Abstract
Rare adipose disorders (RADs) including multiple symmetric lipomatosis (MSL), lipedema and Dercum's disease (DD) may be misdiagnosed as obesity. Lifestyle changes, such as reduced caloric intake and increased physical activity are standard care for obesity. Although lifestyle changes and bariatric surgery work effectively for the obesity component of RADs, these treatments do not routinely reduce the abnormal subcutaneous adipose tissue (SAT) of RADs. RAD SAT likely results from the growth of a brown stem cell population with secondary lymphatic dysfunction in MSL, or by primary vascular and lymphatic dysfunction in lipedema and DD. People with RADs do not lose SAT from caloric limitation and increased energy expenditure alone. In order to improve recognition of RADs apart from obesity, the diagnostic criteria, histology and pathophysiology of RADs are presented and contrasted to familial partial lipodystrophies, acquired partial lipodystrophies and obesity with which they may be confused. Treatment recommendations focus on evidence-based data and include lymphatic decongestive therapy, medications and supplements that support loss of RAD SAT. Associated RAD conditions including depression, anxiety and pain will improve as healthcare providers learn to identify and adopt alternative treatment regimens for the abnormal SAT component of RADs. Effective dietary and exercise regimens are needed in RAD populations to improve quality of life and construct advanced treatment regimens for future generations.
Keywords: adiposis dolorosa, Dercum's disease, lipedema, multiple symmetric lipomatosis, familial multiple lipomatosis, familial partial, lipodystrophy, lymph, lymphatics
Introduction
Lifestyle-induced obesity in children and adults has reached epidemic proportions worldwide. In the United States (US), a third of adults aged 20 years and over are overweight, a third are obese, and over five percent are extremely obese1. The National Health and Nutrition Examination Survey and Pediatric Nutrition Surveillance System reported a tripling of the prevalence of obesity among US school-age children and adolescents over the past three decades2. Numerous published studies validate the weight loss efficacy of lifestyle changes that include decreased amounts and types of food, and improved exercise regimens. Medications used for the treatment of obesity are severely limited3, 4. Bariatric surgery has been exceptional in its ability to induce weight loss and resolve the co-morbidities of obesity, though complications rates can be high5, many people are still obese by body mass index (BMI) after Roux-en-Y gastric bypass (RYGB)6, and weight regain occurs7, 8.
This review aims to demonstrate lymphatic dysfunction as a component of rare adipose disorders (RADs) that increases the amount and alters the location of subcutaneous adipose tissue (SAT) while resisting fat loss after lifestyle changes or bariatric surgery. Lipodystrophies are also discussed as they may be confused with rare adipose disorders (RADs).
Non-lifestyle causes of obesity
Lipodystrophies
Lipodystrophies or fat redistribution syndromes involve a primary lack or loss of SAT; however, increased SAT in other areas can be confused with lifestyle-induced obesity. Human immunodeficiency virus (HIV)-associated lipodystrophy, is well-known, but familial partial lipodystrophies are rare and therefore less well known, and can go undiagnosed for years or are never recognized. Acquired partial lipodystrophy, also rare, with a progressive and symmetrical lipoatrophy of SAT starting from the face and spreading to the upper part of the body, sparing the legs, can be confused with the RAD, lipedema, due to a disproportion between upper and lower body SAT (see below).
Acquired lipodystrophies
Human immunodeficiency virus (HIV)-associated lipodystrophy
HIV-and highly active antiretroviral treatment (HAART)-associated lipodystrophy includes loss of SAT from the face, buttocks, arms, and legs. In men with lipodystrophy, SAT can be increased on the abdomen and chest (gynecomastia), and a dorsocervical fat pad or “buffalo hump” is common9, 10. The SAT in the dorsocervical fat pad is thought to be identical to the SAT in multiple symmetric lipomatosis (MSL), one of the RADs (see below). Upper body fat, including parotid hypertrophy, circumferential enlargement of the neck and a dorsocervical fat pad are associated with insulin resistance in HIV+ men11, 12, 13 as is intermuscular fat and SAT on the legs in HIV+ women14. Large breasts are part of HIV lipodystrophy in Black women and other non-Caucasian ethnicities15. Women with HIV may also develop increased SAT on the upper part of the arm out of context with the usual lipoatrophy in this area in HIV+ men suggesting an estrogen and/or progesterone component to location of the SAT. This upper arm SAT looks visually similar to the SAT in women with the RAD, MSL (see below and Figure 1). In addition to excision of excess SAT as treatment for lipodystrophy16, tesamorelin, a synthetic analogue of human growth hormone-releasing hormone, is FDA-approved for the reduction of excess visceral adipose tissue in HIV-infected patients with lipodystrophy. Visceral adipose tissue was reduced up to 18% during active use of tesamorelin17. The glucagon-like peptide-1 agonist, exenetide, also improved the HIV- and treatment-induced obesity through weight loss in a single case18.
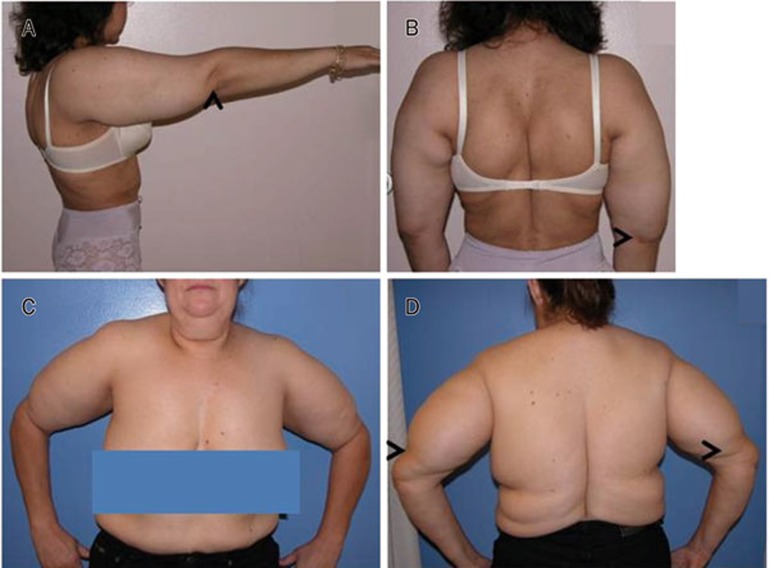
Figure 1.
Multiple symmetric lipomatosis with or without HIV infection. A and B, non-HIV-related MSL Type II; note increased upper arm size and increased fat on back. Not shown is increased fat in the labia majora. C and D, increased arm and back fat, respectively in HIV-and HAART-induced MSL Type II. Arrows point to end of MSL fat on the upper arm. Normal labia majora (not shown).
Acquired partial lipodystrophy (APL; BARRAQUER-SIMONS syndrome)
Acquired partial lipodystrophy is characterized by a regional loss of SAT primarily in children and adolescents starting at the face and extending to the waist, sparing the legs; in fact SAT may be increased on the legs19. Because of the higher amount of SAT in the legs compared to the upper body, APL could be confused with the RAD, lipedema (Figure 2). In lipedema, there is increased fat on the legs but the fat of the upper body is normal or increased (see below). APL is thought to be autoimmune occurring after a febrile (viral) illness20 with low levels of complement factor 3 (C3) and the presence of a circulating autoantibody called complement 3-nephritic factor. Treatment with the thiazolidinedione, rosiglitazone, improved levels of C3 and increased SAT in a participant with APL21.
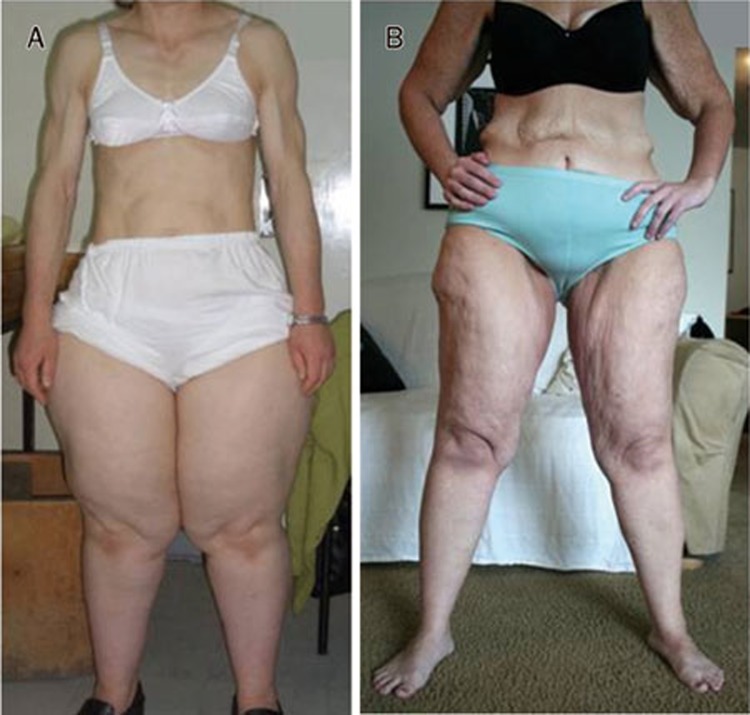
Figure 2.
Acquired partial lipodystrophy and lipedema. A, a 37 year old woman with acquired partial lipodystrophy. C3 level<16.1 mg/dL (normal range: 90-180) and C4 level 23.11 mg/dL (normal range: 10-40). Note the loss of SAT from the upper body to the waist but obesity of the hips and legs (photo by Dr Alper GURLEK). B, a woman with lipedema stage II and a previous history of obesity with a 100 kg weight loss; note redundant skin on arms and abdomen from weight loss of non-RAD fat; note also lipedema in legs.
Familial partial lipodystrophies (FPLD)
FPLD Type 1: FPLD1, also known as Köbberling lipodystrophy, is a lesser known partial lipodystrophy primarily found in women causing a lipodystrophy of the arms, legs, and sometimes breasts, with an increase in fat on the abdomen and remainder of the trunk22. The prevalence of FPLD1 and any genetic mutation remains unknown. There are no blood or urine biomarkers for FPLD1. FPLD1 may go unrecognized if the practitioner does not recognize the lipodystrophy; finding a ledge where SAT ends on the buttocks can help in the diagnosis. Diabetes and hypertriglyceridemia are highly prevalent in FPLD1 while acanthosis nigricans is minimal. Treatment is restricted to usual care of obesity-associated co-morbidities, although, RYGBP should be considered as it improved weight and co-morbidities in a case of FPLD123.
FPLD Type 2: The best studied FPLD is Type 2 (FPLD2), also known as Dunnigan lipodystrophy. In FPLD2, SAT is lost around the time of puberty from the legs, arms, buttocks, abdomen and chest; areas of remaining SAT deposits are on the back, face and chin, giving a Cushingoid appearance; fat is increased in the labia majora in women24; this finding also occurs in women with MSL (Figure 1). Mutations in the lamin A and C gene, LMNA, cause FPLD225. People with FPLD2 have all the co-morbidities associated with obesity. Leptin levels can be very low in lipodystrophies and leptin treatment has shown benefit but remains investigational26. RYGB has also shown benefit in reducing the co-morbidities associated with FPLD227.
FPLD Type 3: Mutations in the peroxisome proliferator-activated receptor gamma (PPARG) gene can cause partial lipodystrophy with abdominal obesity28 known as FPLD329; people with FPLD3 may look very similar to FPLD2. Treatment with thiazolidinediones may be useful in people with PPARG gene mutations30 and other cases of FPLD without identified gene mutations31.
Additional lipodystrophies and single cases of additional types of FPLD have been well reviewed19.
RADs
Multiple Symmetric Lipomatosis (Madelung's disease/syndrome; Launois Bensaude syndrome)
Multiple symmetric lipomatosis (MSL) is a rare syndrome (Table 1) originally described in 184632, characterized by the painless, symmetrical accumulation of abnormal tumor-like SAT. The first systematic treatise was by the German surgeon Dr Otto Madelung who collected 30 cases and reported an additional 3 cases in 1888 under the name “Fetthals” or fat neck33, but the French Physicians Drs Pierre-Emile LAUNOIS and Raoul BENSAUDE gave prominence to and caused recognition of MSL by publishing a detailed account of 65 cases in 189834. There are many synonyms for MSL including benign symmetric lipomatosis but this disorder is anything but benign, arguing against its use. Over 300 adult cases are reported in the literature with an age range of 20–71 years. The early literature on MSL was dominated by research on alcoholic men with a reported incidence of 1/25 000 in the Italian population35. Non-alcoholics and women are also affected36, 37, 38; two cases have been reported in children39, 40.
Table 1. Identifying codes or numbers for SAT Disorders.
| Code or number |
SAT disorder |
||
|---|---|---|---|
| MSL | DD | Lipedema | |
| OMIM | 151800 | 103200 | 614103 |
| Listed by NORD | Yes | Yes | No |
| NLM MESH ID | D008069 | D000274 | NA* |
| ICD-9/10 | 272.8/E88.8 | NA/E88.2 | NA |
| Alternative ICD-9/10 | NA | 338.4/G89.4 | 457.1/189.0 |
| Chronic pain syndrome | Lymphedema, not elsewhere classified | ||
| Orphanet number | 2398 | 36397 | 77243 |
ICD=International Classification of Diseases; MESH=medical subject headings; NLM=national Librar y of Medicine; NORD=National Organization of Rare Disease; OMIM=Online Mendelian Inheritance in Man®;
*Application for a MESH code submitted
Diagnosis
Identification of MSL is by history and clinical exam. There are no blood or urine biomarkers for MSL and the gene(s) remains unknown in a majority of cases. Individuals with MSL have increased SAT, either as discrete non-encapsulated lipomas or as a confluent increase in SAT in a symmetrical distribution on the neck, the back, the chest, the upper arms, or on the thighs; MSL usually spares the distal limbs41 but not in many women with MSL where the altered fat may be global42 (Figure 3). Because the appearance and location of SAT in MSL can vary, MSL has been divided into three types:
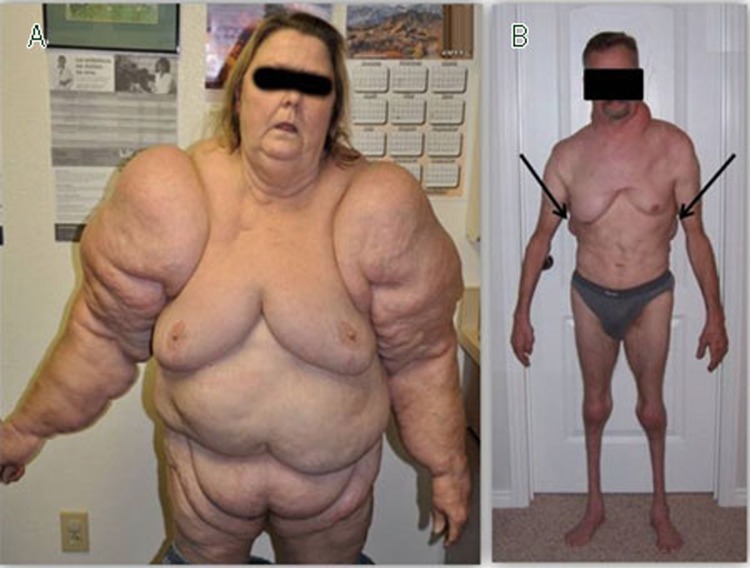
Figure 3.
Whole body MSL and MSL-associated lipodystrophy. While MSL is noted to spare the forearms (see text), the entire body can be clearly affected. A, 60 year old woman with a history of alcohol dependence with global MSL SAT; note prevalent SAT on the forearms (photo by Dr Andy COREN). This type of MSL may be easily confused with global obesity or lipedema stage III. B, 50 year old man with MSL Types I and II with associated muscle and normal fat atrophy (also note the increased back MSL SAT; arrows); this type of MSL may be confused with partial lipodystrophy.
Clinical types of MSL37, 43
Type I, head and/or neck with extension down the back, or only on the back: In rare cases, MSL SAT can invade the lingual muscles of the tongue44, 45, or the vocal cords and compress the recurrent laryngeal nerve causing hoarseness46, or increase periorbital fat47. Tracheal or esophageal compression and the superior vena cava syndrome can be found in 15%–20% of patients48. The presence of a dorsocervical fat pad (buffalo hump) can be found both in MSL41, 49, 50 and HIV-associated lipodystrophy9, 10, 51, 52; it has been proposed that the fat in these two disorders arises from brown adipose tissue located in that area53.
Type II body: Includes the shoulder girdle, the upper arms, the thorax, the back, the abdomen and upper buttocks. In one case, fat grew around the testicles in the scrotum and was contiguous with MSL tissue in the perineum and the root of the penis54. Also rare is growth of the MSL fat on the hands55. Many individuals have a combination of Types II and III. While the MSL fat grows, normal fat and muscle can undergo wasting which can be confused with a partial lipodystrophy (Figure 3).
Type III, thigh (female type): Rarest type. MSL type III is clinically similar to and may be instead, the RAD, lipedema (see below). Women tend to have Type II and III MSL with widespread altered SAT41.
MSL inheritance
MSL is thought to be inherited through mitochondrial mutations in a few familial cases including multiple deletions of mitochondrial DNA, and the myoclonus epilepsy and ragged-red fibers (MERRF) tRNA(Lys) A>G(8344) mutation56, 57. Klopstock et al found mitochondrial mutations in only 2 of 12 patients studied50. Chalk et al found no mitochondrial pathology or mutations in four siblings with MSL with a pattern favoring autosomal recessive58. The phenotype of MSL may require a combined effect of alcohol (or other insult) and a currently unknown genetic mutation.
Histology of MSL fat
Individual fat cells have been described as smaller than normal38, 49, 59 or normal sized38. MSL SAT is thought to be derived from brown adipose tissue (BAT) or as white adipose tissue (WAT) that transdifferentiates into BAT49, 60, 61. Ultrastructurally, brown adipocytes have numerous large mitochondria packed with cristae. Under light microscopy, brown adipocytes have cytoplasmic lipids arranged as numerous small droplets (multilocularity), while white adipocytes have cytoplasmic lipids arranged in a unique vacuole (unilocularity). In BAT, the metabolic reactions of mitochondria are uncoupled from ATP synthesis by uncoupling protein (UCP)-1 so that energy produced is released as heat62. Infants and even adolescents have a substantial amount of BAT, especially between the shoulder blades. BAT persists throughout adulthood in the perirenal, omental, mesenteric, pericardial, intercostal, axillary, cervical, and interscapular fat, embedded within WAT63, 64 with an approximated ratio of 1 brown adipocyte for every 200 white adipocytes65, 66.
By light microscopy, adipocytes in MSL SAT are monovacuolar67, 68 or multivacuolar69. By electron microscopy of long-term primary cultures from the stromal vascular fraction (SVF), containing stem and immune cells, cells were polymorphic with thin microfilaments suggestive of elevated metabolic activity69, were multivacuolar, and had large mitochondria packed with cristae suggesting a more BAT phenotype in MSL60, 68.
Physiology of MSL SAT
MSL SAT may arise from a stem cell population either destined to form BAT, or WAT that transdifferentiates to BAT; in either case, UCP-1 levels help track BAT features. SAT cells from subjects with MSL express UCP-1 suggesting its origin as BAT61, 70, but this is not substantiated in all cases71. Adrenergic receptors (AR) that respond to sympathetic input, such as the three subtypes of β-AR, β1-, β2- and β3-, promote lipolysis and energy expenditure. Cells isolated from the MSL SVF did not increase UCP-1 in response to noradrenaline even though MSL cells express all three β-AR61. Resting energy expenditure (REE) may be expected to be higher in MSL with BAT; indeed REE when normalized to fat free mass was mildly higher in MSL subjects than normal, suggestive of energy uncoupling and heat generation72, however, REE in other subjects with MSL was within normal limits38. In MSL cell culture, catecholamines did not increase lipolysis, expression of inducible nitric oxide synthase (iNOS) or PPARγ coactivator-1α (PGC-1α), a coregulator of nuclear receptors that control metabolic pathways in BAT61, 73, 74, 75. Two other groups found a normally reactive adenylate cyclase system and a normal number of a- and β-adrenergic receptors in MSL SAT76, 77. Cytokine and adipokine levels in MSL are also mixed37, 61. While the multilocularity of MSL SAT is suggestive of BAT, more data in a larger number of subjects of well characterized participants are needed to substantiate the cell type of origin and functionality of pathways.
The increase in MSL fat is extensive and deforming, compressing tissue structures and vessels. Early, MSL SAT is watery but later becomes fibrotic and scars easily78. Similarly in obesity, excess fat physically impedes lymph collection and flow, protein-rich lymphatic fluid collects in SAT, resulting in lymphedema and tissue hypoxia79. SAT also grows in the presence of lymphedema80. Further accumulation of fluid in the setting of decreased oxygen tension leads to fibrosis81. Interestingly, ischemia activates the growth of adipose-derived progenitor cells82. Congestion of lymph nodes by other means, such as lymphoma in the neck, induces fat growth similar to MSL83. Increased volumes of SAT in MSL, like obesity, may therefore be sufficient to externally compress vasculature and lymphatics inducing further growth of SAT as seen in other localized fat collections84. Impedance of lymph flow into lymph collectors is a local effect and does not affect flow in larger lymph trunks, therefore the role of lymphoscintigraphy in MSL is questionable85.
Conditions associated with MSL
Alcohol-induced liver disease is common in MSL. Hyperlipidemia, hyperuricemia, hypothyroidism, and diabetes mellitus have been reported but are not consistent amongst those affected86, 87. People with MSL I or II should be tested for sleep apnea87. Cancerous transformation of the SAT is uncommon; development of myxoid liposarcoma was reported in one case88. Slowly progressive axonal sensory and autonomic peripheral neuropathies have been reported to occur after the development of MSL fat and impairment of autonomic function has been suggested as a cause of sudden death86, 89. The neuropathology is a distal axonal demyelination different from that associated with alcohol intake58, 86, 90; this impairment seems to be prevalently parasympathetic48, 91. In a ten year follow-up, ∼10% of 31 patients died from sudden death due to autonomic neuropathy36. Surgical placement of a cardiac pacemaker may be needed.
MSL treatment recommendations
Primary recommendations
1) Alcohol abstinence: Abstinence from alcohol may arrest further progression of the MSL SAT but does not cause regression of the SAT deformities92.
2) Lymphatic decongestive therapy (LDT): Includes manual lymphatic drainage (MLD), wrapping of the limbs, compression garments, exercise such as pool therapy and other non-impact exercise (so as to avoid lactic acid accumulation in tissue due to poor lymph flow), dietary recommendations, and skin care. Manual lymphatic drainage works well to reduce MSL SAT before fibrosis93.
3) Surgery: Surgical resection and liposuction provide the only means of dramatically decreasing the MSL SAT94, 95, 96, 97. In a majority of cases of MSL Type II and III, resection or liposuction of the lipomatosis is considered cosmetic and insurance companies are reluctant to cover this procedure98. Unfortunately, the fat usually penetrates and surrounds deeper structures such as muscle and bone, making total excision of the abnormal tissue difficult92; the lipomatosis can, therefore, recur after liposuction or excision87, 99, 100; in three of eleven patients in one series101.
Additional considerations for MSL treatment
4) β2-Adrenergic Agonist: After demonstrating an intact lipolytic response of the MSL fat to catecholamines, an oral β2- AR specific drug, salbutamol, 15 mg per day in divided doses, reversed the rapid accumulation of the MSL fat and increased REE in a man with MSL, but was effective only during active use102.
5) Fibric acid: A man with MSL Type II with a past history of hypertriglyceridemia was treated with fenofibrate 200mg daily. The circumference of his abdomen decreased 119 cm (46.9 in) to 108 cm (42.7 in) within a year. Fibric acids are PPARα agonists. Activation of the PPARα receptor may suppress expression of proteins involved in the architecture of BAT, thereby maintaining BAT in a quiescent state103.
6) Growth hormone: Growth hormone (GH) treatment has been suggested in the community of individuals with MSL as a treatment option (personal communication) but GH levels were normal in one subject with MSL during a glucose tolerance test49 and in three other subjects104 suggesting a normal GH axis. Testing for GH deficiency should be undertaken and replacement considered only for those deficient in this hormone.
7) Lifestyle: Lifestyle improvements provide no resolution of the MSL SAT105.
8) Local SAT injections: Corticosteroid injections have been suggested as treatment for lipomatosis such as MSL SAT106 but there are a number of cases demonstrating the development of lipomatosis after steroid use107, 108. Local injection with thyroxine107, enoxaparin109, deoxycholate110, and phosphatidylcholine78 have also been proposed for treatment of lipomas but the latter require multiple injections and use of thyroxine injections in the presence of autonomic dysfunction would be dangerous. In addition, the extent of the SAT in MSL does not allow for single site injections, limiting these treatments to lipomas.
Lipedema (lipoedema; lipalgia; adiposis dolorosa; lipomatosis dolorosa of the legs; lipohypertrophy dolorosa; painful column leg)
Lipedema is generally unknown to medical providers, is easily confused as obesity, does not have a MESH term in the National Library of Medicine, and does not have an International Classification of Diseases (ICD) code; it does have an Online Mendelian Inheritance in Man code, and is recognized by Orphanet (a European website providing information about orphan drugs and rare diseases (Table 1). Drs Allen and Hines Jr from the Mayo clinic labeled this condition as lipedema in 1940111. Outside the US, lipedema is known as “lipoedema”, meaning edema of the fat. This disorder is likely very common but underdiagnosed.
Diagnosis
The diagnosis of lipedema is made clinically by history, visual inspection and physical exam as extensive deposition of SAT between the iliac crest and the malleoli and approximately 30% of the time, on the arm42. When the fat is palpated, it will be tender and feel like round peas in a plastic bag or a “beanie baby”111, 112. Larger nodules, lumps, lipomas or angiolipomas may also be found in the SAT. There are no blood or urine biomarkers for lipedema and the gene(s) is unknown. The skin and SAT is thicker in lipedema compared to healthy controls and muscle mass is not edematous as it is in lymphedema113. The skin is also less elastic and striae are common in lipedema.
In 1951, Wold, Hines and Allen analyzed 119 cases and provided the diagnostic criteria for lipedema114:
1) Almost exclusive occurrence in women developing by the third decade of life. Prevalence within the population remains grossly under diagnosed115. According to an epidemiologic study by Földi E and Földi M116, lipedema affects 11% of the female population. At least seven cases have been reported in men with testosterone or GH deficiency, or liver disease114, 115, 117.
2) Bilateral and symmetrical nature with minimal involvement of the feet, resulting in an ''inverse shouldering'' or ''bracelet” effect at the ankle
3) Minimal pitting edema (non-pitting edema is present)
4) Pain, tenderness, and easy bruising
5) Persistent enlargement despite elevation of the extremities or weight loss
6) Increased vascular fragility; easy bruising
Often women note that the lipedema appears or is exacerbated at the time of puberty, pregnancy118 or menopause suggesting an estrogen component; that few men have this condition except those with hypogonadism or hyperestrogenemia supports this hypothesis.
There are five types of lipedema119
Type I: Pelvis, buttocks and hips (saddle bag phenomenon)
Type II: Buttocks to knees, with formation of folds of fat around the inner side of the knee
Type III: Buttocks to ankles
Type IV: Arms
Type V: Lower leg
There may be a mixture of lipedema types in one person, for example Type II and IV. Only the arms may be affected in 3% of lipedema cases (Type IV)42. The importance of knowing the different lipedema types is to improve recognition, and identification of differences ie, all people with lipedema do not look alike; treatment is similar amongst the types. In addition to types of lipedema, the lipedema progresses through stages; the progression varies greatly amongst those affected and there is no data suggesting everyone need progress through all stages.
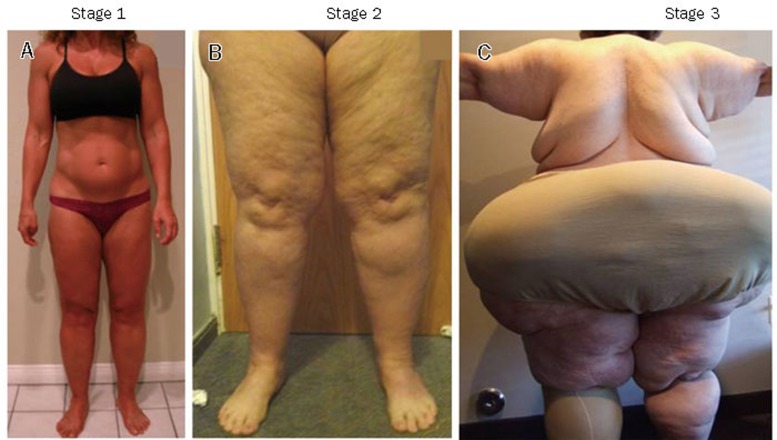
There are three stages of lipedema (Figure 4)112, 120
Figure 4.
The three stages of lipedema. A, Stage I with little alteration of the skin surface. B, In stage 2, the surface of the skin takes on the appearance of a mattress with lipomas in the fat. C, In stage III lipedema, there are much larger fat extrusions.
Stage 1: Normal skin surface with enlarged hypodermis
Stage 2: Uneven skin with indentations in the fat121; larger mounds of tissue grow as unencapsulated masses, lipomas and angiolipomas
Stage 3: Large extrusions of tissue causing deformations especially on the thighs and around the knees
Stage 4: Lipedema with lymphedema (lipolymphedema)
Progression to lipolymphedema can develop during stage II-III. The description and representative pictures of Type III MSL41 are that of lipedema stage II38; no study has formerly differentiated these two SAT disorders. Synonyms for lipedema also include adiposis dolorosa, which is another name for the RAD, Dercum's disease (see below). However, according to Cornely, the trunk, hands and feet are not involved in lipedema “Thus, lipedema differs clearly from Dercum's disease”122. As lipedema progresses to lipolymphedema, the hands, feet, trunk and head develop excess SAT making this statement incorrect. Because lymphatic dysfunction is a part of Dercum's disease and many early cases of Dercum's disease are visually and descriptively lipedema (see below), the two SAT disorders are at a minimum, in the same spectrum. Lipedema may also be confused with APL, however, in APL there is a lack of SAT on the face and upper body while in lipedema, SAT is normal or increased in these areas (Figure 1).
Inheritance of lipedema
Inheritance has been noted up to 60% of people with lipedema118, 123, 124 but is likely higher due to under diagnosis. In six families over three generations with lipedema, the inheritance pattern was autosomal dominant with incomplete penetrance115.
Histology of lipedema SAT
The gross description of the fat in lipedema is similar to that of MSL with “free fluid fat” in biopsy specimens125. Histological exam is not unlike that found for cellulite with dilation of subdermal blood capillaries, perivascular cells, fibrosis of arterioles, fibrosis and dilation of venules, and hypertrophy and hyperplasia of adipocytes126, 127. Histochemical studies show adipocytes death and stem cell regeneration128. There are also increased numbers of blood vessels especially capillaries and prominent venules116. Large clusters of macrophages are found around multiple fat cells (not isolated crown-like structures129), surrounding blood vessels and forming oil cysts in lipedema SAT116, 130; macrophages may also be a prominent component of cellulite131. The histology of lipedema SAT can also appear as normal125.
Physiology of lipedema SAT
The elasticity of the skin and fascia is decreased in lipedema132 which in Stage III may progress to abnormally clumped elastic fibers or pseudoxanthoma133. The skin loses its role as an abutment for the skeletal muscle venous pump and the increased compliance of the SAT results in an increase in capillary compliance116, 124. The permeable capillaries release excess protein-rich fluid into the interstitium along with blood42, 116, 134, 135. The veno-arteriolar reflex in lipedema is also absent so that under orthostatic conditions (standing), there is limited vasoconstriction and increased net filtration driving edema116. Early on, lymphatic transport increases to accommodate the increased fluid flux from the capillaries136, 137. During this time, visualization of lymphatic vessels on a gross level by lymphoscintigraphy is normal138, 139. As lipedema progresses, microaneurysms appear in the lymphatics in the skin139, 140 which eventually leak125, 136. It is during this time that hypertrophy and hyperplasia of fat cells accelerates138 further altering the microlymphatic architecture and increasing venous congestion. The resultant edema increases hydrostatic pressure in the tissue and pain123, 141.
As an example of what happens in SAT when lymph leaks, mutation of prospero homeoboxprotein 1, encoded by the PROX1 gene, causes leakage from lymphatics and resultant obesity in heterozygote mice142. Lymph placed on adipocytes in culture also induces robust growth; in essence, “lymph makes you fat”143. Although PROX1 mutations are not known to be associated with lipedema, it is clear that fat grows in response to lymph80. Eventually, the microlymphatics may become obliterated in lipedema144 leading to backflow and an overall dynamic insufficiency of the lymphatic system42. The increased tissue pressure and lymphatic vessel leakage lead to the development of lipolymphedema136, 145, 146. While lymphedema does not usually develop with cellulite in women, the pathophysiology of cellulite development is similar to that in lipedema, and LDT (see treatments below) improves the cosmetic appearance of cellulite147, 148. Lipedema may therefore be an extreme form of cellulite.
Conditions associated with lipedema
Depression and anxiety are very common in people with lipedema for many reasons including the lengthy time to diagnosis, repeated counseling on diet and exercise by the healthcare community when neither is particularly effective and because of the massive and sometimes rapid body metamorphosis over a lifetime. In one clinic, women with lipedema were found to be more depressed than patients with paralysis112. Painful SAT is a chronic problem in lipedema111, 114. The excess tissue fluid weakens nearby structures leading to the development of joint pains; with progression of lipedema, arthritis develops149. Capillary fragility, ecchymosis, hematomas and venous varicosities are common150. The Kaposi-Stemmer sign is negative in lipedema (the skin cannot be pinched as a fold by the fingers) until the development of lipolymphedema. Idiopathic edema (IE) is similar to lipedema by description and has been identified in women with lipedema116, 134. Other changes in skin include dryness, fungal infections, cellulitis, and slow wound healing. Free fatty acids may be different in both blood and the lipedema SAT125.
Lipedema treatments recommendations
Primary recommendations
1) Lymphatic Decongestive therapy (LDT) is the standard of care for lipedema. Includes manual lymphatic drainage (MLD), wrapping of the limbs, compression garments, movement therapy, dietary recommendations, and skin care. LDT has been shown to improve skin elasticity, restore the veno-arteriolar reflex, increase pre-lymph drainage and lymph transport in lymphatic vessels116, 151, and reduce capillary fragility in lipedema152. Intermittent pneumatic compression may not improve limb size over MLD alone153 but may be effective alone when MLD is not available154. Compression is most effective when tissue edema is present155 as in its absence, it has little effect156. That compression was effective in lipedema was noted by Hines in a woman with lipedema whose fat and edema were absent under the area covered by her “high-topped shoes”157.
2) Exercise: Aqua lymphatic therapy (pool hydrotherapy) significantly reduces limb volume in lymphedema158. In addition to improving strength and bone mineral density, whole body vibration (WBV) improves peripheral circulation159, 160 and increases lymph flow, raising the threshold level for edema formation in the legs161. During WBV, the user simply stands (or stretches/exercises) on a platform for 10-15 min. making this a very accessible exercise modality.
3) Pain Control: Must be individually optimized; liposuction improves pain (see below).
4) Psychological support: Many women with lipedema are left on their own to find their diagnosis, convince their healthcare providers about lipedema and then seek treatment, all complicated by depression, anxiety and eating disorders; counseling and support during treatment are necessary when any of these are present116. Counseling reduces anxiety by 50% in people with secondary lymphedema162.
5) Surgery: Liposuction works effectively for lipedema to reduce SAT and pain122, 163, 164. In patients who have lipolymphedema, it may be prudent to undergo lymphoscintigraphy to confirm the absence of large lymph vessel damage before pursuing liposuction116. Bariatric surgery is ineffective in uncomplicated lipedema (without obesity or lymphedema)165, 166 but effective in lipedema and lymphedema associated with obesity as long as LDT is performed before and after bariatric surgery167.
Additional considerations for lipedema treatment
6) Beta-adrenergic agonist: Modeling treatment after capillary leak syndrome, terbutaline sulfate, 5 mg five times daily, and theophylline, 200 mg twice daily, were given to a woman with lipedema (called lymphedema in the paper) and after 10 months a weight loss of 20 kg was noted. Cessation or lowering the medication allowed weight regain168.
7) Corticosteroids: Corticosteroids produce a fast reduction in swelling and pain but increase the risk of infection, capillary fragility and SAT growth. A series of corticosteroid joint injections is usually well-tolerated without exacerbation of lipedema.
8) Diuretics: Diuretics can quickly deplete lymphedema fluid but concentrate protein in edematous tissue promoting fibrosclerosis169. Use of diuretics in lipedema before lymphedema may result in the development of pseudo Barrter's syndrome characterized by hypokalemic-hypochloremic alkalosis, hyperactivity of the renin-angiotensin-aldosterone system and elevation of atrial natriuretic peptide116, 170.
9) Flavonoids: Daflon is a flavonoid that has been used to treat lymphedema171, 172, 173; it may be expensive and is unlikely available by prescription. Other flavonoids such as those for venous disease174 have not been formerly tested in lipedema participants. The International Society of Lymphology does not endorse the use of flavonoids as a substitute for LDT.
10) Lifestyle: Obesity can occur along with lipedema especially in Stage III when the lipedema limits movement, but can also occur when movement is limited by pain in earlier stages; lifestyle improvements should always be considered but are not the cause of lipedema175. Lipedema SAT is unaffected by caloric restriction alone175.
11) Selenium: Sodium selenite (selenium) has proven effective for reduction of secondary lymphedema169, 176, 177, 178, 179, 180, 181. The US National Research Council has defined the individual maximum safe dietary intake for selenium as 600 μg daily and the no adverse effect level as 800 μg daily.
12) Shock wave therapy: One report suggests that shock wave therapy functions similarly to LDT in reducing oxidative stress of the tissues and in smoothing the dermis and hypodermis182 which may be useful as part of a treatment plan and when lymphatics are still functioning.
Dercum's disease (adiposis dolorosa; Morbus Dercums)
Dercum's disease (DD) was recognized in 1892 as a clinical entity called “adiposis dolorosa”, meaning painful fatty deposits, when Dr Francis X DERCUM from the University of Pennsylvania published on three cases183. This sentinel publication was preceded by a report of a single case in 1888184 and followed by the published autopsy of that case185. Numerous case studies, case series and descriptions of DD have been published with such a wide variety of locations for the fatty deposits, including misdiagnoses of obvious cases of lipedema, familial multiple lipomatosis and MSL186 that, unless one is an expert in SAT disorders, it would be difficult to diagnose this often misunderstood syndrome. DD is currently considered to be a rare disorder (Table 1).
Diagnosis
Diagnosis of DD is made by history and physical exam. Dercum's disease occurs primarily in women with a ratio of females to males of 5-7:1186, 187, 188; the average age of development in one series was 35 years188 but it has been reported to develop in children188, 189, 190, 191 and in adults up to age 80 years186. One in a 1,000 are affected in Sweden187. Many cases of peri- or post-menopausal women with DD have been reported suggesting a hormonal component to the development of DD192. In addition to painful SAT, there are many other signs and symptoms associated with DD so a lengthy review of systems is helpful (Table 2).
Table 2. Cardinal and accessory signs and symptoms of Dercum's disease with checkbox.
| Cardial Signs/Symptoms | Details | √ |
|---|---|---|
| Fat deposits | Nodules (lipomas) in fat ranging in size from rice grains to a fist or larger | |
| Pain in fat deposits for at least 3 months | Pain exacerbated by stress, strenuous exercise, trauma, changes in weather, or other; pain can be spontaneous or on palpation; may wax and wane or move around | |
| Fatigue (asthenia) | Exacerbated by activities of daily living or exercise | |
| ≥2 accessory symptoms | ||
| Cognitive change(s) | Memory difficulties; difficulty forming thoughts; “brain fog” | |
| Weight gain | Difficult to lose fatty deposits with lifestyle changes | |
| Vascular involvement | Visible vascularity near lipomas; telangiectasias; multiple cherry angiomas, multiple petechiae; easy bruising; flushing; hematuria of unknown etiology; heavy or prolonged menstrual bleeding; epistaxis | |
| SAT edema | Non-pitting | |
| Gastrointestinal complaints | Gastroesophageal reflux disease, irritable bowel syndrome, bloating, abdominal pain, early satiety | |
| Joint pain and/or stiffness | Increased in areas of fat deposits | |
| Muscle pain/stiffness | Especially on awakening or the day after physical activity | |
| Shortness of breath | In the presence of normal oxygen saturation or as part of the need for oxygen supplementation | |
| Tachycardia | Varies from palpitations to supraventricular tachycardia requiring beta-blockade |
There are three types of DD187, 193:
Type I, juxta-articular (around the joint): Painful folds or nodular fat on the inside of the knees and/or on the hips; in rare cases only evident in the upper-arm fat (similar to Type IV lipedema).
Type II, diffuse, generalized type: Widespread pain from fatty tissue found anywhere from head to the soles of the feet.
Type III, nodular type: Intense pain in and around multiple “lipomas”, sometimes in the absence of obesity.
Interestingly, the painful lumps of fat first noticed around joints in DD Type 1 occur in locations of lymph nodes, for example around the knee (popliteal nodes), the elbow (cubit nodes), hips and thighs (inguinal nodes), upper arm (axillary nodes) and supraclavicular. As Dr Kling reported in 112 cases of Type I DD, “Juxta-articular adiposis dolorosa is regarded as the initial and intermediate stage of generalized adiposis dolorosa”194. Dercum's disease Type I is therefore, the first stage of DD, and Type II a stage with more widespread dysfunction. Type I DD around the knees is visually consistent with Type IV and Type II lipedema Stages 2–3.
Type III DD is likely a variant of familial multiple lipomatosis (FML) in which men present mainly with lipomas and/or angiolipomas predominating on the lower and upper arms, the lower trunk and thighs and women present with lipomas, angiolipomas and obesity188, 195. Angiolipomas can be found in up to 30% of people with DD188, 196. The lipomas are generally not painful in FML except if they are growing or traumatized frequently, however, they are painful in DD Type 3. In a DD family, family members may have lipomas without pain195. Even if a person with FML has non-painful lipomas, at some point in time a lipoma can become painful, followed by generalized pain in all lipomas. Pack and Ariel197 described this as lipoma dolorosa, distinct from DD. It is unclear why the authors make this distinction as others ascribe the same pathological process to both FML and DD Type III, with pain in the latter due to “local conditions”198. The “local conditions” may be increased tissue tension from fluid accumulation. In two cases of DD Type III, pain was relieved after local hemorrhage186. The underlying pathophysiology of DD needs to be elucidated to further differentiate or group the three types of DD.
DD inheritance: Thought to be autosomal domin-ant188, 195, 199, 200. In two families, females were more affected than males suggesting a sex-specific influence on the expression of the DD phenotype195.
Histology of DD
Some of the unilocular adipocytes are extremely large in DD SAT compared to weight matched controls187. Dr Dercum and others found an infiltration of nerves (neuritis)185, 201 but this has not been substantiated. Increased connective tissue around nerves, blood vessels and as thickened septae has been noted185, 202, 203. Perivascular cells203, giant cells204, and granulomas suggestive of a foreign body reaction are apparent in some areas205. The histology of DD SAT can also appear normal194, 206, 207, 208, 209.
Physiology of DD
The physiology of DD is unknown and many etiologies have been advanced. These include thyroid dysfunction185, pituitary dysfunction, polyglandular disease, infection, neuritis, alcohol, trauma, a defect in the synthesis of long chain fatty acids205, lower resting energy expenditure202, and altered responses to norepinephrine and insulin.210 Ballet may have been closest to the actual etiology when he stated that it is a “chronic intoxication of endogenous origin”211. The evidence currently points to an underlying vascular and lymphatic dysfunction in DD Types I/ II similar to lipedema (Birgher Fagher, personal communication) for the following reasons:
1) Vascular dysfunction as hematemesis212, 213, epis-taxis213, 214, hemaochezia212, heavy menses215, 216, varicose veins194, and altered vasoconstrictor responses217 is common in both lipedema and DD. Perivascular infiltration of immune cells have been found in DD tissue218 suggesting damage to or repair of blood vessels, and brain vasculitis in DD has been reported219.
2) LDT has been reported to be beneficial in DD220 as in lipedema.
3) Multiple lipomas can develop in lipedema as in DD221.
4) Fibrosis secondary to lymphedema222 is common in lipedema112 and DD202.
5) In the presence of lymphatic and vascular dysfunction in lipedema, the fat is painful112, similar to DD.
6) In the German literature, lipedema is known as adiposis dolorosa, another name for DD118.
7) Original descriptions of DD match descriptions of lipedema. For example, Spiller described a woman with painful fat as follows: “The obesity was marked over the thighs, calves, abdomen, nates (buttocks), and back. It was also very great in the arms, less marked in the forearms, and absent in the feet and hands”223. Dr Collins noted that “The fatty accumulations have not been noticed in the hands, face or feet, and frequently the contrast between the feet which preserve their normal outline and contour and the legs, when the latter are involved, is most striking”214. These cases are similar to lipedema in terms of the pattern of painful fat (less likely early on in the hands, feet, face, and forearms) and the latter case describes well the distinct “bracelet” of fat seen at the base of the leg above the foot that is classic in lipedema112. The published photographs of the columnar legs with the cuff of fat above the foot, or the mass of tender fat inferomedial to the knee, and the enlarged upper arms in DD are consistent with lipedema112, 183, 184, 224, 225.
8) The nodular “beans in a bag” feel of the fat in lipedema is the same as in DD Types 1 and 2188, 226.
9) Dr Dercum described DD as a disorder of the “haemolymph system”227 though the importance of these structures in humans is unclear. Hemolymph nodes are structures resembling a lymph node, but which can have blood in the sinuses; erythrocytes enter the hemolymph nodes through afferent lymphatics228. There are few reports on the function of hemolymph glands in humans.
10) Dr Mills reported “In one case studied carefully with Dr Dercum, there was a general disease of the lymphatic system”229.
The data suggest that the vascular and lymph system are dysfunctional in both lipedema and DD, that pre-lymph remains in the tissue longer, inducing fat growth and the characteristic beans in a bag feel to the fat. In both lipedema and DD there is a hereditary component195, 199. Also in both cases, estrogen and/or progesterone likely play a role resulting in the predominance of women with lipedema and/or DD; lipedema is known to occur with the onset puberty and pregnancy, and DD with menopause, both times of changing hormone levels. In DD, a more widespread insult to the vascular and lymphatic system may occur compared to lipedema. Many of the early reported cases of DD had syphilis213, 216, 230, well known to affect the lymph nodes, consumed alcohol214, 231 which acutely increases mesenteric lymphatic pumping but decreases lymphatic myogenic tone232, or had antecedent trauma which may have affected lymphatic function207, 213, 233. Many patients with DD Type I or II noticed their first painful area of fat after a viral flu, severe pneumonia or trauma188, 213, 234. Data are needed on lymphatic function in DD to confirm these hypotheses.
Conditions associated with DD
In addition to the two cardinal symptoms of fatty deposits and pain proposed by Dercum184, Vitaut added the third cardinal symptom of DD, asthenia (abnormal physical weakness or lack of energy)193. Accessory symptoms in DD are found in the psychiatric, motor, sensory and sympathetic nervous systems186 as well as the pulmonary, endocrine, gastrointestinal and rheumatological systems187, 188, 235 (Table 2).
Thyroid dysfunction has been suggested as one etiology of DD. While a few cases of DD benefited from thyroid treatment216, 236 many cases of DD failed to improve192, 215, 230, 237, 238 and DD generally continues to progress during adequate thyroid replacement188. Others have suggested multiple endocrine dysfunction as a cause for DD201 (reviewed186) but ACTH and pituitary extract did not improve signs and symptoms associated with DD192, 239 and hormone testing was normal in other cases210, 240. If hypercholesterolemia is present, severe and generalized vascular disease may be found241.
DD treatment recommendations
Primary recommendations
1) Exercise: Similar to lipedema. Supporting the use of WBV as exercise in DD, WBV slowed the acquisition of fat in female rats242 and improved pain and fatigue in women with fibromyalgia243.
2) LDT: LDT220 and “massage”225 are known to be beneficial for DD Types I and II; recommendations are similar to lipedema (see above).
3) Pain control: Must be individually optimized; only published or important anecdotal reports are included here:
(a) Chemotherapy: A patient with DD had improved pain and growth of DD SAT slowed on methotrexate combined with infliximab244. One case had resolution of her lipomas and pain with paclitaxel and carboplatin (unpublished); once the paclitaxel was discontinued because of neuropathy, the pain and lumps returned.
(b) Cyclic Variations in Adaptive Conditioning (CVAC™): A novel therapy that reduces tissue fluid by variable patterning of different atmospheric pressures around a person sitting in an altitude simulator. Peri-corporal pressure patterns vary from sea level to four sequential altitude levels: 3200 m (10.5K ft), 4419 m (14.5K ft), 5638 m (18.5K ft), and 6858 m (22.5K ft). This 'body conditioning' reduced fluid and pain in 10 DD participants245 and improved VO2max in healthy men246.
(c) Lidocaine: Intravenous (IV) lidocaine has been used with some success to treat the intractable pain associated with DD217, 247, 248, 249, 250, 251, 252, 253. Many individuals with DD obtain good local pain relief using lidocaine patches, cream, gel or EMLA248, 254.
(d) Mexilitene: Mexilitene (an antiarrhythmic drug) has been used for the effective treatment of pain in DD196.
(e) Pregabalin: LDT combined with pregabalin (an anticonvulsant drug used for neuropathic pain) has been used to treat the pain associated with DD220.
4) Psychological support: See lipedema (above).
5) Surgery: Liposuction is one of the accepted methods of treatment for DD196, 255, 256, 257 resulting in decreased pain258, 259. When asked specifically about liposuction in a series of 110 patients, 83 respondents (75.5%) reported having had liposuction; of these, 50.6% reported that the painful fat depots grew back188. Surgical resection and liposuction should be preceded by LDT and compression to support all vasculature and decrease the risk of seroma and hematoma formation. DD may be one reason why RYGBP without LDT failed to result in weight loss in a published case260.
Additional recommendations
6) Aminoacetic acid (glycine) and prostigmine: In three women with DD, a diet consisting of 70 grams protein, 70 grams of fat and 100 grams carbohydrate or 1500 calories/day (specifics unavailable), 10 grams glycine and 45 mg prostigmine daily improved weight loss and energy261. If glycine binding in the central nervous system is antagonized, feeding in rats increased262; glycine may therefore be an appetite suppressant while prostigmine improves asthenia.
7) Corticosteroids (oral): Cortisone treatment has been shown to help with pain but with none of the other features of DD263; cortisone treatment can also induce DD264. A series of corticosteroid joint injections is usually well-tolerated without exacerbation of symptoms and signs in DD.
8) Hormonal testing: Testing for thyroid function and assessing a complete panel of pituitary hormones at least once after diagnosis of DD and when symptoms change is prudent so as not to miss accompanying hormonal dysfunction, which should be treated with usual methods. Adipose tissue is a very hormonally responsive tissue265, so estrogen, progesterone and testosterone levels should be monitored regularly on any replacement regimen so as to regulate high and low levels and avoid wide fluctuations.
9) Lifestyle: While obesity is prominent in DD, the DD SAT is resistant to loss with lifestyle changes261, 266 while normal SAT as part of obesity can be lost261.
10) Oxygen therapy: Many people with DD feel short of breath188. This can progress on to the need for continuous oxygen therapy. It is unclear why the shortness of breath occurs but it is likely a combination of increased interstitial fluid moving cells away from their oxygen source and a weakened diaphragm. Pulmonary function testing should be performed on everyone with DD that has shortness of breath even if it serves simply as a baseline for future changes or symptoms. Similar to MSL, if a patient has increased thickened fat around the chest or neuropathy, DD patients with shortness of breath and/or edema should be evaluated for thoracic outlet syndrome, sleep apnea and/or autonomic dysfunction.
Co-morbidities associated with obesity in DD are treated as usual.
Conclusions
Obesity is very common and in the limited time allotted to patient care, it may be easy to misdiagnose a patient with a lipodystrophy or a RAD disorder as having simple obesity, and prescribe lifestyle changes only. The widespread increase in abnormal SAT in MSL, DD or lipedema Type II or III can easily masquerade as global obesity (Table 3). The loss of normal fat and muscle in MSL or the disproportion of fat in lipedema can also be confused with lipodystrophy; lipedema Type I is usually overlooked. Lifestyle changes and bariatric surgery work effectively for the obesity component of FPLD and RADs but not for the abnormal SAT tissue in RADs. The RAD SAT likely results from the growth of a brown stem cell population that secondarily compresses lymphatics and vessels (in MSL) or a primarily vascular and lymphatic dysfunction with secondary growth of SAT (in lipedema and DD), neither of which respond well to caloric limitation. Academic testing of various dietary regimens, mechanical treatments, surgery, medications, and supplements is needed for RADs. Understanding the genomics of the RADs is also important to help differentiate lipedema, MSL and DD especially in women where the three disorders can look so much alike, and to assess for RADs in obesity. Improved recognition of RADs may also prove that lipedema and DD are not RADs at all but common disorders and that understanding the underlying pathophysiology of RADs may improve our understanding of refractory obesity. Lymphatic drainage methods used for RADs should be considered in resistant obesity cases or before bariatric surgery, low to very low calorie diets or other methods that induce rapid weight loss requiring optimal lymphatic function.
Table 3. Comparison of RAD characteristics.
| Characteristic | MSL | RADs DD | Lipedema |
|---|---|---|---|
| Abnormal SAT location | upper* | Global | Legs±Arms |
| Diet-resistant SAT | Yes | Yes | Yes |
| Lipomas | Common in males | Common | Large nodular fat masses |
| Time of SAT change | Adult | Child to adult | Puberty; by third decade |
| Painful SAT | Not usually | Yes | Yes |
| Sex predominance | Male | Female | Female |
| Lymphatic dysfunction | Secondary | Primary | Primary |
| Look-alike conditions | Obesity; HIV lipodystrophy | Obesity; FML | Obesity; APL |
| Associated conditions | Neuropathy | Autoimmune; diabetes | Lymphedema |
| Population frequency | Rare | Likely common | Likely common |
| Inheritance pattern | Autosomal dominant or recessive | Autosomal dominant; sex-specific influence | Autosomal dominant; incomplete penetrance |
| Known gene | tRNALys mutations uncommon | None | None |
| Known biomarkers | No | No | No |
| Alcohol association | Yes | No | No |
*Can be global especially in women; APL=acquired partial lipodystrophy; FML=familial multiple lipomatosis; RAD=rare adipose disorder; SAT=subcutaneous adipose tissue
Conflicts of interest
This study was approved by the University of California, San Diego Human Research Protection Program and the Research and Development Committee at the Veterans Affairs San Diego Healthcare System. All subjects described herein completed an informed consent process prior to enrollment.
Acknowledgments
This study received research support from the UCSD NIDDK Diabetes and Endocrinology Research Center Grant P30 DK063491 and the UCSD General Clinical Research Center by Public Health Grant 5M01RR000827. This paper is dedicated to people with RADs and to Birgher Fagher, MD, who died in April of 2011.
References
- Ogden CL, Carroll MD.Prevalence of overweight, obesity, and extreme obesity among adults: united states, trends 1960–1962 through 2007–2008 . http://www.cdc.gov/nchs/fastats/overwt.htm .
- Orsi CM, Hale DE, Lynch JL. Pediatric obesity epidemiology. Curr Opin Endocrinol Diabetes Obes. 2011;18:14–22. doi: 10.1097/MED.0b013e3283423de1. [DOI] [PubMed] [Google Scholar]
- James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–17. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- Sam AH, Salem V, Ghatei MA. Rimonabant: From RIO to Ban. J Obes. 2011;2011:432607. doi: 10.1155/2011/432607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobowicz M, Lehmann A, Orlowski M, Lech P, Michalik M. Preliminary outcomes 1 year after laparoscopic sleeve gastrectomy based on bariatric analysis and reporting outcome system (BAROS) Obes Surg. 2011. p. 15. [DOI] [PMC free article] [PubMed]
- Butner KL, Nickols-Richardson SM, Clark SF, Ramp WK, Herbert WG. A review of weight loss following Roux-en-Y gastric bypass vs restrictive bariatric surgery: impact on adiponectin and insulin. Obes Surg. 2010;20:559–68. doi: 10.1007/s11695-010-0089-z. [DOI] [PubMed] [Google Scholar]
- Ray JB, Ray S. Safety, efficacy, and durability of laparoscopic adjustable gastric banding in a single surgeon U.S. community practice. Surg Obes Relat Dis. 2011;7:140–4. doi: 10.1016/j.soard.2010.08.011. [DOI] [PubMed] [Google Scholar]
- D'Hondt M, Vanneste S, Pottel H, Devriendt D, Van Rooy F, Vansteenkiste F. Laparoscopic sleeve gastrectomy as a single-stage procedure for the treatment of morbid obesity and the resulting quality of life, resolution of comorbidities, food tolerance, and 6-year weight loss. Surg Endosc. 2011;25:2498–504. doi: 10.1007/s00464-011-1572-x. [DOI] [PubMed] [Google Scholar]
- Engelson ES. HIV lipodystrophy diagnosis and management. Body composition and metabolic alterations: diagnosis and management. AIDS Read. 2003;13:S10–14. [PubMed] [Google Scholar]
- Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. “Buffalo hump” in men with HIV-1 infection. Lancet. 1998;351:867–70. doi: 10.1016/S0140-6736(97)11443-X. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Rimland D, Gibert CL, Powderly WG, Sidney S, Shlipak MG, et al. Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immune Defic Syndr. 2007;46:283–90. doi: 10.1097/qai.0b013e31814b94e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon PW, Wand H, Law M, Miller J, Cooper DA, Carr A. Buffalo hump seen in HIV-associated lipodystrophy is associated with hyperinsulinemia but not dyslipidemia. J Acquir Immune Defic Syndr. 2005;38:156–62. doi: 10.1097/01.qai.0000147527.64863.1a. [DOI] [PubMed] [Google Scholar]
- Tierney EP, Hanke CW. “Bullfrog neck,” a unique morphologic trait in HIV lipodystrophy: case series and review of the literature. Arch Dermatol. 1279;146:1279–82. doi: 10.1001/archdermatol.2010.341. [DOI] [PubMed] [Google Scholar]
- Albu JB, Kenya S, He Q, Wainwright M, Berk ES, Heshka S, et al. Independent associations of insulin resistance with high whole-body intermuscular and low leg subcutaneous adipose tissue distribution in obese HIV-infected women. Am J Clin Nutr. 2007;86:100–6. doi: 10.1093/ajcn/86.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andany N, Raboud JM, Walmsley S, Diong C, Rourke SB, Rueda S, et al. Ethnicity and gender differences in lipodystrophy of HIV-positive individuals taking antiretroviral therapy in Ontario, Canada. HIV Clin Trials. 2011;12:89–103. doi: 10.1310/hct1202-89. [DOI] [PubMed] [Google Scholar]
- Abrams H, Herbst KL.Novel liposuction techniques for the treatment of HIV-associated dorsocervical fat pad and parotid hypertrophyIn: Serdev N, ed. Advanced techniques in liposuction and fat transfer. Vol In press. Rijeka, Croatia: InTech; 2011
- Falutz J, Potvin D, Mamputu JC, Assaad H, Zoltowska M, Michaud SE, et al. Effects of tesamorelin, a growth hormone-releasing factor, in HIV-infected patients with abdominal fat accumulation: a randomized placebo-controlled trial with a safety extension. J Acquir Immune Defic Syndr. 2011;53:311–22. doi: 10.1097/QAI.0b013e3181cbdaff. [DOI] [PubMed] [Google Scholar]
- Oriot P, Hermans MP, Selvais P, Buysschaert M, de la Tribonniere X. Exenatide improves weight loss insulin sensitivity and beta-cell function following administration to a type 2 diabetic HIV patient on antiretroviral therapy. Ann Endocrinol. 1016;72:244–6. doi: 10.1016/j.ando.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Garg A. Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–25. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurugol Z, Ulger Z, Berk O, Tugral O. Acquired partial lipodystrophy associated with varicella. Turk J Pediatr. 2009;51:617–20. [PubMed] [Google Scholar]
- Walker UA, Kirschfink M, Peter HH. Improvement of acquired partial lipodystrophy with rosiglitazone despite ongoing complement activation. Rheumatology. 2003;42:393–4. doi: 10.1093/rheumatology/keg076. [DOI] [PubMed] [Google Scholar]
- Herbst KL, Tannock LR, Deeb SS, Purnell JQ, Brunzell JD, Chait A. Kobberling type of familial partial lipodystrophy: an underrecognized syndrome. Diabetes Care. 2003;26:1819–24. doi: 10.2337/diacare.26.6.1819. [DOI] [PubMed] [Google Scholar]
- Utzschneider KM, Trence DL. Effectiveness of gastric bypass surgery in a patient with familial partial lipodystrophy. Diabetes Care. 2006;29:1380–2. doi: 10.2337/dc06-0130. [DOI] [PubMed] [Google Scholar]
- Donadille B, Lascols O, Capeau J, Vigouroux C. Etiological investigations in apparent type 2 diabetes: when to search for lamin A/C mutations. Diabetes Metab. 2005;31:527–32. doi: 10.1016/s1262-3636(07)70227-6. [DOI] [PubMed] [Google Scholar]
- Decaudain A, Vantyghem MC, Guerci B, Hécart AC, Auclair M, Reznik Y, et al. New metabolic phenotypes in laminopathies: LMNA mutations in patients with severe metabolic syndrome. J Clin Endocrinol Metab. 2007;92:4835–44. doi: 10.1210/jc.2007-0654. [DOI] [PubMed] [Google Scholar]
- Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2009;53:27–35. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- McGrath NM, Krishna G. Gastric bypass for insulin resistance due to lipodystrophy. Obes Surg. 2006;16:1542–4. doi: 10.1381/096089206778869988. [DOI] [PubMed] [Google Scholar]
- Ludtke A, Buettner J, Wu W, Muchir A, Schroeter A, Zinn-Justin S, et al. Peroxisome proliferator-activated receptor-gamma C190S mutation causes partial lipodystrophy. J Clin Endocrinol Metab. 2007;92:2248–55. doi: 10.1210/jc.2005-2624. [DOI] [PubMed] [Google Scholar]
- Jeninga EH, van Beekum O, van Dijk AD, Hamers N, Hendriks-Stegeman BI, Bonvin AM, et al. Impaired peroxisome proliferator-activated receptor gamma function through mutation of a conserved salt bridge (R425C) in familial partial lipodystrophy. Mol Endocrinol. 2007;21:1049–65. doi: 10.1210/me.2006-0485. [DOI] [PubMed] [Google Scholar]
- Ludtke A, Buettner J, Schmidt HH, Worman HJ. New PPARG mutation leads to lipodystrophy and loss of protein function that is partially restored by a synthetic ligand. J Med Genet. 2007;44:e88. doi: 10.1136/jmg.2007.050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanishi M, Ebihara K, Kusakabe T, Chen W, Ito J, Masuzaki H, et al. Clinical characteristics and efficacy of pioglitazone in a Japanese diabetic patient with an unusual type of familial partial lipodystrophy. Metabolism. 2009;58:1681–7. doi: 10.1016/j.metabol.2009.04.043. [DOI] [PubMed] [Google Scholar]
- Brodie B. London, Longman; 1846. Lectures illustrative of various subjects in pathology and surgery. [Google Scholar]
- Madelung O. Uber den Fetthals. Langenbecks Archiv Klin Chirurg. 1888;37:106. [Google Scholar]
- Lanois P F FB. L'adénolipomatose symmetrique. Bull Soc méd Hop Paris. 1898;1:289. [Google Scholar]
- Enzi G, Biondetti PR, Fiore D, Mazzoleni F. Computed tomography of deep fat masses in multiple symmetrical lipomatosis. Radiology. 1982;144:121–4. doi: 10.1148/radiology.144.1.7089242. [DOI] [PubMed] [Google Scholar]
- Enzi G, Busetto L, Ceschin E, Coin A, Digito M, Pigozzo S. Multiple symmetric lipomatosis: clinical aspects and outcome in a long-term longitudinal study. Int J Obes Relat Metab Disord. 2002;26:253–61. doi: 10.1038/sj.ijo.0801867. [DOI] [PubMed] [Google Scholar]
- Harsch IA, Michaeli P, Hahn EG, Ficker JH, Konturek PC. Launois-Bensaude syndrome in a female with type 2 diabetes. Med Sci Monit. 2003;9:CS5–8. [PubMed] [Google Scholar]
- Nielsen S, Levine J, Clay R, Jensen MD. Adipose tissue metabolism in benign symmetric lipomatosis. J Clin Endocrinol Metab. 2001;86:2717–20. doi: 10.1210/jcem.86.6.7557. [DOI] [PubMed] [Google Scholar]
- Kratz C, Lenard HG, Ruzicka T, Gartner J. Multiple symmetric lipomatosis: an unusual cause of childhood obesity and mental retardation. Eur J Paediatr Neurol. 2000;4:63–7. doi: 10.1053/ejpn.2000.0264. [DOI] [PubMed] [Google Scholar]
- Nounla J, Rolle U, Grafe G, Kraling K. Benign symmetric lipomatosis with myelomeningocele in an adolescent: an uncommon association-case report. J Pediatr Surg. 2001;36:E13. doi: 10.1053/jpsu.2001.24776. [DOI] [PubMed] [Google Scholar]
- Busetto L, Strater D, Enzi G, Coin A, Sergi G, Inelmen EM, et al. Differential clinical expression of multiple symmetric lipomatosis in men and women. Int J Obes Relat Metab Disord. 2003;27:1419–22. doi: 10.1038/sj.ijo.0802427. [DOI] [PubMed] [Google Scholar]
- Herpertz U. Das lipödem. Lymphologie. 1995;19:1–11. [PubMed] [Google Scholar]
- Donhauser G, Vieluf D, Ruzicka T, Braun-Falco O. Benign symmetric Launois-Bensaude type III lipomatosis and Bureau-Barriere syndrome. Hautarzt. 1991;42:311–4. [PubMed] [Google Scholar]
- Ettl T, Gaumann A, Ehrenberg R, Reichert TE, Driemel O. Encapsulated lipomas of the tongue in benign symmetric lipomatosis. J Dtsch Dermatol Ges. 2009;7:441–4. doi: 10.1111/j.1610-0387.2008.06977.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Ceres A, Aguilar-Lizarralde Y, Villalobos Sanchez A, Prieto Sanchez E, Valiente Alvarez A. Benign symmetric lipomatosis of the tongue in Madelung's disease. J Craniomaxillofac Surg. 2006;34:489–93. doi: 10.1016/j.jcms.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Birnholz JC, Macmillan AS., Jr Advanced laryngeal compression due to diffuse, symmetric lipomatosis (Madelung's disease) Br J Radiol. 1973;46:245–9. doi: 10.1259/0007-1285-46-544-245. [DOI] [PubMed] [Google Scholar]
- Laure B, Sury F, Tayeb T, Corre P, Goga D. Launois-Bensaude syndrome involving the orbits. J Craniomaxillofac Surg. 2011;39:21–3. doi: 10.1016/j.jcms.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Enzi G. Multiple symmetric lipomatosis: an updated clinical report. Medicine (Baltimore) 1984;63:56–64. doi: 10.1097/00005792-198401000-00004. [DOI] [PubMed] [Google Scholar]
- Kodish ME, Alsever RN, Block MB. Benign symmetric lipomatosis: functional sympathetic denervation of adipose tissue and possible hypertrophy of brown fat. Metabolism. 1974;23:937–45. doi: 10.1016/0026-0495(74)90043-2. [DOI] [PubMed] [Google Scholar]
- Klopstock T, Naumann M, Seibel P, Shalke B, Reiners K, Reichmann H. Mitochondrial DNA mutations in multiple symmetric lipomatosis. Mol Cell Biochem. 1997;174:271–5. [PubMed] [Google Scholar]
- Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000;14:F25–32. doi: 10.1097/00002030-200002180-00001. [DOI] [PubMed] [Google Scholar]
- Heath KV, Hogg RS, Chan KJ, Harris M, Montessori V, O'Shaughnessy MV, et al. Lipodystrophy-associated morphological, cholesterol and triglyceride abnormalities in a population-based HIV/AIDS treatment database. AIDS. 2001;15:231–9. doi: 10.1097/00002030-200101260-00013. [DOI] [PubMed] [Google Scholar]
- Urso R, Gentile M. Are 'buffalo hump' syndrome, Madelung's disease and multiple symmetrical lipomatosis variants of the same dysmetabolism. Aids. 2001;15:290–1. doi: 10.1097/00002030-200101260-00028. [DOI] [PubMed] [Google Scholar]
- Poggi G, Moro G, Teragni C, Delmonte A, Saini G, Bernardo G. Scrotal involvement in Madelung disease: clinical, ultrasound and MR findings. Abdom Imaging. 2006;31:503–5. doi: 10.1007/s00261-005-0401-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ichimiya M, Hamamoto Y, Muto M. Benign symmetrical lipomatosis of the hands. J Dermatol. 2000;27:748–9. doi: 10.1111/j.1346-8138.2000.tb02271.x. [DOI] [PubMed] [Google Scholar]
- Klopstock T, Naumann M, Schalke B, Bischof F, Seibel P, Kottlors M, et al. Multiple symmetric lipomatosis: abnormalities in complex IV and multiple deletions in mitochondrial DNA. Neurology. 1994;44:862–6. doi: 10.1212/wnl.44.5.862. [DOI] [PubMed] [Google Scholar]
- Berkovic SF, Andermann F, Shoubridge EA, Carpenter S, Robitaille Y, Andermann E, et al. Mitochondrial dysfunction in multiple symmetrical lipomatosis. Ann Neurol. 1991;29:566–9. doi: 10.1002/ana.410290519. [DOI] [PubMed] [Google Scholar]
- Chalk CH, Mills KR, Jacobs JM, Donaghy M. Familial multiple symmetric lipomatosis with peripheral neuropathy. Neurology. 1990;40:1246–50. doi: 10.1212/wnl.40.8.1246. [DOI] [PubMed] [Google Scholar]
- Enzi G, Favaretto L, Martini S, Fellin R, Baritussio A, Baggio G, et al. Metabolic abnormalities in multiple symmetric lipomatosis: elevated lipoprotein lipase activity in adipose tissue with hyperalphalipoproteinemia. J Lipid Res. 1983;24:566–74. [PubMed] [Google Scholar]
- Zancanaro C, Sbarbati A, Morroni M, Carraro R, Cigolini M, Enzi G, et al. Multiple symmetric lipomatosis. Ultrastructural investigation of the tissue and preadipocytes in primary culture. Lab Invest. 1990;63:253–8. [PubMed] [Google Scholar]
- Nisoli E, Regianini L, Briscini L, et al. Multiple symmetric lipomatosis may be the consequence of defective noradrenergic modulation of proliferation and differentiation of brown fat cells. J Pathol. 2002;198:378–87. doi: 10.1002/path.1212. [DOI] [PubMed] [Google Scholar]
- Rial E, Nicholls DG. The mitochondrial uncoupling protein from guinea-pig brown adipose tissue. Synchronous increase in structural and functional parameters during cold-adaptation. Biochem J. 1984;222:685–93. doi: 10.1042/bj2220685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany T, Gharehpapagh E, Kamel E, Buck A, Himms-Hagen J, von Schulthess G. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nuclear Med Mol Imaging. 2002;29:1393–8. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- Seemayer TA, Knaack J, Wang NS, Ahmed MN. On the ultrastructure of hibernoma. Cancer. 1975;36:1785–93. doi: 10.1002/1097-0142(197511)36:5<1785::aid-cncr2820360533>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Krief S, Lonnqvist F, Raimbault S, Baude B, Van Spronsen A, Arner P, et al. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest. 1993;91:344–9. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberkofler H, Dallinger G, Liu YM, Hell E, Krempler F, Patsch W. Uncoupling protein gene: quantification of expression levels in adipose tissues of obese and non-obese humans. J Lipid Res. 1997;38:2125–33. [PubMed] [Google Scholar]
- Morelli F, De Benedetto A, Toto P, Tulli A, Feliciani C. Alcoholism as a trigger of multiple symmetric lipomatosis. J Eur Acad Dermatol Venereol. 2003;17:367–9. doi: 10.1046/j.1468-3083.2003.00792_15.x. [DOI] [PubMed] [Google Scholar]
- Dwells AR, Plans AB, Sarasura JG.Cervical LipomatosisManual de Cirugia Plastica. Vol 39: Sociedad de Espanola de Cirugia; 2000
- Cinti S, Enzi G, Cigolini M, Bosello O. Ultrastructural features of cultured mature adipocyte precursors from adipose tissue in multiple symmetric lipomatosis. Ultrastruct Pathol. 1983;5:145–52. doi: 10.3109/01913128309141834. [DOI] [PubMed] [Google Scholar]
- Vila MR, Gamez J, Solano A, Playán A, Schwartz S, Santorelli FM, et al. Uncoupling protein-1 mRNA expression in lipomas from patients bearing pathogenic mitochondrial DNA mutations. Biochem Biophys Res Commun. 2000;278:800–2. doi: 10.1006/bbrc.2000.3828. [DOI] [PubMed] [Google Scholar]
- Kazumi T, Ricquier D, Maeda T, Masuda T, Hozumi T, Ishida Y, et al. Failure to detect brown adipose tissue uncoupling protein mRNA in benign symmetric lipomatosis (Madelung's disease) Endocr J. 1994;41:315–8. doi: 10.1507/endocrj.41.315. [DOI] [PubMed] [Google Scholar]
- Coin A, Sergi G, Enzi G, Busetto L, Pigozzo S, Lupoli L, et al. Total and regional body composition and energy expenditure in multiple symmetric lipomatosis. Clin Nutr. 2005;24:367–74. doi: 10.1016/j.clnu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Dorigo P, Prosdocimi M, Carpenedo F, Caparrotta L, Tessari F, Enzi G. Multiple symmetric lipomatosis. A defect in adrenergic stimulated lipolysis II. Pharmacol Res Commun. 1980;12:625–38. doi: 10.1016/s0031-6989(80)80100-7. [DOI] [PubMed] [Google Scholar]
- Enzi G, Inelmen EM, Baritussio A, Dorigo P, Prosdocimi M, Mazzoleni F. Multiple symmetric lipomatosis: a defect in adrenergic-stimulated lipolysis. J Clin Invest. 1977;60:1221–9. doi: 10.1172/JCI108881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai KS, Zinman B, Steiner G. Multiple symmetric lipomatosis (Launois-Bensaude syndrome) - adipose tissue insensitivity to cyclic AMP. J Clin Endocrinol Metab. 1979;49:307–9. doi: 10.1210/jcem-49-2-307. [DOI] [PubMed] [Google Scholar]
- Kather H, Schroder F. Adrenergic regulation of fat-cell lipolysis in multiple symmetric lipomatosis. Eur J Clin Invest. 1982;12:471–4. doi: 10.1111/j.1365-2362.1982.tb02227.x. [DOI] [PubMed] [Google Scholar]
- Pecquery R, Malagrida L, Giudicelli Y. Adipocyte adenylate cyclase and alpha- and beta-adrenergic receptors in one case of multiple symmetric lipomatosis. Biomedicine. 1980;33:64–6. [PubMed] [Google Scholar]
- Bechara FG, Sand M, Sand D, Rotterdam S, Stücker M, Altmeyer P, et al. Lipolysis of lipomas in patients with familial multiple lipomatosis: an ultrasonography-controlled trial. J Cutan Med Surg. 2006;10:155–9. doi: 10.2310/7750.2006.00040. [DOI] [PubMed] [Google Scholar]
- Mogilevskii IL, Osmolovskaia NN, Deputovich SA. Microcirculatory disorders of the arm in post-mastectomy edema. Sov Med. 1989. pp. 15–20. [PubMed]
- Brorson H, Ohlin K, Olsson G, Svensson B, Svensson H. Controlled compression and liposuction treatment for lower extremity lymphedema. Lymphology. 2008;41:52–63. [PubMed] [Google Scholar]
- Garcia Hidalgo L. Dermatological complications of obesity. Am J Clin Dermatol. 2002;3:497–506. doi: 10.2165/00128071-200203070-00006. [DOI] [PubMed] [Google Scholar]
- Suga H, Eto H, Aoi N, Kato H, Araki J, Doi K, et al. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg. 2010;126:1911–23. doi: 10.1097/PRS.0b013e3181f4468b. [DOI] [PubMed] [Google Scholar]
- Becker ST, Wiltfang J, Klapper W, Repp R, Sinis N, Warnke PH. Massive swelling of the cervical region: an uncommon manifestation of B cell chronic lymphocytic leukemia. Oral Maxillofac Surg. 2008;12:205–8. doi: 10.1007/s10006-008-0128-2. [DOI] [PubMed] [Google Scholar]
- Dwyer TM, Mizelle HL, Cockrell K, Buhner P. Renal sinus lipomatosis and body composition in hypertensive, obese rabbits. Int J Obes Relat Metab Disord. 1995;19:869–74. [PubMed] [Google Scholar]
- Vasileiou AM, Bull R, Kitou D, Alexiadou K, Garvie NJ, Coppack SW. Oedema in obesity; role of structural lymphatic abnormalities. Int J Obes (London) 2011;35:1247–50. doi: 10.1038/ijo.2010.273. [DOI] [PubMed] [Google Scholar]
- Pollock M, Nicholson GI, Nukada H, Cameron S, Frankish P. Neuropathy in multiple symmetric lipomatosis. Madelung's disease. Brain. 1988;111:1157–71. doi: 10.1093/brain/111.5.1157. [DOI] [PubMed] [Google Scholar]
- Chen XM, Li WY, Ni DF, Wei BJ, Xu CX, Gao ZQ, et al. Diagnosis and treatment of Madelung's disease. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2006;41:524–7. [PubMed] [Google Scholar]
- Tizian C, Berger A, Vykoupil KF. Malignant degeneration in Madelung's disease (benign lipomatosis of the neck): case report. Br J Plast Surg. 1983;36:187–9. doi: 10.1016/0007-1226(83)90089-9. [DOI] [PubMed] [Google Scholar]
- Taylor LM, Beahrs OH, Fontana RS. Benign symmetric lipomatosis. Proc Staff Meet Mayo Clin. 1961;36:96–100. [PubMed] [Google Scholar]
- Uglesic V, Knezevic P, Milic M, Jokic D, Kosutic D. Madelung syndrome (benign lipomatosis): clinical course and treatment. Scand J Plast Reconstr Surg Hand Surg. 2004;38:240–3. doi: 10.1080/02844310410025295. [DOI] [PubMed] [Google Scholar]
- Fedele D, Bellavere F, Bosello G, Cardone C, Girardello L, Ferri M, et al. Impairment of cardiovascular autonomic reflexes in multiple symmetric lipomatosis. J Auton Nerv Syst. 1984;11:181–8. doi: 10.1016/0165-1838(84)90075-4. [DOI] [PubMed] [Google Scholar]
- Lee HW, Kim TH, Cho JW, Ryu BY, Kim HK, Choi CS. Multiple symmetric lipomatosis: Korean experience. Dermatol Surg. 2003;29:235–40. doi: 10.1046/j.1524-4725.2003.29056.x. [DOI] [PubMed] [Google Scholar]
- Foldi M, Foldi E.Benign symmetric lipomatosisIn: Foldi M, Foldi E, Strossenreyther R, S K, eds. Földi's textbook of lymphology Germany: Elsevier, Urban & Fischer Verlag; 2007. p430–31. [Google Scholar]
- Guilemany JM, Romero E, Blanch JL. An aesthetic deformity: Madelung's disease. Acta Otolaryngol. 2005;125:328–30. doi: 10.1080/00016480410022903. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia R, Rodriguez-Campo FJ, Sastre-Perez J, Munoz-Guerra MF. Benign symmetric lipomatosis (Madelung's disease): case reports and current management. Aesthetic Plast Surg. 2004;28:108–12. discussion 113. doi: 10.1007/s00266-004-3123-5. [DOI] [PubMed] [Google Scholar]
- Verhelle NA, Nizet JL, Van den Hof B, Guelinckx P, Heymans O. Liposuction in benign symmetric lipomatosis: sense or senseless. Aesthetic Plast Surg. 2003;27:319–21. doi: 10.1007/s00266-003-2016-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Escribano JA, Gonzalez R, Quecedo E, Febrer I. Efficacy of lipectomy and liposuction in the treatment of multiple symmetric lipomatosis. Int J Dermatol. 1999;38:551–4. doi: 10.1046/j.1365-4362.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- Faga A, Valdatta LA, Thione A, Buoro M. Ultrasound assisted liposuction for the palliative treatment of Madelung's disease: a case report. Aesthetic Plast Surg. 2001;25:181–3. doi: 10.1007/s002660010118. [DOI] [PubMed] [Google Scholar]
- Horl C, Biemer E. Benign symmetrical lipomatosis. Lipectomy and liposuction in the treatment of Madelung disease. Handchir Mikrochir Plast Chir. 1992;24:93–6. [PubMed] [Google Scholar]
- Smith PD, Stadelmann WK, Wassermann RJ, Kearney RE. Benign symmetric lipomatosis (Madelung's disease) Ann Plast Surg. 1998;41:671–3. doi: 10.1097/00000637-199812000-00016. [DOI] [PubMed] [Google Scholar]
- Constantinidis J, Steinhart H, Zenk J, Bohlender J, Iro H. Surgical therapy of Madelung's disease in the head and neck area. HNO. 2003;51:216–20. doi: 10.1007/s00106-002-0683-z. [DOI] [PubMed] [Google Scholar]
- Leung NW, Gaer J, Beggs D, Kark AE, Holloway B, Peters TJ. Multiple symmetric lipomatosis (Launois-Bensaude syndrome): effect of oral salbutamol. Clin Endocrinol (Oxf) 1987;27:601–6. doi: 10.1111/j.1365-2265.1987.tb01190.x. [DOI] [PubMed] [Google Scholar]
- Zeitler H, Ulrich-Merzenich G, Richter DF, Vetter H, Walger P. Multiple benign symmetric lipomatosis — a differential diagnosis of obesity. Is there a rationale for fibrate treatment. Obes Surg. 2008;18:1354–6. doi: 10.1007/s11695-008-9491-1. [DOI] [PubMed] [Google Scholar]
- Mirouze J, Orsetti A, Vidal F. Application of 2 radioimmunological methods for the determination of growth hormone. Application to various dysmorphic syndromes. Ann Endocrinol (Paris) 1970;31:237–46. [PubMed] [Google Scholar]
- Harsch IA, Wiedmann R, Bergmann T, Hahn EG, Wiest GH. Unspecified gain of weight. Internist (Berl) 2005;46:1265–9. doi: 10.1007/s00108-005-1500-z. [DOI] [PubMed] [Google Scholar]
- Botwin KP, Sakalkale DP. Epidural steroid injections in the treatment of symptomatic lumbar spinal stenosis associated with epidural lipomatosis. Am J Phys Med Rehabil. 2004;83:926–30. doi: 10.1097/01.phm.0000143397.02251.56. [DOI] [PubMed] [Google Scholar]
- McCullen GM, Spurling GR, Webster JS. Epidural lipomatosis complicating lumbar steroid injections. J Spinal Disord. 1999;12:526–9. [PubMed] [Google Scholar]
- Taille C, Fartoukh M, Houel R, Kobeiter H, Remy P, Lemaire F. Spontaneous hemomediastinum complicating steroid-induced mediastinal lipomatosis. Chest. 2001;120:311–3. doi: 10.1378/chest.120.1.311. [DOI] [PubMed] [Google Scholar]
- Fischer M, Wohlrab J, Taube KM, Marsch WC. Intralesional injection of enoxaparin in benign symmetrical lipomatosis: an alternative to surgery. Br J Dermatol. 2001;144:629–30. doi: 10.1046/j.1365-2133.2001.04102.x. [DOI] [PubMed] [Google Scholar]
- Rotunda AM, Ablon G, Kolodney MS. Lipomas treated with subcutaneous deoxycholate injections. J Am Acad Dermatol. 2005;53:973–8. doi: 10.1016/j.jaad.2005.07.068. [DOI] [PubMed] [Google Scholar]
- Allen EV, Hines EAJ. Lipedema of the legs:A syndrome characterised by fat legs and orthostatic edema. Proc Staff Meet Mayo Clin. 1940;15:184–7. [Google Scholar]
- Fife CE, Maus EA, Carter MJ. Lipedema: a frequently misdiagnosed and misunderstood fatty deposition syndrome. Adv Skin Wound Care. 2010;23:81–92. 93–84. doi: 10.1097/01.ASW.0000363503.92360.91. [DOI] [PubMed] [Google Scholar]
- Dimakakos PB, Stefanopoulos T, Antoniades P, Antoniou A, Gouliamos A, Rizos D. MRI and ultrasonographic findings in the investigation of lymphedema and lipedema. Int Surg. 1997;82:411–6. [PubMed] [Google Scholar]
- Wold LE, Hines EA, Jr, Allen EV. Lipedema of the legs; a syndrome characterized by fat legs and edema. Ann Intern Med. 1951;34:1243–50. doi: 10.7326/0003-4819-34-5-1243. [DOI] [PubMed] [Google Scholar]
- Child AH, Gordon KD, Sharpe P, Brice G, Ostergaard P, Jeffery S, et al. Lipedema: an inherited condition. Am J Med Genet A. 2010;152A:970–6. doi: 10.1002/ajmg.a.33313. [DOI] [PubMed] [Google Scholar]
- Foldi E, Foldi M.LipedemaIn: Foldi M, Foldi E, eds. Foldi's Textbook of Lymphology. Munich (Germany): Elsevier GmbH; 2006. p417–27. [Google Scholar]
- Chen SG, Hsu SD, Chen TM, Wang HJ. Painful fat syndrome in a male patient. Br J Plast Surg. 2004;57:282–6. doi: 10.1016/j.bjps.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Schmeller W, Meier-Vollrath I. Lipödem-aktuelles zu einem weitgehend unbekannter Krankheitsbild. Aktuelle Dermatologie. 2007;33:1–10. [Google Scholar]
- Meier-Vollrath I, Schneider W, Schmeller W. Das Lipodem: neue Möglichkeiten der Therapie. Schweiz Med Forum. 2007;7:150–5. [Google Scholar]
- Meier-Vollrath I, Schmeller W. Lipoedema — current status, new perspectives. J Dtsch Dermatol Ges. 2004;2:181–6. doi: 10.1046/j.1439-0353.2004.04051.x. [DOI] [PubMed] [Google Scholar]
- Pascucci A, Lynch PJ. Lipedema with multiple lipomas. Dermatol Online J. 16(9):4. [PubMed] [Google Scholar]
- Cornely ME.Lipedema and Lymphatic EdemaIn: Shiffman MA, Di Giuseppe A, eds. Liposuction. Berlin Heidelberg: Springer; 200610–4. [Google Scholar]
- Greer KE. Lipedema of the legs. Cutis. 1974;14:98. [Google Scholar]
- Földi E, Földi M.Das LipödemIn: Földi M, Földi E, Kubik S, eds. Lehrbuch der Lymphologie für Mediziner, Masseure und Physiotherapeuten. Munich: Elsevier, Urban & Fischer; 2005. p443–53. [Google Scholar]
- Stallworth JM, Hennigar GR, Jonsson HT, Jr, Rodriguez O. The chronically swollen painful extremity. A detailed study for possible etiological factors. JAMA. 1974;228:1656–9. [PubMed] [Google Scholar]
- Curri SB, Merlen JF. Microvascular disorders of adipose tissue. J Mal Vasc. 1986;11:303–9. [PubMed] [Google Scholar]
- Merlen JF, Curri SB, Sarteel AM. Cellulitis, a conjunctive microvascular disease. Phlebologie. 1979;32:279–82. [PubMed] [Google Scholar]
- Suga H, Araki J, Aoi N, Kato H, Higashino T, Yoshimura K. Adipose tissue remodeling in lipedema: adipocyte death and concurrent regeneration. J Cutan Pathol. 2009;3:3. doi: 10.1111/j.1600-0560.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- Kaiserling E.Morphological changes in lymphedema and tumorsIn: Foldi M, Foldi E, eds. Foldi's Textboof of Lymphology. Munich (Germany): Elsevier, GmbH; 2006322–90. [Google Scholar]
- Kligman AM. Cellulite: facts and fiction. J Geriatric Dermatol. 1997;5:136–9. [Google Scholar]
- Jagtman BA, Kuiper JP, Brakkee AJ. Measurements of skin elasticity in patients with lipedema of the Moncorps “rusticanus” type. Phlebologie. 1984;37:315–9. [PubMed] [Google Scholar]
- Taylor NE, Foster WC, Wick MR, Patterson JW. Tumefactive lipedema with pseudoxanthoma elasticum-like microscopic changes. J Cutan Pathol. 2004;31:205–9. doi: 10.1111/j.0303-6987.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- Szolnoky G.Chapter 17: Differential Diagnosis - LipedemaIn: Lee BB, Bergan J, Rockson SG, eds. Lymphedema: A Concise Compendium of Theory and Practice. London: Springer-Verlag; 2011. p125–35. [Google Scholar]
- Wienert V, Leeman S. Lipedema. Hautarzt. 1991;42:484–6. [PubMed] [Google Scholar]
- Partsch H, Stoberl C, Urbanek A, Wenzel-Hora BI. Clinical use of indirect lymphography in different forms of leg edema. Lymphology. 1988;21:152–60. [PubMed] [Google Scholar]
- Tiedjen KU, Schultz-Ehrenburg U.Isotopenlymphographische Befunde beim LipödemIn: Holzmann H, Altmeyer P, Hör G, eds. Dermatologie und Nuklearmedizin. Berlin: Springer-Verlag; 1985. p432–438. [Google Scholar]
- van Geest AJ, Esten SCAM, Cambier J-PRA, et al. Lymphatic disturbances in lipedema. Phlebologie. 2003;32:138–42. [Google Scholar]
- Amann-Vesti BR, Franzeck UK, Bollinger A. Microlymphatic aneurysms in patients with lipedema. Lymphology. 2001;34:170–175. [PubMed] [Google Scholar]
- Bollinger A, Amann-Vesti BR. Fluorescence microlymphography: diagnostic potential in lymphedema and basis for the measurement of lymphatic pressure and flow velocity. Lymphology. 2007;40:52–62. [PubMed] [Google Scholar]
- Harwood CA, Bull RH, Evans J, Mortimer PS. Lymphatic and venous function in lipoedema. Br J Dermatol. 1996;134:1–6. [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–81. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- Schneider M, Conway EM, Carmeliet P. Lymph makes you fat. Nat Genet. 2005;37:1023–4. doi: 10.1038/ng1005-1023. [DOI] [PubMed] [Google Scholar]
- Bollinger A. Microlymphatics of human skin. Int J Microcirc Clin Exp. 1993;12:1–15. [PubMed] [Google Scholar]
- Brautigam P, Foldi E, Schaiper I, Krause T, Vanscheidt W, Moser E. Analysis of lymphatic drainage in various forms of leg edema using two compartment lymphoscinctigraphy. Lymphology. 1998;31:43–55. [PubMed] [Google Scholar]
- Bilancini S, Lucchi M, Tucci S, Eleuteri P. Functional lymphatic alterations in patients suffering from lipedema. Angiology. 1995;46:333–9. doi: 10.1177/000331979504600408. [DOI] [PubMed] [Google Scholar]
- de Godoy JM, de Godoy Mde F. Treatment of cellulite based on the hypothesis of a novel physiopathology. Clin Cosmet Investig Dermatol. 4:55–9. doi: 10.2147/CCID.S20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayrakci Tunay V, Akbayrak T, Bakar Y, Kayihan H, Ergun N. Effects of mechanical massage, manual lymphatic drainage and connective tissue manipulation techniques on fat mass in women with cellulite. J Eur Acad Dermatol Venereol. 2009;24:138–42. doi: 10.1111/j.1468-3083.2009.03355.x. [DOI] [PubMed] [Google Scholar]
- Wenczl E, Daroczy J. Lipedema, a barely known disease: diagnosis, associated diseases and therapy. Orv Hetil. 2008;149:2121–7. doi: 10.1556/OH.2008.28490. [DOI] [PubMed] [Google Scholar]
- Tsukanov Iu T, Tsukanov A. Syndrome of lower limb volume enlargement in varicosity: causes and medical approaches. Angiol Sosud Khir. 2007;13:85–91. [PubMed] [Google Scholar]
- Tan IC, Maus EA, Rasmussen JC, Marshall MV, Adams KE, Fife CE, et al. Assessment of lymphatic contractile function after manual lymphatic drainage using near-infrared fluorescence imaging. Arch Phys Med Rehabil. 1992:756–764. e751. doi: 10.1016/j.apmr.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szolnoky G, Nagy N, Kovacs RK, Dósa-Rácz E, Szabó A, Bársony K, et al. Complex decongestive physiotherapy decreases capillary fragility in lipedema. Lymphology. 2008;41:161–6. [PubMed] [Google Scholar]
- Szolnoky G, Borsos B, Barsony K, Balogh M, Kemeny L. Complete decongestive physiotherapy with and without pneumatic compression for treatment of lipedema: a pilot study. Lymphology. 2008;41:40–4. [PubMed] [Google Scholar]
- Adams KE, Rasmussen JC, Darne C, Tan IC, Aldrich MB, Marshall MV, et al. Direct evidence of lymphatic function improvement after advanced pneumatic compression device treatment of lymphedema. Biomed Opt Express. 2010;1:114–25. doi: 10.1364/BOE.1.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninson J, Edelglass JW. Lipedema — the non-lymphatic masquerader. Angiology. 1984;35:506–10. doi: 10.1177/000331978403500806. [DOI] [PubMed] [Google Scholar]
- Bauke J. Kritische Gegenüberstellung der verschiedenen behandlungsmethoden der adipositas. Med Welt. 1983;34:201–3. [PubMed] [Google Scholar]
- Hines EA., Jr Lipedema and physiologic edema. Proc Staff Meet Mayo Clin. 1952;27:7–9. [PubMed] [Google Scholar]
- Tidhar D, Katz-Leurer M. Aqua lymphatic therapy in women who suffer from breast cancer treatment-related lymphedema: a randomized controlled study. Support Care Cancer. 2009;18:383–92. doi: 10.1007/s00520-009-0669-4. [DOI] [PubMed] [Google Scholar]
- Lohman EB, 3rd, Petrofsky JS, Maloney-Hinds C, Betts-Schwab H, Thorpe D. The effect of whole body vibration on lower extremity skin blood flow in normal subjects. Med Sci Monit. 2007;13:CR71–76. [PubMed] [Google Scholar]
- Kerschan-Schindl K, Grampp S, Henk C, Resch H, Preisinger E, Fialka-Moser V, et al. Whole-body vibration exercise leads to alterations in muscle blood volume. Clin Physiol. 2001;21:377–82. doi: 10.1046/j.1365-2281.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Karman C, Montgomery LD, McLeod KJ. Plantar vibration improves leg fluid flow in perimenopausal women. Am J Physiol Regul Integr Comp Physiol. 2005;288:R623–629. doi: 10.1152/ajpregu.00513.2004. [DOI] [PubMed] [Google Scholar]
- Gulias S, Nieto S. Psychological assistaqnce and its importance in the medical treatment of lymphedema. J Soc Phlebol Lymphol. 2007;2:179–87. [Google Scholar]
- Schmeller W, Meier-Vollrath I. Tumescent liposuction: a new and successful therapy for lipedema. J Cutan Med Surg. 2006;10:7–10. doi: 10.1007/7140.2006.00006. [DOI] [PubMed] [Google Scholar]
- Warren AG, Janz BA, Borud LJ, Slavin SA. Evaluation and management of the fat leg syndrome. Plast Reconstr Surg. 2007;119:9e–15e. doi: 10.1097/01.prs.0000244909.82805.dc. [DOI] [PubMed] [Google Scholar]
- Ray C. Caring for the Bariatric Patient with lymphedema and obesity. Bariatrics Today. 2004. pp. 48–50.
- Foldi E, Foldi M.LipedemaIn: Foldi M, Foldi E, Kubik S, eds. Foldi's Textbook of Lymphology. Munich (Germany): Elsevier GmbH; 2006. p417–427. [Google Scholar]
- Williams A. Amy's Butterfly Journey: Living My Life with Lymphedema and Obesity. Obesity Help. 2004. pp. 43–4.
- Moore JC, Ballas ZK. A novel therapy for lymphedema. Arch Intern Med. 2009;169:201–2. doi: 10.1001/archinternmed.2008.580. [DOI] [PubMed] [Google Scholar]
- Bruns F, Micke O, Bremer M. Current status of selenium and other treatments for secondary lymphedema. J Supportive Oncol. 2003;1:121–30. [PubMed] [Google Scholar]
- Sasaki H, Okumura M, Kawasaki T, Kangawa K, Matsuo H. Indomethacin and atrial natriuretic peptide in Pseudo-Bartter's syndrome. N Engl J Med. 1987;316:167. doi: 10.1056/NEJM198701153160314. [DOI] [PubMed] [Google Scholar]
- Pecking AP, Fevrier B, Wargon C, Pillion G. Efficacy of Daflon 500 mg in the treatment of lymphedema (secondary to conventional therapy of breast cancer) Angiology. 1997;48:93–8. doi: 10.1177/000331979704800115. [DOI] [PubMed] [Google Scholar]
- Pecking AP. Evaluation by lymphoscintigraphy of the effect of a micronized flavonoid fraction (Daflon 500 mg) in the treatment of upper limb lymphedema. Int Angiol. 1995;14:39–43. [PubMed] [Google Scholar]
- Casley-Smith JR, Casley-Smith JR. The effects of diosmin (a benzo-pyrone) upon some high-protein oedemas: lung contusion, and burn and lymphoedema of rat legs. Agents Actions. 1985;17:14–20. doi: 10.1007/BF01966674. [DOI] [PubMed] [Google Scholar]
- Suter A, Bommer S, Rechner J. Treatment of patients with venous insufficiency with fresh plant horse chestnut seed extract: a review of 5 clinical studies. Adv Ther. 2006;23:179–90. doi: 10.1007/BF02850359. [DOI] [PubMed] [Google Scholar]
- Cornely ME.Lipedema and Lymphatic EdemaIn: Shiffman MA, Di Giuseppe A, eds. Liposuction Principles and Practice. Berlin Heidelberg: Springer; 2006 [Google Scholar]
- Conley SM, Bruhn RL, Morgan PV, Stamer WD. Selenium's effects on MMP-2 and TIMP-1 secretion by human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2004;45:473–9. doi: 10.1167/iovs.03-0767. [DOI] [PubMed] [Google Scholar]
- Obenheimer H, Jankowiak P, Berlemann K, Hermann V, Diethelm A.Clinical and biological effects of selenium in edemaPaper presented at: Proceedings of the International Symposium: Lymphedema — New Perspectives in Research and Treatment. 1976Zaragossa, Spain [Google Scholar]
- Schrauzer GN. Selenium in the therapy of chronic lymphedema—mechanistic perspectives and practical applications. Z Lymphol. 1997;21:16–9. [Google Scholar]
- Micke O, Bruns F, Schäfer U, Kisters K, Hesselmann S, Willich N. Selenium in the treatment of acute and chronic lymphedema. Trace Elements and Electrolytes. 2000;17:206–9. [Google Scholar]
- Horvathova M, Jahnova E, Gazdik F. Effect of selenium supplementation in asthmatic subjects on the expression of endothelial cell adhesion molecules in culture. Biol Trace Elem Res. 1999;69:15–26. doi: 10.1007/BF02783912. [DOI] [PubMed] [Google Scholar]
- Kiremidjian-Schumacher L, Roy M, Glickman R, Schneider K, Rothstein S, Cooper J, et al. Selenium and immunocompetence in patients with head and neck cancer. Biol Trace Elem Res. 2000;73:97–111. doi: 10.1385/BTER:73:2:97. [DOI] [PubMed] [Google Scholar]
- Siems W, Grune T, Voss P, Brenke R. Anti-fibrosclerotic effects of shock wave therapy in lipedema and cellulite. Biofactors. 2005;24:275–82. doi: 10.1002/biof.5520240132. [DOI] [PubMed] [Google Scholar]
- Dercum FX. Three cases of a hitherto unclassified affection resembling in its grosser aspects obesity, but associated with special nervous symptoms - adiposis dolorosa. Am J Med Sci. 1892;civ:521. [Google Scholar]
- Dercum FX. A subcutaneous connective-tissue dystrophy of the arms and back, associated with symptoms resembling myxoedema. Univ Med Magazine Philadelphia. 1888. pp. 140–50.
- Dercum FX. Autopsy in a case of adiposis dolorosa, with microscopical examination. J Nerv Ment Dis. 1900;XXVII(8):419–29. [Google Scholar]
- Lyon IP. Adiposis and lipomatosis: Considered in reference to their constitutional relations and symptomatology. Arch Int Med. 1910;VI:28–120. [Google Scholar]
- Brorson H, Fagher B. Dercum's disease. Fatty tissue rheumatism caused by immune defense reaction. Lakartidningen. 1996;93:1430, 1433–6. [PubMed] [Google Scholar]
- Herbst KL, Asare-Bediako S. Adiposis Dolorosa is More than Painful Fat. The Endocrinologist. 2007;17:326–44. [Google Scholar]
- Walser WC. Adiposis dolorosa in an infant. Boston Med Surg J. 1910;CLXII:906–7. [Google Scholar]
- Bruning H, Walter FK. Zur frage der Adipositas dolorosa (Dercumshe Krankheit) in Kindersalter. Ztscyhr f Kinderh. 1919;24:183. [Google Scholar]
- Loening K, Fuss S. Schildrusenveranderungen bei Adipositas dolorosa. Verhandl d Cong f inn Med. 1906. p. 222.
- Steiger WA, Litvin H, Lasche EM, Durant TM. Adiposis dolorsa (Dercum's disease) N Engl J Med. 1952;247:393–6. doi: 10.1056/NEJM195209112471104. [DOI] [PubMed] [Google Scholar]
- Vitaut L. Maladie de Dercum (adiposis dolorosa) Loire med St Etienne. 1901;20:254. [Google Scholar]
- Kling DH. Juxta-articular adiposis dolorosa, its significance and relation to Dercum's disease and osteo-arthritis. J Arch Surg. 1937;34:599–630. [Google Scholar]
- Lynch HT, Harlan WL. Hereditary factors in adiposis dolorosa (Dercum's disease) Am J Hum Genet. 1963;15:184–90. [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Schiltz K, Heidenreich F, Weissenborn K. Lipomatosis dolorosa — a frequently overlooked disease picture. Nervenarzt. 2002;73:183–7. doi: 10.1007/s00115-001-1240-9. [DOI] [PubMed] [Google Scholar]
- Pack GT, Ariel IM. New York: Hoeber-Harper; 1958. Tumors of the soft somatic tissues: a clinical treatise. [Google Scholar]
- Thimm P. Adipositas dolorosa und schmerzende symmetrische. Lipome Montash f prakt Dermatol. 1903;xxxvi:282. [Google Scholar]
- Campen R, Mankin H, Louis DN, Hirano M, Maccollin M. Familial occurrence of adiposis dolorosa. J Am Acad Dermatol. 2001;44:132–6. doi: 10.1067/mjd.2001.110872. [DOI] [PubMed] [Google Scholar]
- Cantu JM, Ruiz-Barquin E, Jimenez M, Castillo L, Macotela-Ruiz E. Autosomal dominant inheritance in adiposis dolorosa (Dercum's disease) Humangenetik. 1973;18:89–91. doi: 10.1007/BF00279037. [DOI] [PubMed] [Google Scholar]
- Winkelman NW, Eckel JL. Adiposis dolorosa (Dercum's disease) a clinicopathologic study. J Am Med Assoc. 1925;85:1935–9. [Google Scholar]
- Herbst KL, Coviello AD, Chang A, Boyle DL. Lipomatosis-associated inflammation and excess collagen may contribute to lower relative resting energy expenditure in women with adiposis dolorosa. Int J Obes (Lond) 2009;33:1031–8. doi: 10.1038/ijo.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpilä J, Ripatti N. Adipositas dolorosa juxtaarticularis (Dercum's disease) och dess behandling [in Swedish] Nord Med. 1958;59:358–60. [PubMed] [Google Scholar]
- Hovesen E. Adiposis dolorosa (Dercum's syndrome) Nord Med. 1953;50:971. [PubMed] [Google Scholar]
- Blomstrand R, Juhlin L, Nordenstam H, Ohlsson R, Werner B, Engstrom J. Adiposis dolorosa associated with defects of lipid metabolism. Acta Derm Venereol. 1971;51:243–50. [PubMed] [Google Scholar]
- Page IH. Chemiache Untersuchungen bel der Dercumschen Krankheit. Virchow Arch Path Anat. 1930;279:262. [Google Scholar]
- Mella BA. Adiposis dolorosa. Univ Michigan Med Center J. 1967;33:79–81. [PubMed] [Google Scholar]
- Held JL, Andrew JA, Kohn SR. Surgical amelioration of Dercum's disease: a report and review. J Dermatol Surg Oncol. 1989;15:1294–6. doi: 10.1111/j.1524-4725.1989.tb03150.x. [DOI] [PubMed] [Google Scholar]
- Campen RB, Sang CN, Duncan LM. Case records of the Massachusetts General Hospital. Case 25-2006. A 41-year-old woman with painful subcutaneous nodules. N Engl J Med. 2006;355:714–22. doi: 10.1056/NEJMcpc069018. [DOI] [PubMed] [Google Scholar]
- Pimenta WP, Paula FJ, Dick-de-Paula I, Piccinato CE, Monteiro CM, Brandão-Neto J, et al. Hormonal and metabolic study of a case of adiposis dolorosa (Dercum's disease) Braz J Med Biol Res. 1992;25:889–93. [PubMed] [Google Scholar]
- Ballet G. L'adipose, douloureuse (maladie de Dercum) Presse méd Par. 1903;i:285–8. [Google Scholar]
- Price GE. Adiposis dolorosa: a clinical and pathological study, with the report of two cases with necropsy. Am J Med Sci. 1909;137:705–14. [Google Scholar]
- Eshner AA. A case of adiposis dolorosa. J Am Med Assoc. 1898;XXXI:1156–60. [Google Scholar]
- Collins J.Adiposis dolorosaIn: Dercum FX, ed. Textbook of Nervous Diseases. Lea Brothers & Co; 1895. p898–200. [Google Scholar]
- Alger EM. Clinical memoranda: A case of adiposis dolorosa. Med News (1882-1905) 1901;78:91–2. [Google Scholar]
- Frankenheimer JB. Adiposis dolorosa. J Am Med Assoc. 1908;L:1012–3. [Google Scholar]
- Skagen K, Petersen P, Kastrup J, Norgaard T. The regulation of subcutaneous blood flow in patient with Dercum's disease. Acta Derm Venereol. 1986;66:337–9. [PubMed] [Google Scholar]
- Falta W.Endocrine diseasesIn: Meyers MK, ed. 2 ed. Philadelphia: Blakiston's Son & Co; 1916575 [Google Scholar]
- Moi L, Canu C, Pirari P, Mura MN, Piludu G, Del Giacco GS. Dercum's disease: a case report. Ann Ital Med Int. 2005;20:187–91. [PubMed] [Google Scholar]
- Lange U, Oelzner P, Uhlemann C. Dercum's disease (Lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29:17–22. doi: 10.1007/s00296-008-0635-3. [DOI] [PubMed] [Google Scholar]
- Pascucci A, Lynch PJ. Lipedema with multiple lipomas. Dermatol Online J. 2010;16:4. [PubMed] [Google Scholar]
- Rockson SG. The unique biology of lymphatic edema. Lymphat Res Biol. 2009;7:97–100. doi: 10.1089/lrb.2009.7202. [DOI] [PubMed] [Google Scholar]
- Spiller WG. Clinical Memoranda: Report of three cases of adiposis dolorosa. Med News. 1898. pp. 268–70.
- Eisman J, Swezey RL. Juxta-articular adiposis dolorosa: what is it? Report of 2 cases. Ann Rheum Dis. 1979;38:479–82. doi: 10.1136/ard.38.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White WH. A case of adiposis dolorosa. Br Med J. 1899;2:1533–4. doi: 10.1136/bmj.2.2031.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr CW. A case of adiposis dolorosa with necropsy. J Nervous Mental Disease. 1900;XXVII:519–25. [Google Scholar]
- Dercum FX, McCarthy DJ. Autopsy in a case of adiposis dolorosa. Am J Med Sci. 1902;(124):994. [Google Scholar]
- Abu-Hijleh MF, Scothorne RJ. Studies on haemolymph nodes. IV. Comparison of the route of entry of carbon particles into parathymic nodes after intravenous and intraperitoneal injection. J Anat. 1996;188:565–73. [PMC free article] [PubMed] [Google Scholar]
- Mills CK. A case of adeno lipomatosis: With some remarks on the differential diagnosis of the affectation from adiposis dolorosa and other diseases. J Nervous Mental Disease. 1918;36:106–8. [Google Scholar]
- Stolkind E. Cases of adiposis dolorosa (Dercum's disease) Proc Royal Soc Med. 1923;16 (Clinical Section):45–7. doi: 10.1177/003591572301600336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JA. An instance of adiposis dolorosa in two sisters. Br Med J. 1904;2:121. doi: 10.1136/bmj.2.2272.121-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Smith FM, Kurtz KM, Molina PE, Breslin JW. Adaptation of mesenteric collecting lymphatic pump function following acute alcohol intoxication. Microcirculation. 2010;17:514–24. doi: 10.1111/j.1549-8719.2010.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dercum FX. Note on a case of adiposis dolorosa in which there was present also spasticity and contracture involving the extremities. J Nervous Mental Disease. 1918;36:159–62. [Google Scholar]
- Price GE, Bird JT. Adiposis dolorosa: A report of a case with increased sugar tolerance and epileptiform convulsions. J Am Med Association. 1925;84:247–8. [Google Scholar]
- Wortham NC, Tomlinson IP. Dercum's disease. Skinmed. 2005;4:157–162. quiz 163–4. doi: 10.1111/j.1540-9740.2005.03675.x. [DOI] [PubMed] [Google Scholar]
- Bergeron PN. A case of adiposis dolorosa with involvement of the large nerve trunks. J Nerv Mental Disease. 1918;36:159. [Google Scholar]
- Kirpila J, Ripatti N. Adiposis dolorosa juxta-articularis: Dercum's disease & its therapy. Nordisk medicin March. 1958;59:358–60. [PubMed] [Google Scholar]
- Taylor EW, Luce DS. A case of adiposis dolorosa. Boston Medical and Surgical Journal. CLIV:187–90. [Google Scholar]
- Myers B. Case of adiposis dolorosa. Proceedings of the Royal Society of Med. 1923;16 (Clinical Section):12–3. doi: 10.1177/003591572301600312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ED. Dercum's disease: adiposis dolorosa. Am Fam Physician. 1981;24:155–7. [PubMed] [Google Scholar]
- Szypula I, Kotulska A, Szopa M, Pieczyrak R, Kucharz EJ. Adiposis dolorosa with hypercholesterolemia and premature severe generalized atherosclerosis. Wiad Lek. 2009;62:64–65. [PubMed] [Google Scholar]
- Maddalozzo GF, Iwaniec UT, Turner RT, Rosen CJ, Widrick JJ. Whole-body vibration slows the acquisition of fat in mature female rats. Int J Obes (Lond) 2008;32:1348–54. doi: 10.1038/ijo.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alentorn-Geli E, Padilla J, Moras G, Lazaro Haro C, Fernandez-Sola J. Six weeks of whole-body vibration exercise improves pain and fatigue in women with fibromyalgia. J Altern Complement Med. 2008;14:975–81. doi: 10.1089/acm.2008.0050. [DOI] [PubMed] [Google Scholar]
- Singal A, Janiga J, Bossenbroek N, Lim H. Dercum's disease (adiposis dolorosa): a report of improvement with infliximab and methotrexate. J Eur Acad Dermatol Venereol. 2007;21:717. doi: 10.1111/j.1468-3083.2006.02021.x. [DOI] [PubMed] [Google Scholar]
- Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147–53. doi: 10.2147/JPR.S12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzler RK, Sargent RW, Kimura IK, et al. The effect of a cyclic variable altitude conditioning program on arterial oxygen saturation acclimationPaper presented at: 53rd Annual Meeting, American College of Sporte Med, 2006. Denver, CO.
- Juhlin L. Long-standing pain relief of adiposis dolorosa (Dercum's disease) after intravenous infusion of lidocaine. J Am Acad Dermatol. 1986;15:383–5. doi: 10.1016/s0190-9622(86)70184-9. [DOI] [PubMed] [Google Scholar]
- Desai MJ, Siriki R, Wang D. Treatment of pain in Dercum's disease with Lidoderm (lidocaine 5% patch): a case report. Pain Med. 2008;9:1224–6. doi: 10.1111/j.1526-4637.2008.00417.x. [DOI] [PubMed] [Google Scholar]
- Petersen P, Kastrup J. Dercum's disease (adiposis dolorosa) Treatment of the severe pain with intravenous lidocaine. Pain. 1987;28:77–80. doi: 10.1016/0304-3959(87)91062-1. [DOI] [PubMed] [Google Scholar]
- Devillers AC, Oranje AP. Treatment of pain in adiposis dolorosa (Dercum's disease) with intravenous lidocaine: a case report with a 10-year follow-up. Clin Exp Dermatol. 1999;24:240–1. doi: 10.1046/j.1365-2230.1999.00466.x. [DOI] [PubMed] [Google Scholar]
- Atkinson RL. Intravenous lidocaine for the treatment of intractable pain of adiposis dolorosa. Int J Obes. 1982;6:351–7. [PubMed] [Google Scholar]
- Taniguchi A, Okuda H, Mishima Y, Nagata I, Oseko F, Hara M, et al. A case of adiposis dolorosa: lipid metabolism and hormone secretion. Int J Obes. 1986;10:277–81. [PubMed] [Google Scholar]
- Iwane T, Maruyama M, Matsuki M, Ito Y, Shimoji K. Management of intractable pain in adiposis dolorosa with intravenous administration of lidocaine. Anesth Analg. 1976;55:257–9. doi: 10.1213/00000539-197603000-00028. [DOI] [PubMed] [Google Scholar]
- Reggiani M, Errani A, Staffa M, Schianchi S. Is EMLA effective in Dercum's disease. Acta Derm Venereol. 1996;76:170–1. doi: 10.2340/0001555576170171. [DOI] [PubMed] [Google Scholar]
- Berntorp E, Berntorp K, Brorson H, Frick K. Liposuction in Dercum's disease: impact on haemostatic factors associated with cardiovascular disease and insulin sensitivity. J Intern Med. 1998;243:197–201. doi: 10.1046/j.1365-2796.1998.00264.x. [DOI] [PubMed] [Google Scholar]
- DeFranzo AJ, Hall JH, Jr, Herring SM. Adiposis dolorosa (Dercum's disease): liposuction as an effective form of treatment. Plast Reconstr Surg. 1990;85:289–92. [PubMed] [Google Scholar]
- Brorson H, Aberg M, Fagher B. Liposuction in adiposis dolorosa (morbus Dercum) — an effective therapy. Ugeskr Laeger. 1992;154:1914–5. [PubMed] [Google Scholar]
- Wollina U, Goldman A, Heinig B. Microcannular tumescent liposuction in advanced lipedema and Dercum's disease. G Ital Dermatol Venereol. 2010;145:151–9. [PubMed] [Google Scholar]
- Hansson E, Svensson H, Brorson H. Liposuction may reduce pain in Dercum's disease (adiposis dolorosa) Pain Med. 2011;12:942–52. doi: 10.1111/j.1526-4637.2011.01101.x. [DOI] [PubMed] [Google Scholar]
- Tsang C, Aggarwal R, Bonanomi G. Dercum's disease as a cause of weight loss failure after gastric bypass surgery. Surg Obes Relat Dis. 2011;7:243–5. doi: 10.1016/j.soard.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Wohl MG, Pastor N. Adipositas dolorosa (Dercum's disease) JAMA. 1938;110:1261–4. [Google Scholar]
- Sorrels TL, Bostock E. Induction of feeding by 7-chlorokynurenic acid, a strychnine-insensitive glycine binding site antagonist. Brain Res. 1992;572:265–8. doi: 10.1016/0006-8993(92)90481-n. [DOI] [PubMed] [Google Scholar]
- Spota BB, Brage D. Cortisona Y Enfermedad de Dercum. Dia med B Air. 1952;24:1930. [PubMed] [Google Scholar]
- Greenbaum SS, Varga J. Corticosteroid-induced juxta-articular adiposis dolorosa. Arch Dermatol. 1991;127:231–3. [PubMed] [Google Scholar]
- Rebuffe-Scrive M, Lonnroth P, Marin P, Wesslau C, Bjorntorp P, Smith U. Regional adipose tissue metabolism in men and postmenopausal women. Int J Obes. 1987;11:347–55. [PubMed] [Google Scholar]
- de Médicis Sajous CE.The Internal secretions and the principles of medicineVol 1. 9th ed. Philadelphia: FA Davis Co; 1920 [Google Scholar]