Abstract
The microtubule-associated protein (MAP) tau has been implicated in the pathology of numerous neurodegenerative diseases. In the past decade, the hyperphosphorylated and aggregated states of tau protein have been important targets in the drug discovery field for the potential treatment of Alzheimer’s disease. Although several compounds have been reported to reduce the hyperphosphorylated state of tau or impact the stabilization of tau, their therapeutic activities are still to be validated. Recently, reduction of total cellular tau protein has emerged as an alternate intervention point for drug development and a potential treatment of tauopathies. We have developed and optimized a homogenous assay, using the AlphaLISA and HTRF assay technologies, for the quantification of total cellular tau protein levels in the SH-SY5Y neuroblastoma cell line. The signal-to-basal ratios were 375 and 5.3, and the Z’ factors were 0.67 and 0.60 for the AlphaLISA and HTRF tau assays, respectively. The clear advantages of this homogeneous tau assay over conventional total tau assays, such as ELISA and Western blot, are the elimination of plate wash steps and miniaturization of the assay into 1536-well plate format for the ultra–high-throughput screening of large compound libraries.
Keywords: Alzheimer’s disease, FRET-based assay, high throughput screening assay, prions, neurodegenerative diseases, Tau protein
INTRODUCTION
Abnormalities associated with the tau protein, such as hyperphosphorylation and formation of aggregates (referred to as neurofibrillary tangles or NFTs), have been implicated in a variety of neuronal diseases including but not limited to Alzheimer’s disease (AD), frontotemporal dementias (FTDs), traumatic brain injury (TBI), chronic traumatic encephalopathy (CTE), progressive supranuclear palsy (PSP) and Niemann-Pick type C (NPC) (1, 2). Tau is a cytosolic protein that binds to microtubules in the cell and is mainly expressed in neuronal cells. Tau is generally considered to be an intrinsically disordered protein, which may augment its potential to aggregate under pathological conditions. Under normal conditions, tau is modestly phosphorylated at serine and threonine residues in the brain. In contrast, tau is frequently hyperphosphorylated under pathological conditions (1, 3). The deposition of hyperphosphorylated tau aggregates in the form of NFTs in the brain is a common and frequent response to brain injury (4). Much evidence suggests that aggregation of the tau protein in the brain is not simply a marker of the disease, but actually causes neuronal dysfunction (5).
The role of the tau protein in the pathogenesis of AD was resolved when mutations in the tau gene were found to cause heritable tauopathies including familial FTD, inherited PSP, and Pick’s disease, but not familial AD (6-9). Aggregates formed from truncated recombinant human tau composed of residues 242–364 were shown to enter C17 cells and seed the polymerization of endogenous tau (10). These important studies were extended using HEK293 cells expressing full-length, wild-type (wt) human tau(2N4R) as well as truncated and mutant human tau(P301S) (11). The transmission of mutant tau(P301S) prions produced in transgenic mice to recipient mice expressing wild type human tau was also demonstrated (12). After approximately 6 months, the inoculated transgenic mice showed wild type tau aggregates that had spread from the site of inoculation to neighboring regions. This transmission argues that tau can form prions, or infectious protein states. Like the Aβ amyloid plaques of AD, NFTs also spread along neuroanatomical pathways (13). Presumably, the tau prions spread transynaptically as they move from one neuron to another. Recent transgenic mouse models expressing human tau in the entorhinal cortex show spread along neuroanatomically defined pathways to hippocampal pyramidal neurons especially in CA1 and dentate gyrus granule cells (14-16).
In the past decade, the focal point of drug discovery efforts for tau has been the alleviation of the aggregated and hyperphosphorylated states of the tau protein through the stabilization of tau and inhibition of kinases, respectively (Table 1). While the kinase inhibitor approach has been challenging due to the large number of kinases involved in tau phosphorylation, numerous compounds, such as thiazine red R, Evans blue, crystal violet, adriamycin, and aminothienopyridazines, have been reported to inhibit tau protein aggregation. Valproic acid has been reported to reduce levels of tau phosphorylation and has been tested in clinical trials, although no data is available at this time (Clinicaltrials.gov). To our knowledge, few, if any, compounds have resulted in promising clinical efficacy, thus new drug discovery targets for the treatment of tau-related diseases are needed. Based on the observation that elimination of tau protein expression is well tolerated in tau-knockout mice (17), reduction of total tau protein levels in neuronal cells by small molecule inhibitors renders a promising drug development target. Our hypothesis is that the reduction of total tau protein levels in a patient’s brain is likely to reverse and/or arrest the formation of neurotoxic tau aggregates.
Table 1.
Assays and compounds related to drug discovery on tau proteins.
| MOA | Target | Assay | Representative Compounds |
Reference |
|---|---|---|---|---|
| Tau aggregation and stabilizers |
Tau protein | Fluorescence, FP | Thiazine red R, Evans blue, Crystal violet, Adriamycin, Aminothienopyridazines |
Pickhardt et al., 2005 (29) Bulic et al., 2010 (30) Honson et al, 2007 (31) Crowe et al, 2009 (32) |
| Tau hyperphosphorylation |
Kinases | Transgenic mice [γ-33P]-ATP |
APS, CTIU | Liu et al., 2002 (33) Liu et al, 2008 (34) |
| Tau splicing | Exon 10 of MAPT* gene |
Fluorescence | Digoxin, Tyrphostin, 5- Iodotubercidin |
Stoilov et al, 2008 (35) |
| Tau ubiquitination and degradation |
HSP70 and HSP90 |
Malachite green, westerns |
Chaperonin stabilizers (inhibitors) |
Dickey et al, 2005 (36) Jinwal et al, 2009 (24) |
MAPT (microtubule-associated protein tau)
Discovering compounds that specifically lower total tau protein expression levels is a challenge. Because tau protein is natively unstructured, using computational approaches for the rational design of molecules with target specificity to tau would be nearly impossible. Thus, we have focused on establishing an assay for the screening of large compound libraries, with the goal of finding inhibitors capable of reducing total cellular tau protein levels. We envision at least three possible mechanisms of action for tau-lowering compounds. One is the destabilization of tau, leading to increased clearance via the proteasome through an ubiquitination-independent pathway (18). The second is explicit inhibition of tau gene (denoted MAPT) transcription via binding of a compound to an enhancer/promoter region of the gene. This approach may be challenging because compounds that are general inhibitors of transcription or translation are unlikely to be good drug candidates since numerous other proteins are probably simultaneously affected. The third mechanism for reducing tau expression is through perturbation of tau synthesis and/or degradation pathways. Reports have shown that tau protein has a long half-life in cells, indicating that its expression and turnover are tightly coordinated (18, 19). Compounds that disrupt this regulation (such as activating a protease that clears tau) may lower tau levels in neurons.
We report here a homogenous compound screening assay for specifically monitoring total cellular tau protein expression levels. This assay has several advantages over traditional ELISA and Western blot assay formats. A homogenous assay format eliminates the need for plate-washing steps, making it amenable to miniaturization into 1536-well plate format for the ultra–high-throughput screening (HTS) of large compound libraries. In addition, the homogenous tau protein assay is compatible with both the amplified luminescence proximity homogenous assay (AlphaLISA) and homogenous time-resolved fluorescence energy transfer (HTRF) detection technologies; two highly sensitive and robust HTS assay methods that are reproducible and quantitative for the accurate measurement of compound potencies. Since AlphaLISA and HTRF methods use two different detection mechanisms, chemiluminescence and time-resolved fluorescence resonance energy transfer (TR-FRET), respectively, they can be used in parallel or as counter-screens for one another for eliminating detection-related artifacts.
MATERIALS AND METHODS
Reagents
The HTRF Tau Assay kit (Catalog No. 63IDC000) was obtained from Cisbio Bioassays US (Bedford, MA). The AlphaLISA Tau kit (Catalog No. AL271C) was obtained from PerkinElmer Health Sciences, Inc. (Shelton, CT). All tissue culture flasks were obtained from Corning (Lowell, MA). Polypropylene compound plates (Catalog No.789173-F) and white sterile tissue culture–treated polystyrene plates (Catalog No.781073 and 789270-C) were purchased from Greiner Bio-One (Monroe, NC).
Cell line and cell culture
The SH-SY5Y neuroblastoma cell line (Catalog No. CRL-2266) was obtained from American Type Culture Collection (ATCC; Manassas, VA). The cell culture medium was composed of a 1:1 mixture of Eagle’s Minimum Essential Medium and F-12K Medium (Catalog No. 30-2003 and 30-2004, respectively) purchased from ATCC supplemented with 10% FBS (Catalog No. SH-30071.03, Thermo Scientific, Logan, UT). Chinese hamster ovary (CHO) cells (Catalog No. CCL-61) were obtained from ATCC. CHO-K1 cell culture media was composed of F-12K media, supplemented with 10% fetal bovine serum (FBS). Human skin fibroblast line (Catalog No. GM05659) as obtained from Coriell Cell Repository (Camden, NJ) and cultured in DMEM medium (catalog # 11995-040, Invitrogen) supplemented with 10% FBS, 100 unit/ml penicillin and 100 μg/ml streptomycin in a humidified incubator with 5% CO2 at 37 °C. The SH-SY5Y cells were suspended into 20 ml of SH-SY5Y cell culture media, plated in T-75 cm2 flasks, and grown to confluence. Cells were then cultured and passaged in T-225 flasks every 7–10 days. Media was changed daily, as required for the robust culturing of the SH-SY5Y cell line. CHOK1 cells and skin fibroblasts were maintained according to protocols provided by their respective vendors.
Cytotoxicity assay
Cells were plated at a seeding density of 5,000 cells per well in 1536-well assay plates using a Multidrop Combi dispenser (Thermo Scientific, Logan, UT) and incubated for 4 h at 37 °C with 5% CO2. Compound libraries were transferred in a volume of 23 nl per well using a NX-TR pin tool (WAKO, San Diego, CA), and incubated for an additional 72 h at 37 °C with 5% CO2. The ATP content reagent (ATPlite, PerkinElmer) was added at 5 μl/well using a BioRAPTR dispenser (Beckman Coulter, Brea CA), and the plates were incubated at room temperature for 10 min. The assay plates were then detected in luminescence mode using a ViewLux plate reader (PerkinElmer).
HTRF total tau protein assay
All optimization experiments were performed in 1536-well plate format. SH-SY5Y, CHO, and skin fibroblasts were plated at a seeding density of 1,250, 2,500, and 5,000 cells in 4 μl per well using the Multidrop Combi dispenser. After incubation at 37 °C with 5% CO2 for 4 h, the compounds were added to the assay plates and incubated for an additional 3 days. A customized HTRF tau assay kit, modified from the original tau biomarker kit, was developed for the homogenous detection of total tau protein levels in cells, where we eliminated steps involving medium aspiration, cell washing, and transferring of cell lysate. Briefly, 1 μl/well of 4 × cell lysis buffer was added to the 1536-well assay plates after 3-days’ incubation with compounds, and plates were incubated for 10 min at 37 °C, followed by the addition of 2 μl/well of 1 × anti-tau–d2 antibody prepared in detection buffer and incubation at room temperature for 1 h. Then, 2 μl/well of 1 × europium-conjugated anti-tau antibody prepared in detection buffer was added and incubated for 2 h at room temperature. All the kit reagents were dispensed using the BioRAPTR dispenser. The resulting plates were read in RT-FRET mode (Ex= 320 nm, Em= 665 and 615 nm) on an EnVision plate reader (PerkinElmer).
AlphaLISA total tau protein assay
Cells were plated and treated similar to the HTRF assay described above in 1536-well assay plates. A customized AlphaLISA tau assay kit, a modified version of the original tau biomarker kit, was utilized for detecting tau protein levels in assay plates after three-day incubation in the presence or absence of compound. Briefly, 1 μl/well of a 5 × cell lysis buffer was added to the assay plates that were incubated for 10 min at 37 °C with 5% CO2, followed by the addition of 2.5 μl/well mixture of the anti-tau acceptor beads (40 μg/ml) and biotinylated anti-tau antibody (4 nM) prepared in 2 × HiBlock buffer, and incubation at room temperature with subdued lighting for 1 h. Subsequently, 2.5 μl/well of streptavidin-coated donor beads (160 μg/ml) in 2 × HiBlock buffer was added to the plates, and incubated at room temperature with subdued lighting for an additional 2 h. All kit reagents described above were dispensed using the BioRAPTR dispenser (Indianapolis, IN). The resulting plates were read using the AlphaLISA detection mode (Ex= 665 nm, Em= 615 nm) on the EnVision plate reader.
Compound library and instruments for liquid handling
The library of pharmacologically active compounds (LOPAC) containing 1,280 compounds was purchased from Sigma-Aldrich. Compounds were dissolved in 100% DMSO as 10 mM stock solution, and were further diluted in 384-well plates to 7 concentrations at a 1:5 ratio followed by reformatting into 1536-well compound plates. A CyBi®-Well dispensing station with a 384-well head (Cybio Inc., Woburn, MA) was used to reformat compounds from 384-well plate to 1536-well plate. The 4 μl/well of cell suspension was plated in 1536-well assay plates using the Multidrop Combi dispenser. The 1 to 4 μl/well kit reagents, both HTRF and AlphaLISA, were dispensed into 1536-well assay plates using the BioRAPTR dispenser. Compounds in DMSO solution were transferred to 1536-well assay plates at 23 nl/well using a NX-TR pin tool workstation (WAKO, San Diego, CA).
Data analyses
IC50 values, the concentration required to inhibit tau levels by half, were calculated from signal intensity using the Prism software (Graphpad Software, Inc., San Diego, CA). Signal to background ratio was calculated as total signal in SH-SY5Y cells divided by the signal in the wells without cells. The Z’ factor index of assay quality control was determined by Z’=1-(3*SDhigh+3*SDlow)/(Meanhigh-Meanlow). All values were expressed as mean ± SD.
RESULTS
Assay development
Total tau protein level in the cerebrospinal fluid (CSF) has been used as a biomarker for Alzheimer’s disease (20). Traditionally, total tau protein levels have been measured using one specific anti-tau antibody in either Western blot or ELISA assay formats. Although both assay formats are acceptable for qualitative analysis, these assays have limited screening throughput and are not favorable for quantification. Recently, total tau protein kits in both the AlphaLISA and HTRF assay formats were developed for the measurement of total tau protein level as a biomarker in CSF samples (personal communications). Taking advantage of commercially available kits, we have applied the AlphaLISA and HTRF assay technologies for the measurement of total tau protein levels in SH-SY5Y cells and developed a homogenous assay for compound screening. In contrast to the total tau ELISA and Western blot assays, two specific antibodies recognizing different sites of the tau protein are used in both the HTRF and AlphaLISA total tau protein kits (Fig. 1). One antibody is labeled with a fluorescence donor (Eu3+ cryptate in the HTRF assay) or AlphaLISA donor bead, while the second antibody is labeled with a fluorescence acceptor (d2 in the HTRF assay) or AlphaLISA acceptor bead. The binding of the two antibodies to the tau protein brings the labels to close proximity that, in turn, yields a TR-FRET signal in the HTRF assay, or a chemiluminescence signal in the AlphaLISA assay. The resulting assay signal is directly proportional to total tau protein levels in the cell lysate. Because detection sensitivity for both the HTRF and AlphaLISA assays are comparable in 1536-well plates to those observed in 96-well and 384-well plates, 1536-well plates were directly utilized in the assay development stage to help reduce reagent consumption and increase screening throughput.
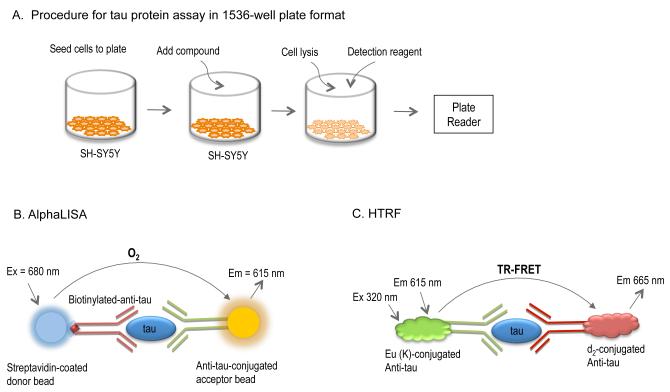
Fig. 1.
Schematic illustration of a quantitative tau protein assay for the AlphaLISA and HTRF assay formats. (A) Step-by-step procedure for the tau protein assay. SH-SY5Y cells are seeded in the assay plate and incubated for 4-h followed by compound addition. After a 3-day incubation with compounds, cells are lysed and detection reagents (either AlphaLISA or HTRF) are added. After a 2-h incubation, the signal is captured using a plate reader. (B) AlphaLISA tau assay principle. The anti-tau acceptor beads and biotinylated–anti-tau antibody are added simultaneously in as a suspension, to the cell lysate allowing for the two antibodies to bind to their respective sites on the tau molecule. The streptavidin-donor beads are then added and they are able to capture the biotinylated-anti-tau antibody. Singlet oxygen molecules are generated upon excitation of donor bead by laser light source. In return, the singlet oxygen excites the acceptor bead due to close proximity of two tau-specific antibodies to the same molecule resulting in light emission for detection. (C) HTRF assay principle. A d2 (fluorescent dye) conjugated anti-tau antibody-1 binds to a specific site on the tau protein, and a europium cryptate–labeled anti-tau antibody-2 captures a second specific site on the same tau protein, resulting in the TR-FRET signal due to the close proximity of the dye conjugates (d2 and Europium).
We tested several cell lines, including neuronal cells and others, for total tau expression levels and found that the SH-SY5Y cell line derived from a human neuroblastoma had superior tau expression levels (Joel Gever et al., unpublished data). We found that the total tau levels in the control cells lines, CHO and fibroblast cells, were similar to the signal in the wells without cells. Since a control inhibitor was not available for defining basal signal for the assay window, we used the signal determined from wells in the absence of cells.
The assay procedure established for both the HTRF and AlphaLISA assays are similar; both consist of five reagent addition steps and five incubation times devoid of plate-washing steps (Table 2). Briefly, the SH-SY5Y cells were plated in 1536-well plates at 4 μl/well and cultured for 4 h to allow cell adhesion in plates. Compounds prepared in 100% DMSO solution were added to cells in 1536-well plates, with a final DMSO solution of 0.57%, and incubated for an additional 72 h. Cells were then lysed with a lysis solution for 10 min, followed by the sequential addition of two labeled anti-tau antibodies. After a 2-h incubation of cell lysate with detection antibodies, the HTRF signal or chemiluminescence signal in AlphaLISA assay was read using a plate reader for total tau expression levels in SH-SY5Y cells.
Table 2.
Cell-based tau protein assay in 1536-well plate format.
| Step | Parameter | Value | Description | |
|---|---|---|---|---|
| AlphaScreen | HTRF | |||
| 1 | Cell plating | 4 μl/well | 4 μl/well | final density of 5,000 cells/well |
| 2 | Incubation | 4 h | 4 h | at 37 °C with 5% CO2 |
| 3 | Compound addition | 0.02 μl/well | 0.02 μl/well | in DMSO solution |
| 4 | Incubation | 72 h | 72 h | at 37 °C and 5% CO2 |
| 5 | Lysis solution addition | 1 μl/well | 1 μl/well | lyse cells |
| 6 | Incubation | 10 min | 10 min | at 37 °C |
| 7 | Reagent-1 addition | 2 μl/well | 2.5 μl/well | see note (1) below |
| 8 | Incubation | 1 h | 1 h | room temperature |
| 9 | Reagent-2 addition | 2 μl/well | 2.5 μl/well | see note (2) below |
| 10 | Incubation | 2 h | 2 h | room temperature |
| 11 | Plate reading | Ex=665 nm | Ex=540 nm | Envision plate reader |
| Em=520-620 nm | Em=665/615 nm | |||
Note: (1) Reagent-1. AlphaLISA tau assay: anti-tau acceptor beads and biotinylated–anti-tau antibody (40 μg/ml and 4 nM, respectively) prepared in 2 × HiBlock buffer. HTRF tau assay: 1 × Anti-tau–d2 antibody prepared in detection buffer. (2) Reagent-2. AlphaLISA tau assay: Streptavidin-donor beads (160 μg/ml) prepared in 2 × HiBlock buffer; HTRF tau assay: 1 × K–anti-tau antibody prepared in detection buffer.
Cell lines and cell density titration
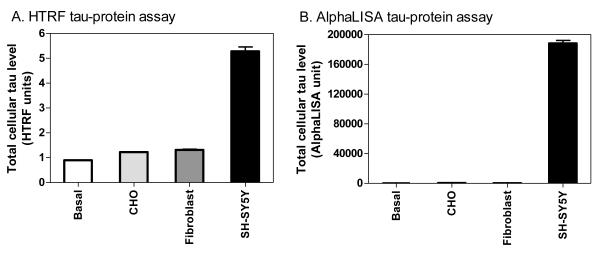
The HTRF signal representing total tau levels in SH-SY5Y cells were 5-fold higher than that of control wells containing no cells. In contrast, the CHO and fibroblast cell lines produced a HTRF signal only 20% higher than the no-cell control (Fig. 2). Similar results were obtained in the AlphaLISA, where high levels of total tau protein were observed in the SH-SY5Y cells, while the CHO and fibroblast cells displayed negligible levels of total tau. Thus, the SH-SY5Y cells were chosen as the cellular model for the screening of compounds that could potentially reduce total cellular tau protein levels while the CHO cells line was used as a negative control.
Fig. 2.
Total tau protein levels in CHO, human skin fibroblasts and SH-SY5Y cells determined by AlphaLISA and HTRF methods. Three cell lines were plated at a seeding density of 5,000 per well in 1536-well plates. The basal signal (Basal) was determined in the control wells in the absence of cells. Total tau protein levels were substantially higher in SH-SY5Y cells, measured by both the HTRF (A) and AlphaLISA (B) methods, compared to CHO and fibroblast cells.
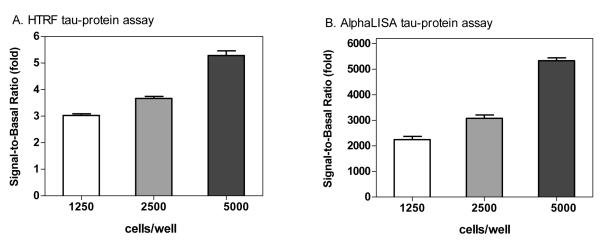
We then determined the cell density effect on the assay window. The signal-to-basal ratios in the HTRF tau assay were 3.02 ± 0.24, 3.66 ± 0.43 and 5.28 ± 0.71 fold for cell densities of 1,250, 2,500 and 5,000 cells/well, respectively (Fig. 3A). For the AlphaLISA tau assay, the signal-to-basal ratios were 2,248 ± 500, 3,077 ± 516 and 5,332 ± 451 for cell densities of 1,250, 2,500 and 5,000 cells/well, respectively (Fig. 3B). The data demonstrated that both assay technologies have a robust assay window and are suitable for HTS in 1536-well plate format. The results also indicated that the cell density of 5,000 cell/well was optimal for subsequent experiments, as it has a sufficient assay window. The cell confluence at 5,000 cell/well was 70–80% after the 3-day incubation time.
Fig. 3.
Signal-to-basal ratios of tau protein assays determined for a seeding density of 1,250, 2,500 or 5,000 cells per well in 1536-well plates. The SH-SY5Y cells at 5,000 cells per well yielded better assay windows in both the HTRF (A) and AlphaLISA (B) methods.
Time course of reagent incubation
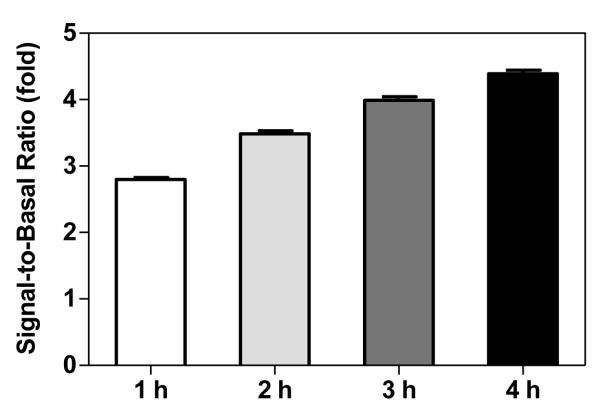
We then measured the appropriate incubation time of the cell lysate with labeled detection antibodies that would result in the optimal assay signal window. The calculated signal-to-basal ratios for the HTRF tau assay were 2.8-, 3.5-, 4.0- and 4.4-fold after 1-, 2-, 3- and 4-h of incubation, respectively (Fig. 4). Because the HTRF tau assay is a ratiometric assay and a signal-to-basal ratio greater than 3-fold is considered robust for compound screening purposes, we selected the 2-h incubation time of cell lysate with the labeled detection antibodies as the optimal condition for all subsequent experiments. Similarly, the 2-h incubation time of antibody reagents with the cell lysate was selected for the AlphaLISA tau screen (data not shown).
Fig. 4.
Effect of incubation times on the HTRF tau-protein assay. After addition of detection reagents, the assay plate was incubated for 1, 2, 3, and 4 h before measurement of tau levels in a plate reader.
Compound screen of LOPAC library
The LOPAC library of 1,280 compounds has been used in many test screens to validate an assay’s performance before application in the screening of large compound collections. The HTRF total tau protein assay was selected for the primary screening because the ratiometric readout not only minimizes well-to-well variation but also provides additional information for compound cytotoxicity. The utilization of the fluorescence signals of both the acceptor and donor beads can help to eliminate the false-positive compounds due to compound cytotoxicity or a fluorescence-quenching effect. Additionally, the labels used in the HTRF tau assay are stable, which is suitable for robotic screening operation in the large-scale compound screening. Conversely, the AlphaLISA donor beads are sensitive to light, and special precautions need to be taken where subdued lighting should be used for dispensing both types of beads. Despite of this, the AlphaLISA tau protein assay has a robust assay window with a chemiluminescence readout that should be a secondary assay for hit confirmation to help further eliminate other false-positive compounds.
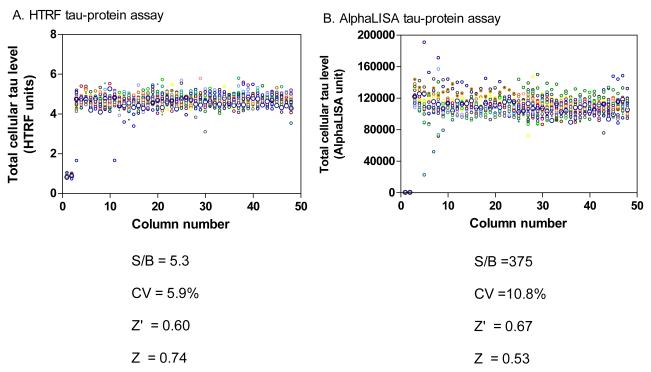
A DMSO plate test was first carried out to assess the parameters of assay performance for both the HTRF and AlphaLISA tau assays. In the HTRF tau assay, the signal-to-basal ratio was 4.3-fold, the coefficient of variation (CV) was 5.9% and Z’ factor was 0.60. In the AlphaLISA tau assay, the signal-to-basal ratio, CV and Z’ factor were 375-fold, 10.8% and 0.53, respectively (Fig. 5). The results demonstrated that both the HTRF and AlphaLISA screens are robust and thus suitable for HTS.
Fig. 5.
Results from a DMSO plate screening in scatter plots. The wells in columns 1 and 2 of the 1536-well assay plates contained CHO cells as a control (0% response) while all other wells were added with an equal amount of DMSO (0.6%). (A) The signal-to-basal ratio (S/B) was 5.3-fold and the Z’ factor was 0.60 in the HTRF tau-protein assay. (B) The S/B was 375-fold and the Z’ factor was 0.67 in the AlphaLISA tau-protein assay.
We then screened 1,280 compounds in the LOPAC library at 4 concentrations ranging from 80 nM to 10 μM in a 1:5 compound dilution with the HTRF total tau protein assay. The average signal-to-basal ratio was 5.3-fold and the Z’ factor was approximately 0.5. An ATP content assay was carried out in parallel to help eliminate false-positive compounds due to compound cytotoxicity (data not shown). Since the multiple compound concentrations were used in the primary screening, the potencies of primary hits were available after the data analysis. A total of 33 compounds were identified as primary hits with a selection criteria of IC50 <10 μM and maximal inhibition > 20%.
Confirmation and counter-screen
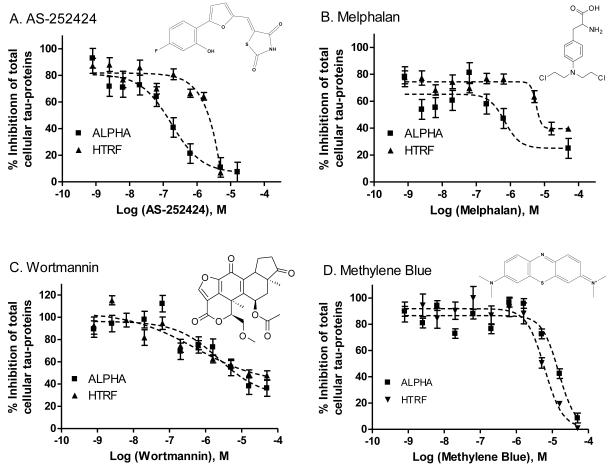
To confirm compound activity, the primary hits were selected and rescreened in the HTRF tau assay. The same set of compounds was also screened in the AlphaLISA tau protein assay. Because the AlphaLISA assay has a chemiluminescence readout that is different from the fluorescence ratiometric readout of the HTRF tau assay, false-positive compounds associated with the HTRF tau assay (e.g., compounds that interfere with or quench fluorescence), would be less likely to appear in the AlphaLISA tau assay. Four compounds were found to be active in both the HTRF and AlphaLISA total tau protein assays: AS-252424, melphalan, Wortmannin and methylene blue (Fig. 6). In addition, the results from the compound cytotoxicity assay measured by the ATP content showed that these compounds were cytotoxic to the SH-SY5Y cells at similar inhibitory concentrations observed for the AlphaLISA and HTRF total tau assays. Thus, the apparent reduction of total tau levels in the SH-SY5Y cells may be related to the cytotoxicity of these compounds and thus, they cannot be lead compounds.
Fig. 6.
Structures and concentration-response curves of 4 compounds determined in the tau assays using both HTRF (squares) and AlphaLISA (triangles) methods in SH-SY5Y cells. (A) AS-252424 (NCGC00185997-01), (B) melphalan (NCGC00015653-06), (C) Wortmannin, (NSC627609, NCGC00016094-07), and (D) methylene blue (NCGC00167496-01).
DISCUSSION
The tau protein has been a target of drug development for the treatment of neurodegenerative diseases for the past decade. Previous efforts focused on inhibitors specific to tau protein aggregation and hyperphosphorylation as well as chaperones responsible for tau protein folding (Table 1). Despite the tremendous efforts put forth on identifying efficacious tau compounds, none have been proven to be useful drug candidates for further development. Therefore, searching for alternate compound intervention points and identifying new lead compounds targeting tau are essential, and this effort may result in the identification of a new drug candidate for the treatment of neurodegenerative diseases. We hypothesized that a reduction of total tau protein levels in neuronal cells may diminish the formation of tau prions under pathological conditions for Alzheimer’s disease and the frontotemporal dementias (8). A total tau protein assay described here may lead to the identification of a new class of compounds for targeting total cellular tau expression levels for drug development.
To select an appropriate cell line for compound library screening, we have evaluated several cell lines for total tau protein expression levels. We found that the SH-SY5Y neuroblastoma cell line is appealing because it is a human neuronal cell line that expresses high total tau protein levels, while many other cell lines including CHO and skin fibroblasts cells express extremely low levels of total tau protein. Therefore, the SH-SY5Y cell line is a useful cellular model for the identification of small molecule inhibitors that suppress total tau protein levels in cells. The SH-SY5Y cell line is a thrice-cloned subline from a human neuroblastoma case and has been used in many compound screens. In addition, SH-SY5Y cells have been used as a recipient cell line for the transfection of target DNA as a compound screening tool. A recent report showed that SH-SY5Y cells were used to establish a stable cell line expressing mutant tau441 to screen for compounds inhibiting tau protein phosphorylation (21). However, the SH-SY5Y cells were not modified or differentiated in our experiments.
Several assay formats are available for the measurement of total and phosphorylated cellular tau protein levels. Similarly as for many other proteins, Western blot is a classical method used for the detection of total and phosphorylated tau protein through the use of specific antibodies. Although it has been used extensively for the identification of the phosphorylation state and total tau protein levels, Western blot is generally not an ideal method for the estimate of compound potency (e.g., IC50) and is not used in HTS of compound collections. The enzyme-linked immunosorbent assay (ELISA) was developed in the 1970s using a specific antibody and an enzyme reporting system for the detection of protein or other molecules in a sample. Although the detection sensitivity and screening throughput are much improved in comparison to the Western blot assay, the ELISA is not ideal for HTS of large compound collections because of the time-consuming and laborious plate-coating and multiple washing steps. ELISAs are usually performed in 96-well plates and difficult to miniaturize to higher density plate formats.
Therefore, a few quantitative assays for the measurement of various cellular proteins have been developed in the last 10 years for HTS of large compound libraries (22). A homogenous assay format (i.e., no washing steps necessary) for detecting total tau protein levels in cells with high assay sensitivity and cost effectiveness is needed for the screening of large compound collections. We found that the HTRF, utilizing a time-resolved fluorescence resonance energy transfer readout, and the AlphaLISA technologies met the criteria discussed above (23). In addition, we modified and customized the commercially available HTRF and AlphaLISA tau assay kits, originally developed for the detection of total tau protein levels in CSF samples of clinical patients, for the cell-based compound screening assay. Both HTRF and AlphaLISA assay methods are amenable to a homogeneous format and have been miniaturized to 1536-well plate format. The assay principles for both the HTRF and AlphaLISA total tau protein kits are similar in that both use two specific anti-tau antibodies for the recognition of total tau proteins in cells, though two different detection methods are used for the HTRF and AlphaLISA technologies (TR-FRET versus chemiluminescence). Thus, they are inclined to have high target specificity due to the use of two specific antibodies against two different sites on the tau protein, excellent dynamic range and low limits of detection. As a result, one assay format (such as HTRF) can be used for the primary screening of compounds, while the second (such as AlphaLISA) can be used for confirmation of primary screen hits to help eliminate detection of assay technology–related, false-positive compounds.
Of the four confirmed compounds reducing tau levels in our studies, two (methylene blue and Wortmannin) have been reported previously with respect to tau and two (melphalan and AS-232424) are compounds with no previous association with tau. Methylene blue has been previously reported to reduce cellular tau expression levels through the inhibition of HSP70 (24) using a Western blot assay in transfected HeLa cell lines. Wortmannin has been previously reported to induce tau hyper-phosphorylation in cells (25). Melphalan is an alkylating agent used in chemotherapy (26) and AS-252424 has been developed as a PI3K inhibitor (27, 28). Because all these four compounds were found to have cytotoxicity at the similar concentrations needed to reduce total tau protein levels in SH-SY5Y cells, they are not considered useful for further studies for targeting tau protein. However, the robust assay feature obtained from the test screen with the LOPAC library demonstrates that this total tau protein assay in AlphaLISA and HTRF formats is a valid approach for future HTS of large compound collections. A screening of large compound collections may identify high quality lead compounds for further drug development.
In summary, we have developed a homogenous HTS assay for the quantification of total tau levels in SH-SY5Y neuroblastoma cells. This assay has been miniaturized to 1536-well plate format and has been validated for future use in HTS of large compound collections. Because this total tau protein assay is available in HTRF and AlphaLISA detection formats, one assay format can be used for primary screening of large compound collections, while the other can be used for the confirmation of the primary screen hits to eliminate false active compounds. Therefore, the further screening of large compound collections may identify new lead compounds that suppress total cellular tau protein level for drug development.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the Therapeutics for Rare and Neglected Diseases, National Center for Advancing Translational Sciences, National Institutes of Health. We would thank the compound management team in NCATS for their support. This work was also supported by research grants from the NIH to S.B.P. (AG021601 and AG031220). Additionally, S.B.P. gratefully acknowledges the support of the Tau Consortium.
Abbreviations
- AlphaLISA
amplified luminescence proximity homogenous assay
- HTRF
homogenous time-resolved fluorescence energy transfer
- NFTs
neurofibrillary tangles
- TR-FRET
time-resolved fluorescence resonance energy transfer
Footnotes
CONFLICT of INTEREST None
REFERENCES
- [1].Stoothoff WH, Johnson GV. Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta. 2005;1739:280–97. doi: 10.1016/j.bbadis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- [2].Yoshiyama Y, Uryu K, Higuchi M, Longhi L, Hoover R, Fujimoto S, et al. Enhanced neurofibrillary tangle formation, cerebral atrophy, and cognitive deficits induced by repetitive mild brain injury in a transgenic tauopathy mouse model. J Neurotrauma. 2005;22:1134–41. doi: 10.1089/neu.2005.22.1134. [DOI] [PubMed] [Google Scholar]
- [3].Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986;83:4044–8. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Smith DH, Chen XH, Nonaka M, Trojanowski JQ, Lee VM, Saatman KE, et al. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J Neuropathol Exp Neurol. 1999;58:982–92. doi: 10.1097/00005072-199909000-00008. [DOI] [PubMed] [Google Scholar]
- [5].Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–9. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- [6].Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- [7].Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI, et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science. 1998;282:1914–7. doi: 10.1126/science.282.5395.1914. [DOI] [PubMed] [Google Scholar]
- [8].Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8:423–34. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Prusiner SB. A unifying role for prions in neurodegenerative diseases. Science. 2012;336:1511–3. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–52. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guo JL, Lee VM-Y. Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem. 2011;286:15317–31. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–84. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- [14].Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–97. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Polymenidou M, Cleveland DW. Prion-like spread of protein aggregates in neurodegeneration. J Exp Med. 2012;209:889–93. doi: 10.1084/jem.20120741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gomez de Barreda E, Perez M, Gomez Ramos P, de Cristobal J, Martin-Maestro P, Moran A, et al. Tau-knockout mice show reduced GSK3-induced hippocampal degeneration and learning deficits. Neurobiol Dis. 2010;37:622–9. doi: 10.1016/j.nbd.2009.11.017. [DOI] [PubMed] [Google Scholar]
- [18].David DC, Layfield R, Serpell L, Narain Y, Goedert M, Spillantini MG. Proteasomal degradation of tau protein. J Neurochem. 2002;83:176–85. doi: 10.1046/j.1471-4159.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- [19].Keck S, Grafe A, Ullrich O, Grund T. Degradation of tau - Does the proteasomal system play a role? Neurobiol Aging. 2004;25:S422–S. [Google Scholar]
- [20].Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–45. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- [21].Loffler T, Flunkert S, Taub N, Schofield EL, Ward MA, Windisch M, et al. Stable mutated tau441 transfected SH-SY5Y cells as screening tool for Alzheimer’s disease drug candidates. J Mol Neurosci. 2012;47:192–203. doi: 10.1007/s12031-012-9716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Inglese J, Johnson RL, Simeonov A, Xia M, Zheng W, Austin CP, et al. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3:466–79. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- [23].Inglese J, Johnson RL, Simeonov A, Xia MH, Zheng W, Austin CP, et al. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3:466–79. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- [24].Jinwal UK, Miyata Y, Koren J, 3rd, Jones JR, Trotter JH, Chang L, et al. Chemical manipulation of hsp70 ATPase activity regulates tau stability. J Neurosci. 2009;29:12079–88. doi: 10.1523/JNEUROSCI.3345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Deng YQ, Xu GG, Duan P, Zhang Q, Wang JZ. Effects of melatonin on wortmannin-induced tau hyperphosphorylation. Acta Pharmacol Sin. 2005;26:519–26. doi: 10.1111/j.1745-7254.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- [26].Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–18. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- [27].Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- [28].Pomel V, Klicic J, Covini D, Church DD, Shaw JP, Roulin K, et al. Furan-2-ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase gamma. J Med Chem. 2006;49:3857–71. doi: 10.1021/jm0601598. [DOI] [PubMed] [Google Scholar]
- [29].Pickhardt M, von Bergen M, Gazova Z, Hascher A, Biernat J, Mandelkow EM, et al. Screening for inhibitors of tau polymerization. Curr Alzheimer Res. 2005;2:219–26. doi: 10.2174/1567205053585891. [DOI] [PubMed] [Google Scholar]
- [30].Bulic B, Pickhardt M, Mandelkow EM, Mandelkow E. Tau protein and tau aggregation inhibitors. Neuropharmacology. 2010;59:276–89. doi: 10.1016/j.neuropharm.2010.01.016. [DOI] [PubMed] [Google Scholar]
- [31].Honson NS, Johnson RL, Huang W, Inglese J, Austin CP, Kuret J. Differentiating Alzheimer disease-associated aggregates with small molecules. Neurobiol Dis. 2007;28:251–60. doi: 10.1016/j.nbd.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Crowe A, Huang W, Ballatore C, Johnson RL, Hogan AM, Huang R, et al. Identification of aminothienopyridazine inhibitors of tau assembly by quantitative high-throughput screening. Biochemistry. 2009;48:7732–45. doi: 10.1021/bi9006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu SJ, Wang JZ. Alzheimer-like tau phosphorylation induced by wortmannin in vivo and its attenuation by melatonin. Acta Pharmacol Sin. 2002;23:183–7. [PubMed] [Google Scholar]
- [34].Liu M, Choi S, Cuny GD, Ding K, Dobson BC, Glicksman MA, et al. Kinetic studies of Cdk5/p25 kinase: phosphorylation of tau and complex inhibition by two prototype inhibitors. Biochemistry. 2008;47:8367–77. doi: 10.1021/bi800732v. [DOI] [PubMed] [Google Scholar]
- [35].Stoilov P, Lin CH, Damoiseaux R, Nikolic J, Black DL. A high-throughput screening strategy identifies cardiotonic steroids as alternative splicing modulators. Proc Natl Acad Sci U S A. 2008;105:11218–23. doi: 10.1073/pnas.0801661105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dickey CA, Eriksen J, Kamal A, Burrows F, Kasibhatla S, Eckman CB, et al. Development of a high throughput drug screening assay for the detection of changes in tau levels -- proof of concept with HSP90 inhibitors. Curr Alzheimer Res. 2005;2:231–8. doi: 10.2174/1567205053585927. [DOI] [PubMed] [Google Scholar]