolate-containing food in female BALB/c mice. J Agric Food Chem. 2010;58(7):4529–36. doi: 10.1021/jf9039843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coldham NG, et al. Absolute bioavailability of [14C] genistein in the rat; plasma pharmacokinetics of parent compound, genistein glucuronide and total radioactivity. Eur J Drug Metab Pharmacokinet. 2002;27(4):249–58. doi: 10.1007/BF03192335. [DOI] [PubMed] [Google Scholar]
- 43.Doerge DR, et al. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos. 2000;28(3):298–307. [PubMed] [Google Scholar]
- 44.Shelnutt SR, et al. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002;76(3):588–94. doi: 10.1093/ajcn/76.3.588. [DOI] [PubMed] [Google Scholar]
- 45.Hosoda K, et al. Plasma profiling of intact isoflavone metabolites by high-performance liquid chromatography and mass spectrometric identification of flavone glycosides daidzin and genistin in human plasma after administration of kinako. Drug Metab Dispos. 2008;36(8):1485–95. doi: 10.1124/dmd.108.021006. [DOI] [PubMed] [Google Scholar]
- 46.Zhou S, et al. Dose-dependent absorption, metabolism, and excretion of genistein in rats. J Agric Food Chem. 2008;56(18):8354–9. doi: 10.1021/jf801051d. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, et al. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J Nutr. 1999;129(2):399–405. doi: 10.1093/jn/129.2.399. [DOI] [PubMed] [Google Scholar]
- 48.Rimbach G, et al. Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim Biophys Acta. 2004;1670(3):229–37. doi: 10.1016/j.bbagen.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Kgomotso T, Chiu F, Ng K. Genistein- and daidzein 7-O-beta-D-glucuronic acid retain the ability to inhibit copper-mediated lipid oxidation of low density lipoprotein. Mol Nutr Food Res. 2008;52(12):1457–66. doi: 10.1002/mnfr.200800010. [DOI] [PubMed] [Google Scholar]
- 50.Andrade JE, et al. Absolute bioavailability of isoflavones from soy protein isolate-containing food in female BALB/c mice. J Agric Food Chem. 58(7):4529–36. doi: 10.1021/jf9039843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Busby MG, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr. 2002;75(1):126–36. doi: 10.1093/ajcn/75.1.126. [DOI] [PubMed] [Google Scholar]
- 52.Hosoda K, et al. Identification and quantification of daidzein-7-glucuronide-4'-sulfate, genistein-7-glucuronide-4'-sulfate and genistein-4',7-diglucuronide as major metabolites in human plasma after administration of kinako. Anal Bioanal Chem. 2010;397(4):1563–72. doi: 10.1007/s00216-010-3714-8. [DOI] [PubMed] [Google Scholar]
- 53.Fischer L, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer. 2004;48(2):160–70. doi: 10.1207/s15327914nc4802_5. [DOI] [PubMed] [Google Scholar]
- 54.Gu L, et al. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J Nutr. 2006;136(5):1215–21. doi: 10.1093/jn/136.5.1215. [DOI] [PubMed] [Google Scholar]
- 55.Shelnutt SR, et al. Urinary pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein. Cancer Epidemiol Biomarkers Prev. 2000;9(4):413–9. [PubMed] [Google Scholar]
- 56.Adlercreutz H, et al. Lignan and isoflavonoid conjugates in human urine. J Steroid Biochem Mol Biol. 1995;52(1):97–103. doi: 10.1016/0960-0760(94)00146-d. [DOI] [PubMed] [Google Scholar]
- 57.Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–81. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 58.Hur HG, et al. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch Microbiol. 2000;174(6):422–8. doi: 10.1007/s002030000222. [DOI] [PubMed] [Google Scholar]
- 59.Kwon SH, et al. Comparison of oral bioavailability of genistein and genistin in rats. Int J Pharm. 2007;337(1–2):148–54. doi: 10.1016/j.ijpharm.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 60.Rothwell JA, Day AJ, Morgan MR. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J Agric Food Chem. 2005;53(11):4355–60. doi: 10.1021/jf0483669. [DOI] [PubMed] [Google Scholar]
- 61.Wu JG, et al. Solubility of Genistein in Water, Methanol, Ethanol, Propan-2-ol, 1-Butanol, and Ethyl Acetate from (280 to 333) K. J. Chem. Eng. Data. 2010;55:5286–5288. [Google Scholar]
- 62.Ungar Y, Osundahunsi OF, Shimoni E. Thermal stability of genistein and daidzein and its effect on their antioxidant activity. J Agric Food Chem. 2003;51(15):4394–9. doi: 10.1021/jf034021z. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Lin H, Hu M. Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J Pharmacol Exp Ther. 2003;304(3):1228–35. doi: 10.1124/jpet.102.046409. [DOI] [PubMed] [Google Scholar]
- 64.Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos. 2002;30(4):370–7. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 65.Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175(3):880–5. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- 66.Pade V, Stavchansky S. Link between drug absorption solubility and permeability measurements in Caco-2 cells. J Pharm Sci. 1998;87(12):1604–7. doi: 10.1021/js980111k. [DOI] [PubMed] [Google Scholar]
- 67.Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol. 2007;72(4):967–75. doi: 10.1124/mol.107.034751. [DOI] [PubMed] [Google Scholar]
- 68.Alvarez AI, et al. Bioavailability of the Glucuronide and Sulfate Conjugates of Genistein and Daidzein in Bcrp1 Knockout Mice. Drug Metab Dispos. 2011 doi: 10.1124/dmd.111.040881. [DOI] [PubMed] [Google Scholar]
- 69.Andlauer W, et al. Absorption and metabolism of genistein in isolated rat small intestine. J Nutr. 2000;130(4):843–6. doi: 10.1093/jn/130.4.843. [DOI] [PubMed] [Google Scholar]
- 70.Chen J, et al. Disposition of flavonoids via recycling: comparison of intestinal versus hepatic disposition. Drug Metab Dispos. 2005;33(12):1777–84. doi: 10.1124/dmd.105.003673. [DOI] [PubMed] [Google Scholar]
- 71.Coldham NG, Sauer MJ. Pharmacokinetics of [(14)C]Genistein in the rat: gender-related differences, potential mechanisms of biological action, and implications for human health. Toxicol Appl Pharmacol. 2000;164(2):206–15. doi: 10.1006/taap.2000.8902. [DOI] [PubMed] [Google Scholar]
- 72.Steensma A, et al. Bioavailability of genistein and its glycoside genistin as measured in the portal vein of freely moving unanesthetized rats. J Agric Food Chem. 2006;54(21):8006–12. doi: 10.1021/jf060783t. [DOI] [PubMed] [Google Scholar]
- 73.Lu LJ, Anderson KE. Sex and long-term soy diets affect the metabolism and excretion of soy isoflavones in humans. Am J Clin Nutr. 1998;68(6 Suppl):1500S–1504S. doi: 10.1093/ajcn/68.6.1500S. [DOI] [PubMed] [Google Scholar]
- 74.Chang YC, Nair MG. Metabolism of daidzein and genistein by intestinal bacteria. J Nat Prod. 1995;58(12):1892–6. doi: 10.1021/np50126a014. [DOI] [PubMed] [Google Scholar]
- 75.Kulling SE, Honig DM, Metzler M. Oxidative metabolism of the soy isoflavones daidzein and genistein in humans in vitro and in vivo. J Agric Food Chem. 2001;49(6):3024–33. doi: 10.1021/jf0012695. [DOI] [PubMed] [Google Scholar]
- 76.Heinonen SM, et al. Metabolism of the soy isoflavones daidzein, genistein and glycitein in human subjects. Identification of new metabolites having an intact isoflavonoid skeleton. J Steroid Biochem Mol Biol. 2003;87(4–5):285–99. doi: 10.1016/j.jsbmb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Strassburg CP, Manns MP, Tukey RH. Expression of the UDP-glucuronosyltransferase 1A locus in human colon. Identification and characterization of the novel extrahepatic UGT1A8. J Biol Chem. 1998;273(15):8719–26. doi: 10.1074/jbc.273.15.8719. [DOI] [PubMed] [Google Scholar]
- 78.Riches Z, et al. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: the SULT “pie”. Drug Metab Dispos. 2009;37(11):2255–61. doi: 10.1124/dmd.109.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Izukawa T, et al. Quantitative analysis of UDP-glucuronosyltransferase (UGT) 1A and UGT2B expression levels in human livers. Drug Metab Dispos. 2009;37(8):1759–68. doi: 10.1124/dmd.109.027227. [DOI] [PubMed] [Google Scholar]
- 80.Zhang L, Zuo Z, Lin G. Intestinal and hepatic glucuronidation of flavonoids. Mol Pharm. 2007;4(6):833–45. doi: 10.1021/mp700077z. [DOI] [PubMed] [Google Scholar]
- 81.Kurkela M, et al. Expression and characterization of recombinant human UDP-glucuronosyltransferases (UGTs). UGT1A9 is more resistant to detergent inhibition than other UGTs and was purified as an active dimeric enzyme. J Biol Chem. 2003;278(6):3536–44. doi: 10.1074/jbc.M206136200. [DOI] [PubMed] [Google Scholar]
- 82.Setchell KD, et al. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr. 2003;133(4):1027–35. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 83.Hosoda K, Furuta T, Ishii K. Simultaneous determination of glucuronic acid and sulfuric acid conjugated metabolites of daidzein and genistein in human plasma by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(7–8):628–36. doi: 10.1016/j.jchromb.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 84.Hosoda K, Furuta T, Ishii K. Metabolism and disposition of isoflavone conjugated metabolites in humans after ingestion of kinako. Drug Metab Dispos. 2011 doi: 10.1124/dmd.111.038281. [DOI] [PubMed] [Google Scholar]
- 85.Bursztyka J, et al. Comparison of genistein metabolism in rats and humans using liver microsomes and hepatocytes. Food Chem Toxicol. 2008;46(3):939–48. doi: 10.1016/j.fct.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 86.Pritchett LE, et al. Glucuronidation of the soyabean isoflavones genistein and daidzein by human liver is related to levels of UGT1A1 and UGT1A9 activity and alters isoflavone response in the MCF-7 human breast cancer cell line. J Nutr Biochem. 2008;19(11):739–45. doi: 10.1016/j.jnutbio.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 87.Tang L, et al. Structure and concentration changes affect characterization of UGT isoform-specific metabolism of isoflavones. Mol Pharm. 2009;6(5):1466–82. doi: 10.1021/mp8002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakamura A, et al. Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab Dispos. 2008;36(8):1461–4. doi: 10.1124/dmd.108.021428. [DOI] [PubMed] [Google Scholar]
- 89.Sfakianos J, et al. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr. 1997;127(7):1260–8. doi: 10.1093/jn/127.7.1260. [DOI] [PubMed] [Google Scholar]
- 90.Wang SW, et al. Disposition of flavonoids via enteric recycling: UDP-glucuronosyltransferase (UGT) 1As deficiency in Gunn rats is compensated by increases in UGT2Bs activities. J Pharmacol Exp Ther. 2009;329(3):1023–31. doi: 10.1124/jpet.108.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang SW, et al. Disposition of flavonoids via enteric recycling: structural effects and lack of correlations between in vitro and in situ metabolic properties. Drug Metab Dispos. 2006;34(11):1837–48. doi: 10.1124/dmd.106.009910. [DOI] [PubMed] [Google Scholar]
- 92.Soucy NV, et al. Kinetics of genistein and its conjugated metabolites in pregnant Sprague-Dawley rats following single and repeated genistein administration. Toxicol Sci. 2006;90(1):230–40. doi: 10.1093/toxsci/kfj077. [DOI] [PubMed] [Google Scholar]
- 93.Zhu W, et al. Breast cancer resistance protein (BCRP) and sulfotransferases contribute significantly to the disposition of genistein in mouse intestine. AAPS J. 2010;12(4):525–36. doi: 10.1208/s12248-010-9209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peterson TG, et al. Metabolism of the isoflavones genistein and biochanin A in human breast cancer cell lines. Am J Clin Nutr. 1998;68(6 Suppl):1505S–1511S. doi: 10.1093/ajcn/68.6.1505S. [DOI] [PubMed] [Google Scholar]
- 95.Breinholt VM, et al. In vitro metabolism of genistein and tangeretin by human and murine cytochrome P450s. Pharmacol Toxicol. 2003;93(1):14–22. doi: 10.1034/j.1600-0773.2003.930102.x. [DOI] [PubMed] [Google Scholar]
- 96.Kulling SE, et al. Oxidative in vitro metabolism of the soy phytoestrogens daidzein and genistein. J Agric Food Chem. 2000;48(10):4963–72. doi: 10.1021/jf000524i. [DOI] [PubMed] [Google Scholar]
- 97.Wang XL, et al. Enhanced biosynthesis of dihydrodaidzein and dihydrogenistein by a newly isolated bovine rumen anaerobic bacterium. J Biotechnol. 2005;115(3):261–9. doi: 10.1016/j.jbiotec.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 98.Kulling SE, Lehmann L, Metzler M. Oxidative metabolism and genotoxic potential of major isoflavone phytoestrogens. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777(1–2):211–8. doi: 10.1016/s1570-0232(02)00215-5. [DOI] [PubMed] [Google Scholar]
- 99.Hu M, et al. Identification of CYP1A2 as the main isoform for the phase I hydroxylated metabolism of genistein and a prodrug converting enzyme of methylated isoflavones. Drug Metab Dispos. 2003;31(7):924–31. doi: 10.1124/dmd.31.7.924. [DOI] [PubMed] [Google Scholar]
- 100.Moon YJ, Brazeau DA, Morris ME. Effects of flavonoids genistein and biochanin a on gene expression and their metabolism in human mammary cells. Nutr Cancer. 2007;57(1):48–58. doi: 10.1080/01635580701268196. [DOI] [PubMed] [Google Scholar]
- 101.Bolca S, et al. Disposition of soy isoflavones in normal human breast tissue. Am J Clin Nutr. 2010;91(4):976–84. doi: 10.3945/ajcn.2009.28854. [DOI] [PubMed] [Google Scholar]
- 102.Peterson TG, et al. The role of metabolism in mammary epithelial cell growth inhibition by the isoflavones genistein and biochanin A. Carcinogenesis. 1996;17(9):1861–9. doi: 10.1093/carcin/17.9.1861. [DOI] [PubMed] [Google Scholar]
- 103.Pumford SL, et al. Determination of the isoflavonoids genistein and daidzein in biological samples by gas chromatography-mass spectrometry. Ann Clin Biochem. 2002;39(Pt 3):281–92. doi: 10.1258/0004563021901982. [DOI] [PubMed] [Google Scholar]
- 104.Casanova M, et al. Developmental effects of dietary phytoestrogens in Sprague-Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicol Sci. 1999;51(2):236–44. doi: 10.1093/toxsci/51.2.236. [DOI] [PubMed] [Google Scholar]
- 105.Chang HC, et al. Mass spectrometric determination of Genistein tissue distribution in diet-exposed Sprague-Dawley rats. J Nutr. 2000;130(8):1963–70. doi: 10.1093/jn/130.8.1963. [DOI] [PubMed] [Google Scholar]
- 106.Gilani GS, et al. Distribution of Isoflavones in Samples of Serum, Liver and Mammary Glands of Rats or Pigs Fed Dietary Isoflavones. Ann Nutr Metab. 2011;58(3):171–180. doi: 10.1159/000328771. [DOI] [PubMed] [Google Scholar]
- 107.Orlando L, Schiavone P, Cinieri S. Genistein: the future of prevention and treatment of breast cancer? Cancer Biol Ther. 2011;11(10):918–20. doi: 10.4161/cbt.11.10.15493. [DOI] [PubMed] [Google Scholar]
- 108.Manson MM, et al. Determining the efficacy of dietary phytochemicals in cancer prevention. Biochem Soc Trans. 2007;35(Pt 5):1358–63. doi: 10.1042/BST0351358. [DOI] [PubMed] [Google Scholar]
- 109.Lee KW, Bode AM, Dong Z. Molecular targets of phytochemicals for cancer prevention. Nat Rev Cancer. 2011;11(3):211–8. doi: 10.1038/nrc3017. [DOI] [PubMed] [Google Scholar]
- 110.El Touny LH, Banerjee PP. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009;69(8):3695–703. doi: 10.1158/0008-5472.CAN-08-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hargreaves DF, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84(11):4017–24. doi: 10.1210/jcem.84.11.6152. [DOI] [PubMed] [Google Scholar]
- 112.Maubach J, et al. Distribution of soy-derived phytoestrogens in human breast tissue and biological fluids. Obstet Gynecol. 2004;103(5 Pt 1): 892–8. doi: 10.1097/01.AOG.0000124983.66521.6a. [DOI] [PubMed] [Google Scholar]
- 113.Guy L, et al. Orally administered isoflavones are present as glucuronides in the human prostate. Nutr Cancer. 2008;60(4):461–8. doi: 10.1080/01635580801911761. [DOI] [PubMed] [Google Scholar]
- 114.Hong SJ, et al. Comparative study of concentration of isoflavones and lignans in plasma and prostatic tissues of normal control and benign prostatic hyperplasia. Yonsei Med J. 2002;43(2):236–41. doi: 10.3349/ymj.2002.43.2.236. [DOI] [PubMed] [Google Scholar]
- 115.Rannikko A, et al. Plasma and prostate phytoestrogen concentrations in prostate cancer patients after oral phytoestogen supplementation. Prostate. 2006;66(1):82–7. doi: 10.1002/pros.20315. [DOI] [PubMed] [Google Scholar]
- 116.Farhan H, et al. Isoflavonoids inhibit catabolism of vitamin D in prostate cancer cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777(1–2):261–8. doi: 10.1016/s1570-0232(02)00081-8. [DOI] [PubMed] [Google Scholar]
- 117.King RA, Broadbent JL, Head RJ. Absorption and excretion of the soy isoflavone genistein in rats. J Nutr. 1996;126(1):176–82. doi: 10.1093/jn/126.1.176. [DOI] [PubMed] [Google Scholar]
- 118.Jia X, et al. Disposition of flavonoids via enteric recycling: enzyme-transporter coupling affects metabolism of biochanin A and formononetin and excretion of their phase II conjugates. J Pharmacol Exp Ther. 2004;310(3):1103–13. doi: 10.1124/jpet.104.068403. [DOI] [PubMed] [Google Scholar]
- 119.Hu M, Chen J, Lin H. Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther. 2003;307(1):314–21. doi: 10.1124/jpet.103.053496. [DOI] [PubMed] [Google Scholar]
- 120.Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28(3):249–68. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 121.Mao Q. BCRP/ABCG2 in the placenta: expression, function and regulation. Pharm Res. 2008;25(6):1244–55. doi: 10.1007/s11095-008-9537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zamek-Gliszczynski MJ, et al. The important role of Bcrp (Abcg2) in the biliary excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in mice. Mol Pharmacol. 2006;70(6):2127–33. doi: 10.1124/mol.106.026955. [DOI] [PubMed] [Google Scholar]
- 123.Mizuno N, et al. Evaluation of the role of breast cancer resistance protein (BCRP/ABCG2) and multidrug resistance-associated protein 4 (MRP4/ABCC4) in the urinary excretion of sulfate and glucuronide metabolites of edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one) Drug Metab Dispos. 2007;35(11):2045–52. doi: 10.1124/dmd.107.016352. [DOI] [PubMed] [Google Scholar]
- 124.van de Wetering K, et al. Intestinal breast cancer resistance protein (BCRP)/Bcrp1 and multidrug resistance protein 3 (MRP3)/Mrp3 are involved in the pharmacokinetics of resveratrol. Mol Pharmacol. 2009;75(4):876–85. doi: 10.1124/mol.108.052019. [DOI] [PubMed] [Google Scholar]
- 125.Alvarez AI, et al. Bioavailability of the Glucuronide and Sulfate Conjugates of Genistein and Daidzein in Bcrp1 Knockout Mice. Drug Metab Dispos. doi: 10.1124/dmd.111.040881. [DOI] [PubMed] [Google Scholar]
- 126.van de Wetering K, et al. Targeted metabolomics identifies glucuronides of dietary phytoestrogens as a major class of MRP3 substrates in vivo. Gastroenterology. 2009;137(5):1725–35. doi: 10.1053/j.gastro.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 127.Wang SWJ. Pharmacological and Pharmaceutical Science. University of Houston; Houston: 2007. Investigation into the role and functional capacity of MRP2 efflux transporter and the predominant UDP-glucuronosyltransferases isoform(s) in the intestinal disposition of isoflavones. [Google Scholar]
- 128.Chen J, Lin H, Hu M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol. 2005;55(2):159–69. doi: 10.1007/s00280-004-0842-x. [DOI] [PubMed] [Google Scholar]
- 129.O'Leary KA, et al. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem Pharmacol. 2003;65(3):479–91. doi: 10.1016/s0006-2952(02)01510-1. [DOI] [PubMed] [Google Scholar]
- 130.Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58(2):140–61. doi: 10.1124/pr.58.2.3. [DOI] [PubMed] [Google Scholar]
- 131.Borst P, et al. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92(16):1295–302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 132.Konig J, et al. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461(2):377–94. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 133.Zhu W, et al. Breast Cancer Resistance Protein (BCRP) and Sulfotransferases Contribute Significantly to the Disposition of Genistein in Mouse Intestine. AAPS J. 2010 doi: 10.1208/s12248-010-9209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yue W, et al. Decreased hepatic breast cancer resistance protein expression and function in multidrug resistance-associated protein 2-deficient (TR) rats. Drug Metab Dispos. 2011;39(3):441–7. doi: 10.1124/dmd.110.035188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fanti P, et al. Serum levels and metabolic clearance of the isoflavones genistein and daidzein in hemodialysis patients. J Am Soc Nephrol. 1999;10(4):864–71. doi: 10.1681/ASN.V104864. [DOI] [PubMed] [Google Scholar]
- 136.Yasuda T, et al. Urinary and biliary metabolites of genistein in rats. Biol Pharm Bull. 1996;19(3):413–7. doi: 10.1248/bpb.19.413. [DOI] [PubMed] [Google Scholar]
- 137.Xu X, et al. Bioavailability of soybean isoflavones depends upon gut microflora in women. J Nutr. 1995;125(9):2307–15. doi: 10.1093/jn/125.9.2307. [DOI] [PubMed] [Google Scholar]
- 138.Winter J, Bokkenheuser VD. Bacterial metabolism of natural and synthetic sex hormones undergoing enterohepatic circulation. J Steroid Biochem. 1987;27(4–6):1145–9. doi: 10.1016/0022-4731(87)90201-9. [DOI] [PubMed] [Google Scholar]
- 139.Jeong EJ, et al. Coupling of conjugating enzymes and efflux transporters: impact on bioavailability and drug interactions. Curr Drug Metab. 2005;6(5):455–68. doi: 10.2174/138920005774330657. [DOI] [PubMed] [Google Scholar]
- 140.Liu Z, Hu M. Natural polyphenol disposition via coupled metabolic pathways. Expert Opin Drug Metab Toxicol. 2007;3(3):389–406. doi: 10.1517/17425255.3.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Penza M, et al. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology. 2006;147(12):5740–51. doi: 10.1210/en.2006-0365. [DOI] [PubMed] [Google Scholar]
- 142.Cassidy A, et al. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr. 2006;136(1):45–51. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]
- 143.Zhang Y, et al. Urinary disposition of the soybean isoflavones daidzein, genistein and glycitein differs among humans with moderate fecal isoflavone degradation activity. J Nutr. 1999;129(5):957–62. doi: 10.1093/jn/129.5.957. [DOI] [PubMed] [Google Scholar]
- 144.Sepehr E, et al. Bioavailability of soy isoflavones in rats Part I: application of accurate methodology for studying the effects of gender and source of isoflavones. Mol Nutr Food Res. 2007;51(7):799–812. doi: 10.1002/mnfr.200700083. [DOI] [PubMed] [Google Scholar]
- 145.Loukovaara M, et al. Regulation of sex hormone-binding globulin production by isoflavonoids and patterns of isoflavonoid conjugation in HepG2 cell cultures. Steroids. 1995;60(9):656–61. doi: 10.1016/0039-128x(95)00089-9. [DOI] [PubMed] [Google Scholar]
- 146.Yi MA, et al. Regulation of male sex hormone levels by soy isoflavones in rats. Nutr Cancer. 2002;42(2):206–10. doi: 10.1207/S15327914NC422_9. [DOI] [PubMed] [Google Scholar]
- 147.Buckley DB, Klaassen CD. Mechanism of gender-divergent UDP-glucuronosyltransferase mRNA expression in mouse liver and kidney. Drug Metab Dispos. 2009;37(4):834–40. doi: 10.1124/dmd.108.024224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang H, et al. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am J Physiol Endocrinol Metab. 2006;291(6):E1295–304. doi: 10.1152/ajpendo.00193.2006. [DOI] [PubMed] [Google Scholar]
- 149.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20(2):187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 150.Alvarez AI, et al. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci. 2010;99(2):598–617. doi: 10.1002/jps.21851. [DOI] [PubMed] [Google Scholar]
- 151.Zhang B, et al. Enhanced anticancer effect of gemcitabine by genistein in osteosarcoma: the role of Akt and nuclear factor-kappaB. Anticancer Drugs. 2010;21(3):288–96. doi: 10.1097/CAD.0b013e328334da17. [DOI] [PubMed] [Google Scholar]
- 152.Park SJ, et al. Combined cetuximab and genistein treatment shows additive anti-cancer effect on oral squamous cell carcinoma. Cancer Lett. 2010;292(1):54–63. doi: 10.1016/j.canlet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 153.Li Y, et al. Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 2006;66(9):4816–25. doi: 10.1158/0008-5472.CAN-05-3752. [DOI] [PubMed] [Google Scholar]
- 154.Cheng CW, et al. Evidence-based management of herb-drug interaction in cancer chemotherapy. Explore (NY) 2010;6(5):324–9. doi: 10.1016/j.explore.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 155.Xu H, Chen KJ. Herb-drug interaction: an emerging issue of integrative medicine. Chin J Integr Med. 2010;16(3):195–6. doi: 10.1007/s11655-010-0195-z. [DOI] [PubMed] [Google Scholar]
- 156.Chen J, et al. Pharm Res. Vol. 21. 11: 2004. Potential beneficial metabolic interactions between tamoxifen and isoflavones via cytochrome P450-mediated pathways in female rat liver microsomes; pp. 2095–104. [DOI] [PubMed] [Google Scholar]
- 157.Laurenzana EM, et al. Effect of dietary administration of genistein, nonylphenol or ethinyl estradiol on hepatic testosterone metabolism, cytochrome P-450 enzymes, and estrogen receptor alpha expression. Food Chem Toxicol. 2002;40(1):53–63. doi: 10.1016/s0278-6915(01)00095-3. [DOI] [PubMed] [Google Scholar]
- 158.Sun XY, et al. Increased UDP-glucuronosyltransferase activity and decreased prostate specific antigen production by biochanin A in prostate cancer cells. Cancer Res. 1998;58(11):2379–84. [PubMed] [Google Scholar]
- 159.Ohkimoto K, et al. Characterization of a zebrafish estrogen-sulfating cytosolic sulfotransferase: inhibitory effects and mechanism of action of phytoestrogens. Chem Biol Interact. 2004;147(1):1–7. doi: 10.1016/j.cbi.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 160.Eaton EA, et al. Flavonoids, potent inhibitors of the human P-form phenolsulfotransferase. Potential role in drug metabolism and chemoprevention. Drug Metab Dispos. 1996;24(2):232–7. [PubMed] [Google Scholar]
- 161.Alvarez AI, et al. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci. 99(2):598–617. doi: 10.1002/jps.21851. [DOI] [PubMed] [Google Scholar]
- 162.Zhang S, Yang X, Morris ME. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 2004;21(7):1263–73. doi: 10.1023/b:pham.0000033015.84146.4c. [DOI] [PubMed] [Google Scholar]
- 163.Merino G, et al. In vivo inhibition of BCRP/ABCG2 mediated transport of nitrofurantoin by the isoflavones genistein and daidzein: a comparative study in Bcrp1 (−/−) mice. Pharm Res. 27(10):2098–105. doi: 10.1007/s11095-010-0208-5. [DOI] [PubMed] [Google Scholar]
- 164.Tamaki H, et al. Inhibitory effects of herbal extracts on breast cancer resistance protein (BCRP) and structure-inhibitory potency relationship of isoflavonoids. Drug Metab Pharmacokinet. 2010;25(2):170–9. doi: 10.2133/dmpk.25.170. [DOI] [PubMed] [Google Scholar]
- 165.Imai Y, et al. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64(12):4346–52. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- 166.Takimoto CH, et al. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1213–21. [PubMed] [Google Scholar]
- 167.King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr. 1998;67(5):867–72. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- 168.Okabe Y, Shimazu T, Tanimoto H. Higher bioavailability of isoflavones after a single ingestion of aglycone-rich fermented soybeans compared with glucoside-rich non-fermented soybeans in Japanese postmenopausal women. J Sci Food Agric. 2011;91(4):658–63. doi: 10.1002/jsfa.4228. [DOI] [PubMed] [Google Scholar]
- 169.Watanabe S, et al. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako) J Nutr. 1998;128(10):1710–5. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- 170.Steensma A, Noteborn HP, Kuiper HA. Comparison of Caco-2, IEC-18 and HCEC cell lines as a model for intestinal absorption of genistein, daidzein and their glycosides. Environ Toxicol Pharmacol. 2004;16(3):131–9. doi: 10.1016/j.etap.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 171.Tang L, et al. Use of glucuronidation fingerprinting to describe and predict mono- and dihydroxyflavone metabolism by recombinant UGT isoforms and human intestinal and liver microsomes. Mol Pharm. 2010;7(3):664–79. doi: 10.1021/mp900223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Coldham NG, et al. Biotransformation of genistein in the rat: elucidation of metabolite structure by product ion mass fragmentology. J Steroid Biochem Mol Biol. 1999;70(4–6):169–84. doi: 10.1016/s0960-0760(99)00104-1. [DOI] [PubMed] [Google Scholar]
- 173.Heinonen S, Wahala K, Adlercreutz H. Identification of isoflavone metabolites dihydrodaidzein, dihydrogenistein, 6'-OH-O-dma, and cis-4-OH-equol in human urine by gas chromatography-mass spectroscopy using authentic reference compounds. Anal Biochem. 1999;274(2):211–9. doi: 10.1006/abio.1999.4279. [DOI] [PubMed] [Google Scholar]