Abstract
The hypothesis was examined that physiologic variation of estrogen concentrations during the menstrual cycle can provoke BK virus (BKV) excretion. BKV and JCV viral loads were determined in urine specimens obtained almost daily from 20 healthy, non-pregnant women over 2 months. Asymptomatic urinary shedding of BKV was observed in 123 (12.0%) of 1,021 specimens from 11 (55%) study subjects. Two subjects excreted JCV in their urine, with one subject excreting detectable JCV in all urine specimens. Analysis of 36 complete menstrual cycles revealed no difference in the prevalence of BKV excretion between pre-ovulatory and post-ovulatory phases of the menstrual cycle. The unexpected day-to-day variability in BKV excretion suggests that as yet unidentified factors may contribute to the periodic shedding of BKV by healthy women.
Keywords: polyomavirus, BK virus, menstrual cycle, JC virus
INTRODUCTION
The polyomaviruses JC virus (JCV) and BK virus (BKV) infect the majority of humans asymptomatically, but are an increasing cause of morbidity and mortality in immunocompromised patients. Furthermore, infectious agents, particularly BKV, are an increasing cause of renal allograft loss. Allograft loss due to infectious agents is now more common than loss due to immunologic rejection in some patient populations [Parasuraman et al., 2011]. As such, there is an increasing need to identify risk factors for polyomavirus excretion and disease so that subjects at increased risk can be monitored and treated more aggressively than those who have lower risk.
The prevalence of BKV infection is >90% in the adult population, while JCV infects approximately 50% of adults [Kean et al., 2009; Viscidi et al., 2011]. Despite this difference in prevalence, JCV is more commonly found in the urine of healthy adults, making BKV excretion a more sensitive indicator of immune status. Persistent polyomavirus excretion is common in immune-compromised patients and pregnant women. However, little is known of the dynamics of polyomavirus excretion in the urine of healthy adults. Among pregnant women, the prevalence of BKV excretion has been reported to be as low as 4% and high as 54% in point-prevalence studies [Markowitz et al., 1991; Jin, 1993; Tsai et al., 1997; Bhattacharjee and Chakraborty, 2004; Kalvatchev et al., 2008]. Our recent longitudinal analysis of polyomavirus shedding in pregnancy found that 54.9% of subjects excreted BKV during pregnancy [McClure et al., 2012]. In contrast, JCV excretion was not found to be associated with pregnancy, occurring no more frequently among pregnant than non-pregnant subjects of similar age and ethnicity [McClure et al., 2012].
Pregnancy-associated BKV excretion is a benign phenomenon that is thought to be related to the immunologic and hormonal changes of pregnancy. The presence of estrogen-sensitive promoters in the non-coding control region of BKV and the in vitro increase in BKV replication associated with exogenous estrogen suggest that estrogen could also contribute to BKV excretion in vivo [Moens et al., 1999]. However, the available data on the epidemiology of polyomavirus-associated nephropathy (PVAN) and BKV-associated hemorrhagic cystitis do not suggest that these diseases are more common among women.
The goal of this study was to determine if cyclic changes in estrogen during the menstrual cycle are associated with variations in the urinary excretion of BKV and/or JCV. We hypothesized that BKV excretion will be increased in the pre-ovulatory phase of the menstrual cycle due to a potential effect of estrogen on virus replication. If the excretion of BKV is greater in the pre-ovulatory phase of the menstrual cycle, this would provide a physiologic correlate to prior in vitro observations suggesting that estrogen can increase BKV replication.
MATERIALS AND METHODS
Study Participants
The study protocol was approved by the Institutional Review Board for Human Subjects Research at the Louisiana State University Health Sciences Center—Shreveport (LSUHSC-S). Study participants were identified by sub-investigators (CLK, SEK, and ATW) from students and staff of LSUHSC-S and enrolled between March 21, 2011 and August 19, 2011. The recruiting sub-investigators remained blinded to the personal health information of individual subjects. The principal investigator, study coordinator, and research associates were blinded from the identity of individual subjects in order to maintain strict confidentiality of subjects’ personal health information. Study participants were required to be in good general health, so potential participants were excluded for any of the following: diabetes, prior solid organ, or stem cell transplantation, any medical condition requiring immune suppression, hemoglobinopathy, or renal insufficiency (serum creatinine > 1.5). Each subject completed data collection forms monthly to document contraceptive use, medications, general health information, and the date and duration of her last menstrual period. Per-protocol collection of daily urine specimens for up to 90 days was planned, but not expected due to subjects’ weekends, holidays and vacations away from LSUHSC-S during the study period.
Clinical Specimen Processing
After obtaining informed consent, the study subjects provided urine specimens (8–10 ml) either at LSUHSC-S or at their home. Specimens collected at LSUHSC-S were delivered immediately to a refrigerator (4°C) dedicated for research specimens. Specimens collected at home were frozen immediately at −20°C and delivered to the laboratory in batches. A single serum specimen was obtained mid-study from subjects for serologic testing in order to determine whether subjects had been infected previously with JCV or BKV. All specimens were catalogued, bar-coded, and aliquoted in a restricted laboratory space dedicated for these purposes. One-milliliter of each urine specimen was stored at −80°C prior to DNA extraction using the total nucleic acid extraction kit on a Mag-NAPure LC instrument as directed by the manufacturer (Roche Applied Sciences, Inc., Indianapolis, IN).
Detection of BKV and JCV Viral Loads by Quantitative PCR
Quantitative PCR (QPCR) was performed in duplicate for each specimen using a LC480 LightCycler instrument (Roche Applied Sciences, Inc.) according to the manufacturer’s recommendations and as previously described [McNees et al., 2005; Vanchiere et al., 2005]. Viral loads of less than 250 genomes per milliliter were considered below the reliable limit of accurate detection and were not included in analyses. Based on periodic analyses for quality assurance, the variability in results for specimens and standards is < 0.1 log for repeat analysis using these assays.
BKV and JCV Antibody Testing
Serum specimens were tested for BKV- and JCV-reactive IgG antibodies using an ELISA-based assay, as described previously [Kean et al., 2009]. Serum specimens were tested in triplicate, and laboratory personnel were blinded to the results of PCR studies. Titers of >0.2 units were considered positive, based on prior studies and the testing of control sera.
Statistical Analyses
Descriptive statistical analysis was performed for comparison of continuous and categorical variables for the different study cohorts. Means, medians, and standard deviations (SD) were determined for continuous variables, and prevalence percentages were determined for categorical variables. Comparison of demographic, clinical, and laboratory-determined characteristics was performed using t-test for means and χ2 test for frequencies with differences considered statistically significant for P < 0.05.
RESULTS
Demographic Characteristics of Study Participants
The mean age of subjects was 25.8 ± 3.1 years with a range from 23 to 38 years. All subjects were non-Hispanic Caucasians and in good health, without chronic illnesses or immune suppressive conditions. None of the subjects had previously been pregnant or diagnosed with a sexually transmitted infection (STI) or vaginal yeast infection. Three participants had a history of urinary tract infections and one subject had asthma. All participants were non-smokers at the time of enrollment, but two participants had smoked cigarettes in the past.
The mean duration of the menstrual cycle was 29.1 ± 4.9 days, with a range of 24–60 days. Two subjects reported cycles longer than 30 days. The average duration of menses was 4.6 ± 1.2 days, with a range of 3–7 days. Four subjects reported no use of contraception during the duration of the study. Twelve subjects reported using hormonal contraception for the entirety of the study and four reported using hormonal contraceptives for only one of the two study months. Nineteen (95%) subjects were seropositive for BKV, while six (30%) were seropositive for JCV. Viremia with BKV or JCV was not detected in any of the study participants.
Polyomavirus Shedding
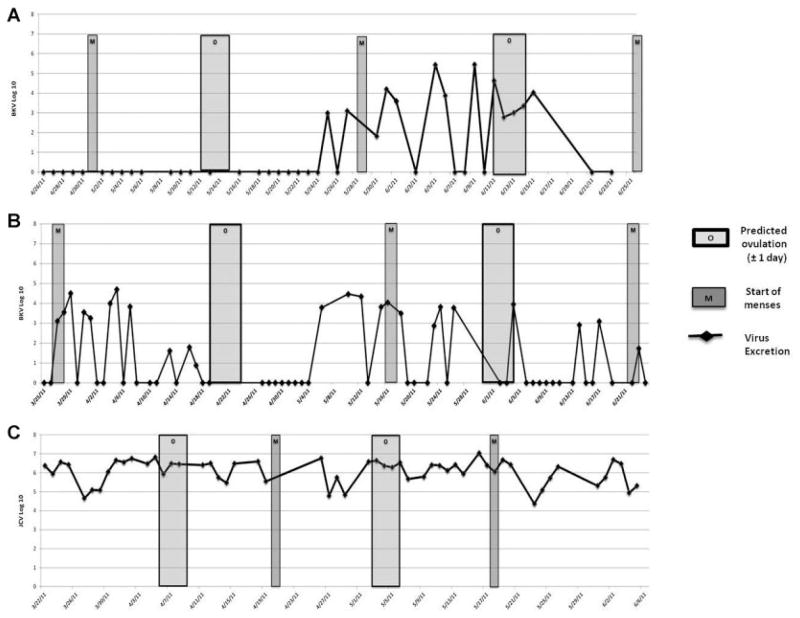
In total, 1,021 urine specimens were collected from 20 subjects, each of whom provided an average of 51 specimens (range: 26–73) over a mean of 66 days (range: 30–87). The overall collection rate for daily urine specimens was 77.8% (range: 59.7–100%). The number of specimens collected from each subject and virus excretion data are summarized in Table I. BKV excretion was detected in 123 (12.0%) specimens from 11 (55%) participants. The mean proportion of positive specimens for BKV-excreting subjects was 21.1% (range: 1.6–59.2%). The mean BKV viral load (log 10) was 3.25 ± 1.13 genomes per milliliter of urine. JCV was detected in 63 (6.2%) specimens from 2 (10%) participants. One JCV-seropositive subject was a constant excretor of JCV, with 54 of 54 JCV-positive specimens (Fig. 1C), while a JCV-seronegative subject was found to have 9 (14.5%) of 62 specimens with detectable JCV. Since only one serum specimen was available for analysis, it is unclear whether this subject may have been recently infected with JCV and had not yet mounted a detectable antibody response. Both JCV excretors were BKV seropositive, one of whom had a single BKV-positive urine sample during the study. The mean JCV viral load (log 10) was 5.50 ± 1.37. There was no significant difference in the BKV viral load based on the site of specimen collection or use of hormonal contraceptives (data not shown).
TABLE I.
Summary of Specimen Collection, Serologic Testing Results, and Polyomavirus Urinary Excretion by Subject
| Subject ID | Number of specimens collected | Time of collection (days) | Seropositivity
|
Polyomaviruria*
|
||
|---|---|---|---|---|---|---|
| BKV | JCV | BKV (%) | JCV (%) | |||
| M101 | 46 | 72 | + | + | 19.6 | 0.0 |
| M102 | 73 | 73 | + | + | 28.8 | 0.0 |
| M103 | 26 | 30 | + | − | 23.1 | 0.0 |
| M104 | 49 | 66 | + | + | 59.2 | 0.0 |
| M105 | 54 | 65 | + | + | 0.0 | 100 |
| M106 | 55 | 71 | + | − | 1.8 | 0.0 |
| M107 | 59 | 64 | + | + | 0.0 | 0.0 |
| M108 | 63 | 63 | + | − | 38.1 | 0.0 |
| M109 | 41 | 64 | + | − | 2.4 | 0.0 |
| M110 | 40 | 63 | + | − | 0.0 | 0.0 |
| M111 | 61 | 64 | + | − | 21.3 | 0.0 |
| M112 | 48 | 60 | + | − | 0.0 | 0.0 |
| M113 | 62 | 82 | + | − | 1.6 | 14.5 |
| M114 | 66 | 88 | + | − | 7.6 | 0.0 |
| M115 | 41 | 60 | + | − | 31.7 | 0.0 |
| M116 | 44 | 82 | + | + | 0.0 | 0.0 |
| M117 | 51 | 60 | + | − | 0.0 | 0.0 |
| M118 | 45 | 60 | + | − | 0.0 | 0.0 |
| M119 | 45 | 61 | − | − | 0.0 | 0.0 |
| M120 | 52 | 87 | + | − | 0.0 | 0.0 |
| Summary | 51.1 | 65.6 | 95% | 30% | 12.1% of specimens, 55% of subjects | 6.2% of specimens, 10% of subjects |
Proportion of positive specimens (%).
Fig. 1.
Polyomavirus excretion by selected subjects, relative to ovulation and menses. A, B: BKV excretion by subjects M115 and M108, respectively; (C) JCV excretion by subject M105. Undetectable virus is plotted as 0. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jmv]
Relationship of Polyomavirus Excretion to the Menstrual Cycle
Menstrual and virologic data were available for 36 complete menstrual cycles from 20 subjects. The time course of BKV and JCV excretion for selected subjects is shown graphically in Figure 1. In order to test the hypothesis that BKV excretion was associated with the preovulatory estrogen peak, we compared the proportion of BKV-positive urine samples in the 7 days prior to predicted ovulation to the proportion of BKV-positive urine specimens in the 7 days after predicted ovulation. For this analysis, the predicted day of ovulation was calculated as 14 days prior to the onset of menses ±1 day. In a subject-by-subject analysis utilizing data from nine subjects found to be excreting BKV at any time during an evaluable menstrual cycle, the mean proportion of pre-ovulatory specimens that were BKV-positive was 42.7%, compared to 45.1% for post-ovulatory specimens, a difference that was not statistically significant. Among all subjects, 25 (14.7%) of 170 urine specimens were BKV-positive in the pre-ovulation period and 27 (14.8%) of 183 specimens were BKV-positive in the post-ovulation period (P > 0.2). Among subjects who excreted BKV one or more times: 25 (19.8%) of 126 specimens were BKV-positive samples during the pre-ovulation period and 27 (19.7%) of 137 specimens were BKV-positive in the post-ovulation period (P > 0.2). The mean pre-ovulation BKV viral load was 3.46 (log 10) genomes per ml (ge/ml) and the mean post-ovulation BKV viral load was 2.85 (log 10) ge/ml (P > 0.2).
DISCUSSION
The primary goal of this study was to compare polyomavirus excretion in the pre-ovulation period with that of the post-ovulation period. The women who participated in this study were all pre-menopausal and thus assumed to have higher basal levels of estrogen than men or post-menopausal women. Therefore, it was hypothesized that these women would have an increased frequency of BKV excretion coincident with or shortly after the pre-ovulatory estrogen surge. The results confirm the null hypothesis that there is no discernable difference in virus excretion in the pre-ovulatory period compared to the post-ovulatory period. This suggests that the variation in estrogen concentrations during the menstrual cycle may not be of sufficient duration or magnitude to elicit BKV excretion.
The overall prevalence of BKV excretion in this study was 55% (11 of 20), much higher than single-specimen reports which have detected BKV excretion in less than 5% of healthy individuals [Sundsfjord et al., 1994; Degener et al., 1997; Ling et al., 2003; Kaneko et al., 2005; Egli et al., 2009]. The overall prevalence of JCV excretion in this study was 10% (2 of 20), which was somewhat less than expected based on prior studies. Possible explanations for these results include: (1) the study of only female subjects may have biased the results compared to prior studies of both male and female subjects; (2) the majority of the participants were medical students (n = 15), and higher levels of stress in these subjects may be more likely to provoke urinary excretion of BKV; and (3) the lability and sporadic/episodic nature of BKV shedding would be difficult to predict from point prevalence and infrequent sampling protocols previously reported. With respect to JCV in particular, the relatively young study population and small number of subjects enrolled likely biased this study against detection of JCV excretion compared to prior reports of the general population where JCV excretion is more common in older subjects.
The proportion of non-pregnant subjects excreting BKV in this study was similar to that of the pregnant cohort reported in our recent longitudinal study of polyomavirus excretion in pregnancy (11 of 20 (55%) vs. 50 of 91 (54.9%), P = 0.81) [McClure et al., 2012]. While differences in the design of the prior study compared to this one preclude direct comparison, the proportion of positive specimens among pregnant women was significantly different than in the longitudinal non-pregnant cohort described here (212 of 649 (32.6%) vs. 123 of 1,021 (12.0%), P < 0.0001; OR = 3.54, 95% CI = 2.76–4.54). This suggests that pregnant women excrete BKV more consistently than non-pregnant women, in whom BKV excretion may be more episodic (see Fig. 1).
This study has several limitations, particularly the small study population of only non-Hispanic Caucasians. Previous studies have suggested that the incidence of polyomavirus shedding may vary due to differences in race/ethnicity. As such, the inclusion of subjects of different races may have yielded different results. Of note is the finding that the proportion of BKV-positive specimens in this study was not significantly different than in the non-pregnant cohort of our prior study, which was predominantly of African–American descent [McClure et al., 2012]. Because we relied on the predicted day of ovulation instead of directly measuring estrogen concentrations, the accuracy of the analysis is limited by the subjects’ reporting of the dates of menstruation. In addition, the 2-month duration of the study limited the ability to detect seasonal variations in polyomavirus excretion, if they exist. Periods of discontinuity in the collection of specimens (e.g., due to vacations or away rotations for students) limited the ability to use all of the data accumulated for some subjects. Finally, we were unable to analyze the potential impact of various stressors (upcoming exams, acute illnesses, and on-call hospital shifts) that may have impacted the daily virus excretion by study subjects who are students.
Despite these limitations, the study provides compelling evidence that the excretion of BKV is a labile phenomenon in healthy young adult women. Future studies in more diverse populations over a longer period of time will be necessary to examine the role of stress, hormonal contraception, and other factors that may contribute to polyomavirus excretion. Such studies will be important in elucidating the factors that influence the natural history of polyomavirus infection and the risk of polyomavirus-associated disease.
Acknowledgments
Grant sponsor: National Center for Research Resources; Grant number: 5P20RR018724-10; Grant sponsor: National Institute of General Medical Sciences; Grant number: 8 P20 GM103433-10; Grant sponsor: National Institutes of Health to the Center for Molecular and Tumor Virology at LSUHSC-S; Grant sponsor: Department of Pediatrics at LSUHSC-S.
Footnotes
The authors have no commercial or other association that might pose a conflict of interest related to this study or its publication.
References
- Bhattacharjee S, Chakraborty T. High reactivation of BK virus variants in Asian Indians with renal disorders and during pregnancy. Virus Genes. 2004;28:157–168. doi: 10.1023/B:VIRU.0000016854.37475.f3. [DOI] [PubMed] [Google Scholar]
- Degener AM, Pietropaolo V, Di TC, Rizzuti V, Ameglio F, Cordiali FP, Caprilli F, Capitanio B, Sinibaldi L, Orsi N. Detection of JC and BK viral genome in specimens of HIV-1 infected subjects. New Microbiol. 1997;20:115–122. [PubMed] [Google Scholar]
- Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- Jin L. Rapid genomic typing of BK virus directly from clinical specimens. Mol Cell Probes. 1993;7:331–334. doi: 10.1006/mcpr.1993.1047. [DOI] [PubMed] [Google Scholar]
- Kalvatchev Z, Slavov S, Shtereva M, Savova S. Reactivation of polyomavirus hominis 1 (BKV) during pregnancy and the risk of mother-to-child transmission. J Clin Virol. 2008;43:328–329. doi: 10.1016/j.jcv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Moriyama T, Tsubakihara Y, Horio M, Imai E. Prevalence of human polyoma virus (BK virus and JC virus) infection in patients with chronic renal disease. Clin Exp Nephrol. 2005;9:132–137. doi: 10.1007/s10157-005-0348-9. [DOI] [PubMed] [Google Scholar]
- Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling PD, Lednicky JA, Keitel WA, Poston DG, White ZS, Peng R, Liu Z, Mehta SK, Pierson DL, Rooney CM, Vilchez RA, Smith EO, Butel JS. The dynamics of herpesvirus and polyomavirus reactivation and shedding in healthy adults: A 14-month longitudinal study. J Infect Dis. 2003;187:1571–1580. doi: 10.1086/374739. [DOI] [PubMed] [Google Scholar]
- Markowitz RB, Eaton BA, Kubik MF, Latorra D, McGregor JA, Dynan WS. BK virus and JC virus shed during pregnancy have predominantly archetypal regulatory regions. J Virol. 1991;65:4515–4519. doi: 10.1128/jvi.65.8.4515-4519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure GB, Gardner JS, Williams JT, Copeland CM, Sylvester SK, Garcea RL, Meinerz NM, Groome LJ, Vanchiere JA. Dynamics of pregnancy-associated polyomavirus urinary excretion: A prospective longitudinal study. J Med Virol. 2012 doi: 10.1002/jmv.23320. in press. [DOI] [PubMed] [Google Scholar]
- McNees AL, White ZS, Zanwar P, Vilchez RA, Butel JS. Specific and quantitative detection of human polyomaviruses BKV, JCV, and SV40 by real time PCR. J Clin Virol. 2005;34:52–62. doi: 10.1016/j.jcv.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Moens U, Van GM, Johansen B, Seternes OM. Concerted expression of BK virus large T- and small t-antigens strongly enhances oestrogen receptor-mediated transcription. J Gen Virol. 1999;80:585–594. doi: 10.1099/0022-1317-80-3-585. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Abouljoud M, Jacobsen G, Reddy G, Koffron A, Venkat KK. Increasing trend in infection-related death-censored graft failure in renal transplantation. Transplantation. 2011;91:94–99. doi: 10.1097/tp.0b013e3181fdd96c. [DOI] [PubMed] [Google Scholar]
- Sundsfjord A, Flaegstad T, Flo R, Spein AR, Pedersen M, Permin H, Julsrud J, Traavik T. BK and JC viruses in human immunodeficiency virus type 1-infected persons: Prevalence, excretion, viremia, and viral regulatory regions. J Infect Dis. 1994;169:485–490. doi: 10.1093/infdis/169.3.485. [DOI] [PubMed] [Google Scholar]
- Tsai RT, Wang M, Ou WC, Lee YL, Li SY, Fung CY, Huang YL, Tzeng TY, Chen Y, Chang D. Incidence of JC viruria is higher than that of BK viruria in Taiwan. J Med Virol. 1997;52:253–257. [PubMed] [Google Scholar]
- Vanchiere JA, White ZS, Butel JS. Detection of BK virus and simian virus 40 in the urine of healthy children. J Med Virol. 2005;75:447–454. doi: 10.1002/jmv.20287. [DOI] [PubMed] [Google Scholar]
- Viscidi RP, Rollison DE, Sondak VK, Silver B, Messina JL, Giuliano AR, Fulp W, Ajidahun A, Rivanera D. Age-specific seroprevalence of merkel cell polyomavirus, BK virus, and JC virus. Clin Vaccine Immunol. 2011;18:1737–1743. doi: 10.1128/CVI.05175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]