Abstract
Objectives
Sulfation via sulfotransferases is an important metabolic pathway in contribution to the low bioavailability of flavonoids. The present study aims to characterize the sulfation of mono-hydroxyflavones (MHF) to obtain useful information on structure-metabolizing relationships on animal species and gender differences.
Methods
Three representative MHF, namely, 7-, 6-, and 4'-MHF, were used by incubating each MHF at different concentrations with various liver S9 fractions (mouse, rat, dog, and human).
Key findings
One mono-sulfate was identified for each MHF. 7-MHF and 4'-MHF usually have greater sulfations than that of 6-MHF. Regardless if the S9 fraction came from a male or female, there was a difference in sulfation in the species as observed for all MHFs and that the highest activities of sulfotransferases was in dog S9 as compared to the other liver S9. Furthermore, gender differences affect sulfation of MHFs significantly. In rats, all sulfations for the three MHF were higher in males than that in females while it was the opposite in mice.
Conclusions
regiospecific-, species-, and gender-dependence exist in the sulfonation of all selected MHFs.
Keywords: mono-hydroxyflavones, regiospecific, species, gender, sulfotransferases
Introduction
Dietary flavonoids include a broad range of compounds that are known to have a variety of claimed healthy benefits such as anti-cancer, antioxidant, anti-aging, and cholesterol lowering[1]. However, there is still no therapeutic agent in the current market because of the poor bioavailabilities of these agents[2]. Glucuronidation and sulfation in the liver and intestine are two of the major barriers to the oral bioavailability[3, 4]. The glucuronidation of flavonoids catalyzed by UDP-glucuronosyltransferases has been extensively demonstrated in many literatures[5, 6]. In contrast, only a few studies have been involved in the sulfation of flavonoids[7, 8].
Previously, the glucuronidation of isoflavones, such as daidezein, glycitein, and prunetin, as well as flavones, such as mono- and di-hydroxyflavones, was shown to be significantly affected by their structure, especially by the hydroxyl group position[9, 10]. However, the relationship between the sulfation of flavonoids and their structures is not known. Additionally, flavone is one of the main subgroups of flavonoids, and the sulfation characteristics of three selected MHFs with similar and simple structural properties, namely, 7-, 6-, and 4'-MHF (Figure 1) were investigated.
Figure 1. Structures of model flavones used in the present study.
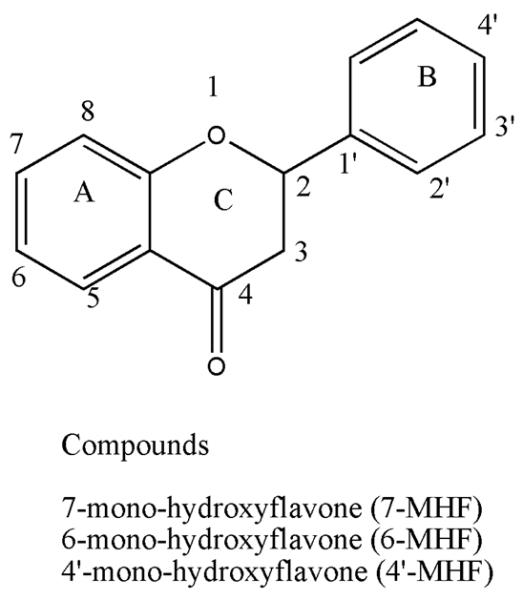
Shown in the scheme are structures of aglycone forms of hydroxyflavone analogs. Three MHFs were chosen in the experiment. Conjugated forms of flavones with the sulfated metabolites maybe attached to the 7-, 6-, and 4' - positions.
Many earlier studies indicated that the main sites of flavonoid metabolism were in the liver and intestine[11]. Therefore, liver S9 fractions were chosen for this study. Reports state that glucuronidation and sulfation of 7-hydroxytcoumarin in liver matrices from humans, dogs, monkeys, rats, and mice were different[12]. Previous studies also indicated that gender may influence the activities of some conjugating enzymes, mainly due to the steroid hormone levels and metabolizing-enzyme levels including glucuronosyltransferase and sulfotransferases[13]. Punt, Delatour et al reported that sulfation was about 30 times more efficient by male rat liver S9 than by human liver S9, whereas the catalytic efficiency by male mouse and human liver S9 was about the same which the results were the same as ours[14]. Our study also indicated that the species differences affected the glucuronidation of emodin in vitro and in vivo[15]. As a result, the mouse, rat, dog, and human liver S9 fractions were used in the present study.
Published literature also showed that gender-divergent Sults are mostly female-predominant and Sult1c1 is the only male-dominant[16]. These studies indicated that there may be difference in the sulfation for the flavones we are interested in. So we also chose pooled female and male liver S9 fractions to investigate the gender difference.
This study has two major objectives: (i) to determine how species and gender affect sulfation by incubating the flavones with liver S9 fractions and cofactors, and (ii) to investigate whether different structures and substrate concentrations of flavones influence the sulfation. This present investigate is novel and will shed light the preclinical study in the sulfation of flavones in different genders and species systematically.
Material and Methods
7-, 6-, and 4'-MHF (purity > 98%), adenosine 3'-phosphate 5'-phosphosulfate lithium salt hydrate (PAPS), and sulfatase were purchased from Sigma-Aldrich (St Louis, MO). CD-1 pooled female and male mouse liver S9, Fischer 344 pooled female and male rat liver S9, pooled female and male dog liver S9, pooled mouse liver S9, pooled rat liver S9, pooled dog liver S9 and pooled human (female 8 and male 16) liver S9 were purchased from BD Bioscience (Woburn, MA). All other materials were of analytical grade or higher.
The sulfation of flavones by liver S9 fractions was essentially the same as those described previously with minor modifications[11] and there is no NADPH (the coenzyme of P450), UDPGA (the glucuronic acid donator of UGTs) or conjugating donators of other metabolizing enzymes in liver S9 sulfation system. Briefly, the incubation mixture contained S9 fractions (final concentration = 0.5 mg/mL in a 50 mM potassium phosphate buffer, pH = 7.4), PAPS (final concentration = 100 μM), and different concentrations of flavones (final volume 200 μL). The mixed reaction system was incubated at 37 °C in a shaking (50 rpm) water bath for 30 to 90 min. Two controls were incubated in the absence of PAPS or without liver S9 fractions. The reactions were terminated by adding 100 μL solution of 94% acetonitrile/6% glacial acetic acid containing 50 μM internal standard testosterone. The supernatants after centrifugation were subjected to assay using ultra performance liquid chromatography (UPLC).
The UPLC conditions for analyzing the MHFs and their conjugates, and the elution program were based on a published method[9]. The main working parameters for the mass spectrometers were: capillary voltage of 3.0 kV, cone voltage of 35 V, ion source temperature of 110°C, and minor adjustments were then made for each MHF. Mono-sulfates were identified by MS and MS2 full scan modes. An MHF standard curve was used for the quantification of MHF sulfate by using a conversion factor (K) because of the lack of MHF sulfate standards. This was done using the similar methods shown previously in our laboratory for flavonoids[9].
All the data were analyzed using Kruskal-Wallis test or one-way ANOVA with Dunnett' multiple comparison (post-hoc) tests. Differences were considered significant when p values were less than 0.05 (p < 0.05).
Results and Discussion
Each of the three flavones has one metabolite which was identified as the sulfate. For all the MHFs, the pseudomolecular ion [M+H]+ of sulfates were at m/z 319, which was 80 Da higher (characteristic of the sulfuric acid addition) than that of MHFs, in which the pseudomolecular ion appeared at m/z 239. Based on these data, they were identified as the sulfate of MHFs (table 1).
Table 1. UPLC and LC/MS/MS characteristics and the conversion factors (K, mean ±SD) of the selected MHF sulfates.
Conversion factors were determined separately at three different concentrations.
| Selected MHFs | Sulfates | Chromatographic Retention Time (min) | Characteristic ions in LC/MS/MS | K (mean ±SD) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| M | MHF | [M+H] | [M-S+H] | |||
| 7-MHF | 7-O-S | 1.82 | 2.50 | 319 | 239 | 0.98±0.04 |
| 6-MHF | 6-O-S | 1.79 | 2.66 | 319 | 239 | 1.59±0.04 |
| 4'-MHF | 4'-O-S | 1.84 | 2.65 | 319 | 239 | 1.21±0.05 |
The species effects were compared and there is significant difference in species. Sulfation rates of the three MHFs at 5 μM, 10 μM, and 40 μM concentrations were measured using mouse, rat, dog, and human pooled liver S9 fractions. For 7-MHF, the rank order of the sulfation rate (nmol min−1 mg−1) (Table 2) at 5 μM , 10 μM and 40 μM was dog (0.1558 ± 0.0093, 0.1997 ± 0.0064 and 0.1475 ± 0.0040) > rat (0.1260 ± 0.0013, 0.1180 ± 0.0013 and 0.1376 ± 0.0026) > human (0.0810 ± 0.0026, 0.0577 ± 0.0011 and 0.0375 ± 0.0010) > mouse (0.0220 ± 0.0001 0.0230 ± 0.0002 and 0.0235 ± 0.0008) (Table 2). For 6-MHF, the sulfation rates in dog (all about 0.0275 – 0.0459 nmol min−1 mg−1) liver S9 fractions were much faster than other species, fallowed by rat, human and mouse at all three substrate concentrations. However, for 4'-MHF, the trend was different (Table 2), the rate (nmol min−1 mg−1) of 4'-O-S formation was always the highest in dog liver S9 at three concentrations, followed by mouse, rat and human at 4μM, At 10 μM and 40μM, the the order was dog > rat > mouse > human (p < 0.05, Kruskal-Wallis with Dunn's test). The above results clearly showed that the metabolic rate in dog liver S9 fractions was the fastest among the four selected liver S9 fractions for all three MHFs at three substrate concentrations.
Table 2. Species-dependent sulfation rates of three flavones (7-MHF, 6-MHF and 4'-MHF) in liver S9 fractions prepared from four different species (mouse, rat, dog, human).
Three different concentrations (5 μM, 10 μM, 40 μM) were used in the experiment (n = 3). Sulfation rates were calculated as nmol min−1 mg−1 protein.
| Sulfation rates (nmol/min/mg, Mean±SD) | Mouse | Rat | Dog | Human | |
|---|---|---|---|---|---|
| 7-MHF | 5μM | 0.0220±0.0001#▲ | 0.1260±0.0013*▲ | 0.1558±0.0093*# | 0.0810±0.0026*#▲ |
| 10μM | 0.0230±0.0002#▲ | 0.1180±0.0013*▲ | 0.1997±0.0064*# | 0.0577±0.0011*#▲ | |
| 40μM | 0.0235±0.0008#▲ | 0.1376±0.0026*▲ | 0.1475±0.0040*# | 0.0375±0.0010*#▲ | |
| 6-MHF | 5μM | 0.0007±0.0001#▲ | 0.0060±0.0004*▲ | 0.0275±0.0005*# | 0.0021±0.0001*#▲ |
| 10μM | 0.0007±0.0001#▲ | 0.0062±0.0001*▲ | 0.0293±0.0010*# | 0.0029±0.0001*#▲ | |
| 40μM | 0.0010±0.0001#▲ | 0.0123±0.0008*▲ | 0.0459±0.0005*# | 0.0067±0.0004*#▲ | |
| 4'-MHF | 5μM | 0.0457±0.0045#▲ | 0.0405±0.0017*▲ | 0.1458±0.0127*# | 0.0384±0.0062*#▲ |
| 10μM | 0.0366±0.0026#▲ | 0.0418±0.0091*▲ | 0.1467±0.0031*# | 0.0302±0.0027*#▲ | |
| 40μ | 0.0312±0.0006#▲ | 0.0358±0.0011*▲ | 0.1388±0.0069*# | 0.0203±0.0008*#▲ | |
Significant differences are marked as follows:
P<0.05 vs Mouse,
P<0.05 vs rat,
P<0.05 vs Dog (p < 0.05, Kruskall–Wallis).
Moreover, the species-dependence was changed according to the substitutional positions of the hydroxyl group and the concentration of drug compounds. The sulfation rates of 7-MHF and 4'-MHF in rat, mouse, and human liver S9 fractions were also observed to be much faster than that of 6-MHF. For 7-MHF, the rate was about 5 – 20 fold faster than that of 6-MHF and for 4'-MHF, the rate was about 5 – 10 fold faster than 6-MHF in rat, mouse, and human liver S9 fractions. Wang et(2006) reported that the Km for 7-hydroxycoumarin -sulfate formation showed no significant difference among humans, monkeys, and rats (approximately 3 microM)[17]. Also, Wang et (2009) reported the liver S9 samples from dogs, monkeys, and humans had higher activities for formation of O-demethyl apixaban sulfate than those of mice, rats, and rabbits[18]. Our data showed significant difference. The explanation could be that the sulfation is regiospecific of the substrates.
We have known that metabolism in species with respect to CYP (cytochrome P450) activity. Lofgren, Hagbjork et al reported that female mice generally had a higher metabolism of bufuralol 1′-hydroxylation and dextromethorphan O-demethylation (human markers for CYP2D activity)[19]. Besides, Hernandez, Chapman et al also reported that greater CYP2B induction in female mice than male[20]. Gender differences in humans were also observed with female HLM possessing greater activity than male HLM[21].The gender effects on the rates of sulfation were also compared in our study. In rat liver S9 fractions (Table 3), the sulfation rates of all MHFs at three concentrations were much higher in males than that in females (p < 0.05, Kruskall–Wallis test). However, in mouse liver S9 fractions (Table 3), the sulfation rates of 4'-MHF and 6-MHF at all three concentrations were much higher in females than in males, while for 7-MHF, statistically significant differences between genders were present only at 10 μM and 40 μM (p<0.05). In dog liver S9 fractions (Table 3), the sulfation rates of the three MHFs had no or there was a small difference between females and males. As a result, gender-dependence exists in the sulfonation metabolism of MHFs.
Table 3. Gender-dependent sulfation rates of three flavones (7-MHF, 6-MHF and 4'-MHF) in liver S9 fractions prepared from the same animal but different genders.
Three different concentrations (5 μM, 10 μM, 40 μM) were used in the experiment (n = 3). Sulfation rates were calculated as nmol min−1 mg−1 protein.
| Sulfation rates (nmol/min/mg, Mean±SD) | Mouse | Rat | Dog | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Female | Male | Female | Male | Female | Male | ||
| 7-MHF | 5μM | 0.0202±0.0006 | 0.0228±0.0003 | 0.0855±0.0015* | 0.1354±0.0018 | 0.1557±0.0023 | 0.1560±0.0061 |
| 10μM | 0.0250±0.0008* | 0.0220±0.0008 | 0.0792±0.0013* | 0.1254±0.0032 | 0.1685±0.0067 | 0.1678±0.0062 | |
| 40μM | 0.0277±0.0007* | 0.0213±0.0006 | 0.1030±0.0022* | 0.1956±0.0122 | 0.1153±0.0174* | 0.1529±0.0041 | |
| 6-MHF | 5μM | 0.0009±0.0001* | 0.0005±0.0001 | 0.0028±0.0002* | 0.0048±0.0004 | 0.0582±0.0003 | 0.0576±0.004 |
| 10μM | 0.0012±0.0001* | 0.0004±0.0001 | 0.0041±0.0002* | 0.0066±0.0001 | 0.0703±0.0049 | 0.0734±0.0003 | |
| 40μM | 0.0016±0.0002* | 0.0007±0.0001 | 0.0061±0.0004* | 0.0099±0.0002 | 0.1365±0.0091* | 0.1150±0.0112 | |
| 4'-MHF | 5μM | 0.0564±0.0098* | 0.0402±0.0019* | 0.0130±0.0010* | 0.0329±0.0065 | 0.0894±0.0029 | 0.0913±0.0021 |
| 10μM | 0.0453±0.0012* | 0.0322±0.0032 | 0.01780±0.0008* | 0.0429±0.0013 | 0.0958±0.0028 | 0.1001±0.0048 | |
| 40μM | 0.0475±0.0021* | 0.0231±0.0003 | 0.0148±0.0025* | 0.0327±0.0035 | 0.1944±0.0211 | 0.1873±0.0017 | |
Significant differences are marked by the *symbol (p < 0.05, Kruskall–Wallis test).
SULTs are soluble in liver cytosol. Liver S9 fractions were prepared from fresh hepatocytes (both cytosol and microsomes). Cytosol Fraction were soluble proteins (phase II enzymes) containing SULT. Despite of these difference, the present investigate by using liver S9 fraction will shed light about any possible sulfation of flavones in different genders and species.
Conclusions
Based on the results, the sulfation of the three MHFs was regiospecific, gender-, and species-dependent. Multiple experiments using in vivo perfusion model can be further used to determine the difference in species and gender for it cannot only supply information on potential rates and metabolism routes but also provide guidelines for species-dependence and gender-dependence that can be used in preclinical studies.
Acknowledgements
This work was mainly supported by the National Basic Research Program of China (973 Program, 2009CB5228008), a key project of the National Natural Science Foundation of China (U0832002), and the International Science and Technology Cooperation Base of Guangdong (2010JD035).
References
- 1.Graf BA, Milbury PE, Blumberg JB. Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food. 2005;8(3):281–90. doi: 10.1089/jmf.2005.8.281. [DOI] [PubMed] [Google Scholar]
- 2.Manach C, et al. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur J Clin Nutr. 2003;57(2):235–42. doi: 10.1038/sj.ejcn.1601547. [DOI] [PubMed] [Google Scholar]
- 3.Liu ZQ, et al. Mechanisms responsible for poor oral bioavailability of paeoniflorin: Role of intestinal disposition and interactions with sinomenine. Pharm Res. 2006;23(12):2768–80. doi: 10.1007/s11095-006-9100-8. [DOI] [PubMed] [Google Scholar]
- 4.Tang L, et al. Structure and concentration changes affect characterization of UGT isoform-specific metabolism of isoflavones. Mol Pharm. 2009;6(5):1466–82. doi: 10.1021/mp8002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otake Y, Hsieh F, Walle T. Glucuronidation versus oxidation of the flavonoid galangin by human liver microsomes and hepatocytes. Drug Metab Dispos. 2002;30(5):576–81. doi: 10.1124/dmd.30.5.576. [DOI] [PubMed] [Google Scholar]
- 6.Hu M. Commentary: bioavailability of flavonoids and polyphenols: call to arms. Mol Pharm. 2007;4(6):803–6. doi: 10.1021/mp7001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, et al. Sulfation of dietary flavonoids by human sulfotransferases. Xenobiotica. 2009;39(4):312–22. doi: 10.1080/00498250802714915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Ho CT. Metabolism of flavonoids. Forum Nutr. 2009;61:64–74. doi: 10.1159/000212739. [DOI] [PubMed] [Google Scholar]
- 9.Tang L, et al. Use of glucuronidation fingerprinting to describe and predict monoand dihydroxyflavone metabolism by recombinant UGT isoforms and human intestinal and liver microsomes. Mol Pharm. 2010;7(3):664–79. doi: 10.1021/mp900223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, et al. Use of isoform-specific UGT metabolism to determine and describe rates and profiles of glucuronidation of wogonin and oroxylin A by human liver and intestinal microsomes. Pharm Res. 2010;27(8):1568–83. doi: 10.1007/s11095-010-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong EJ, Lin H, Hu M. Disposition mechanisms of raloxifene in the human intestinal Caco-2 model. J Pharmacol Exp Ther. 2004;310(1):376–85. doi: 10.1124/jpet.103.063925. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, et al. Glucuronidation and sulfation of 7-hydroxycoumarin in liver matrices from human, dog, monkey, rat, and mouse. In Vitro Cell Dev Biol Anim. 2005;41(3–4):97–103. doi: 10.1290/0501005.1. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka E. Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther. 1999;24(5):339–46. doi: 10.1046/j.1365-2710.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- 14.Punt A, et al. Tandem mass spectrometry analysis of N2-(trans-Isoestragol-3'-yl)-2'-deoxyguanosine as a strategy to study species differences in sulfotransferase conversion of the proximate carcinogen 1'-hydroxyestragole. Chem Res Toxicol. 2007;20(7):991–8. doi: 10.1021/tx600298s. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, et al. Species and gender differences affect the metabolism of emodin via glucuronidation. AAPS J. 2010;12(3):424–36. doi: 10.1208/s12248-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alnouti Y, Klaassen CD. Mechanisms of gender-specific regulation of mouse sulfotransferases (Sults) Xenobiotica. 41(3):187–97. doi: 10.3109/00498254.2010.535923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, et al. Inter-species comparison of 7-hydroxycoumarin glucuronidation and sulfation in liver S9 fractions. In Vitro Cell Dev Biol Anim. 2006;42(1–2):8–12. doi: 10.1007/s11626-006-0004-z. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, et al. Sulfation of o-demethyl apixaban: enzyme identification and species comparison. Drug Metab Dispos. 2009;37(4):802–8. doi: 10.1124/dmd.108.025593. [DOI] [PubMed] [Google Scholar]
- 19.Lofgren S, et al. Metabolism of human cytochrome P450 marker substrates in mouse: a strain and gender comparison. Xenobiotica. 2004;34(9):811–34. doi: 10.1080/00498250412331285463. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez JP, et al. Gender-specific induction of cytochrome P450s in nonylphenol-treated FVB/NJ mice. Toxicol Appl Pharmacol. 2006;216(2):186–96. doi: 10.1016/j.taap.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang J, et al. Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse, and rat liver microsomes. Drug Metab Dispos. 2001;29(9):1201–4. [PubMed] [Google Scholar]


