Abstract
Pancreatic cancer is generally detected at later stages with a poor prognosis and a high-mortality rate. Development of theranostic imaging agents that non-invasively target pancreatic cancer by gene expression and deliver therapies directly to malignant cells could greatly improve therapeutic outcomes. Small-peptide ligands that bind cell-surface proteins and are conjugated to imaging moieties have demonstrated efficacy in cancer imaging. Identification of cancer specific targets is a major bottleneck in the development of such agents. Herein, a method is presented that uses DNA microarray expression profiling of large sets of normal and cancer tissues to identify targets expressed in cancer but not expressed in relevant normal tissues. Identified targets are subsequently validated for protein expression using tissue microarray. Further validations are performed by quantifying expression in pancreatic cancer cells by quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR), by immunocytochemistry and immunohistochemistry and by reviewing data and literature in public databases. Validated targets are selected for ligand development based on the existence of a known ligand or by known structure activity relationships useful for development of novel ligands.
Keywords: Pancreatic adenocarcinoma, cancer target, cell-surface, gene expression profiling, DNA microarray, tissue microarray, imaging, ligand conjugate
1. Introduction
The incidence of pancreatic cancer is increasing worldwide and is associated with a high mortality rate. Since pancreatic adenocarcinoma (PanAdo) is typically diagnosed at later stages of disease, only a small fraction of patients have operable lesions at the time of diagnosis (1). Endoscopic ultrasound and other imaging modalities are currently used for detection of pancreatic cancer in suspect patients (2). Agents that non-invasively target pancreatic cancer by specificity of gene expression have great potential for theranostic use. Patient outcomes could be improved by non-invasively following tumor response to treatment through imaging; by selective delivery of therapy to tumor relative to normal tissue; by aiding in diagnosis through characterization of gene expression; and by providing treatment options for patients with inoperable or disseminated disease.
Development of imaging and therapeutic agents using antibody or small peptide-based ligands has been moderately successful in targeting cancer. Effective treatment of non-Hodgkin’s lymphoma was achieved using radiolabeled monoclonal antibodies targeting cell-surface epitopes (3). However, the use of large conjugated-antibodies to deliver imaging or therapeutic compounds to solid tumors has not been ideal (4,5). Several agents based on the relatively small RGD peptide have been developed to deliver therapeutic and imaging agents to solid tumor vasculature (6), e.g. an [18F]galacto-RGD positron emission tomography (PET) agent was used to reliably image tumor αvβ3-integrin expression and has a favorable pharmacokinetic profile (7,8). Hence, targeting of small peptide conjugates to cell-surface proteins is a solid approach. Known structure activity relationships (SAR) are routinely used to engineer specific binding ligands via computer modeling and high-throughput screening methodologies (9). These small peptide ligands may be developed with binding constants in the nanomolar range and can subsequently be tethered to imaging or therapeutic moieties without significantly altering binding characteristics (10,11).
Although such agents can be readily developed, there remains a critical need for agents that specifically target pancreatic cancer. The question might be asked, why aren’t there a plethora of agents currently in development? Often, too little emphasis in study design has been made on target identification and validation. A target that has been reported in the literature as being “expressed in pancreatic cancer” may or may not be a good target. After further validation, such targets are often found to be commonly expressed in other vital tissues and are thus non-specific. Both expression of a target in cancer and non-expression in normal tissues must be considered. Often targets are identified using contrived cell lines that are grown in a medium that does not accurately reflect conditions found in human tissues and therefore may have an altered expression profile. Additionally, pancreatic cancers are heterogeneous in origin and a given validated target may be useful for only a subset of tumors (12). Hence, multiple targets may be required to cover all pancreatic cancers.
For target identification, it is necessary to quantify the differential expression of protein localized to the cell surface in cancer tissue relative to normal tissues. Unfortunately, the field of proteomics has not yet advanced to the degree that quantitative determinations can be made for the complete set of cell surface proteins in tissue samples with high-throughput (13). Alternatively, gene expression profiling via DNA microarray determination of transcript levels are now routinely performed on tissue samples with robust and quantitative results. Although transcript levels are not necessarily linearly related to the level of translation and subcellular localization of gene product, transcript is required for translation and for a large percentage of gene-products there is a linear relationship. When using mRNA levels to identify putative targets, further validation of protein expression and localization is required.
The following points are general guidelines for identification and validation of cancer targets. Determination and validation of protein expression in a panel of tumor tissues is a requirement for target identification. Furthermore, validation of non-expression in a large panel of vital normal tissues (e.g. heart, lungs, liver, kidneys), and in normal tissues found associated with tumors (e.g. vascular tissues and monocytes), is also a requirement. Limiting a target identification search to cell surface proteins is beneficial during agent development because strategies that enable the construct to cross the plasma membrane are not required. Also, knowledge of the biological function of the target protein may be of interest, but is not a requirement for agent development. For targeting, discovery of a small peptide binding ligand with high affinity is sufficient.
2. Materials
All solutions were made using distilled and deionized H2O and all chemicals were purchased from Sigma-Aldrich, St. Louis, MO, unless stated otherwise.
2.1 Tissue Samples and Cell Lines
For DNA microarray for expression profiling of pancreatic adenocarcinoma (PanAdo), 28 tissue samples were acquired (8 fresh-frozen from the Arizona Cancer Center Tissue Acquisition and Cellular/Molecular Analysis Shared Service, AZCC TACMASS, at the University of Arizona, Tucson, AZ, and 20 sets of array data from the Molecular Profiling Institute, Phoenix, AZ).
For DNA microarray of normal, unaffected tissue, 4 fresh-frozen normal pancreas tissue samples (AZCC TACMASS) and 103 RNA samples from normal tissues representing 28 different organ sites (BioChain, Hayward, CA and Stratagene, La Jolla, CA) were acquired.
For tissue microarray (TMA), paraffin-embedded pancreatic tissues were obtained from the Biospecimen Repository Core of the Pancreatic Cancer P01 project (CA109552) at the Translational Genomics Research Institute (TGen), Phoenix, AZ.
All tissue samples were de-identified and an institutional review board exemption obtained for their use.
For DNA microarray, qRT-PCR and immunocytochemistry, 17 PanAdo cell lines were obtained from American Type Culture Collection (ATCC).
2.2 Cell Culture
Cells were grown using RPMI medium with 10% fetal bovine serum (FBS), 1% penicillin and 1% streptomycin added.
2.3 RNA Extraction and DNA Microarray
RNeasy kit (Qiagen, Valencia, CA).
Agilent low-input RNA fluorescent linear amplification kit (Agilent Technologies, Santa Clara, CA).
Human 1A Microarray Chips (Agilent Technologies, Santa Clara, CA).
GenElute Mammalian Total RNA Miniprep Kit and Amplification Grade DNase I (Sigma-Aldrich, St. Louis, MO) for qRT-PCR of specific targets in pancreatic cancer cell lines.
2.4 Tissue Microarray (TMA)
A tissue microarray, designed and constructed by the TMA Core Facility at TGen, Phoenix, AZ, was comprised of 42 unique PanAdo cases and triple punched using 1.0mm cores. When available, a normal duct (by morphology) was arrayed alongside tumor counterparts. TMA slides for normal tissues (version CHTN2002N1) were provided by the Cooperative Human Tissue Network funded by the National Cancer Institute/NIH.
The TMA block was sectioned at 5 microns and transferred by water floatation to standard charged slides.
TMA slides containing a variety of tumor and normal tissues were used in the antibody optimization process.
2.5 Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
Primer-sets were designed in-house (see Note 1) and purchased from Invitrogen, San Diego, CA.
QuantiTect SYBR Green RT-PCR Kit (Qiagen, Valencia, CA).
2.6 Immunocytochemistry (ICC)
The same primary antibodies used for TMA staining were used for ICC.
Secondary antibodies: Alexafluor 488 goat anti-rabbit and AlexaFluor488 goat anti-mouse (Invitrogen, San Diego, CA).
10× PBS stock solution: 80 g NaCl, 2.0 g KCl, 14.4 g Na2HPO4 and 2.4 g KH2PO4 in 1 L, adjust pH to 7.4 with HCl.
Paraformaldehyde solution made fresh on day of use (see Note 2): 2% paraformaldehyde in 1× PBS. Heat water in microwave, then slowly add powder while stirring, add 10% final volume of 10× PBS. Clarify by adding one drop of 1N NaOH. Filter through paper-lined funnel. Cool on ice until cold to touch.
20× SSC stock solution: 3.0 M NaCl and 0.3 M Na3-citrate, pH to 7.4 with 1N NaOH.
Antibody conjugation buffer: 1× SSC, 2 % goat serum, 1% BSA, 0.05% Triton X-100 and 0.02% NaN3.
Antibody wash buffer: 1× SSC and 0.05% Triton X-100.
Glycine wash for quenching paraformaldehyde: 0.75% glycine, pH to 7.4 with 1N NaOH and store at 4°C.
Permeabilization buffer: 1× SSC, 0.1% Triton X-100. Invert flask several times to mix thoroughly.
Vectashield H-1000 mounting medium for fluorescence (Vector Laboratories, Burlingame, CA).
3. Methods
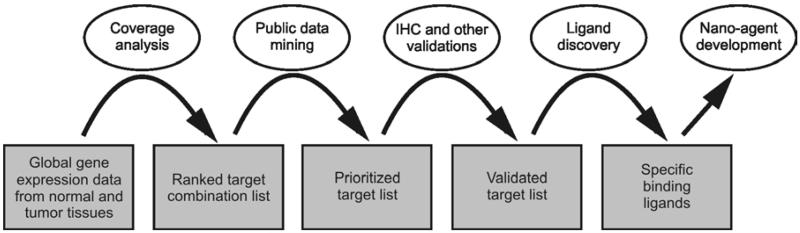
Figure 1 is a flow chart describing the identification and validation of cancer targets, and the subsequent discovery of binding ligands and development of targeting nano-agents. To identify potential pancreatic cancer targets, mRNA was obtained from multiple pancreatic tumor samples and multiple normal tissue samples from 28 organ sites including normal pancreas. DNA microarray data were generated and data for genes with products that are localized to the cell-surface were parsed and analyzed for expression in tumor and normal tissues. To do this, a list of cell-surface genes was compiled and expression versus non-expression threshold values determined for the array data (12). Genes determined to be expressed in tumor, but not expressed in normal tissues were prioritized based on percentage of expression amongst the tumor samples (tumor coverage). Targets intended for imaging were limited to non-expression in normal pancreas and organs associated with drug toxicity and clearance. Targets intended for delivery of therapeutics were limited to non-expression in all normal tissues (see Note 3). Putative targets were ranked and prioritized based on percent coverage amongst the tumor samples and potential for ligand development, i.e. known ligands or SAR. The highest ranked targets were verified by checking expression values reported in public databases, evaluated for availability of known ligands and structure activity relationships (SAR), and selected for further validation. Protein expression was validated via tissue microarray (TMA) for the highest ranked targets that had commercially available antibodies (see Notes 4 & 5) (14-17). TMA validated targets were further validated for expression in pancreatic cancer cell lines via quantitative real-time RT-PCR (qRT-PCR) (18) and immunocytochemistry (ICC) (see Note 6) (19). The most promising fully validated targets were selected for ligand development for future incorporation into targeted agents.
Figure 1.
Flow chart for target identification and validation, ligand discovery, and nano-agent development.
3.1 RNA Extraction
For DNA array, total RNA was isolated from fresh-frozen tissue samples and from pancreatic cancer cells grown to ~80% confluence using the RNeasy kit (Qiagen, Valencia, CA).
For qRT-PCR, RNA was extracted from pancreatic cancer cells using the GenElute Mammalian Total RNA Miniprep Kit and traces of genomic DNA removed using Amplification Grade DNase I (Sigma-Aldrich, St. Louis, MO).
3.2 DNA Microarray and Gene Expression Profiling
CY5 labeled cRNA targets were generated from normal tissue samples (103), pancreatic cancer tissues (28) or PaAdo cell lines using 1 ug total RNA and the Agilent low-input RNA fluorescent linear amplification kit. For use as a reference, CY3 cRNA were generated from normal pancreas tissue.
The concentration and integrity of fluorescence labeled cRNAs were determined using an Agilent 2100 Bioanalyzer.
For each sample, equal amounts of labeled cRNA targets from sample and reference were hybridized onto an Agilent Human 1A oligonucleotide array.
Hybridization signals were acquired and analyzed using Agilent’s feature extraction software (version 7.1).
Feature intensity values were normalized by the array median intensity.
Internal stability of the dataset was determined using data analysis techniques such as Cluster analysis and multi-dimensional scaling analysis (MDS). Multi-dimensional scaling analysis (MDS) was used to view the entire array data projected onto 3-dimensions to verify the consistency of groupings based on phenotypes (see Note 7).
3.3 Compilation of Cell Surface Protein List
The entire Gene Ontology hierarchical vocabulary was manually browsed through using the Cancer Genome Anatomy Project Gene Ontology browser. Each category was followed through to the lowest possible level in order to select lists of genes that may encode proteins containing cell-surface epitopes (6,389 genes combined).
Genes that were not represented on the microarray chips (Agilent Human 1A v1 & v2 arrays) used in our study were removed (4,407 remaining genes).
Each remaining gene was manually checked using existing databases (Genecard, Harvester, Entrez, Protein Database and UniProt), retaining genes that encode or are predicted to encode cell-surface products (2,177 genes total).
3.4 Determination of Cutoff Values for Coverage Analysis (What is zero expression?)
The maximum level of mRNA expression in normal tissues that corresponds to non-expression of protein at the cell surface was estimated. This estimate was used to establish a threshold value for normalized DNA microarray data of normal tissues, below which the corresponding protein is considered to be not expressed at the cell-surface (see Note 8).
The minimum level of mRNA expression in tumor samples that corresponds with protein expression at the cell-surface was estimated (see Note 8).
The above estimates were used to set the most stringent coverage analysis threshold values possible (see Section 3.5 below) that still provided targets (see Note 8).
3.5 Target Identification by Coverage Analysis
An algorithm was prepared, where for a given cell-surface DNA array probe, an expression flag was assigned for each normal tissue and tumor sample (see Notes 9 and 10). Expression flag values for probes on each array were assigned as “0” if the median normalized value was ≤ the threshold value; or as “1” if > the threshold value (see Section 3.4 for determining threshold values).
For each probe, the normal and tumor expression flag values were summed.
The percent normal tissue coverage and pancreatic tumor coverage for each probe was calculated by dividing the summed value by the number of normal or tumor samples (see Note 3).
All probes that had 0% tumor coverage were removed from the analysis.
The remaining probes were sorted by ascending percent normal coverage and descending percent tumor coverage.
Genes with low or 0% normal tissue coverage, but with high tumor coverage were ranked highest.
3.6 Target Selection for Validation
Since targets were identified based on “expression” versus “non-expression,” tumor expression level was considered for the highest ranked targets. Targets with the highest expression in tumor tissues were preferred.
Targets with known small MW peptide binding ligands were given priority (see Note 11).
Targets with known biology or structure activity relationships (SAR) were also preferred in order to enable a directed approach towards small ligand development.
Targets with available monoclonal antibodies that specifically target extracellular epitopes were considered desirable. In particular if the structure was known for the binding region of the antibody.
Published databases and current literature were consulted for agreement, e.g. a given target may be expressed in a specific tissue type that was not represented in the sample set.
Pancreatic tumors display heterogeneity of cell-surface gene expression (12). Thus, multiple targets are required to cover all pancreatic tumor types.
3.7 TMA sectioning and (IHC) Staining / Scoring
The TMA block was sectioned at 5 microns and sections were transferred to standard glass slides by water floatation. Slides were baked 20 minutes at 56 degrees.
IHC optimization included titration of antibodies against regular tissue sections and “tester” TMA slides containing a variety of tumor and normal tissues. TMA slides underwent antigen retrieval by heating at 100 degrees in citrate buffer (0.1mol/L, pH 6.0) for 5 to 30 min depending on the antibody.
Slides were incubated with primary antibody at optimal dilutions for 30 minutes at room temperature with incubation (and washes) with biotinylated secondary antibody were followed by application of streptavidin-peroxidase complex (Vision BioSystems) and resolved with diaminobenzidine chromogen staining. Primary antibodies and dilutions used were anti-cholecystokinin A receptor (CCKAR; R&D Systems), 7 μg/mL; and anti-protein tyrosine phosphatase receptor, type C (PTPRC; R&D Systems), 2 μg/mL.
Slides were then counter-stained with hematoxylin for 5 minutes and rinsed with water. After dehydration with serial concentrations of ethanol (70%, 80%, 90% and 100%), the slides were soaked with xylene. After draining the xylene, mounting media and cover slips were applied to the slides (see Figure 2 for representative immunostained and counterstained TMA cores).
Stained slides were evaluated using light microscopy and scored by a board-certified pathologist (G.H.) with 0 interpreted as negative to 3+ = strongly positive (see Table 1). When applicable, staining localization was listed as nuclear, cytoplasmic or membranous.
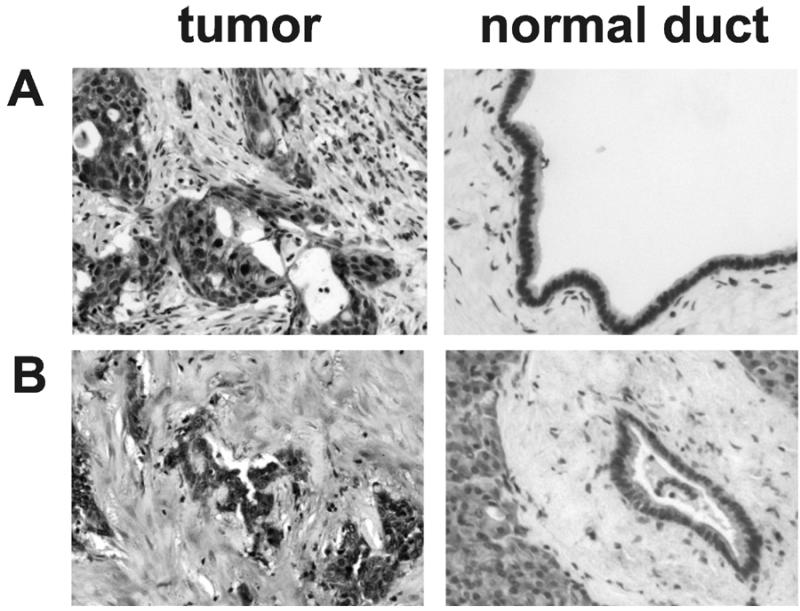
Figure 2.
Representative TMA cores immunostained for CCKAR (A) and PTPRC (B) from pancreatic adenocarcinoma cases (left) and pancreatic ducts with normal morphology (right).
Table 1. Summarized results for TMA.
| Target | Sample classification |
Score | % of cases with ≥ 2+ |
|||
|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | |||
| CCKAR | Adjacent Normal | 8 | 5 | 3 | 0 | 23 |
| Tumor | 5 | 4 | 10 | 2 | 57 | |
| PTPRC | Adjacent Normal | 10 | 5 | 1 | 0 | 7 |
| Tumor | 5 | 6 | 7 | 1 | 42 | |
3.8 Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
Pancreatic cancer cells were grown in 6 well plates to 80% confluence and RNA was extracted using the GenElute Mammalian Total RNA Miniprep Kit. Traces of genomic DNA were subsequently removed using Amplification Grade DNase I (Sigma-Aldrich, St. Louis, MO).
Primer sets were designed to generate cDNA and perform RT-PCR from transcripts of pancreatic cancer cell-surface targets of interest (see Note 1). Fragments lengths were approximately 100 bp long.
PCR conditions were optimized for maximum yield without spurious priming.
Real-time RT-PCR was performed using a Smart Cycler® (Cephid, Sunnyvale, CA) and the QuantiTect SYBR Green RT-PCR Kit (Qiagen).
A no-RT reaction and no-template reaction were included during each experiment as controls.
Melting curves yielded a single melt-peak for all template reactions and a minimal melt peak for the no-template reaction.
A single reaction was performed per extract for each target template, three extracts per cell line. Overall reliability was high, Chronbach’s alpha = 0.93) (18).
Expression values were determined as 2−CT, where CT is the second derivative of the fluorescence curve.
Raw expression values were normalized using ACTB transcript as an internal standard.
3.9 Immunocytochemistry (ICC)
Primary antibody dilutions (typically 1:50) were optimized individually to provide maximal staining without using excess antibody. The same primary antibodies were used for ICC as was used for TMA staining. Secondary antibodies were conjugated with AlexaFluor488 fluorescent dye (Invitrogen) and dilutions (typically 1:200) were optimized to provide maximal staining without background.
Pancreatic cancer cells were grown to 80% confluence on round glass coverslips, 22 mm diameter, in 6-well tissue culture plates.
Paraformaldehyde solution was made fresh on the day of staining (see Section 2.6 above).
Primary antibodies were prepared were diluted to the optimized concentration using antibody conjugation buffer (see Section 2.6 above).
Media was aspirated from the plates and paraformaldehyde solution (see Section 2.6 above) added until coverslips were submerged and incubated 15 minutes.
Paraformaldehyde was quenched with an equal volume of warm 50 mM glycine buffer (see Section 2.6 above & Note 12). The resulting solution was removed using a disposable tip and discarded in a paraformaldehyde waste bottle.
Plates were washed twice for 5 minutes in glycine buffer. The first wash was discarded in a paraformaldehyde waste bottle.
The second glycine wash was discarded and cell membranes permeabilized by incubating for 10-15 minutes in 0.1% Triton X-100 permeabilization buffer (section 2.6).
The permeabilization buffer was discarded and primary antibody solution, 80 μL, dispensed to the top of each coverslip (see Note 13). Plates were covered and incubated at room temperature for 1 hour (see Note 14).
Coverslips were washed three times, 5 minutes per wash, using antibody wash buffer (see Section 2.6 above & Note 15).
Secondary antibodies were prepared during the washes (step 10) using the optimized dilution (step 1) and antibody conjugation buffer (see Section 2.6 above). The tubes were wrapped in aluminum foil in order to prevent quenching of the fluorophore. Secondary antibody solution, 80 μL, was dispensed to the top of each coverslip (see Note 13). Plates were covered and incubated at room temperature in the dark for 45 minutes.
While keeping the plates in the dark as much as possible, coverslips were washed three times, 5 minutes per wash, using antibody wash buffer (see Section 2.6 above & Note 15).
Vectashield H-1000 mounting medium for fluorescence (Vector Laboratories), 10 μL, was dispensed onto the center of one microscope slide per coverslip.
Coverslips were removed from the antibody wash solution using forceps, gently dried on the back side with a kimwipe, and placed cell-side down onto the mounting media (see Note 16).
Excess mounting media was removed from the coverslip and the outside edges sealed using clear fingernail polish.
Slides were air dried for 15 minutes at room temperature in the dark and moved to a −20°C freezer for long-term storage.
Duplicate slides were prepared for each cell line and target, and background was determined by staining only with secondary antibody as a control.
Relative staining intensities were compared to the no-primary antibody control and recorded as +++ = high, ++ = moderate and + = low.
3.10 Verification using Public Databases and Expression Signal Intensity Distributions
In order to further verify expression, or non-expression in normal tissues, additional microarray gene expression datasets were obtained from public databases such as the Gene Expression Omnibus (GEO) and the GeneAtlas by the Genomics Institute of the Novartis Research Foundation (GNF).
Expression signal intensity distribution analyses were performed for each validated target using public data for a broad range of cancers and normal tissues. These analyses are useful to identify expression of a given target in normal tissues that were not included in the set used for DNA microarray identification and TMA validations. Also, these analyses can provide insight into the broader applicability of a given target amongst a range of cancer types.
Acknowledgments
We would like to thank Dr. Michael Bittner and Sonsoles Shack for microarray data acquisition, and Dr. Ronald Lynch for immunocytochemistry advice.
Footnotes
When designing primer sets for real-time RT-PCR, the following should be considered: the optimal product length when using the QuantiTect SYBR Green RT-PCR Kit (Qiagen) is ~100 bp; it is best if primers span an intron so that amplified cDNA can be readily distinguished from genomic DNA; some primer sequences form secondary structures, e.g. stem-loops, or can anneal to sequence from the reverse direction primer (primer-dimerization), these structures can be predicted by available computer algorithms or by eye, and should be avoided; the annealing temperature for both primers in a set should be nearly identical; and NCBI-BLAST database searches should be performed for both primer sequences and no cDNA template other than the intended sequence should be primed by both primers in a set. Optimize PCR conditions for each primer set by varying annealing temperature and Mg2+ concentration if necessary, so that maximum yield without spurious priming is achieved.
Paraformaldehyde is toxic. Wear mask while weighing and handling, and work in the fume hood. Dispose of liquid and dry waste appropriately.
A major hindrance to the development of targeted agents for imaging or therapy of cancer is the difficulty in identifying targets that are specifically expressed in cancer relative to normal unaffected tissue. In the case of targeted therapeutic agents and in order to avoid toxicity and serious side effects, it is best to identify targets that are not expressed in the broadest possible range of normal tissues, but are expressed in cancer. However, since imaging agents generally have significantly lower toxicities, the range of normal tissues surveyed can be decreased to include tissues involved in respiration, clearance and the site of origin of the cancer (normal pancreas). Thus, increasing the likelihood of identifying targets suitable for early detection imaging.
If not available for a specific target of interest, antibodies can be commercially produced for a price.
TMA validations were largely in agreement with DNA microarray assessments, but not for 100% of the cases. Some differences are to be expected due to post-translational regulation of gene product. In addition to determination of expression in tumor tissue, TMA provides useful detail, such as identification of specific cell types expressing the target within the tumor and determination of target expression in surrounding tissues of different pathologies amongst the sample, e.g. tumor versus adjacent normal pathology.
As a substitute for validation of protein expression in cell lines via ICC, Western analyses may be performed. To assure that cell-surface expression is determined both whole cell and plasma membrane extracts can be performed. Other alternatives include ligand binding assay or functional assay if these are available for the given target.
Multi-dimensional scaling analysis is a data analysis technique for mapping data of higher dimensionality (e.g. expression data with a number of genes across multiple samples) to a smaller dimensions (typically 3 or lower) where distance between any two points corresponds to similarities or dissimilarities (20). It allows the visualization of the entire datasets on lower dimensions and the identification of any discrepancies that may result from data acquisition and processing.
- Always allow for a gap between the lower normal tissue cutoff value and the higher tumor cutoff value, e.g. if the lower value is set at say 0.3 of the array median, then set the upper tumor cutoff value to 0.4 of the array median value.
- Perform the coverage analysis (see section 3.5) at a range of lower normal tissue cutoff values, e.g. 0.2 to 0.6 of the array median. If set too low, a large and unmanageable number of targets will be identified, many of which may not be expressed in tumor. If set too high, few targets will be identified, and these targets may be expressed in normal tissues.
- Choose the lowest normal expression threshold value that provides a manageable number of targets, i.e. less than 100 rather than thousands. Further validation of select targets by TMA will provide feedback. For example, if many target proteins are found to be expressed in normal tissues, the normal threshold value may be set too high.
Rather than identifying targets that are differentially expressed in tumor relative to normal, for targeted agents it is vital that the target not be expressed in normal tissues. Hence, representing the data in binary form as “expressed” versus “not-expressed” is required.
Alternatively, in order to eliminate outliers in the normal tissue dataset, all values for a given normal tissue type may first be averaged prior to determining an expression flag value. Thus determining an expression flag value for each normal tissue type rather than for each normal tissue sample.
Small peptide ligands are optimal for attachment to nanoparticles, or attachment of linkers and imaging or therapeutic moieties. Small organic ligands are generally too small for attachment without disruption of binding or biological activity. Peptide ligands can be altered and developed via rational design and high-throughput synthesis and screening to improve the desired properties, e.g. reduced size, incorporation of peptidomimetic residues to decrease degradation, and to increase or decrease binding affinity and agonist or antagonist properties.
Stock glycine wash solution is stored at 4°C. It is important that the glycine solution is warm before quenching paraformaldehyde, so bring the solution to 37°C in a water bath before use.
Alternatively, in order to use less antibody 40-50 μL primary antibody solution per coverslip can be dispensed on parafilm in a petri dish. Coverslips are then removed from the 6-well plate, dabbed with a kimwipe to remove excess permeabilization or wash buffer, and placed cell-side down onto the primary antibody on parafilm making sure that there are no bubbles. Following incubation, carefully remove the coverslip from the parafilm and place them cell side up in a 6-well culture plate for subsequent washes.
Primary antibody incubation time may vary depending on the cells and antibodies used. Another common procedure is to incubate overnight at 4°C. In this case the incubation should be performed on parafilm in a covered petri dish.
To prevent washing cells off the coverslip, wash buffer should be added to the side of the well, not directly on the coverslip. To prevent antibody from being transferred amongst the different slips, use a new pipet tip to remove the antibody wash solution from each coverslip.
If there are bubbles in the mounting medium after placing the slide, move the coverslip to the edge of the slide and gently peel up and replace.
Contributor Information
David L. Morse, Molecular and Functional Imaging, H. Lee Moffitt Cancer Center & Research Institute, Tampa, Florida.
Galen Hostetter, Tissue Microarray Center, Translational Genomics Research Institute, Phoenix, Arizona.
Yoganand Balagurunathan, Computational Biology Division, Translational Genomics Research Institute, Phoenix, Arizona.
Robert J. Gillies, Department of Radiology, H. Lee Moffitt Cancer Center & Research Institute, Tampa, Florida
Haiyong Han, Clinical Cancer Research Division, Translational Genomics Research Institute, Phoenix, Arizona.
References
- 1.Dunphy EP. Pancreatic cancer: A review and update. Clin J Oncol Nurs. 2008;12:735–741. doi: 10.1188/08.CJON.735-741. [DOI] [PubMed] [Google Scholar]
- 2.Klapman J, Malafa MP. Early Detection of Pancreatic Cancer: Why, Who, and How to Screen. Cancer Control. 2008;15:280–287. doi: 10.1177/107327480801500402. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg DM, Sharkey RM. Novel radiolabeled antibody conjugates. Oncogene. 2007;26:3734–3744. doi: 10.1038/sj.onc.1210373. [DOI] [PubMed] [Google Scholar]
- 4.Jhanwar YS, Divgi C. Current status of therapy of solid tumors. J Nucl Med. 2005;46(Suppl 1):141–150S. [PubMed] [Google Scholar]
- 5.Goldenberg DM, Sharkey RM. Advances in cancer therapy with radiolabeled monoclonal antibodies. Q J Nucl Med Mol Imaging. 2006;50:248–264. [PubMed] [Google Scholar]
- 6.Temming K, Schiffelers RM, Molema G, Kok RJ. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist Updat. 2005;8:381–402. doi: 10.1016/j.drup.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Haubner R, Weber WA, Beer AJ, et al. Noninvasive visualization of the activated avh3 integrin in cancer patients by positron emission tomography and [18F]galacto-RGD. PLoS Med. 2005;2:e70. doi: 10.1371/journal.pmed.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beer AJ, Haubner R, Wolf I, et al. PET-based human dosimetry of 18F-galacto-RGD, a new radiotracer for imaging avh3 expression. J Nucl Med. 2006;47:763–769. [PubMed] [Google Scholar]
- 9.Fung S, Hruby VJ. Design of cyclic and other templates for potent and selective peptide α-MSH analogues. Curr Opin Chem Biol. 2005;9:352–358. doi: 10.1016/j.cbpa.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handl HL, Vagner J, Yamamura HI, Hruby VJ, Gillies RJ. Development of a lanthanide-based assay for detection of receptor-ligand interactions at the delta-opioid receptor. Anal Biochem. 2005;343:299–307. doi: 10.1016/j.ab.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 11.Black KC, Kirkpatrick ND, Troutman TS, Xu L, Vagner J, Gillies RJ, Barton JK, Utzinger U, Romanowski M. Gold nanorods targeted to delta opioid receptor: plasmon-resonant contrast and photothermal agents. Mol Imaging. 2008;7:50–57. [PubMed] [Google Scholar]
- 12.Balagurunathan Y, Morse DL, Hostetter G, Shanmugam V, Stafford P, et al. Gene expression profiling-based identification of cell-surface targets for developing multimeric ligands in pancreatic cancer. Mol Cancer Ther. 2008;7:3071–3080. doi: 10.1158/1535-7163.MCT-08-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tangrea MA, Wallis BS, Gillespie JW, Gannot G, Emmert-Buck MR, Chuaqui RF. Novel proteomic approaches for tissue analysis. Expert Rev Proteomics. 2004;1:185–192. doi: 10.1586/14789450.1.2.185. [DOI] [PubMed] [Google Scholar]
- 14.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 15.Andersen CL, Hostetter G, Grigoryan A, Sauter G, Kallioniemi A. Improved procedure for fluorescence in situ hybridization on tissue microarrays. Cytometry. 2001;45:83–86. doi: 10.1002/1097-0320(20011001)45:2<83::aid-cyto1149>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Mousses S, Bubendorf L, Wagner U, et al. Clinical validation of candidate genes associated with prostate cancer progression in the CWR22 model system using tissue microarrays. Cancer Res. 2002;62:1256–1260. [PubMed] [Google Scholar]
- 17.Watanabe A, Cornelison R, Hostetter G. Tissue microarrays:applications in genomic research. Expert Rev Mol Diagn. 2005;5:171–181. doi: 10.1586/14737159.5.2.171. [DOI] [PubMed] [Google Scholar]
- 18.Morse DL, Carroll D, Weberg L, Borgstrom MC, Ranger-Moore J, Gillies RJ. Determining suitable internal standards for mRNA quantification of increasing cancer progression in human breast cells by real-time reverse transcriptase polymerase chain reaction. Anal Biochem. 2005;342:69–77. doi: 10.1016/j.ab.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Lynch RM, Fogarty KE, Fay FS. Modulation of hexokinase association with mitochondria analyzed with quantitative three-dimensional confocal microscopy. J Cell Biol. 1991;112:385–395. doi: 10.1083/jcb.112.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhinik Z, Ben-Dork A, Sampask N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V, Hayward N, Trent J. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature Letters. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]