Abstract
Prions are proteins that acquire alternative conformations that become self-propagating. Transformation of proteins into prions is generally accompanied by an increase in β-sheet structure and a propensity to aggregate into oligomers. Some prions are beneficial and perform cellular functions, whereas others cause neurodegeneration. In mammals, more than a dozen proteins that become prions have been identified and a similar number has been found in fungi. In both mammals and fungi, variations in the prion conformation encipher the biological properties of distinct prion strains. Increasing evidence argues that prions cause many neurodegenerative diseases (NDs), including Alzheimer’s, Parkinson’s, Creutzfeldt-Jakob, and Lou Gehrig’s diseases, as well as the tauopathies. The majority of NDs are sporadic, and 10% to 20% are inherited. The late onset of heritable NDs, like their sporadic counterparts, may reflect the stochastic nature of prion formation; the pathogenesis of such illnesses seems to require prion accumulation to exceed some critical threshold before neurological dysfunction manifests.
PRION, NEURODEGENERATION: INTRODUCTION
Most neurodegenerative diseases (NDs) are age-dependent, i.e., their incidence increases with advancing age. An expanding body of evidence argues that many different NDs are caused by prions, which are formed from normal proteins (146). Prions arise when normal proteins acquire an alternative conformation that becomes self-propagating. The most well-studied mammalian prions are composed of PrPSc proteins that cause Creutzfeldt-Jakob disease (CJD) in humans, scrapie in sheep, chronic wasting disease (CWD) in deer and elk, and mad cow disease. Conformational variants of PrPSc create different regional patterns of PrP accumulation and thus produce different disease phenotypes. Ten to twenty percent of CJD patients have a familial form of the disease due to mutations in the PRNP gene that encodes PrPC. Prions composed of PrPSc are formed from PrPC by a posttranslational process that results in a profound change in conformation. In some CJD patients, PrPSc and smaller fragments are found in amyloid plaques. Although the number of prions identified in mammals and in fungi continues to expand, the existence of prions in other phylogeny remains undetermined. Some mammalian prions perform vital functions and do not cause disease; such nonpathogenic prions include cytoplasmic polyadenylation element binding (CPEB) protein, mitochondrial antiviral-signaling (MAVS) protein, and T-cell-restricted intracellular antigen 1 (TIA-1). The pathogenic mammalian prions include PrP (prion protein), Aβ, tau, α-synuclein, superoxide dismutase 1 (SOD1), and possibly huntingtin, each of which causes a distinct ND (Table 1). All mammalian prion proteins adopt a β-sheet-rich conformation and appear to readily oligomerize as this process becomes self-propagating (Figure 1). Control of the self-propagating state of benign mammalian prions is not well understood but is critical for the well-being of the host. In contrast, pathogenic mammalian prions appear to multiply exponentially, but the mechanisms by which they cause disease are poorly defined. We do not know if prions multiply as monomers or as oligomers; notably, the ionizing radiation target size of PrPSc prions seems to suggest it is a PrP trimer (5). The oligomeric states of pathogenic mammalian prions are thought to be the toxic forms, and assembly into larger polymers, such as amyloid fibrils, seems to be a mechanism for minimizing toxicity. To date, there is not a single medication that halts or even slows one ND caused by prions. This may reflect the unprecedented pathogenic mechanisms that feature in each of the prion diseases and highlights the urgent need to develop informative molecular diagnostics and effective antiprion therapeutics.
Table 1.
Neurodegenerative diseases caused by prions.
| Neurodegenerative diseases | Causative prion proteins | Percent inherited |
|---|---|---|
|
| ||
| Creutzfeldt-Jakob disease | PrPSc | 10–20 |
| Gerstmann-Sträussler-Scheinker | 90 | |
| Fatal insomnia | 90 | |
| Bovine spongiform encephalopathy | 0 | |
| Scrapie | 0 | |
| Chronic wasting disease | 0 | |
|
| ||
| Alzheimer’s disease | Aβ → tau | 10–20 |
|
| ||
| Parkinson’s disease | α-synuclein | 10–20 |
|
| ||
| Frontotemporal dementia (FTD) Posttraumatic FTD, called chronic traumatic encephalopathy |
tau, TDP43, FUS, C9orf72 (progranulin) | 10–20 |
|
| ||
| Amyotrophic lateral sclerosis | SOD1, TDP43, FUS, C9orf72 | 10–20 |
|
| ||
| Huntington’s disease | huntingtin | 100 |
Figure 1.
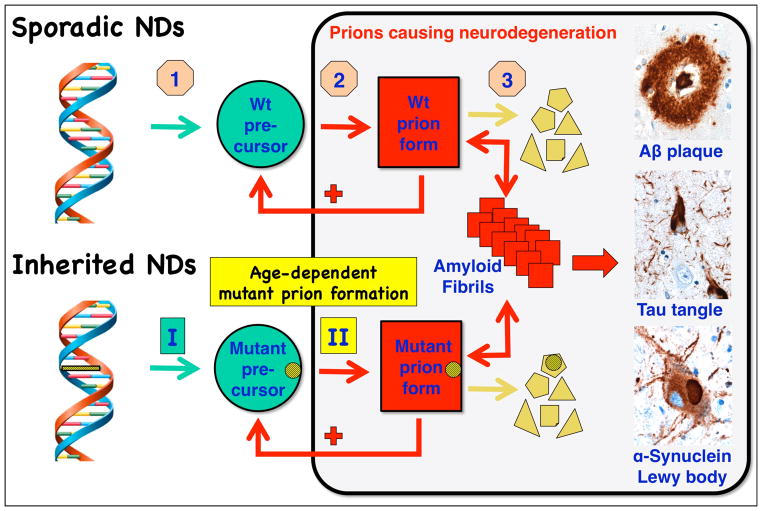
Neurodegeneration caused by prions. (Sporadic NDs) In sporadic neurodegenerative diseases (NDs), wild-type (wt) prions multiply through self-propagating cycles of posttranslational modification during which the precursor protein (green circle) is converted into the prion form (red square), which generally is high in β-sheet content. Pathogenic prions are most toxic as oligomers and less toxic after polymerization into amyloid fibrils. The small polygons (pale yellow) represent proteolytic cleavage products of the prion. Depending on the protein, the fibrils coalesce into Aβ amyloid plaques in Alzheimer’s disease (AD), neurofibrillary tangles in AD and Pick’s disease, or Lewy bodies in Parkinson’s disease and Lewy body dementia. Drug targets for the development of therapeutics (beige octagons): (1) lowering the precursor protein, (2) inhibiting prion formation, and (3) enhancing prion clearance. (Inherited NDs) Late-onset heritable neurodegeneration argues for two discrete events: the first event (green rectangle I) is the synthesis of mutant precursor protein (green circle) and the second event (yellow rectangle II) is the age-dependent formation of mutant prions (red square). The yellow bar with diagonal lines in the DNA structure represents mutation of a base pair within an exon, and the small yellow circles with diagonal lines signify the corresponding mutant amino acid substitution.
MAMMALIAN PRIONS
Discovery of Prions
Following the experimental transmission of the human disorders kuru and CJD to apes and monkeys, the search intensified for a slow-acting virus that causes the analogous disease, called scrapie, in sheep and goats (42, 43, 48, 63). As preparations from the brains of scrapie-infected hamsters were enriched for infectivity, evidence for an essential protein emerged, but no similar data for a nucleic acid could be generated. To the consternation of many, I introduced the term prion in order to distinguish from viruses the proteinaceous infectious particles causing scrapie and CJD (142). Despite numerous attempts to demonstrate a scrapie-specific nucleic acid, none could be found, and as such, those investigations were eventually abandoned (162).
Soon after the introduction of the prion concept, a protein with a molecular weight of 27–30 kDa was found in purified fractions containing high levels of scrapie infectivity (9, 119). This protein was designated prion protein 27–30 (PrP 27–30). Scrapie infectivity and PrP 27–30 were found at the top of sucrose gradients, indicating some of the infectivity was likely to be very small, in agreement with the small ionizing radiation target size, whereas the majority of the infectivity sedimented to the bottom (1, 5, 148, 149). The rod-shaped structures at the bottom of the gradients were shown to be composed of PrP 27–30 and to stain with Congo red dye, establishing that these large aggregates were amyloid (150). This discovery suggested that amyloid deposits in other disorders, such as Alzheimer’s (AD) and Parkinson’s diseases (PD), might be composed of causative proteins, i.e., prions (143).
Plaques, Tangles, and Inclusions
Beginning in the mid-1980s, the proteins in plaques, tangles, and intracellular bodies of the brains of patients who died of neurodegeneration were identified through purification or immunostaining (14, 54, 61, 98, 173, 200). Each of these proteins was found to aggregate into fibrils under some conditions and to form amyloids (Figure 1). The amyloid fibrils in AD were found to contain the Aβ peptide (53, 116), which is cut from the larger amyloid precursor protein (APP). Neurofibrillary tangles (NFTs) in many NDs were found to contain the tau protein. Those same proteins were also identified when genetic studies found that specific mutant genes cause familial forms of neurodegeneration (55, 77, 83, 140). Subsequently, expression of these mutant genes in transgenic (Tg) mice was shown to recapitulate many aspects of the human illnesses (for review, see Reference 145).
Despite these similarities with CJD, many investigators still prefer to think about the other NDs as unrelated because Gajdusek, Gibbs, and their collaborators were unable to transmit AD and several other NDs to apes and monkeys (56, 57). These initial attempts to demonstrate transmissibility of AD and PD to apes and monkeys were made long before the Aβ peptide and α-synuclein, the respective causative proteins, were isolated, so neither could be used as biomarkers. Later, Ridley, Baker, and colleagues demonstrated transmission of disease to marmosets, as indicated by Aβ cerebral amyloidosis, after intracerebral injections of AD brain homogenates (3, 157). The incubation times in those studies exceeded 3.5 years, making confirmation of such experiments impractical. Later, Tg mouse studies would supplant the use of nonhuman primates.
Inherited Neurodegenerative Diseases
The recognition that 10% to 20% of CJD cases occur in families led to the suspicion that heredity might play a role in the pathogenesis of this disorder (Table 1). Subsequently, transmission of familial (f) CJD and Gerstmann-Sträussler-Scheinker disease (GSS) to apes and monkeys was reported, but multiple explanations involving slow viral illnesses in families were offered (115, 160). First, the putative CJD virus might be passed from mother to offspring during childbirth as was later shown for HIV. Second, the CJD virus might be passed to offspring in utero or during childhood when parents and children lived in close proximity. A third possibility was that a chromosomal gene conferred susceptibility to a ubiquitous CJD virus. All three of these explanations proved incorrect.
The identification of a mutation followed by the demonstration of genetic linkage in GSS patients was first reported for the P102L mutation in the PrP gene (77). Subsequently, four more mutations in the PrP gene were linked to fCJD, fatal familial insomnia (FFI), and GSS with NFTs (32, 41, 122, 141). More than 40 different mutations of the PrP gene have been identified, of which 35 are point mutations and the remainder are octapeptide expansions or deletions (see References 95, 121, 190). Virtually all cases of GSS and FFI appear to be caused by germ line mutations in the PrP gene. Some mutations seem to have arisen de novo; whether any of these mutations in PrP are responsible for the development of PrP prion disease remains to be established.
For years, the late-onset presentation of fCJD and GSS presented a conundrum. Despite mutations in PrP being expressed early in life, such prions do not cause disease for decades. In studies of fCJD caused by the E200K mutation in PrP, actuarial analyses showed that if carriers live long enough, they would all develop CJD, i.e., there was complete penetrance (18, 174).
Analogous to discoveries coming from GSS and fCJD, mutant proteins causing other inherited NDs were determined to be the same as those found in disease-specific amyloid deposits within intracellular inclusions, such as NFTs or Lewy bodies, or outside of cells as plaques (for review, see Reference 145). These parallel findings offered support to the early proposal that such common diseases as AD and PD might be caused by prions (143).
Late-Onset Neurodegeneration
Despite the fact that most mutant proteins that cause familial NDs are expressed early in embryogenesis, signs of neurological dysfunction are generally delayed for decades. This finding argues that some event occurs with aging that renders a disease-specific protein pathogenic. Many explanations have been offered to explain the late onset of familial NDs (Table 2), including age-dependent mitochondrial DNA mutations; oxidative modifications of DNA and proteins; proteasome malfunction; diminished innate immunity; exogenous toxins, such as alcohol and drugs; concomitant conditions, such as atherosclerosis; somatic mutations; chaperone malfunction; haploinsufficiency; RNA-DNA differences; expanded repeat segments in proteins and noncoding regions of DNA; and postinfectious syndromes. However, little evidence supports most of these proposed mechanisms. The formation of prions from etiologic proteins provides a plausible explanation for the late-onset presentations of many different NDs and is consistent with the observation that aging appears to be the most important risk factor. These proteins must first refold into prions and then accumulate in sufficient numbers for an infection to become self-sustaining (146). Presumably, the number of prions being generated by this self-propagating process has to reach a threshold before an infection can continue unabated (144). Under these conditions, prion propagation becomes uncontrolled, and eventually central nervous system (CNS) dysfunction results.
Table 2.
Some possible explanations for late-onset neurodegeneration
| 1. Mitochondrial DNA mutations (26, 125) |
| 2. Oxidative modifications of DNA, lipids, or proteins (87, 100) |
| 3. Impaired autophagy (133) |
| 4. Altered apoptosis (201) |
| 5. Posttranslational chemical modification (201) |
| 6. Modified innate immunity (186) |
| 7. Accumulation of exogenous toxins, such as heavy metals, alcohol, drugs, and hormones (19) |
| 8. Concomitant conditions, such as atherosclerosis (96) |
| 9. RNA-DNA differences (107) |
| 10. Chaperone malfunction (111) |
| 11. Somatic mutations (100) |
| 12. Altered regulation of transcription (8) |
| 13. Haploinsufficiency (30, 153) |
| 14. Postinfectious syndromes of the central nervous system, including late polio, subacute sclerosing leukoencephalitis, postencephalitic Parkinson’s disease, and Lyme disease (59, 89, 203) |
| 15. Modifier genes, such as apolipoprotein E and LRRK2 (17, 25) |
| 16. Polyglutamine expansions (101, 196) |
| 17. Dipeptide repeat proteins (127) |
| 18. Cu2+ binding to expanded PrP octarepeat region (177) |
| 19. Prion formation and accumulation (134, 144) |
One fascinating insight into the control of the timing of disease onset comes from studies of ~35 fCJD families with expanded PrP octarepeat regions (27, 177). Wild-type PrPC contains four octarepeats; one to four additional octarepeats cause late-onset fCJD with a mean age of 62 years, and —five to eight additional octarepeats cause early-onset fCJD or GSS with a mean age of 32 years. In other words, the fifth additional octarepeat decreases the age of disease onset by three decades. This profound transition in pathogenesis was found to correlate with a shift in Cu2+ binding to PrP: a high Cu2+ occupancy state shifted to a low one (177). Titrations showed that one Cu2+ ion binds to each histidine in the high occupancy state; each of the four octarepeats contains a single histidine. In the low occupancy state, a single Cu2+ ion coordinated with four histidines. The mechanism by which Cu2+ ions that are bound to mutant PrP with octarepeat expansions participate in the formation of PrPSc remains unknown. Whatever the process that controls the formation of prions in the inherited PrP prion diseases, it may prove relevant to some other age-dependent NDs.
Sporadic Neurodegenerative Diseases
In the absence of a mutation or an environmental cause, such as an infection or a toxin, the NDs are generally referred to as sporadic. Such illnesses are thought to arise stochastically. Although 10% to 20% of cases are inherited, more than 80% are sporadic. This relationship has been found for CJD, AD, PD, the tauopathies, and amyotrophic lateral sclerosis (ALS) but not for Huntington’s disease (HD), which is always inherited (183). Notably, inheritance of the e4 allele of apolipoprotein E is the only well-established genetic risk factor for sporadic AD (69, 112). Presumably, many disease-causing proteins can form prions but most are cleared before they begin to multiply in sufficient numbers to support a sustained infection involving spread to adjacent cells and eventually other regions of the brain. One possible mechanism to explain sporadic prion diseases involves precursor proteins becoming transformed into disease-causing prions through a stochastic process, which most of the time probably represents a dead-end route in which small numbers of prions are cleared via protein degradation pathways.
PrP Prions
Synthetic PrP prions provided indisputable evidence that the disease-causing agent of CJD and other PrP prion diseases is composed solely of protein (23, 45, 102, 114, 193). The first glimmer of a synthetic PrPSc prion came when we produced a synthetic PrP peptide of fifty-five amino acids containing the P101L mutation (mouse numbering) that causes GSS in humans; the peptide was made by coupling individual amino acids together, as was later done for the synthetic Aβ peptide (90). After we mixed the PrP peptide with acetonitrile, which caused it to adopt β-sheet-rich structure, the peptide was injected into a Tg mouse model of GSS (80, 81, 187). Approximately a year later, the inoculated mice became ill, whereas the uninoculated ones remained well for another six months. Some investigators have argued that the subsequent illness in the uninoculated controls showed that the peptide was not a prion but was merely an accelerator of disease. Such arguments persisted for a more than a decade (130). Importantly, we were able to show by serial passage that the brains of the inoculated mice contained a transmissible entity, i.e., a prion (187).
In part on the basis of studies in yeast (172), we assembled recombinant mouse PrP(89–230) made in bacteria into amyloid fibrils and injected them into Tg mice expressing the same N-terminally truncated PrP. When the mice remained well for more than 250 days, we concluded that the experiment was negative and wrote a paper about our experience (4). Unintentionally, the experiment was not terminated and approximately 600 days after inoculation, the mice showed signs of neurological dysfunction and their brains showed the hallmarks of prion disease on neuropathological examination (102). The prion strain found in these mice was more resistant to denaturation at higher concentrations of GdnHCl than that generally seen with naturally occurring strains of prions (103).
When we examined the stability of a half-dozen prion isolates in mice, including one of the synthetic prions, we found that the more stable strains, which resisted denaturation, produced longer incubation times, and the less stable ones yielded shorter incubation times (104). On the basis of this finding, we produced synthetic prions under conditions that created PrP preparations that were highly resistant to denaturation as well as those that were quite susceptible and those that were intermediate. The stable preparations produced long incubation times, whereas the more labile ones yielded much shorter incubation times (23, 45). Although these investigations confirmed and extended our earlier work, they remained very expensive because the incubation times exceeded 500 days on first passage. More extensive analyses in cell culture and Tg mice have demonstrated the evolution of prions toward strains causing shorter incubation times and harboring an unglycosylated, protease-resistant PrPSc fragment (PrP 27–30) of 21 kDa (45, 46, 105).
Aβ Prions
In a series of incisive experiments, Mathias Jucker and Lary Walker collaborated on the transmission of Aβ prions to weanling Tg(APP23) mice expressing mutant human APP. Much of their work was performed with brain homogenates from aged Tg(APP23) mice that spontaneously develop Aβ amyloid plaques. The inoculum prepared from these old mice dramatically accelerated the deposition of nascent Aβ amyloid, and immunoabsorption of Aβ in the inoculum prevented the accelerated deposition of Aβ (123). These investigators also reported transmission of Aβ prions after intraperitoneal injections (35). More recently, Claudio Soto and colleagues, as well as Jucker and Walker, reported that brain homogenates from AD patients could also transmit disease to Tg mice expressing wild-type human APP and Tg rats expressing mutant APP, neither of which develops cerebral Aβ deposits spontaneously (126, 161).
Using bigenic mice expressing mutant APP and luciferase under the control of the murine Thy-1.2 and Gfap promoters, respectively, my colleagues and I found brain homogenates from aged Tg(APP23) mice transmitted Aβ prions, as reflected by increased bioluminescence and Aβ deposits (194). Subsequently, we found that fractions highly enriched for Aβ produced an increase in bioluminescence after approximately five months, which was approximately six weeks more rapid than that seen with the crude brain homogenates. Most important, synthetic Aβ peptides became prions during polymerization into amyloid, establishing that some hypothetical contaminate was not responsible for the change in bioluminescence (178).
Remarkably, Heiko Braak not only described the spreading of Aβ amyloid plaques but also showed the concurrent deposition of NFTs composed of the tau protein in the brains of autopsied AD patients (11, 12). Recent studies have traced the spread of tau prions using functional magnetic resonance imaging (fMRI) intrinsic connectivity analysis (202). Such spreading of prions was described much earlier for scrapie prions in ovines and rodents (38, 39, 64, 92, 165, 181, 182).
Familial Alzheimer’s Disease
Soon after the identification of mutant PrP genes, missense mutations in the APP gene were discovered in familial AD (fAD) followed by mutations in the presenilin 1 and 2 genes (PS1 and PS2) encoding the catalytic subunits of γ-secretase; these mutations result in elevated levels of wild-type Aβ peptide (Table 1) (55, 68, 163, 164, 175, 176). In addition, more than a dozen mutations within the Aβ peptide have been discovered (6, 192), most of which also cause fAD. All of the mutations causing fAD, which account for 10% to 20% of AD cases, are autosomal dominant and produce one or two amino acid substitutions.
In contrast to APP mutations causing fAD, the A673T mutation in APP identified in a genome-wide study of Icelandic people seems to protect against late-onset AD (88). This missense mutation is located at the β-secretase cleavage site and presumably inhibits the production of the Aβ peptide. Diminished wild-type Aβ levels would seem likely to reduce the chances of sustained Aβ prion formation.
Although no authentic animal models of AD exist, much has been learned from mice, and more recently Tg rats (22), that express mutant human APP transgenes. The initial Tg mouse models of fAD used human APP harboring mutations that elevate the level of wild-type Aβ (44, 78); these mice exhibited numerous amyloid plaques filled with fibrils composed of the Aβ peptide as well as astrocytic gliosis and behavioral changes. Mice were constructed expressing mutant human APP, presenilin, and tau in the hope of building a more authentic model of AD; these mice were designated triple Tgs, or 3×Tg-AD, mice (131). Such Tg models have been used to assess the therapeutic efficacy of antibodies for the clearance of the Aβ peptide (29). Unfortunately, attempts to treat AD patients with anti-Aβ antibodies have yet to produce an FDA-approved therapy (167).
Tau Prions
The tauopathies sit at an interface between psychiatry and neurology. These disorders often present after age 40 as psychiatric illnesses. Often, psychiatrists see patients with frontotemporal dementia (FTD) for several years before they refer them to neurologists with the diagnosis of AD. Tau lesions in the frontal lobes can produce behavioral disinhibition, apathy, inappropriate social interactions, depression, and insomnia as well as reduced executive function (152). Later, semantic dementia, drug abuse, alcoholism, and sometimes suicide are seen. As behavioral and language problems worsen, these inherited and sporadic neurological disorders are frequently classified clinically as behavioral variants of FTD (bvFTD). Different phenotypes of tauopathy, including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and Pick’s disease, may be due to conformational variants of tau prions producing lesions in different neuronal circuits (166).
In an important series of studies over the past four decades, contact sport athletes, including boxers and football and hockey players, have symptoms similar to those of FTD patients. Numerous NFTs have been found in the frontal lobes of contact sports athletes (24), some of whom have committed suicide. The first football player identified with a tauopathy was the Pittsburgh Steeler Mike Webster, who played professional football for 16 seasons and died at age 50 (136). Subsequently, fifty other football players in high school, college, and the NFL who committed suicide were identified with tauopathies (118). Recently, a 27-year-old Marine, who suffered multiple episodes of traumatic brain injury (TBI) during explosions of roadside bombs in Iraq, was found to suffer from a tauopathy. Following the diagnosis of posttraumatic stress disorder (PTSD), he was honorably discharged, divorced his wife, and became an alcoholic. Eight months after his honorable discharge, he committed suicide by hanging; at autopsy, numerous NFTs were found in his frontal lobes (135). Many similar cases of military veterans with the symptoms of PTSD have been reported (118).
US Army studies show that multiple episodes of mild TBIs from exposure to blasts from improvised explosive devices increase the risk for PTSD (73). The latest estimates suggest that ~450,000 men of the 2 million who have served in Iraq or Afghanistan will develop PTSD. How many of these will develop a posttraumatic tauopathy [or chronic traumatic encephalopathy (CTE)] caused by tau prions is unknown. Although concussive forces presumably stimulate tau prion formation in the human brain, the mechanism by which this occurs remains unknown.
Aggregates formed from truncated recombinant human tau composed of residues 242–364 (K18 fragment) in the presence of arachidonic acid were shown to enter C17 cells and seed the polymerization of endogenous tau (40). These studies were extended using HEK293 cells expressing full-length wild-type human tau(2N4R) as well as truncated and mutant human tau(P301S) (62, 91). Michel Goedert, Markus Tolnay, and their colleagues described the transmission of mutant tau(P301S) prions produced in Tg mice to recipient mice expressing wild-type human tau (21). After approximately six months, the inoculated Tg mice showed wild-type tau aggregates that had spread from the site of inoculation to neighboring regions. Synthetic tau fibrils were formed from full-length tau(2N4R) with the P301S mutation and from the truncated K18 fragment with the P301L mutation expressed in Escherichia coli, which were purified and polymerized into fibrils in the presence of heparin. The fibrils were inoculated intracerebrally into young Tg(MAPT*P301S) mice overexpressing mutant human tau (84). These inoculations produced NFT-like inclusions that propagated from injected sites to connected brain regions in a time-dependent manner. Interestingly, injection of the tau fibrils into either the hippocampus or the striatum, along with the overlaying cortex, gave rise to a distinct pattern of spreading. Unlike tau pathology that spontaneously develops in older Tg(MAPT*P301S) mice, the tau deposits resembled NFTs as reflected by their acetylation, thioflavin S positivity, and resistance to limited proteinase K digestion.
As with the Aβ amyloid plaques of AD, the NFTs also spread along defined neuroanatomical pathways (10). Presumably, the tau prions spread transsynaptically as they move from one neuron to another. Recent Tg mouse models expressing human tau in the entorhinal cortex show spread along neuroanatomically defined pathways to hippocampal pyramidal neurons, especially in CA1 and dentate gyrus granule cells (28, 108, 139).
Inherited Tauopathies
Familial forms of FTDs result from mutations in the MAPT gene that encodes the tau protein; these disorders include PSP, Pick’s disease, frontotemporal lobar degeneration (FTLD), CBDs, and argyrophilic grain disease (AGD) (Table 3). FTD with parkinsonism has been linked to mutations in the MAPT gene on chromosome 17 and is often referred to as FTDP-17, in which behavioral and personality changes, cognitive impairment, and signs of motor dysfunction are present. In addition to the gene encoding tau, mutations in other genes encoding TDP43, FUS, UPS, progranulin, and C9orf72 have been linked to familial forms of the FTDs. As with CJD and AD, familial FTDs account for 10% to 20% of FTD cases. Most of the FTD cases appear to be sporadic, seem likely to occur spontaneously, and are likely to be caused by tau prions or other prions formed from TDP43 or FUS. Both progranulin and C9orf72 are thought to cause disease through haploinsufficiency (127, 153). Interestingly, the expanded hexanucleotide repeat upstream of the C9orf72 coding region appears to encode several dipeptides that are translated into proteins, which deposit as neuronal cytoplasmic inclusions, suggesting an alternative pathogenic mechanism.
Table 3.
Genetic correlates of the neuropathological subtypes of frontotemporal lobar degeneration (FTLD)a
| Molecular classification | Neuropathological subtypeb | Mutated genesb |
| FTLD-tau | Pick’s disease | MAPT |
| Corticobasal degeneration | MAPT | |
| Progressive supranuclear palsy | MAPT | |
| Argyrophilic grain disease | MAPT | |
| Neurofibrillary tangle–predominant dementia | MAPT | |
| Multiple system tauopathy with dementia | MAPT | |
| White matter tauopathy with globular glial inclusions | MAPT | |
| FTLD–TAR DNA-binding protein 43 | Type A | GRN |
| C9orf72 | ||
| Type B | C9orf72 | |
| Type C | ||
| Type D | VCP | |
| FTLD–fused in sarcoma | Atypical FTLD with ubiquitinated inclusions | FUS |
| Neuronal intermediate filament inclusion disease | FUS | |
| Basophilic inclusion body disease | FUS | |
| FTLD–ubiquitin proteasome system | Frontotemporal dementia linked to chromosome 3 | CHMP2B |
Adapted from Reference 154.
Characteristic pattern of pathology, not the clinical syndrome.
In the FTDs caused by tau prions, NFTs are the neuropathological hallmark of these illnesses. Finding NFTs composed of tau fibrils in many CNS disorders led to the conclusion that tangles are nonspecific, unimportant histopathological entities. The discovery of mutations in the tau gene in patients suffering from familial FTDs brought remarkable clarity to a once rather Byzantine area of neurology (75, 83, 154). As with PrPSc, conformational variants of tau prions are strains that create different regional patterns of tau accumulation. These distinct patterns of tau prion accumulation produce different disease phenotypes.
The primary tauopathies encompass both the familial and sporadic forms of the FTDs, in which pathologic deposits of tau accumulate as either NFTs or collections of fibrils within neurons. In contrast, the secondary tauopathies have many causes, the most common of which is AD. Importantly, ablation of the MAPT gene encoding tau ameliorated disease in AD mouse models expressing mutant APP (158). Other disorders in which tau-laden NFTs accumulate include viral illnesses, such as rabies, subacute sclerosing panencephalitis, and postencephalitic PD; inherited disorders such as Nieman-Pick disease and myotonic dystrophy; and a disease of unknown etiology called Guam ALS-PD with dementia. In addition, the F198S point mutation and an octarepeat expansion in PrP have been reported to cause GSS and fCJD with NFTs, respectively (79, 99).
Synuclein Prions
In the late 1990s, fetal brain cells taken from the substantia nigra of aborted fetuses were transplanted into patients with advanced PD to mitigate the loss of dopaminergic neurons. A decade later, Lewy bodies were found in the grafted fetal brain cells when the PD patients died (97, 106). The surface of a Lewy body is covered with fibrils composed of β-sheet-rich, α-synuclein proteins. The normal form of α-synuclein seems to be either unstructured or high in α-helical structure, but like other prion precursor proteins, α-synuclein can adopt a β-sheet-rich conformation. Although unproven, it seems likely that β-sheet-rich α-synuclein prions crossed from the patient’s own neurons into the grafted cells and induced a change in the structure of α-synuclein (134). Once established, this process became self-propagating, as is the case for all pathogenic prions. This scenario is consistent with the findings of Braak and coworkers, who have mapped the spread of aggregated α-synuclein called Lewy neurites from the gut into the brainstem and throughout the cerebral hemispheres (11, 13, 139).
Inherited Parkinson’s Disease
Genetic linkage of the A53T mutation in the α-synuclein gene (140) led to the identification of α-synuclein in Lewy bodies that are found in both inherited and sporadic forms of PD (173). Duplication and triplication of the α-synuclein gene have also been reported in PD patients (66). Additionally, the G2019S variant in the leucine-rich repeat kinase 2 (LRRK2) protein imposes an increased risk for sporadic late-onset PD (71). Although mutations in other proteins have been identified as the causes of other forms of inherited PD, the relevance, if any, of these polypeptides to sporadic PD is unclear.
Tg mice expressing mutant human α-synuclein(A53T) were found to exhibit neurological disease at ~400 days of age (47). When brains from these ill mice were homogenized and injected into weanlings, the recipient mice became ill at ~200 days of age (110, 128). Recombinant α-synuclein was purified and induced to form fibrillar aggregates in vitro. After the introduction of aggregated α-synuclein into cultured cells, in Tg mice expressing mutant human α-synuclein(A53T), and in non-Tg mice, the aggregates of α-synuclein underwent self-propagation and, as such, became prions (109, 110, 117, 191).
Inherited Amyotrophic Lateral Sclerosis and SOD1 Prions
Studies of the progressive spread of motor neuron lesions along the neuraxis suggest an orderly and active process in ALS, also known as Lou Gehrig’s disease (155). More than 150 different mutations in the gene encoding human SOD1 have been found to cause familial forms of ALS (189). Aggregates of mutant human SOD1(H46R) protein have been used to initiate self-propagation in cultured cells, which can continue indefinitely; as such, mutant SOD1(H46R) forms prions (129). In another study, two human SOD1 constructs harboring either the G127X or G85R mutation induced wild-type SOD1 to misfold and aggregate in human neural cell lines (58).
In addition to mutant SOD1, mutations in two RNA-binding proteins, TDP-43 and FUS, have been identified in patients with familial ALS. As well as being involved in RNA metabolism, TDP-43 and FUS form aggregates in neurons in some cases of ALS and FTD (139, 188). Recent evidence indicates that both TDP-43 and FUS contain fungal prion–like domains rich in glutamine and asparagine residues, and in the case of TDP-43, this domain contains a substantial number of disease-causing mutations.
Huntingtin Prions
Unlike the aforementioned NDs, HD is always inherited. The wild-type huntingtin protein harbors an N-terminal region of ~35 glutamine residues. An additional 5 to 20 glutamines, resulting from mutations in the huntingtin gene, are found in most patients with HD, but many more have also been recorded (101). The length of the polyglutamine expansion is inversely proportional to the age of onset of HD.
Expanded polyglutamine repeats in a fragment of the huntingtin protein show spontaneous aggregation that self-propagates in cultured cells; in other words, they may prove to be prions (156). The idea of huntingtin prions is attractive because it would explain why people with 5 to 10 additional glutamines do not become ill until they are 40 to 50 years of age even though the mutant protein is produced beginning in embryogenesis.
NONPATHOGENIC MAMMALIAN PRIONS
Importantly, some prions, such as CPEB and MAVS, in mammals are not known to cause disease but perform important cellular functions (76, 169, 170). Both CPEB and MAVS contain glutamine-rich domainssimilar to those found in most yeast prions. The CPEB prion seems to control localized gene transcription in long-term memory, and the MAVS prion features in the immune response to infection by some RNA viruses. Unexpectedly, the biologically active forms of CPEB and MAVS are the oligomeric prion states and not the monomeric precursor proteins. Another nonpathogenic prion may be TIA-1, which is an RNA-binding protein that promotes translational arrest and the assembly of stress granules under conditions of cellular stress, including acute energy starvation (51). TIA-1 contains a carboxyterminal, glutamine-rich, prion-related domain that can undergo aggregation and thus functions as a metabolic switch. When this glutamine-rich domain of TIA-1 aggregates, stress granule formation ceases, but the process appears to be reversible.
FUNGAL PRIONS
Yeast prions have been invaluable in defining the spectrum of prions (20, 65, 197). The most extensively studied yeast prion is [PSI+], in which the Sup35 protein becomes a prion and its polypeptide chain termination function is diminished. In other words, stop codons are ignored, and proteins are abnormally synthesized with carboxyterminal extensions. The prion domain of Sup35, which is rich in Gln and Asn residues, is located near the amino terminus. Studies of the prion domains of two [PSI+] strains demonstrated that the more stable and more slowly replicating strain showed an extended, tightly packed β-sheet structure (184). The HET-s prion of Podospora anserina, which is a filamentous fungus, controls a programmed cell death reaction termed heterokaryon incompatibility (60). Although yeast prions are not infectious in the sense of being released into the culture medium and infecting other yeast, they are transmissible from mother to daughter cells and thus can readily multiply. Many of the lessons learned from studies of fungal prions are applicable to the NDs, such as AD, PD, and FTDs, in which prion infectivity is not readily demonstrable. As with fungi, special conditions, including cultured cells and generally Tg mice, must be used to demonstrate the existence and propagation of prions.
AMYLOIDS
The term amyloid was initially used to describe waxy substances that accumulate in tissues in a variety of systemic and CNS human disorders. The tinctorial properties of amyloid accumulation in tissues resembled those of some long-chain polysaccharides from plants referred to as starch. Features common to all amyloid fibrils are their nonbranched ultrastructure, their birefringence in polarized light after staining with Congo red dye, their shift in the fluorescence emission wavelength after staining with thioflavin T dye, and their crossed β-pleated sheet structure (31, 36, 52, 159, 185). Some of the most well-studied amyloids include the abnormal immunoglobulins, or paraproteins, that are produced by neoplastic plasma cells in multiple myeloma. Some of these aberrant immunoglobins,such as the Bence-Jones light chains, readily polymerize into amyloid fibrils. The accumulation of Bence-Jones paraproteins in the kidney leads to renal failure. Another protein, such as mutant transthyretin (TTR), shows diminished stability as a native tetramer and readily polymerizes into amyloid fibrils. TTR is a plasma protein that carries thyroxine and retinol-binding protein; it is also found in cerebrospinal fluid. The deposition of TTR amyloid fibrils within myelin sheaths and cardiac muscle causes polyneuropathy and cardiomyopathy, respectively. Recently, the drug Tafamidis [2-(3,5-dichloro-phenyl)-benzoxazole-6-carboxylic acid] was developed to stabilize the tetrameric structure of mutant TTR and is now being used to treat familial amyloidotic polyneuropathy (86).
In CNS disorders, amyloid plaques are a prominent feature in such diseases as kuru and AD (2, 31, 94). Subsequently, the proteins that polymerize into the fibrils of kuru and AD were identified as PrPSc and Aβ, respectively (9, 54). Unlike the Bence-Jones immunoglobulin light chains, both PrPSc and Aβ are prions in that each can acquire a conformation that becomes self-propagating. Although amyloid plaques are an essential feature of AD, they are nonobligatory features of kuru, CJD, scrapie, and other PrPSc disorders (151). Notably, a mouse scrapie prion strain was isolated that consistently produced PrP amyloid plaques (16). As with many mammalian prions, most yeast prions have a high β-sheet content and can polymerize into amyloid fibrils (93).
It is important to distinguish between prions and amyloids (138, 199); as noted above, prions need not polymerize into amyloid fibrils and can undergo self-propagation as oligomers (1, 5, 120, 171). The self-propagation of an alternative conformational state is a key feature of all prions. In contrast, amyloids are linear polymers that increase in number as new seeds are generated. It can be argued that any prion oligomer has the potential to become an amyloid seed; I contend that the polymerization of prions into amyloid fibrils represents a sequestration process whereby the brain seeks to minimize neurotoxicity. Although some investigators argue that any protein can be induced to polymerize into an amyloid fibril (33), only a small subset of proteins seems to become prions. Amyloid fibrils are not only pathogenic but they may also act as storage depots for secreted proteins (113).
CONCLUSIONS
The convergence of studies on these common neurodegenerative maladies has been remarkable (Table 1). Although many mysteries are now explicable within the framework of the prion concept, the science of prions is still in its infancy. Many unanticipated discoveries seem likely to emerge from the future study of prions.
Evidence that Prions Cause Many Different Neurodegenerative Disorders
Over the past few years, there has been a persuasive accumulation of new experimental data arguing that AD, PD, and the tauopathies are caused by prions. This advance in our understanding of the pathogenesis of these maladies will undoubtedly lead to new diagnostics and therapeutics. Notably, studies of NDs represent a curious mixture of outstanding advances and disappointing failures. Discovery of the Aβ peptide in cerebrovascular amyloid and plaques as well as the tau protein in tangles remains central to our understanding of AD pathogenesis. However, the current lack of any therapy that halts or even slows one ND is a colossal tragedy.
Studies of PrP prions are likely to be helpful in developing improved models and bioassays for Aβ, tau, and α-synuclein prions. The use of bigenic mice expressing mutant human APP and luciferase under control of the Gfap promoter (178, 194) is based on earlier studies of bigenic mice expressing luciferase and many different PrP transgenes (179, 195). The strategy for using mutant transgenes overexpressed in cells and mice involves increasing the likelihood that a particular protein will adopt a β-sheet-rich conformation that becomes self-propagating. In bigenic mice expressing mutant APP, the use of bioluminescence is proving to be an invaluable adjunct because such mice do not develop overt clinical signs of neurological dysfunction.
Although no cultured cell systems for propagating Aβ prions have been developed, some excellent systems for propagating tau and α-synuclein prions are now available. Tau prions have been formed using tau fragments and the full-length protein in vitro by exposure to arachidonic acid. They are then introduced into cells where they recruit wild-type and/or mutant tau to become prions. Similar experimental protocols have been adopted for studies of α-synuclein prions.
Many aspects of NDs can be explained by prions. The relentless and continuous spread of prions in the CNS provides a plausible explanation for the uninterrupted progression of the NDs. Prions also provide a unifying mechanism by which late-onset neurodegeneration can be understood. Although germ line mutations are present from the beginning of embryogenesis in familial cases of AD, PD, and the tauopathies, a second event is needed to explain the late onset of these disorders. That event seems likely to be the generation of enough prions to create self-sustaining replication.
Among the NDs, the tauopathies offer a remarkably wide spectrum of illnesses with respect to clinical presentations as well as progression. These diseases include PSP, Pick’s, CBD, FTLD, and AGD. Using fMRI to map connectivity networks, the progression of these different tauopathies can be defined in vivo. The most plausible explanations for these clinically distinct tauopathies seem to be different strains of tau prions with distinct neurotropisms.
Some Arguments Against Prions Causing Many Neurodegenerative Diseases
In contrast to CJD and kuru, there are no examples of person-to-person transmissions for AD, PD, and the tauopathies; on the basis of the lack of transmission, some investigators argue that prions do not cause these disorders (85). They also cite the inability of extracts from the brains of patients to transmit these disorders to wild-type animals as additional evidence against a prion etiology. Notably, α-synuclein prions have been transmitted to wild-type mice (108, 117). Choosing narrow criteria for defining prion diseases seems rather artificial and unhelpful in advancing our knowledge of the NDs. Moreover, this constricted definition ignores much of the information accumulated about prions over the past three decades. Although some prions replicate more rapidly and accumulate outside of cells, others do not. Fungal prions are not secreted into the media bathing yeast cells; rather, they remain intracellular and pass from mother to daughter cells as the yeast multiply (20, 198). Passage of yeast prions from an infected cell to an uninfected cell requires cytoduction, in which cytoplasmic mixing of the two fungal cells occurs. As such, fungal prions may be a better model for understanding the properties of prions causing many of the NDs.
It is not surprising that cultured cells and Tg mice that overexpress mutant transgenes are needed to demonstrate the transmission of some prions causing NDs. Elevated transgene expression generally shortens the incubation times in Tg mice infected with prions but there occasional exceptions (49, 151, 180). Human point mutations in the transgenes appear to facilitate transmission of Aβ, tau, and α-synuclein prions, not unlike the incidence of the NDs. As noted above, the penetrance of familial forms of the NDs approaches 100%, whereas the prevalence of the sporadic forms of these disorders vary from one per million for CJD to 1 per 100 people for AD in the United States. Using transgene-encoded mutant proteins demands that the inoculated mice develop detectable neurodegeneration well before spontaneous disease in uninoculated controls is evident.
Implications of Prion Etiologies for Patient Care
The discovery that prions are not confined to a small group of disorders in which PrPSc prions accumulate has important implications for the development of informative molecular diagnostics and effective therapeutics. Early diagnosis will likely require molecular reporters, such as PET ligands, to identify prions long before symptoms appear. Such PET ligands would need to distinguish the normal protein precursors from the prion forms whether the proteins are PrP, Aβ, tau, or α-synuclein. Although there is considerable interest in using the levels of Aβ and tau in cerebrospinal fluid for the diagnosis of AD, changes in the levels of these proteins are generally small and may prove problematic in monitoring responses to therapeutic interventions. Additionally, meaningful treatments are likely to require cocktails of drugs that diminish the precursor protein, interfere with the conversion of precursors into prions, and/or enhance the clearance of prions.
Some investigators have expressed concern that the transmissibility of prions will result in patients with AD receiving less attention than they need, particularly those who are incontinent and those who have trouble handling their own saliva (67). Although a recent study argues that there is no evidence for the person-to-person transmission of Aβ, tau, or α-synuclein prions (85), it may prove wise to institute some minimal precautions in care of AD and PD patients that resemble in some ways those followed in the care of patients with CJD and HIV until definitive investigations can be performed. The history of CJD and AIDS is full of regrets where earlier vigilance might have prevented many deaths. It would be a mistake to deny a prion etiology for these common illnesses under the guise of facilitating the care of patients.
Equally important is the issue of prion contamination of biologics prepared from patients with AD, PD, and the tauopathies. The contamination of cadaveric human growth hormone preparations with CJD prions is a sad chapter in the development of therapeutics for children with short stature (124). Four people with variant (v) CJD, contracted from eating tainted beef prepared from “mad” cows, unknowingly transmitted vCJD prions to recipients of blood transfusions (70, 72, 137). Dura mater grafts and cornea transplants have also transmitted CJD prions (166). Inadequately sterilized surgical instruments and implanted electrodes have transmitted sporadic (s) CJD prions (7). PrPSc prions have been shown to bind tightly to stainless steel wires and retain infectivity (50, 204). Aβ prions also bind to steel wires and induce Aβ deposition in the brains of Tg mice (34).
The risk of transmitting Aβ, tau, α-synuclein, or even SOD1 prions through biologics, organ grafts, or inadequately sterilized surgical instruments has not been well studied because the transmissibility of these proteins has only been recently appreciated and the bioassays are not well developed (74). Endpoint titrations, incubation times, and bioluminescence imaging have been used to measure PrP prion titers in fractions prepared from prion-infected mammals (37, 82, 147, 179). Overexpression of wild-type PrP transgenes has generally been found to decrease the incubation times as measured from inoculation to a sustained increase in the BLI signals or to the onset of progressive neurological dysfunction (15, 151, 180, 195), although there are exceptions (49). Purified recombinant tau and α-synuclein have been induced to aggregate in vitro and used to initiate prion replication in cultured cells, but such systems have not yet been used to measure prion titers. Although Tg mice have been used to detect the presence of Aβ, tau, and α-synuclein prions in mouse and human brains, quantitative assays need to be developed. Creating such measurement methods is important in assessing the risk of biologics and organ transplants prepared from patients with neurodegeneration as well as in determining the number of such prions that may contaminate surgical instruments and prosthetics, including chronically implanted electrodes. Of particular concern may be the increasing number of patients with PD, who are having neurosurgical procedures for implantation of deep brain stimulation electrodes (132). It remains uncertain whether more stringent guidelines for such procedures need to be established.
Acknowledgments
The author acknowledges support from NIH grants AG021601, AG002132, AG010770; the Sherman Fairchild Foundation, and the Rainwater Charitable Foundation. The author also thanks Joel Watts and David Ramsay for their helpful comments on the manuscript.
Acronyms
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- APP
amyloid precursor protein
- CJD
Creutzfeldt-Jakob disease
- CTE
chronic traumatic encephalopathy
- CWD
chronic wasting disease
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- GSS
Gerstmann–Sträussler–Scheinker disease
- HD
Huntington’s disease
- ND
neurodegenerative disease
- NFT
neurofibrillary tangle
- PD
Parkinson’s disease
- Prions
proteins that acquire alternative conformations that become self-propagating
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Alper T, Haig DA, Clarke MC. The exceptionally small size of the scrapie agent. Biochem Biophys Res Commun. 1966;22:278–84. doi: 10.1016/0006-291x(66)90478-5. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer A. Ueber eine eigenartige Erkrankung der Hirnrinde. Cent Nervenheilk Psychiat. 1907;30:177–79. [Google Scholar]
- 3.Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Induction of β(A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Mol Neurobiol. 1994;8:25–39. doi: 10.1007/BF02778005. [DOI] [PubMed] [Google Scholar]
- 4.Baskakov IV, Legname G, Baldwin MA, Prusiner SB, Cohen FE. Pathway complexity of prion protein assembly into amyloid. J Biol Chem. 2002;277:21140–48. doi: 10.1074/jbc.M111402200. [DOI] [PubMed] [Google Scholar]
- 5.Bellinger-Kawahara CG, Kempner E, Groth DF, Gabizon R, Prusiner SB. Scrapie prion liposomes and rods exhibit target sizes of 55,000 Da. Virology. 1988;164:537–41. doi: 10.1016/0042-6822(88)90569-7. [DOI] [PubMed] [Google Scholar]
- 6.Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–57. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 7.Bernoulli C, Siegfried J, Baumgartner G, Regli F, Rabinowicz T, et al. Danger of accidental person-to-person transmission of Creutzfeldt-Jakob disease by surgery. Lancet. 1977;309:478–79. doi: 10.1016/s0140-6736(77)91958-4. [DOI] [PubMed] [Google Scholar]
- 8.Bithell A, Johnson R, Buckley NJ. Transcriptional dysregulation of coding and non-coding genes in cellular models of Huntington’s disease. Biochem Soc Trans. 2009;37:1270–75. doi: 10.1042/BST0371270. [DOI] [PubMed] [Google Scholar]
- 9.Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–11. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Braak E. Staging of Alzheimer’s disease–related neurofibrillary changes. Neurobiol Aging. 1995;16:271–84. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J. Pattern of brain destruction in Parkinson’s and Alzheimer’s diseases. J Neural Transm. 1996;103:455–90. doi: 10.1007/BF01276421. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Del Tredici K. Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 2011;121:589–95. doi: 10.1007/s00401-011-0825-z. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–36. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 14.Brion J-P, Passareiro H, Nunez J, Flament-Durand J. Mise en évidence immunologique de la protéine tau au niveau des lésions de dégénérescence neurofibrillaire de la maladie d’Alzheimer. Arch Biol. 1985;95:229–35. [Google Scholar]
- 15.Browning SR, Mason GL, Seward T, Green M, Eliason GA, et al. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol. 2004;78:13345–50. doi: 10.1128/JVI.78.23.13345-13350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruce ME, Dickinson AG. Genetic control of amyloid plaque production and incubation period in scrapie-infected mice. J Neuropathol Exp Neurol. 1985;44:285–94. doi: 10.1097/00005072-198505000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman J, Ben-Israel J, Goldhammer Y, Korczyn AD. The risk of developing Creutzfeldt-Jakob disease in subjects with the PRNP gene codon 200 point mutation. Neurology. 1994;44:1683–86. doi: 10.1212/wnl.44.9.1683. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y-C, Prescott CA, Walsh D, Patterson DG, Riley BP, et al. Different phenotypic and genotypic presentations in alcohol dependence: age at onset matters. J Stud Alcohol Drugs. 2011;72:752–62. doi: 10.15288/jsad.2011.72.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chien P, Weissman JS, DePace AH. Emerging principles of conformation-based prion inheritance. Annu Rev Biochem. 2004;73:617–56. doi: 10.1146/annurev.biochem.72.121801.161837. [DOI] [PubMed] [Google Scholar]
- 21.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric Abeta, and frank neuronal loss. J Neurosci. 2013;33:6245–56. doi: 10.1523/JNEUROSCI.3672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colby DW, Giles K, Legname G, Wille H, Baskakov IV, et al. Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci USA. 2009;106:20417–22. doi: 10.1073/pnas.0910350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3:270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- 25.Corti O, Lesage S, Brice A. What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol Rev. 2011;91:1161–218. doi: 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- 26.Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, et al. Systemic mitochondrial dysfunction and the etiology of Alzheimer’s disease and Down syndrome dementia. J Alzheimers Dis. 2010;20(Suppl 2):S293–310. doi: 10.3233/JAD-2010-100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croes EA, Theuns J, Houwing-Duistermaat JJ, Dermaut B, Sleegers K, et al. Octapeptide repeat insertions in the prion protein gene and early onset dementia. J Neurol Neurosurg Psychiatry. 2004;75:1166–70. doi: 10.1136/jnnp.2003.020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–97. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeMattos RB, Lu J, Tang Y, Racke MM, DeLong CA, et al. A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer’s disease mice. Neuron. 2012;76:908–20. doi: 10.1016/j.neuron.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Deutschbauer AM, Jaramillo DF, Proctor M, Kumm J, Hillenmeyer ME, et al. Mechanisms of haploinsufficiency revealed by genome-wide profiling in yeast. Genetics. 2005;169:1915–25. doi: 10.1534/genetics.104.036871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Divry P. Etude histochimique des plaques seniles. J Belge Neurol Psychiatr. 1927;27:643–54. [Google Scholar]
- 32.Dlouhy SR, Hsiao K, Farlow MR, Foroud T, Conneally PM, et al. Linkage of the Indiana kindred of Gerstmann-Sträussler-Scheinker disease to the prion protein gene. Nat Genet. 1992;1:64–67. doi: 10.1038/ng0492-64. [DOI] [PubMed] [Google Scholar]
- 33.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–90. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 34.Eisele YS, Bolmont T, Heikenwalder M, Langer F, Jacobson LH, et al. Induction of cerebral β-amyloidosis: intracerebral versus systemic Aβ inoculation. Proc Natl Acad Sci USA. 2009;106:12926–31. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, et al. Peripherally applied Aβ-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–82. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eklund CM, Hadlow WJ, Kennedy RC. Some properties of the scrapie agent and its behavior in mice. Proc Soc Exp Biol Med. 1963;112:974–79. [Google Scholar]
- 38.Fraser H. Neuronal spread of scrapie agent and targeting of lesions within the retino-tectal pathway. Nature. 1982;295:149–50. doi: 10.1038/295149a0. [DOI] [PubMed] [Google Scholar]
- 39.Fraser H, Dickinson AG. The sequential development of the brain lesions of scrapie in three strains of mice. J Comp Pathol. 1968;78:301–11. doi: 10.1016/0021-9975(68)90006-6. [DOI] [PubMed] [Google Scholar]
- 40.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–52. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabizon R, Rosenmann H, Meiner Z, Kahana I, Kahana E, et al. Mutation and polymorphism of the prion protein gene in Libyan Jews with Creutzfeldt-Jakob disease (CJD) Am J Hum Genet. 1993;53:828–35. [PMC free article] [PubMed] [Google Scholar]
- 42.Gajdusek DC. Unconventional viruses and the origin and disappearance of kuru. Science. 1977;197:943–60. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- 43.Gajdusek DC, Gibbs CJ, Jr, Alpers M. Experimental transmission of a kuru-like syndrome to chimpanzees. Nature. 1966;209:794–96. doi: 10.1038/209794a0. [DOI] [PubMed] [Google Scholar]
- 44.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–27. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 45.Ghaemmaghami S, Colby DW, Nguyen H-OB, Hayashi S, DeArmond SJ, Prusiner SB. Convergent replication of synthetic mouse prion strains. Am J Pathol. 2013;182(3):866–74. doi: 10.1016/j.ajpath.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghaemmaghami S, Watts JC, Nguyen H-O, Hayashi S, DeArmond SJ, Prusiner SB. Conformational transformation and selection of synthetic prion strains. J Mol Biol. 2011;413:527–42. doi: 10.1016/j.jmb.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron. 2002;34:521–33. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 48.Gibbs CJ, Jr, Gajdusek DC, Asher DM, Alpers MP, Beck E, et al. Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science. 1968;161:388–89. doi: 10.1126/science.161.3839.388. [DOI] [PubMed] [Google Scholar]
- 49.Giles K, De Nicola GF, Patel S, Glidden DV, Korth C, et al. Identifying I137M and other mutations that modulate incubation periods for two human prion strains. J Virol. 2012;86:6033–41. doi: 10.1128/JVI.07027-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giles K, Glidden DV, Beckwith R, Seoanes R, Peretz D, et al. Resistance of bovine spongiform encephalopathy (BSE) prions to inactivation. PLoS Pathog. 2008;4:e1000206. doi: 10.1371/journal.ppat.1000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, et al. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383–98. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glenner GG, Eanes ED, Bladen HA, Linke RP, Termine JD. Beta-pleated sheet fibrils: a comparison of native amyloid with synthetic protein fibrils. J Histochem Cytochem. 1974;22:1141–58. doi: 10.1177/22.12.1141. [DOI] [PubMed] [Google Scholar]
- 53.Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–35. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 54.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–90. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 55.Goate A, Chartier-Harlin M-C, Mullan M, Brown J, Crawford F, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–6. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 56.Godec MS, Asher DM, Masters CL, Kozachuk WE, Friedland RP, et al. Evidence against the transmissibility of Alzheimer’s disease. Neurology. 1991;41:1320. doi: 10.1212/wnl.41.8.1320. [DOI] [PubMed] [Google Scholar]
- 57.Goudsmit J, Morrow CH, Asher DM, Yanagihara RT, Masters CL, et al. Evidence for and against the transmissibility of Alzheimer’s disease. Neurology. 1980;30:945–50. doi: 10.1212/wnl.30.9.945. [DOI] [PubMed] [Google Scholar]
- 58.Grad LI, Guest WC, Yanai A, Pokrishevsky E, O’Neill MA, et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc Natl Acad Sci USA. 2011;108:16398–403. doi: 10.1073/pnas.1102645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenfield JG, Matthews WB. Post-encephalitic parkinsonism with amyotrophy. J Neurol Neurosurg Psychiatry. 1954;17:50–56. doi: 10.1136/jnnp.17.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greenwald J, Buhtz C, Ritter C, Kwiatkowski W, Choe S, et al. The mechanism of prion inhibition by HET-S. Mol Cell. 2010;38:889–99. doi: 10.1016/j.molcel.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grundke-Iqbal I, Iqbal K, Tung Y-C, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–17. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo JL, Lee VM-Y. Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. 2011;286:15317–31. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hadlow WJ. Scrapie and kuru. Lancet. 1959;274:289–90. [Google Scholar]
- 64.Hadlow WJ, Eklund CM, Kennedy RC, Jackson TA, Whitford HW, Boyle CC. Course of experimental scrapie virus infection in the goat. J Infect Dis. 1974;129:559–67. doi: 10.1093/infdis/129.5.559. [DOI] [PubMed] [Google Scholar]
- 65.Halfmann R, Jarosz DF, Jones SK, Chang A, Lancaster AK, Lindquist S. Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature. 2012;482:363–68. doi: 10.1038/nature10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hardy J, Cai H, Cookson MR, Gwinn-Hardy K, Singleton A. Genetics of Parkinson’s disease and parkinsonism. Ann Neurol. 2006;60:389–98. doi: 10.1002/ana.21022. [DOI] [PubMed] [Google Scholar]
- 67.Hardy J, Revesz T. The spread of neurodegenerative disease. N Engl J Med. 2012;366:2126–28. doi: 10.1056/NEJMcibr1202401. [DOI] [PubMed] [Google Scholar]
- 68.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–56. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 69.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–93. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Health Protection Agency. Variant CJD and plasma products. London: Public Health Engl; 2009. http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1195733818681?p=1225960597236. [Google Scholar]
- 71.Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7:583–90. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hewitt PE, Llewelyn CA, Mackenzie J, Will RG. Creutzfeldt-Jakob disease and blood transfusion: results of the UK Transfusion Medicine Epidemiological Review study. Vox Sang. 2006;91:221–30. doi: 10.1111/j.1423-0410.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- 73.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358:453–63. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 74.Holmes BB, Diamond MI. Amyotrophic lateral sclerosis and organ donation: Is there risk of disease transmission? Ann Neurol. 2012;72:832–36. doi: 10.1002/ana.23684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282:1914–17. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- 76.Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–61. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsiao K, Baker HF, Crow TJ, Poulter M, Owen F, et al. Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature. 1989;338:342–45. doi: 10.1038/338342a0. [DOI] [PubMed] [Google Scholar]
- 78.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 79.Hsiao K, Dlouhy S, Farlow MR, Cass C, Da Costa M, et al. Mutant prion proteins in Gerstmann-Sträussler-Scheinker disease with neurofibrillary tangles. Nat Genet. 1992;1:68–71. doi: 10.1038/ng0492-68. [DOI] [PubMed] [Google Scholar]
- 80.Hsiao KK, Groth D, Scott M, Yang S-L, Serban H, et al. Serial transmission in rodents of neurodegeneration from transgenic mice expressing mutant prion protein. Proc Natl Acad Sci USA. 1994;91:9126–30. doi: 10.1073/pnas.91.19.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsiao KK, Scott M, Foster D, Groth DF, DeArmond SJ, Prusiner SB. Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science. 1990;250:1587–90. doi: 10.1126/science.1980379. [DOI] [PubMed] [Google Scholar]
- 82.Hunter GD, Gibbons RA, Kimberlin RH, Millson GC. Further studies of the infectivity and stability of extracts and homogenates derived from scrapie affected mouse brains. J Comp Pathol. 1969;79:101–8. doi: 10.1016/0021-9975(69)90033-4. [DOI] [PubMed] [Google Scholar]
- 83.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 84.Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci. 2013;33:1024–37. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Irwin DJ, Abrams JY, Schonberger LB, Leschek EW, Mills JL, et al. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol. 2013;70:462–68. doi: 10.1001/jamaneurol.2013.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW. The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug. J Mol Biol. 2012;421:185–203. doi: 10.1016/j.jmb.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johri A, Beal MF. Antioxidants in Huntington’s disease. Biochim Biophys Acta. 2012;1822:664–74. doi: 10.1016/j.bbadis.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 89.Jubelt B. Post-polio syndrome. Curr Treat Options Neurol. 2004;6:87–93. doi: 10.1007/s11940-004-0018-3. [DOI] [PubMed] [Google Scholar]
- 90.Kaneko K, Ball HL, Wille H, Zhang H, Groth D, et al. A synthetic peptide initiates Gerstmann-Sträussler-Scheinker (GSS) disease in transgenic mice. J Mol Biol. 2000;295:997–1007. doi: 10.1006/jmbi.1999.3386. [DOI] [PubMed] [Google Scholar]
- 91.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of tau aggregation by fibrillar species. J Biol Chem. 2012;287:19440–51. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kimberlin RH, Walker CA. Pathogenesis of mouse scrapie: dynamics of agent replication in spleen, spinal cord and brain after infection by different routes. J Comp Pathol. 1979;89:551–62. doi: 10.1016/0021-9975(79)90046-x. [DOI] [PubMed] [Google Scholar]
- 93.King C-Y, Tittman P, Gross H, Gebert R, Aebi M, Wüthrich K. Prion-inducing domain 2–114 of yeast Sup35 protein transforms in vitro into amyloid-like filaments. Proc Natl Acad Sci USA. 1997;94:6618–22. doi: 10.1073/pnas.94.13.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klatzo I, Gajdusek DC, Zigas V. Pathology of kuru. Lab Invest. 1959;8:799–847. [PubMed] [Google Scholar]
- 95.Kong Q, Surewicz WK, Petersen RB, Zou W, Chen SG, et al. Inherited prion diseases. In: Prusiner SB, editor. Prion Biology and Diseases. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 2004. pp. 673–775. [Google Scholar]
- 96.Korczyn AD, Vakhapova V, Grinberg LT. Vascular dementia. J Neurol Sci. 2012;322:2–10. doi: 10.1016/j.jns.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–6. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 98.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA. 1986;83:4044–48. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar N, Boeve BF, Boot BP, Orr CF, Duffy J, et al. Clinical characterization of a kindred with a novel 12-octapeptide repeat insertion in the prion protein gene. Arch Neurol. 2011;68:1165–70. doi: 10.1001/archneurol.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Larsson N-G. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem. 2010;79:683–706. doi: 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- 101.Lee JM, Ramos EM, Lee JH, Gillis T, Mysore JS, et al. CAG repeat expansion in Huntington disease determines age at onset in a fully dominant fashion. Neurology. 2012;78:690–95. doi: 10.1212/WNL.0b013e318249f683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Legname G, Baskakov IV, Nguyen H-OB, Riesner D, Cohen FE, et al. Synthetic mammalian prions. Science. 2004;305:673–76. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 103.Legname G, Nguyen H-OB, Baskakov IV, Cohen FE, DeArmond SJ, Prusiner SB. Strain-specified characteristics of mouse synthetic prions. Proc Natl Acad Sci USA. 2005;102:2168–73. doi: 10.1073/pnas.0409079102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Legname G, Nguyen H-OB, Peretz D, Cohen FE, DeArmond SJ, Prusiner SB. Continuum of prion protein structures enciphers a multitude of prion isolate–specified phenotypes. Proc Natl Acad Sci USA. 2006;103:19105–10. doi: 10.1073/pnas.0608970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C. Darwinian evolution of prions in cell culture. Science. 2010;327:869–72. doi: 10.1126/science.1183218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li JY, Englund E, Holton JL, Soulet D, Hagell P, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–3. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 107.Li M, Wang IX, Li Y, Bruzel A, Richards AL, et al. Widespread RNA and DNA sequence differences in the human transcriptome. Science. 2011;333:53–58. doi: 10.1126/science.1207018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu L, Drouet V, Wu JW, Witter MP, Small SA, et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–53. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012;209:975–86. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Macario AJL, Conway de Macario E. Sick chaperones, cellular stress, and disease. N Engl J Med. 2005;353:1489–501. doi: 10.1056/NEJMra050111. [DOI] [PubMed] [Google Scholar]
- 112.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5644–51. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–32. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Makarava N, Kovacs GG, Bocharova O, Savtchenko R, Alexeeva I, et al. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol. 2010;119:177–87. doi: 10.1007/s00401-009-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Masters CL, Gajdusek DC, Gibbs CJ., Jr Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome. Brain. 1981;104:559–88. doi: 10.1093/brain/104.3.559. [DOI] [PubMed] [Google Scholar]
- 116.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–49. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, et al. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136:1128–38. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McKinley MP, Bolton DC, Prusiner SB. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983;35:57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- 120.McKinley MP, Braunfeld MB, Bellinger CG, Prusiner SB. Molecular characteristics of prion rods purified from scrapie-infected hamster brains. J Infect Dis. 1986;154:110–20. doi: 10.1093/infdis/154.1.110. [DOI] [PubMed] [Google Scholar]
- 121.Mead S. Prion disease genetics. Eur J Hum Genet. 2006;14:273–81. doi: 10.1038/sj.ejhg.5201544. [DOI] [PubMed] [Google Scholar]
- 122.Medori R, Tritschler H-J, LeBlanc A, Villare F, Manetto V, et al. Fatal familial insomnia, a prion disease with a mutation at codon 178 of the prion protein gene. N Engl J Med. 1992;326:444–49. doi: 10.1056/NEJM199202133260704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, et al. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–84. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 124.Mills JL, Schonberger LB, Wysowski DK, Brown P, Durako SJ, et al. Long-term mortality in the United States cohort of pituitary-derived growth hormone recipients. J Pediatr. 2004;144:430–36. doi: 10.1016/j.jpeds.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 125.Morais VA, De Strooper B. Mitochondria dysfunction and neurodegenerative disorders: cause or consequence. J Alzheimers Dis. 2010;20:S255–63. doi: 10.3233/JAD-2010-100345. [DOI] [PubMed] [Google Scholar]
- 126.Morales R, Duran-Aniotz C, Castilla J, Estrada LD, Soto C. De novo induction of amyloid-β deposition in vivo. Mol Psychiatry. 2012;17:1347–53. doi: 10.1038/mp.2011.120. [DOI] [PubMed] [Google Scholar]
- 127.Mori K, Weng SM, Arzberger T, May S, Rentzsch K, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339:1335–38. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- 128.Mougenot A-L, Nicot S, Bencsik A, Morignat E, Verchère J, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging. 2012;33:2225–28. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 129.Münch C, O’Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci USA. 2011;108:3548–53. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nazor KE, Kuhn F, Seward T, Green M, Zwald D, et al. Immunodetection of disease-associated mutant PrP, which accelerates disease in GSS transgenic mice. EMBO J. 2005;24:2472–80. doi: 10.1038/sj.emboj.7600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 132.Okun MS. Deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2012;367:1529–38. doi: 10.1056/NEJMct1208070. [DOI] [PubMed] [Google Scholar]
- 133.Olanow CW, McNaught KS. Ubiquitin-proteasome system and Parkinson’s disease. Mov Disord. 2006;21:1806–23. doi: 10.1002/mds.21013. [DOI] [PubMed] [Google Scholar]
- 134.Olanow CW, Prusiner SB. Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci USA. 2009;106:12571–72. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Omalu B, Hammers JL, Bailes J, Hamilton RL, Kamboh MI, et al. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg Focus. 2011;31:E3. doi: 10.3171/2011.9.FOCUS11178. [DOI] [PubMed] [Google Scholar]
- 136.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128–34. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- 137.Peden A, McCardle L, Head MW, Love S, Ward HJ, et al. Variant CJD infection in the spleen of a neurologically asymptomatic UK adult patient with haemophilia. Haemophilia. 2010;16:296–304. doi: 10.1111/j.1365-2516.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 138.Piro JR, Wang F, Walsh DJ, Rees JR, Ma J, Supattapone S. Seeding specificity and ultrastructural characteristics of infectious recombinant prions. Biochemistry. 2011;50:7111–16. doi: 10.1021/bi200786p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Polymenidou M, Cleveland DW. Prion-like spread of protein aggregates in neurodegeneration. J Exp Med. 2012;209:889–93. doi: 10.1084/jem.20120741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–47. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 141.Poulter M, Baker HF, Frith CD, Leach M, Lofthouse R, et al. Inherited prion disease with 144 base pair gene insertion. 1 Genealogical and molecular studies. Brain. 1992;115:675–85. doi: 10.1093/brain/115.3.675. [DOI] [PubMed] [Google Scholar]
- 142.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–44. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 143.Prusiner SB. Some speculations about prions, amyloid, and Alzheimer’s disease. N Engl J Med. 1984;310:661–63. doi: 10.1056/NEJM198403083101021. [DOI] [PubMed] [Google Scholar]
- 144.Prusiner SB. Scrapie prions. Annu Rev Microbiol. 1989;43:345–74. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- 145.Prusiner SB. Shattuck lecture: neurodegenerative diseases and prions. N Engl J Med. 2001;344:1516–26. doi: 10.1056/NEJM200105173442006. [DOI] [PubMed] [Google Scholar]
- 146.Prusiner SB. A unifying role for prions in neurodegenerative diseases. Science. 2012;336:1511–13. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Prusiner SB, Cochran SP, Groth DF, Downey DE, Bowman KA, Martinez HM. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982;11:353–58. doi: 10.1002/ana.410110406. [DOI] [PubMed] [Google Scholar]
- 148.Prusiner SB, Groth DF, Cochran SP, Masiarz FR, McKinley MP, Martinez HM. Molecular properties, partial purification, and assay by incubation period measurements of the hamster scrapie agent. Biochemistry. 1980;21:4883–91. doi: 10.1021/bi00562a028. [DOI] [PubMed] [Google Scholar]
- 149.Prusiner SB, Hadlow WJ, Garfin DE, Cochran SP, Baringer JR, et al. Partial purification and evidence for multiple molecular forms of the scrapie agent. Biochemistry. 1978;17:4993–97. doi: 10.1021/bi00616a021. [DOI] [PubMed] [Google Scholar]
- 150.Prusiner SB, McKinley MP, Bowman KA, Bolton DC, Bendheim PE, et al. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983;35:349–58. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- 151.Prusiner SB, Scott M, Foster D, Pan K-M, Groth D, et al. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990;63:673–86. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- 152.Rabinovici GD, Miller BL. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24:375–98. doi: 10.2165/11533100-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rademakers R, Baker M, Gass J, Adamson J, Huey ED, et al. Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C–>T (Arg493X) mutation: an international initiative. Lancet Neurol. 2007;6:857–68. doi: 10.1016/S1474-4422(07)70221-1. [DOI] [PubMed] [Google Scholar]
- 154.Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8:423–34. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology. 2009;73:805–11. doi: 10.1212/WNL.0b013e3181b6bbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–25. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ridley RM, Baker HF, Windle CP, Cummings RM. Very long term studies of the seeding of β-amyloidosis in primates. J Neural Transm. 2006;113:1243–51. doi: 10.1007/s00702-005-0385-2. [DOI] [PubMed] [Google Scholar]
- 158.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, et al. Reducing endogenous tau ameliorates amyloid β–induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–54. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 159.Rogers DR. Screening for amyloid with the thioflavin-t fluorescent method. Am J Clin Pathol. 1965;44:59–61. doi: 10.1093/ajcp/44.1.59. [DOI] [PubMed] [Google Scholar]
- 160.Roos R, Gajdusek DC, Gibbs CJ., Jr The clinical characteristics of transmissible Creutzfeldt-Jakob disease. Brain. 1973;96:1–20. doi: 10.1093/brain/96.1.1. [DOI] [PubMed] [Google Scholar]
- 161.Rosen RF, Fritz JJ, Dooyema J, Cintron AF, Hamaguchi T, et al. Exogenous seeding of cerebral β-amyloid deposition in βAPP-transgenic rats. J Neurochem. 2012;120:660–66. doi: 10.1111/j.1471-4159.2011.07551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Safar JG, Kellings K, Serban A, Groth D, Cleaver JE, et al. Search for a prion-specific nucleic acid. J Virol. 2005;79:10796–806. doi: 10.1128/JVI.79.16.10796-10806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schellenberg GD, Bird TD, Wijsman EM, Orr HT, Anderson L, et al. Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science. 1992;258:668–71. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- 164.Schellenberg GD, Montine TJ. The genetics and neuropathology of Alzheimer’s disease. Acta Neuropathol. 2012;124:305–23. doi: 10.1007/s00401-012-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Scott JR, Davies D, Fraser H. Scrapie in the central nervous system: neuroanatomical spread of infection and Sinc control of pathogenesis. J Gen Virol. 1992;73:1637–44. doi: 10.1099/0022-1317-73-7-1637. [DOI] [PubMed] [Google Scholar]
- 166.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Selkoe DJ. Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med. 2011;17:1060–65. doi: 10.1038/nm.2460. [DOI] [PubMed] [Google Scholar]
- 168.Shimizu S, Hoshi K, Muramoto T, Homma M, Ironside JW, et al. Creutzfeldt-Jakob disease with florid-type plaques after cadaveric dura mater grafting. Arch Neurol. 1999;56:357–62. doi: 10.1001/archneur.56.3.357. [DOI] [PubMed] [Google Scholar]
- 169.Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell. 2010;140:421–35. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 170.Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003;115:879–91. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- 171.Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, et al. The most infectious prion protein particles. Nature. 2005;437:257–61. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sparrer HE, Santoso A, Szoka FC, Jr, Weissman JS. Evidence for the prion hypothesis: induction of the yeast [PSI+] factor by in vitro–converted Sup35 protein. Science. 2000;289:595–99. doi: 10.1126/science.289.5479.595. [DOI] [PubMed] [Google Scholar]
- 173.Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 174.Spudich S, Mastrianni JA, Wrensch M, Gabizon R, Meiner Z, et al. Complete penetrance of Creutzfeldt-Jakob disease in Libyan Jews carrying the E200K mutation in the prion protein gene. Mol Med. 1995;1:607–13. [PMC free article] [PubMed] [Google Scholar]
- 175.St George-Hyslop P, Haines J, Rogaev E, Mortilla M, Vaula G, et al. Genetic evidence for a novel familial Alzheimer’s disease locus on chromosome 14. Nat Genet. 1992;2:330–34. doi: 10.1038/ng1292-330. [DOI] [PubMed] [Google Scholar]
- 176.StGeorge-Hyslop PH. Molecular genetics of Alzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. 2 Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 311–26. [Google Scholar]
- 177.Stevens DJ, Walter ED, Rodriguez A, Draper D, Davies P, et al. Early onset prion disease from octarepeat expansion correlates with copper binding properties. PLoS Pathog. 2009;5:e1000390. doi: 10.1371/journal.ppat.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stöhr J, Watts JC, Mensinger ZL, Oehler A, Grillo SK, et al. Purified and synthetic Alzheimer’s amyloid beta (Aβ) prions. Proc Natl Acad Sci USA. 2012;109:11025–30. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tamgüney G, Francis KP, Giles K, Lemus A, DeArmond SJ, Prusiner SB. Measuring prions by bioluminescence imaging. Proc Natl Acad Sci USA. 2009;106:15002–6. doi: 10.1073/pnas.0907339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tamgüney G, Giles K, Bouzamondo-Bernstein E, Bosque PJ, Miller MW, et al. Transmission of elk and deer prions to transgenic mice. J Virol. 2006;80:9104–14. doi: 10.1128/JVI.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Taraboulos A, Jendroska K, Serban D, Yang S-L, DeArmond SJ, Prusiner SB. Regional mapping of prion proteins in brains. Proc Natl Acad Sci USA. 1992;89:7620–24. doi: 10.1073/pnas.89.16.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tatzelt J, Groth DF, Torchia M, Prusiner SB, DeArmond SJ. Kinetics of prion protein accumulation in the CNS of mice with experimental scrapie. J Neuropathol Exp Neurol. 1999;58:1244–49. doi: 10.1097/00005072-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 183.The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 184.Toyama BH, Kelly MJ, Gross JD, Weissman JS. The structural basis of yeast prion strain variants. Nature. 2007;449:233–37. doi: 10.1038/nature06108. [DOI] [PubMed] [Google Scholar]
- 185.Toyama BH, Weissman JS. Amyloid structure: conformational diversity and consequences. Annu Rev Biochem. 2011;80:557–85. doi: 10.1146/annurev-biochem-090908-120656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–28. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tremblay P, Ball HL, Kaneko K, Groth D, Hegde RS, et al. Mutant PrPSc conformers induced by a synthetic peptide and several prion strains. J Virol. 2004;78:2088–99. doi: 10.1128/JVI.78.4.2088-2099.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Udan M, Baloh RH. Implications of the prion-related Q/N domains in TDP-43 and FUS. Prion. 2011;5:1–5. doi: 10.4161/pri.5.1.14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Valentine JS, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–93. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 190.van der Kamp MW, Daggett V. The consequences of pathogenic mutations to the human prion protein. Protein Eng Des Sel. 2009;22:461–68. doi: 10.1093/protein/gzp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Walsh DM, Selkoe DJ. Aβ oligomers: a decade of discovery. J Neurochem. 2007;101:1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 193.Wang F, Wang X, Yuan C-G, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–35. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Watts JC, Giles K, Grillo SK, Lemus A, DeArmond SJ, Prusiner SB. Bioluminescence imaging of Aβ deposition in bigenic mouse models of Alzheimer’s disease. Proc Natl Acad Sci USA. 2011;108:2528–33. doi: 10.1073/pnas.1019034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Watts JC, Giles K, Stöhr J, Oehler A, Bhardwaj S, et al. Spontaneous generation of rapidly transmissible prions in transgenic mice expressing wild-type bank vole prion protein. Proc Natl Acad Sci USA. 2012;109:3498–503. doi: 10.1073/pnas.1121556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wheeler VC, Gutekunst CA, Vrbanac V, Lebel LA, Schilling G, et al. Early phenotypes that presage late-onset neurodegenerative disease allow testing of modifiers in Hdh CAG knock-in mice. Hum Mol Genet. 2002;11:633–40. doi: 10.1093/hmg/11.6.633. [DOI] [PubMed] [Google Scholar]
- 197.Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–69. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 198.Wickner RB, Edskes HK, Shewmaker F, Nakayashiki T. Prions of fungi: inherited structures and biological roles. Nat Rev Microbiol. 2007;5:611–18. doi: 10.1038/nrmicro1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wille H, Bian W, McDonald M, Kendall A, Colby DW, et al. Natural and synthetic prion structure from x-ray fiber diffraction. Proc Natl Acad Sci USA. 2009;106:16990–95. doi: 10.1073/pnas.0909006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wood JG, Mirra SS, Pollock NJ, Binder II. Neurofibrillary tangles of Alzheimer’s disease share antigenic determinants with the axonal microtubule-associated protein tau. Proc Natl Acad Sci USA. 1986;83:4040–43. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–9. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 202.Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73:1216–27. doi: 10.1016/j.neuron.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zilber N, Rannon L, Alter M, Kahana E. Measles, measles vaccination, and risk of subacute sclerosing panencephalitis (SSPE) Neurology. 1983;33:1558–64. [PubMed] [Google Scholar]
- 204.Zobeley E, Flechsig E, Cozzio A, Enari M, Weissmann C. Infectivity of scrapie prions bound to a stainless steel surface. Mol Med. 1999;5:240–43. [PMC free article] [PubMed] [Google Scholar]