Abstract
Aim:
To explore the role of the glucagon-like peptide 1 receptor (GLP-1R) in geniposide regulated insulin secretion in rat INS-1 insulinoma cells.
Methods:
Rat INS-1 insulinoma cells were cultured. The content of insulin in the culture medium was measured with ELISA assay. GLP-1R gene in INS-1 cells was knocked down with shRNA interference. The level of GLP-1R protein in INS-1 cells was measured with Western blotting.
Results:
Geniposide (0.01–100 μmol/L) increased insulin secretion from INS-1 cells in a concentration-dependent manner. Geniposide (10 μmol/L) enhanced acute insulin secretion in response to both the low (5.5 mmol/L) and moderately high levels (11 mmol/L) of glucose. Blockade of GLP-1R with the GLP-1R antagonist exendin (9–39) (200 nmol/L) or knock-down of GLP-1R with shRNA interference in INS-1 cells decreased the effect of geniposide (10 μmol/L) on insulin secretion stimulated by glucose (5.5 mmol/L).
Conclusion:
Geniposide increases insulin secretion through glucagon-like peptide 1 receptors in rat INS-1 insulinoma cells.
Keywords: geniposide, glucagon-like peptide 1 receptor, insulin secretion, type 2 diabetes mellitus, RNA interference
Introduction
Type 2 diabetes mellitus involves defects in both insulin secretion and insulin sensitivity. Multiple insulin secretion defects could occur, including the absence of pulsatility1, loss of the early phase of insulin secretion after glucose stimulation2, decreased basal and stimulated concentrations of insulin in the plasma3, excess secretion of pro-hormone, and progressive decreases in insulin secretory capacity4. Thus, the regulation of insulin secretion in pancreatic β cells has long been considered important for the prevention and treatment of type 2 diabetes mellitus4.
Emerging data have shown that GLP-1 functions through its receptor to regulate insulin secretion and glucose metabolism and is, therefore, a potentially important target in the treatment of type 2 diabetes. GLP-1 is able to improve insulin secretion in subjects with impaired glucose tolerance and type 2 diabetes5. Studies have demonstrated that increased expression or activity of GLP-1R is beneficial to β cell functions, including insulin synthesis/secretion6, proliferation7, neogenesis7, and anti-apoptosis8. Unfortunately, GLP-1 has a half-life of less than 2 minutes in the circulatory system owing to rapid degradation by dipeptidyl peptidase-4 (DPP IV)9.
Utilizing a high-throughput screening assay, we previously identified geniposide as a potent agonist of GLP-1R and possessed neurotrophic and neuroprotective properties10, 11, 12. However, it is unknown whether geniposide is able to improve insulin secretion in pancreatic β cells. In the present study we examined the effect of geniposide on insulin secretion in INS-1 cells. The results demonstrate that geniposide increased insulin secretion in a dose-dependent manner and enhanced glucose-stimulated insulin secretion in INS-1 cells. We have further observed that exendin (9–39), an antagonist of GLP-1R, and GLP-1R siRNA decreased the effect of geniposide on glucose-stimulated insulin secretion in INS-1 cells.
Materials and methods
Cell culture
An INS-1 rat insulinoma cell line was purchased from the CCTCC (China Center for Type Culture Collection). The cells were grown in a humidified atmosphere with 5% CO2 at 37 °C. The culture medium was RPMI-1640 containing 11 mmol/L glucose, which was supplemented with 10% FBS, 10 mmol/L HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, and 50 μmol/L mercaptoethanol. Cells were passaged every week following trypsinization.
Geniposide induces insulin secretion in a concentration-dependent manner
To investigate the effect of geniposide on insulin secretion, INS-1 cells were seeded onto 12-well plates and cultured for 24 h. The cells were washed twice with Krebs-Ringer bicarbonate buffer (KRBH; 129 mmol/L NaCl, 4.8 mmol/L KCl, 1.2 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 2.5 mmol/L CaCl2, 5 mmol/L NaHCO3, 0.1% BSA, 10 mmol/L HEPES; pH 7.4) and starved for 2 h in KRBH buffer. Then, the cells were incubated with fresh KRBH containing different concentrations of geniposide (0, 0.01, 0.1, 1.0, 10, or 100 μmol/L) for 1 h. The supernatants were collected to determine the insulin content using commercial kit (Linco Research, Inc, Missouri, USA) according to the kit' instructions.
Effect of geniposide on insulin secretion stimulated by glucose
To explore the influence of geniposide on glucose-stimulated insulin secretion, INS-1 cells were seeded onto 12-well plates and cultured for 24 h. After the cells were washed two times with fresh KRBH and starved for 2 h in fresh KRBH, the cells were incubated with 10 μmol/L geniposide in the absence or presence of indicated concentrations of glucose for 1 h. The supernatants were collected and analyzed for insulin concentration using commercial kit (Linco Research, Inc, Missouri, USA) according to the kit' instructions.
The role of GLP-1R in geniposide-inducing insulin secretion
To probe the role of GLP-1R, we investigated the effect of an antagonist for GLP-1R on insulin secretion in the presence of a low concentration of glucose. INS-1 cells were starved for 2 h in fresh KRBH, placed for 1 h in fresh KRBH containing 200 nmol/L exendin (9–39), and then treated for 1 h with 5.5 mmol/L glucose in the presence or absence of 10 μmol/L geniposide. The supernatants were collected to probe the insulin concentration using commercial kits.
To further explore the role of GLP-1R in the regulatory effects of geniposide on insulin secretion, we constructed a stable INS-1 cell line with the GLP-1R silenced by a shGLP-1R plasmid, which was optimized in our previous work11. The level of GLP-1R expression was analyzed with Western blot and real-time PCR. The primers were 5′-TATTGGCTCATCATACGCTTG-3′ (GLP-1R sense), 5′-GTCTGCATTTG ATGTCGGTCT-3′ (GLP-1R antisense), 5′-AAGTTCAACGGCACAGTCAAG-3′ (GAPDH sense) and 5′-CCAGTAGACTCCACGACATACTCA-3′ (GAPDH antisense). The mRNA and protein levels of GLP-1R are about 35.4% and 21% those of the control, respectively. Using the same protocol as introduced in Materials and Methods, we determined the insulin secretion induced by geniposide in INS-1 cells in which GLP-1R had been knocked down.
Statistical analysis
Data are expressed as the means±SD from at least three independent experiments. When significance was detected, the differences between the treatments were analyzed using a one-way ANOVA with the Origin 8.0 Pair-Sample t-Test. The significance level was set at P<0.05.
Results
Geniposide regulates insulin secretion in INS-1 cells
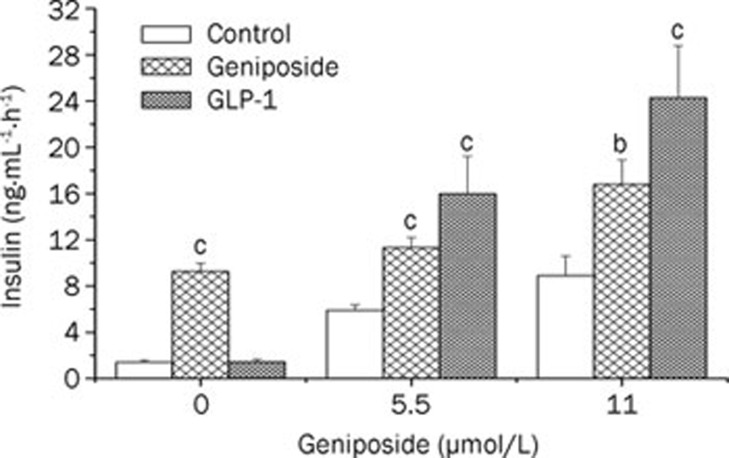
As shown in Figure 1, geniposide elicited concentration-dependent increases in insulin secretion in β cells. Similar to GLP-1, geniposide also increased acute insulin secretion in response to both low and moderately high glucose levels (Figure 2).
Figure 1.
Geniposide induces insulin secretion in a concentration-dependent manner in INS-1 cells. The INS-1 cells at passages of 68–73 were seeded at 5×105 cells/well in 12-well-plates. The next day, the cells were washed twice with KRBH buffer and starved in KRBH buffer for 2 h. The media was changed with fresh KRBH containing different concentrations of geniposide (0, 0.01, 0.1, 1.0, 10, or 100 μmol/L). Following 1-h incubation, the medium was collected and analyzed for insulin concentration. Data are expressed as means±SD from 4 independent experiments. cP<0.01 vs control by one-way ANOVA followed by the Pair-Sample t-Test using Origin 8.0 software.
Figure 2.
Geniposide potentiates glucose-stimulated insulin secretion in INS-1 cells. INS-1 cells were seeded in 12-well-plates and continued to incubate for 24 h; the cells were washed twice with fresh KRBH and starved in KRBH buffer for 2 h. The media was changed with fresh KRBH containing different concentrations of glucose (0, 5.5, or 11 mmol/L) with or without 10 μmol/L geniposide or GLP-1 (330 nmol/L). Following 1-h incubation, the medium was collected and analyzed for insulin concentration. Data are expressed as means±SD from 6 independent experiments. bP<0.05, cP<0.01 vs control at the same glucose concentration by one-way ANOVA followed by the Pair-Sample t-Test using Origin 8.0 software.
In normoglycemic subjects, glucose-stimulated insulin secretion is characterized by a biphasic pattern, with the peak of the first phase rising abruptly 3–5 min after glucose stimulation and lasting for about 10 min4. To determine whether geniposide induces changes in both phases of insulin secretion, we tested the influence of geniposide on biphasic insulin secretion in INS-1 cells in the absence or presence of low glucose levels. It was observed that geniposide enhanced insulin secretion in both phases when glucose was present (Figure 3).
Figure 3.
Effects of geniposide on the biphasic glucose-stimulated insulin secretion in INS-1 cells. INS-1 cells were treated with 10 μmol/L geniposide in the presence or absence of 5.5 mmol/L glucose (G5.5) for 20 min. The media were collected in a 30-s intermediate to determine the insulin secretion with ELISA. Data are expressed as mean of two independent experiments.
GLP-1R plays an important role in geniposide-induced insulin secretion in INS-1 cells.
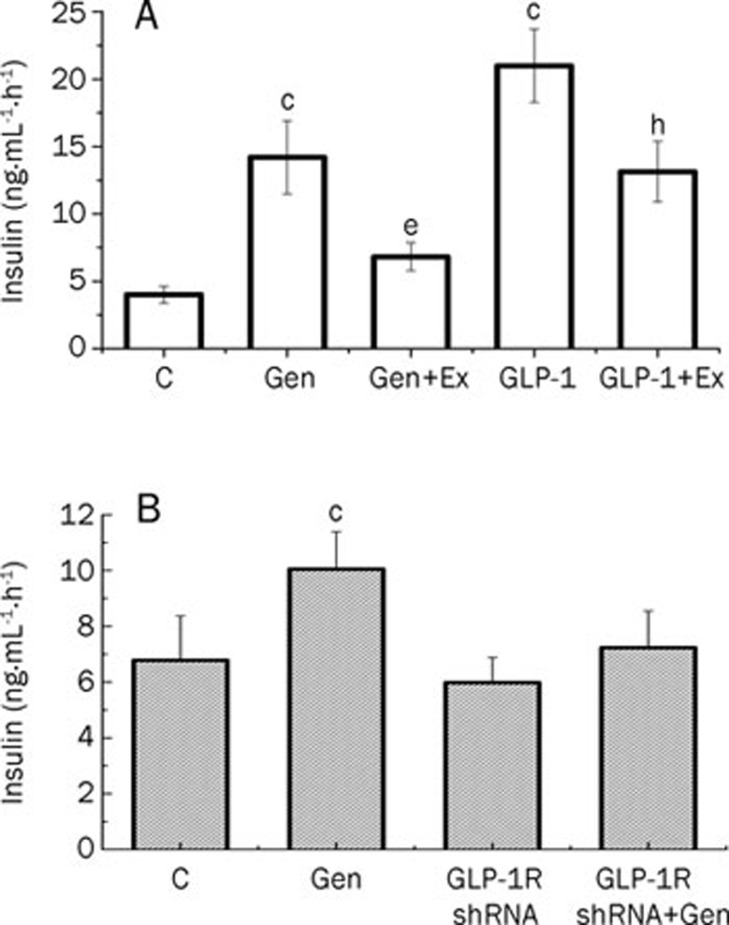
Our previous work showed that geniposide was an agonist of GLP-1R10, 11, 12. To investigate the role of GLP-1R in glucose-stimulated insulin secretion, we determined the effect of geniposide on insulin secretion in the presence or absence of a GLP-1R antagonist, exendin (9–39) amide. The data demonstrate that pretreatment with 200 nmol/L exendin (9–39) for 1 h prevented the geniposide-induced increase in insulin secretion in INS-1 cells (Figure 4A).
Figure 4.
GLP-1R plays a critical role in geniposide-induced insulin secretion in INS-1 cells. (A) After the attached INS-1 cells were washed twice and starved for 2 h, the media was changed with KRBH buffer containing 5.5 mmol/L glucose, and INS-1 cells were treated with geniposide (10 μmol/L) or GLP-1 (330 nmol/L) in the presence or absence of GLP-1 receptor antagonist, exendin (9–39) (Ex, 200 nmol/L) for 1 h. (B) GLP-1R-interferred INS-1 cells and control cells were treated with 5.5 mmol/L glucose for 1 h in the presence or absence of 10 μmol/L geniposide, respectively. The media were collected to determine the level of insulin secretion. Data are expressed as means±SD from 6 independent experiments. cP<0.01 vs control. eP<0.05 vs geniposide group; hP<0.05 vs GLP-1 group by one-way ANOVA followed by the Pair-Sample t-Test using Origin 8.0 software.
We also checked the effect of GLP-1R interference on geniposide-induced insulin secretion in INS-1 cells. The results showed that, in INS-1 cells in which GLP-1R had been knocked down, the level of insulin secretion induced by 5.5 mmol/L glucose did not differ from that of the control cells. These results suggest that glucose-stimulated insulin secretion is not dependent upon GLP-1R. However, in contrast to normal cultured INS-1 cells, geniposide failed to enhance glucose stimulated insulin secretion in GLP-1R- knockdown INS-1 cells (Figure 4B). Taken together with the effect of exendin (9–39) on geniposide regulation of glucose-stimulated insulin secretion, these results indicate that GLP-1R plays an important role in geniposide regulation of insulin secretion in INS-1 cells.
Discussion
Type 2 diabetes mellitus is a metabolic disorder that manifests mainly as abnormalities in pancreatic insulin secretion and the peripheral actions of insulin. During the progression of diabetes, the mass of β cells is reduced, with a concomitant decrease in the amount of insulin secreted. An effective therapeutic option is to use drugs to boost the production and release of insulin by pancreatic islets. For example, sulfonylurea-based drugs that act on the β-cell secretory apparatus augment the release of insulin, overcoming peripheral insulin resistance and restoring normoglycemia in type 2 diabetic patients. Nonetheless, treatment with sulfonylureas is associated with side-effects, such as non-optimal normoglycemic control, which triggers protracted hypoglycemic episodes, worsens myocardial function, and potentially causes weight gain13, 14.
Alternatively, natural hypoglycemic compounds may offer advantages over the drugs currently used for diabetes. For example, plant-derived iridoids, of which many are dietary constituents, may serve as promising candidates for the development of therapeutics to prevent or treat diabetes. Jin et al reported that aucubin lowers blood glucose and protects the pancreas from oxidative stress in streptozotocin-induced diabetes. Thus, aucubin might have potential as a safe preventive agent against diabetes15. In the present study, we examined the effect of geniposide, one of the main iridoid glucoside of Gardenia fruit, on insulin secretion and GLP-1R. We found, for the first time, that geniposide induces insulin secretion in β-cells in a dose-dependent manner and that geniposide has a synergistic effect on insulin secretion in INS-1 cells in the presence of low and moderately high doses of glucose. We also observed that geniposide directly induced insulin secretion in INS-1 cells in the absence of glucose. These results may be a consequence of the activation of GLP-1R by geniposide, which regulates the concentration of Ca2+ through a signal pathway, or changes in the energy balance of INS-1 cells, which alters the ATP ratio and further regulates the activity of ATP-sensitive K+ channels.
GLP-1 is an incretin, which is released in response to carbohydrate and fat intake and enhances glucose-stimulated insulin secretion16. An increasing number of studies have demonstrated the benefits of GLP-1 for increasing pancreatic β cell mass and function. Because GLP-1 exerts its beneficial effects through binding and activating its receptor, GLP-1R is a valuable target for the treatment of diabetes17. However, GLP-1 has an extremely short half-life owing to rapid degradation by DPP IV, which limits the therapeutic value of exogenous GLP-19. Therefore, it is important to develop small molecules that are able to activate GLP-1R. The ability of geniposide to activate GLP-1R has previously been demonstrated in neurons10, 12. However, the effects of this compound on the regulation of insulin secretion and GLP-1R are poorly understood. It is conceivable that GLP-1R agonists can play a role as anti-diabetic agents and as tools for researching islet metabolism. Nevertheless, our findings about the action of geniposide on β-cell insulin secretion warrant investigation into the molecular mechanisms involved. Moreover, we also observed that both exendin (9–39) and GLP-1R siRNA off-set the effect of geniposide on insulin secretion, suggesting that GLP-1R plays a critical role in the geniposide-induced increase in insulin secretion by INS-1 cells.
Additionally, the observed increase in glucose-stimulated insulin secretion in INS-1 cells following incubation with geniposide was especially evident during the first-phase of insulin secretion. In type 2 diabetes mellitus, the first-phase of insulin secretion is abolished and late phase secretion is reduced and delayed18. The early phase of insulin secretion is pivotal in the transition from the fasting state to the fed state with a different function19. A reduction in the first-phase of insulin secretion has been found both in patients with overt type 2 diabetes mellitus and at the early stage of disease development, which involves impaired glucose tolerance and a slightly elevated fasting glucose20. The effect of geniposide on insulin secretion through activation of GLP-1R suggests that geniposide may be a potential therapeutic agent for the prevention or treatment of type 2 diabetes.
Author contribution
Jian-hui LIU and Zhi-ning XIA designed the research; Li-xia GUO, Zhi-ning XIA, Xue GAO, Fei YIN, and Jian-hui LIU performed the research; and Jian-hui LIU and Li-xia GUO analyzed the data and wrote the paper.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30973576), the Key Project of the Chinese Ministry of Education (209098), the Chongqing Science & Technology committee (CSTC, 2009BA5069, 2009BB5373, 2011jjA1396), the Chongqing Municipal Education Committee (KJ090710; KJ090733) and the Innovative Research Team Development Program at the University of Chongqing (No KJTD201020). The authors are grateful to Prof Yan-wen WANG from the Institute for Nutrisciences and Health, and the National Research Council of Canada for providing suggestions and corrections during the preparation of this manuscript.
References
- Bergsten P. Pathophysiology of impaired pulsatile insulin release. Diabetes Metab Res Rev. 2000;16:179–91. doi: 10.1002/1520-7560(200005/06)16:3<179::aid-dmrr115>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kano Y, Kanatsuna T, Nakamura N, Kitagawa Y, Mori H, Kajiyama S, et al. Defect of the first-phase insulin secretion to glucose stimulation in the perfused pancreas of the nonobese diabetic (NOD) mouse. Diabetes. 1986;35:486–90. doi: 10.2337/diab.35.4.486. [DOI] [PubMed] [Google Scholar]
- Hartter E, Svoboda T, Ludvik B, Schuller M, Lell B, Kuenburg E, et al. Basal and stimulated plasma levels of pancreatic amylin indicate its co-secretion with insulin in humans. Diabetologia. 1991;34:52–4. doi: 10.1007/BF00404025. [DOI] [PubMed] [Google Scholar]
- Guillausseau PJ, Meas T, Virally M, Laloi-Michelin M, Medeau V, Kevorkian JP. Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes Metab. 2008;34 Suppl 2:S43–8. doi: 10.1016/S1262-3636(08)73394-9. [DOI] [PubMed] [Google Scholar]
- Arulmozhi DK, Portha B. GLP-1 based therapy for type 2 diabetes. Eur J Pharm Sci. 2006;28:96–108. doi: 10.1016/j.ejps.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Kielgast U, Holst J, Madsbad S. Treatment of type 1 diabetic patients with glucagon-like peptide-1 (GLP-1) and GLP-1R agonists. Curr Diabetes Rev. 2009;5:266–75. doi: 10.2174/157339909789804413. [DOI] [PubMed] [Google Scholar]
- Egan JM, Bulotta A, Hui H, Perfetti R. GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes Metab Res Rev. 2003;19:115–23. doi: 10.1002/dmrr.357. [DOI] [PubMed] [Google Scholar]
- Cornu M, Thorens B. GLP-1 protects beta-cells against apoptosis by enhancing the activity of an IGF-2/IGF1-receptor autocrine loop. Islets. 2009;1:280–2. doi: 10.4161/isl.1.3.9932. [DOI] [PubMed] [Google Scholar]
- Ahren B, Winzell MS, Wierup N, Sundler F, Burkey B, Hughes TE. DPP-4 inhibition improves glucose tolerance and increases insulin and GLP-1 responses to gastric glucose in association with normalized islet topography in mice with beta-cell-specific overexpression of human islet amyloid polypeptide. Regul Pept. 2007;143:97–103. doi: 10.1016/j.regpep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Liu JH, Zheng XX, Yin F, Hu YH, Guo LX, Deng X, et al. Neurotrophic property of geniposide for inducing the neuronal differentiation of PC12 cells. Int J Dev Neurosci. 2006;24:419–24. doi: 10.1016/j.ijdevneu.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Yin F, Liu JH, Zheng XX, Guo LX. GLP-1 receptor plays a critical role in geniposide-induced expression of heme oxygenase-1 in PC12 cells. Acta Pharmacol Sin. 2010;31:540–5. doi: 10.1038/aps.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JH, Yin F, Zheng XX, Jing JJ, Hu YH. Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem Int. 2007;51:361–9. doi: 10.1016/j.neuint.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Martin S, Kolb H, Beuth J, van Leendert R, Schneider B, Scherbaum WA. Change in patients' body weight after 12 months of treatment with glimepiride or glibenclamide in type 2 diabetes: a multicentre retrospective cohort study. Diabetologia. 2003;46:1611–7. doi: 10.1007/s00125-003-1238-x. [DOI] [PubMed] [Google Scholar]
- Jennings AM, Wilson RM, Ward JD. Symptomatic hypoglycemia in NIDDM patients treated with oral hypoglycemic agents. Diabetes Care. 1989;12:203–8. doi: 10.2337/diacare.12.3.203. [DOI] [PubMed] [Google Scholar]
- Jin L, Xue HY, Jin LJ, Li SY, Xu YP. Antioxidant and pancreas-protective effect of aucubin on rats with streptozotocin-induced diabetes. Eur J Pharmacol. 2008;582:162–7. doi: 10.1016/j.ejphar.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A. 2000;97:6874–9. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspohl EJ. Novel therapeutics for type 2 diabetes: incretin hormone mimetics (glucagon-like peptide-1 receptor agonists) and dipeptidyl peptidase-4 inhibitors. Pharmacol Ther. 2009;124:113–38. doi: 10.1016/j.pharmthera.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Rayman G, Grenfell A, Gray IP, Day JL, Hales CN. Loss of the first phase insulin response to intravenous glucose in subjects with persistent impaired glucose tolerance. Diabet Med. 1994;11:432–6. doi: 10.1111/j.1464-5491.1994.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Del Prato S, Marchetti P, Bonadonna RC. Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes. 2002;51:S109–16. doi: 10.2337/diabetes.51.2007.s109. [DOI] [PubMed] [Google Scholar]
- Widen EI, Eriksson JG, Ekstrand AV, Groop LC. The relationship between first-phase insulin secretion and glucose metabolism. Acta Endocrinol (Copenh) 1992;127:289–93. doi: 10.1530/acta.0.1270289. [DOI] [PubMed] [Google Scholar]