Abstract
Creativity requires the rapid combination and recombination of existing mental representations to create novel ideas and ways of thinking. The hippocampal system, through its interaction with neocortical storage sites, provides a relational database necessary for the creation, updating, maintenance, and juxtaposition of mental representations used in service of declarative memory. Given this functionality, we hypothesized that hippocampus would play a critical role in creative thinking. We examined creative thinking, as measured by verbal and figural forms of the Torrance Tests of Creative Thinking (TTCT), in a group of participants with hippocampal damage and severe declarative memory impairment as well as in a group of demographically matched healthy comparison participants. The patients with bilateral hippocampal damage performed significantly worse than comparison participants on both the verbal and figural portions of the TTCT. These findings suggest that hippocampus plays a role critical in creative thinking, adding to a growing body of work pointing to the diverse ways the hallmark processing features of hippocampus serve a variety of behaviors that require flexible cognition.
Keywords: hippocampus, creativity, representational flexibility, relational binding, amnesia
Ancient Grecian and Roman scholars and philosophers attributed creativity to divine inspiration (Albert & Runco, 1999). Today creativity is considered a quintessential and uniquely human characteristic of considerable interest to researchers in psychology and cognitive neuroscience (e.g., Andreasen, 2005; Damasio, 2001; Dietrich, 2004; Heilman, 2005). A precise definition of creativity is not universally agreed upon, yet several researchers have characterized aspects of creativity thinking. Creativity has been thought of as the ability to produce ideas or responses that are both novel (i.e. original, rare, and unexpected) and appropriate (i.e. adaptive and useful given task constraints) (Flaherty, 2005; Sternberg & Lubart, 1999). Creativity has also been described as the “epitome of cognitive flexibility” (Dietrich, 2004, p. 1014), requiring the rapid combination and recombination of existing mental representations to create novel ideas and ways of thinking (Bristol & Viskontas, 2006). Similarly, Damasio (2001) states that the processes of creativity include the generation of representational diversity, manipulation of this representational diversity, and recognition of novel representations that when combined result in creative thinking.
Much of the work linking creativity to the brain has focused on the frontal lobes, which is understandable given the known roles of the frontal lobes in processes such as cognitive flexibility, fluency, and abstract reasoning (cf. Gläscher et al., 2012). For example, there is evidence of changes in creativity in psychiatric conditions where frontal lobe pathology is observed (e.g., schizophrenia; Folley & Park, 2005) and cognitive studies of creativity point to the collection of abilities putatively associated with the frontal lobes (e.g., working memory, abstraction, fluency, reasoning, flexibility) (Bogousslavsky, 2005; Dietrich, 2004; Runco, 2004). fMRI studies also report prefrontal cortex activation in tasks of creativity (e.g., Dietrich & Kanso, 2010; Kowatari et al., 2009).
Another less acknowledged brain structure that appears well suited to us to contribute to creativity is hippocampus and that is the focus of the current study. For example, we have been impressed that definitions of creativity typically refer to processes such as the rapid generation, combination, and recombination of existing mental representations to create novel ideas and ways of thinking (Bristol & Viskontas, 2006; Damasio, 2001). These descriptions are remarkably similar to various processing features of hippocampal function. Hippocampus has been described as the critical structure in the brain that serves as a relational database to create, update, and juxtapose mental representations that form the basis of declarative memory (Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001). Characteristic features of hippocampal processing include the ability to form arbitrary relations and bind together distinct aspects of experience, in addition to interacting with neocortical storage sites to support integration and flexible use of representations to optimize performance under a variety of circumstances (Bunsey & Eichenbaum, 1996; Eichenbaum & Cohen, 2001; Gabrieli, 1998; O’Keefe & Nadel, 1978; Squire, 1992).
The role of hippocampus is well established in forming and recollecting new declarative memories; however, recent evidence suggests the hippocampus also contributes to maintenance and on-line processing of relational information. Participants with hippocampal amnesia show deficits across minimal delays and even when all the necessary information is immediately available (e.g., Barense, Gaffan, & Graham, 2007; Hannula, Tranel & Cohen, 2006; Warren, Duff, Tranel, & Cohen, 2011; Rubin, Brown-Schmidt, Duff, Tranel & Cohen, 2011). These results converge with fMRI findings of hippocampal activation for declarative memory over the same short delays (e.g., Hannula & Ranganath, 2008; Ranganath & D’Esposito, 2001). Such findings encourage the idea that hippocampus processes relational information on the time-scale necessary to rapidly generate, combine, and recombine mental representations, which are essential aspects of creative thinking (Bristol & Viskontas, 2006; Damasio, 2001). Although creative thinking has not been formally examined in hippocampal amnesia, other work links hippocampal damage to impairments in imagining events (Hassabis, Kumaran, Vann, & Maguire, 2007) and to disruptions in the creative use of language (Duff, Hengst, Tranel, & Cohen, 2009). We hypothesize that the characteristic relational processing features of hippocampus support aspects of creativity, which is tested here in formal assessment of creative thinking.
We used a neuropsychological approach to test this proposal. Five patients with bilateral hippocampal damage (hereafter, AM group, for “amnesic”) completed the nationally normed Torrance Tests of Creative Thinking (TTCT). Table 1 presents demographic, anatomical, and neuropsychological information for the amnesic participants. Etiologies of the amnesic patients included anoxia/hypoxia (1846, 2363, 2563), resulting in bilateral hippocampal damage, and herpes simplex encephalitis (HSE) (1951, 2308), resulting in more extensive bilateral medial temporal lobe damage affecting hippocampus, amygdala, and surrounding cortices. In four patients (1846, 1951, 2363, 2308), high-resolution volumetric MRI analyses revealed hippocampal volumes significantly reduced for each patient, with the studentized residual differences in hippocampal volume relative to a matched comparison group down by at least 2.6 and as much as 8.1 z-scores (Table 1; Allen et al., 2006; Buchanan et al., 2005). Patient 2563 wears a pacemaker and was unable to undergo MRI examination; the anatomical analysis for this patient was based on a computerized tomography scan, and the only visible damage was in the hippocampal region.
Table 1. Amnesic Participant's Demographic, Anatomical and Neuropsychological Characteristics.
| Demographic | Anatomical | Intelligence | Memory | Language | Perception | EF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Ed | Etiology | Lesion | HC Volume | WAIS- III FSIQ | WAIS-IIIVIQ | WAIS-IIIPIQ | WMS-IIIGMI | BNT | TT | COWA | CFTCopy | WCTPE | WCTCat | |
| 1846 | 46 | 14 | Anoxia | Bilateral HC | -4.23 | 84 | 88 | 86 | 57 | 43 | 41 | 24 | 28 | 6 | 6 |
| 1951 | 57 | 16 | HSE | Bilateral HC + other MTL | -8.10 | 106 | 107 | 106 | 57 | 49 | 44 | 40 | 32 | 16 | 6 |
| 2308 | 53 | 16 | HSE | Bilateral HC + other MTL | N/A | 95 | 96 | 78 | 45 | 52 | 44 | 16 | 32 | N/A | N/A |
| 2363 | 53 | 16 | Anoxia | Bilateral HC | -2.64 | 98 | 112 | 91 | 73 | 58 | 44 | 26 | 26 | 12 | 6 |
| 2563 | 54 | 16 | Anoxia | Bilateral HC | N/A | 94 | 91 | 103 | 63 | 52 | 44 | 21 | 36 | 6 | 6 |
| Mean(SD) | 52.6(4.0) | 15.6(1.7) | 95.4(7.9) | 98.8(10.3) | 94.8(14.9) | 59.0(10.2) | 50.8(5.5) | 43.4(1.3) | 25.4(9.0) | 30.8(3.9) | 10.0(4.9) | 6(0.0) | |||
Note. Ed. = education; HSE = Herpes Simplex Encephalitis; HC = Hippocampus; MTL = Medial Temporal Lobe; HC Volume = hippocampal volumes obtained using high-resolution volumetric MRI (as studentised residual differences with hippocampal volume z-scores), revealing significantly reduced hippocampal volumes relative to a matched comparison group (Allen, Tranel, Bruss, & Damasio, 2006; Buchanan, Tranel, & Adolphs, 2005); Bolded scores are impaired, defined as 2 standard deviations from the mean on each test; WAIS-III = Wechsler Adult Intelligence Scale-III; FSIQ = Full Scale Intelligence Quotient; VIQ = Verbal Intelligence Quotient; PIQ = Performance Intelligence Quotient; WMS-III = Wechsler Memory Scale-III; GMI = General Memory Index; BNT = Boston Naming Test; TT = Token Test; COWA = Controlled Oral Word Association; COWA score is summed over F, A, and S; CFT = Complex Figure Test; EF = Executive Functions; WCT = Wisconsin Card Sorting Task; PE = Perseverative errors; Cat = Number of categories achieved out of six.
Neuropsychological testing revealed a severe and selective declarative memory impairment in the context of generally intact performance on measures of executive function, intelligence, language, and perception (also see Konkel, Warren, Duff, Tranel, & Cohen, 2008). We also collected data from 10 healthy comparison participants (hereafter, NC group, for “normal comparisons”) matched to amnesic participants on age, sex, handedness, and education. The comparison participants were 56.1 years old, on average, and had 14 years of education, on average. There were no significant differences between groups on age (t(13) = 1.18, p = 0.26) or education (t(13) = 1.86, p = 0.10).
The TTCT has been used widely in the study of creativity (Colangelo & Davis, 1991; Lissitz & Willhoft, 1985) and has undergone significant investigation (e.g., Chase, 1985; Clapham, 1998; Dixon, 1979; Heausler, & Thompson, 1988). The TTCT has a verbal and figural form (booklet A for both tests was administered). The verbal form consists of six timed (five to ten minutes) subtests requiring participants to use written language to ask questions about a picture of an event, guess causes and consequences (immediate or long-term) of an action in a picture, generate ways to improve a toy so that it is more fun to play with, generate alternative uses for a common object (e.g., a cardboard box), and generate hypotheses about potential benefits or problems related to an improbable situation (e.g., if clouds had strings attached to them). For each task, participants listed as many responses as they could during the time allotted.
The figural form consists of three timed (ten minutes) subtests requiring participants to create novel drawings constructed from varying degrees of partial information (e.g., one large oval-shaped figure; ten novel partially incomplete line contours; 30 repeated parallel line segments). For each task, participants were instructed to create meaningful drawings from the incomplete figure and give each drawing a unique title. For both the verbal and figural forms, instructions and materials were visible at all times (eliminating explicit demands on memory). If a participant stopped or indicated they could not think of anything else, the examiner informed the participant they had more time and encouraged them to keep thinking. To ensure consistency and unbiased scoring, test forms were sent to the publisher for standardized scoring. Standard scores for overall verbal and figural performance were calculated, including various dimensions of verbal (e.g., fluency, flexibility, originality) and figural (e.g., fluency, resistance to premature closure, elaboration, abstractness of title, and originality) performance1.
On the verbal portion, the composite verbal score for healthy comparison participants (M = 101.7; SD = 24.6) was significantly higher than amnesic participants (M = 57.0; SD = 8.3) (F(1, 13) = 15.17, p = 0.002; see Figure 1; Table 2). This same pattern was observed for all three of the verbal dimensions including: fluency (NC: M = 101.3; SD = 29.3; AM: M = 54.6; SD = 8.6; F(1, 13) = 11.76, p = 0.004), flexibility (NC: M = 93.9; SD = 21.4; AM: M = 49.4; SD = 9.8; F(1, 13) = 19.05, p = 0.0008), and originality (NC: M = 110.7; SD = 24.6; AM: M = 63.0; SD = 10.6; F(1, 13) = 16.32, p = 0.001). Representative examples are included to illustrate the difference in performance between the groups (see Figure 2). For example, when asked to think of creative uses for cardboard boxes, healthy comparison participant matched to amnesic participant 2363 produced 26 uses, 23 of which were determined to be unique (e.g. including building a suit of armor). In sharp contrast, amnesic participant 2363 produced only 2 uses (e.g. recycling the boxes and making a fort).
Figure 1. Performance on Verbal and Figural Forms of the TTCT.
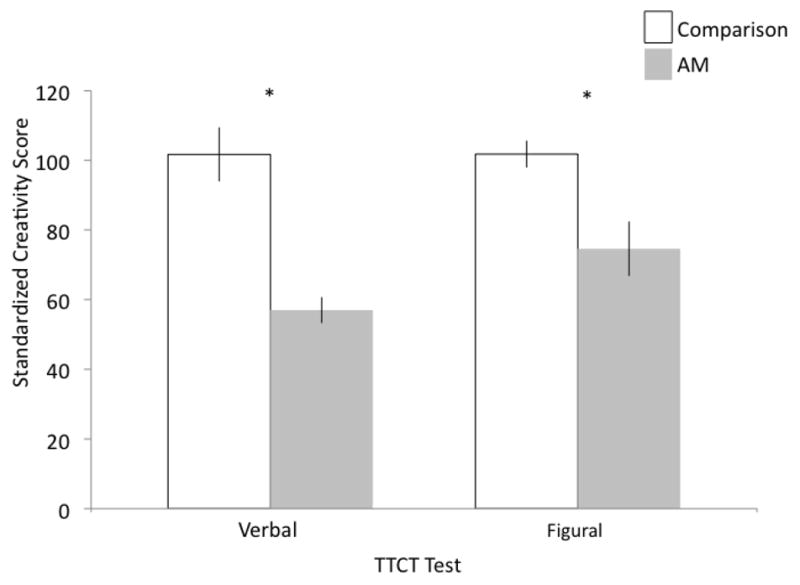
Means and standard error; *Indicates significant (p < 0.05) differences between groups.
Table 2. Individual TTCT Verbal and Figural Scores of Amnesic Participants.
| Verbal | Figural | |||
|---|---|---|---|---|
| SS | %tile | SS | %tile | |
| 1846 | 67 | 7 | 97 | 42 |
| 1951 | 64 | 5 | 73 | 4 |
| 2308 | 47 | 1 | 51 | 1 |
| 2363 | 52 | 1 | 85 | 16 |
| 2563 | 55 | 2 | 67 | 2 |
| Mean(SD) | 57.0(8.3) | 3.2(2.9) | 74.6(17.5) | 13(17.3) |
Note: SS = Standard Score; %tile = national percentile.
Figure 2. Verbal Form Example: Unusual Uses (Cardboard Boxes).

Note: A) Comparison participant – 26 responses B) Amnesic participant 2363 – 2 responses: Recycle the boxes; Make a Fort for kids w/ Ronald McDonald's participation
On the figural portion, the composite figural score for healthy comparison participants (M = 101.8; SD = 12.2) was significantly higher than amnesic participants (M = 74.6; SD = 17.5) (F(1, 13) = 12.48, p = 0.004; see Figure 1; Table 2). This pattern was observed on four of the five figural dimensions including: fluency (NC: M = 97.3; SD = 18.6; AM: M = 68.4; SD = 21.0; F(1, 13) = 7.41, p = 0.02), originality (NC: M = 92.6; SD = 18.01; AM: M = 64.8; SD = 17.2; F(1, 13) = 8.1, p = 0.01), titles (NC: M = 110.0; SD = 10.1; AM: M = 78.0; SD = 28.7; F(1, 13) = 10.5, p = 0.006), and elaboration (NC: M = 105.2; SD = 14.2; AM: M = 71.8; SD = 10.0; F(1, 13) = 21.91, p = 0.0004). On the resistance to premature closure dimension, there was no significant difference (F(1, 13) = 2.1, p = 0.17) between comparison (M = 104.1; SD =14.7) and amnesic (M = 89.6; SD = 24.4) participants. Again, representative examples are included to illustrate the difference in performance between the groups. In the figural subtest where participants were presented with a large oval-shape figure and asked to think of a picture that includes this shape, adding new ideas to make the picture tell as interesting and exciting a story as possible drawings from two amnesic participants (1951 and 1846) and their matched healthy comparison participants are presented in Figure 3. One comparison participant used the oval as part of a golf course complete with signs for parking and the clubhouse, the CBS sports truck, the fairway, a sand trap, and Tiger Woods with his caddy. The other comparison participant made the oval into a giant tick or “tick-mobile” that, similar to hot air balloons, takes people for rides above the city where people stood in line for the ride. In sharp contrast, given the same stimulus and the full 10 minutes, amnesic participant 1951 turned the oval into a bug and amnesic participant 1846 used the shape as an egg and drew a chicken above it.
Figure 3. Figural Form Example: Picture Construction from Oval Stimulus.
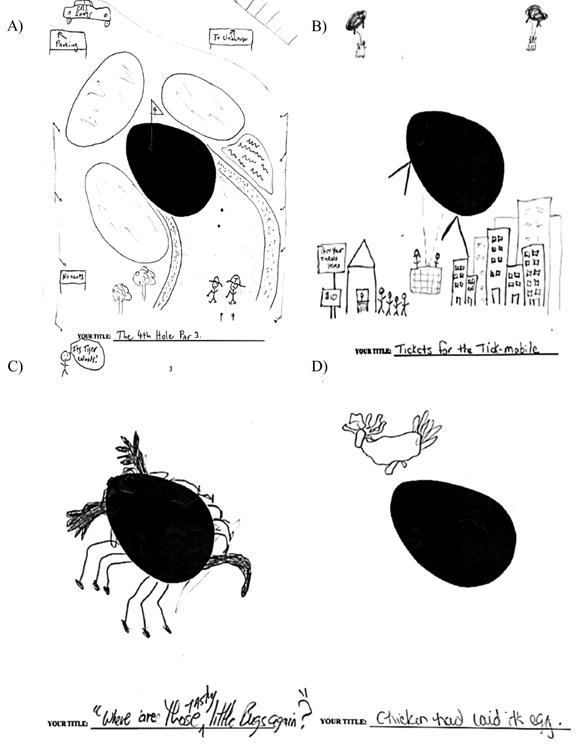
Note: A: Comparison participant – Title: The 4th Hole Par 3; notations read from upper left clockwise: To parking; To clubhouse; Its Tiger Woods!; No carts; B: Comparison participant – Title: Tickets for the tick mobile; notations read Get your tickets here; $10; C: Amnesic participant 1951 – Title: Where are those tasty little buggers?; D: Amnesic participant 1846 – Title: Chicken had laid its egg
The poor performance of patients with hippocampal amnesia relative to comparison participants on both the verbal and figural portions of the TTCT provides preliminary support for our proposal that the hippocampus plays a critical role in creative thinking. The difference between the richness of the picture constructions and associated contextual details produced by the healthy comparison participants and the impoverished picture constructions of the amnesic participants is very striking (see Fig. 3), and is reminiscent of how relatively impoverished are the narratives generated, and spatial details provided, by amnesic patients asked to imagine new experiences (Hassabis et al., 2007). Just as striking is how devoid of ideas the amnesic patients were, relative to the comparison group, about possible functions or uses for a cardboard box, in the verbal portion of the TTCT (see Fig. 2). The magnitude of the deficit seen here for each of these versions of the creative thinking test is comparable to that seen in many tests of declarative or episodic memory.
Decades of research have linked the functionality of the hippocampus to the formation, retrieval and flexible use of declarative memory (e.g., Bunsey & Eichenbaum, 1996; Cohen & Eichenbaum, 1993; Eichenbaum & Cohen, 2001; Gabrieli, 1998; O’Keefe & Nadel, 1978; Squire, 1992). Just as the deficit in declarative memory has long been known to be modality-general, including impairing the ability to learn new relations, whether spatial, temporal, or associational (Konkel et al., 2008), across a range of different stimulus materials (Hannula et al., 2006), here we observed a deficit in creative thinking following hippocampal damage that likewise applies across different classes of stimulus materials.
We propose that the same processing features of the hippocampus that are used in service of declarative or episodic memory for new experiences are used in service of creative thinking, including the ability to rapidly generate, combine, and recombine existing mental representations in the moment to create something new. The deficits seen here in formal testing of creativity extend previous work showing the critical role of hippocampal-dependent processes in multiple cognitive domains across different classes of stimulus materials. Examples run the gamut from inferential reasoning, such as the associative inference task requiring that elements of existing memories (e.g., of face-building pairings or other object-object relations) be retrieved and recombined in order to discover novel associative relations (Zeithamova, Schlichting, & Preston, 2012), to future imagining (e.g., Hassabis et al., 2007) or other examples of future thinking (e.g., Addis & Schacter, 2012), in which memory of previous experience may be reconstructed or re-imagined into novel scenes and scenarios (Hassabis & Maguire, 2007), and verbal play (Duff et al., 2009) or other aspects of verbal processing and the use of language (Duff & Brown-Schmidt, 2012).
Could other aspects of amnesic participants’ neuropsychological profiles account for the current findings? For example, some previous work has suggested a connection between IQ and creativity (e.g., Barron, 1963). While we cannot address this directly with our entire data set (we do not have IQ data on all comparison participants), the TTCT is a nationally normed test with data from tens of thousands of participants. Assuming a normal distribution of IQ scores across this large sample, amnesic participants (who all have IQs in the normal range as measured by the Wechsler Adult Intelligence Scale) are still significantly impaired relative to the national sample. Compared to national norms, on the verbal portion of the TTCT, amnesic participants (as a group) have a standard score of 57 placing them in only the 3rd percentile nationally (range = 1st-7th percentile). On the figural portion of the TTCT amnesic participants have a standard score of 74 placing them in the 13th percentile nationally (range = 1st - 42nd percentile). Looking closer at the individual data from our amnesic sample, patient 1846 has the lowest IQ (WAIS-III FSIQ = 84) among all patients, yet scores the highest on both the verbal (score = 67) and figural (score = 97) tests of creativity. In addition, patient 1951 has the highest IQ (WAIS-III FSIQ = 106) among all patients, yet scores nearly identical to patient 1846 on the verbal (score = 64 and 67, respectively) portion and significantly poorer than 1846 on the figural portion (score = 73 and 97, respectively). Furthermore, there was not a significant correlation between amnesic participants’ IQ and their performance on either the verbal (r(3) = -0.33, p = 0.58) or figural (r(3) = -0.61, p = 0.27) portions of the TTCT. Thus, here, IQ is not a significant factor in creative thinking, an outcome consistent with a recent meta-analysis of 21 studies reporting a negligible relationship between creativity and IQ (Kim, 2005).
Other aspects of cognition frequently linked to creativity (e.g., generation, construction, abstraction, fluency) can be captured by some standardized neuropsychological assessments (Lezak, Howieson, Bigler, & Tranel, 2012). While neuropsychological measures reveal disproportionate memory impairments in amnesia (see Table 1), it is not surprising to see lower performance on certain measures thought to be components of creativity. For example, three of the five amnesic participants (one anoxic and the two HSE participants) have depressed scores on verbal category fluency (COWA), a measure of the ability to generate a number of relevant or related items (e.g., words that begin with the same letter). This appears very much akin to the amnesic participants’ significantly lower scores on the TTCT fluency dimension (verbal and figural). Fluency, or the quantity of responses produced, however, is only one aspect of creative thinking. Individual responses, even a few, could be deemed creative if highly unusual or richly detailed and elaborated (perhaps captured by the TTCT dimensions of originality or elaboration). Indeed, amnesic participant 1846 has a COWA score two standard deviations below the mean (see Table 1), yet scores the highest among all amnesic participants on both verbal and figural creativity tests.
Creativity is among the most complex of human behaviors. Our findings suggest that hippocampus plays a role in the rapid generation, combination, and recombination of existing mental representations that are available for and processed in concert with other neural systems in service of creative thinking. Linking deficits in creative thinking to hippocampal dysfunction adds to a growing body of work pointing to the diverse ways the characteristic processing features of hippocampus serve a variety of cognitive domains. It also reminds us of the emphasis that some have placed on the creative and reconstructive nature of memory itself (e.g., Bartlett, 1932; Neisser & Fivush, 1994). Finally, the results support the idea that the neural basis of creativity extends beyond the more oft-mentioned frontal lobes to include the hippocampus.
Acknowledgments
This study was supported by NIDCD F32 DC008825 to MCD; NIMH RO1 MH062500 to NJC; and NINDS P01 NS19632 to DT.
Footnotes
Fluency = number of interpretable, meaningful and relevant ideas; Flexibility = the number of different categories of relevant responses; Originality = the number of statistically infrequent ideas; Resistance to premature closure = the number of times someone goes beyond the most logical way to complete a figure; Elaboration = the number of added ideas; Abstractness of titles = extent to which titles reflect content beyond labeling (i.e. abstraction of thought) (see Kim, 2006).
References
- Addis DR, Schacter DL. The hippocampus and imagining the future: Where do we stand? Frontiers in Human Neuroscience. 2012;5:173. doi: 10.3389/fnhum.2011.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert RS, Runco MA. A history of research on creativity. In: Sternberg RJ, editor. History of creativity. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Allen JS, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. Journal of Clinical and Experimental Neuropsychology. 2006;28(4):457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- Andreasen N. The creating brain: the neuroscience of genius. Washington, DC: Dana Press; 2005. [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45:2963–2974. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Barron F. Creativity and psychological health. Princeton, NY: Van Nostrand; 1963. [Google Scholar]
- Bartlett LC. Remembering: A study in experimental and social psychology. Cambridge, England: 1932. [Google Scholar]
- Bogousslavsky J. Artistic creativity, style and brain disorders. European Neurology. 2005;54(2):103–111. doi: 10.1159/000088645. [DOI] [PubMed] [Google Scholar]
- Bristol A, Viskontas I. Dynamic processes within associative memory stores. In: Kaufman J, Baer J, editors. Creativity and Reason in Cognitive Development. 2006. pp. 60–80. [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Emotional autobiographical memories in amnesic patients with medial temporal lobe damage. The Journal of Neuroscience. 2005;25(12):3151–3160. doi: 10.1523/JNEUROSCI.4735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Chase CI. Review of the Torrance Tests of Creative Thinking. In: Mitchell JV Jr, editor. The ninth mental measurements yearbook. Lincoln: Buros Institute of Mental Measurements, University of Nebraska; 1985. pp. 1631–1632. [Google Scholar]
- Clapham MM. Structure of figural forms A and B of the Torrance Tests of Creative Thinking. Educational & Psychological Measurement. 1998;58:275–283. [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia and the hippocampal system. Cambridge, M.A: MIT Press; 1993. [Google Scholar]
- Colangelo N, Davis G, editors. Handbook of gifted education. Boston, MA: Allyn & Bacon; 1991. [Google Scholar]
- Damasio AR. Some notes on brain, imagination, and creativity. In: Pfenninger KH, Shubik VR, editors. The origins of creativity. Oxford: Oxford University Press; 2001. [Google Scholar]
- de Souza LC, Volle E, Bertoux M, Czernecki V, Funkiewiez A, Allali G, Levy R. Poor creativity in frontotemporal dementia: a window into the neural bases of the creative mind. Neuropsychologia. 2010;48(13):3733–3742. doi: 10.1016/j.neuropsychologia.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Dietrich A. The cognitive neuroscience of creativity. Psychonomic Bulletin & Review. 2004;11(6):1011–1026. doi: 10.3758/bf03196731. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychological Bulletin. 2010;136(5):822–848. doi: 10.1037/a0019749. [DOI] [PubMed] [Google Scholar]
- Dixon J. Quality versus quantity: the need to control for the fluency factor in originality scores from the Torrance Tests. Journal for the education of the gifted. 1979;2:70–79. [Google Scholar]
- Duff MC, Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Frontiers in Human Neuroscience. 2012 doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Hippocampal amnesia disrupts verbal play and the creative use of language in social interaction. Aphasiology. 2009;23(7):926–939. doi: 10.1080/02687030802533748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. Oxford University Press; New York: 2001. [Google Scholar]
- Flaherty A. Frontotemporal and dopaminergic control of idea generation and creative drive. Journal of Computational Neurology. 2005;493(1):147–153. doi: 10.1002/cne.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folley B, Park S. Verbal creativity and schizotypal personality in relation to prefrontal hemispheric laterality: A behavioral and near-infrared optical imaging study. Schizophrenia Research. 2005;80:271–282. doi: 10.1016/j.schres.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD. Cognitive neuroscience of human memory. Annual review of psychology. 1998;49(1):87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gansler DA, Moore DW, Susmaras TM, Jerram MW, Sousa J, Heilman KM. Cortical morphology of visual creativity. Neuropsychologia. 2011;49(9):2527–2532. doi: 10.1016/j.neuropsychologia.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D. Lesion mapping of cognitive control and value-based decision-making in the prefrontal cortex. Proceedings of the National Academy of Sciences. 2012;109:14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. The Journal of neuroscience. 2008;28(1):116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. The Journal of Neuroscience. 2006;26(32):8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences. 2007;104(5):1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends in cognitive sciences. 2007;11(7):299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Heausler NL, Thompson B. Structure of the Torrance Tests of creative Thinking. Educational and Psychological Measurement. 1988;48:463–468. [Google Scholar]
- Heilman K. Creativity and the brain. New York: New York: 2005. [Google Scholar]
- Kim K. Can only intelligent people be creative? The Journal of Secondary Gifted Education. 2005;26(2/3):57–66. [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Frontiers in Human Neuroscience. 2008;2 doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowatari Y, Lee S, Yamamura H, Nagamori Y, Levy P, Yamane S, Yamamoto M. Neural networks involved in artistic creativity. Human Brain Mapping. 2009;30(5):1678–1690. doi: 10.1002/hbm.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. Fifth. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Lissitz RW, Willhoft JL. A methodological study of the Torrance tests of creativity. Journal of Educational Measurement. 1985;22:1–11. [Google Scholar]
- Neisser U, Fivush R. The remembering self: Construction and accuracy in the self-narrative. Cambridge, England: 1994. [Google Scholar]
- Ranganath C, D'Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31(5):865. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Rubin R, Brown-Schmidt S, Duff MC, Tranel D, Cohen NJ. How do I remember that I know you know that I know? Psychological Science. 2011;22(12):1574–1582. doi: 10.1177/0956797611418245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runco M. Creativity, Annual Review of Psychology. 2004;55:657–687. doi: 10.1146/annurev.psych.55.090902.141502. [DOI] [PubMed] [Google Scholar]
- Squire L. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Sternberg R, Lubart T. The concept of creativity: Prospects and paradigms. Handbook of Creativity. 1999:3–15. [Google Scholar]
- Warren DE, Duff MC, Tranel D, Cohen NJ. Observing degradation of visual representations over short intervals when medial temporal lobe is damaged. Journal of Cognitive Neuroscience. 2011;23(12):3862–3873. doi: 10.1162/jocn_a_00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Schlichting ML, Preston AR. The hippocampus and inferential reasoning: Building memories to navigate future decisions. Frontiers in Human Neuroscience. 2012;6:70. doi: 10.3389/fnhum.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]


