Abstract
Aim:
Sulodexide, a glycosaminoglycan, could reduce albuminuria in diabetic patients. The aim of this study was to determine whether sulodexide could be used to treat chronic kidney failure in rats.
Methods:
Sixty Wistar rats undergone 5/6 nephrectomy, then were randomly divided into 4 groups: the model group, sulodexide group (sulodexide 5 mg/kg per day, im), irbesartan group irbesartan (20 mg/kg per day, ig) and sulodexide plus irbesartan group. Another 12 rats were enrolled into the sham operation group. After the treatments for 4, 8 and 12 weeks, urinary protein and serum creatinine levels were measured. After 12 weeks, serum cholesterin and triglycerides levels were measured, and the degrees of glomerular sclerosis and renal tubulointerstitial fibrosis were scored. The expression of aminopeptidase P (JG-12) in the renal tissue was examined using immunohistochemical staining. The renal expressions of endothelial nitric oxide synthase (eNOS) and tissue type plasminogen activator (tPA) were detected with RT-PCR and Western blot.
Results:
Proteinuria was markedly attenuated in the sulodexide-treated groups. After 4 and 8 weeks only the sulodexide-treated groups showed significant reduction in serum creatinine; while after 12 weeks all the three treatment groups showed significant reduction in serum creatinine. Furthermore, all the three treatment groups showed significant reduction in the scores of glomerular sclerosis and tubulointerstitial fibrosis. The glomerular expression of JG-12 was increased in both the sulodexide group and the sulodexide plus irbesartan group, but not in the irbesartan group. The eNOS mRNA and protein expression was decreased and the tPA mRNA and protein expression was significantly increased in the model group compared with Sham group. Sulodexide, irbesartan, and their combination reversed the decrease of eNOS expression but increased the tPA expression much more compared with model group.
Conclusion:
Sulodexide was similar to irbesartan that can decrease proteinuria and attenuate renal lesions in 5/6 nephrectomy rats. The renal protection by sulodexide might be achieved via its impact on renal vascular endothelial cells.
Keywords: chronic renal failure, 5/6 nephrectomy, sulodexide, irbesartan, endothelial nitric oxide synthase (eNOS), tissue type plasminogen activator (tPA), aminopeptidase P(JG-12), vascular endothelium
Introduction
Chronic renal failure (CRF) is a disease that seriously endangers human health. Currently, significant progress has been made in delaying chronic renal failure (CRF) progression with therapy focusing on the blockade of the renin-angiotensin-aldosterone system (RAAS) and on blood pressure control. However, these measures are not sufficient to halt the progression of CRF1. It has remained a scientifically significant objective to find other agents to delay CRF progression. Renal vascular endothelium injury is one of the factors contributing to the progression of CRF pathological changes. Promoting the repair of injured endothelium can achieve the effects of stabilizing renal function and delaying the progression of CRF which is not related to the control of blood pressure and proteinuria2.
Sulodexide is a compound created from the fractional precipitation of glycosaminoglycans extracted from the mucosa of swine intestines, and it consists mainly of fast-moving heparin (FMH) and dermatan sulfate (DS)3. Some research has shown that sulodexide can improve endothelial dysfunction in diabetic rats4, and it has been proved that sulodexide can reduce cell proliferation and matrix accumulation in the kidneys5. Sulodexide has been shown to reduce proteinuria in patients with diabetic nephropathy6. Sulodexide also has anti-coagulant7,8, anti-inflammatory9,10, and anti-oxidative effects, as well as the effect of regulating blood fat11,12,13. These functions are related to the progression of CRF. Thus, we explored the efficacy of sulodexide in a CRF rat model using irbesartan, a commonly used ARB drug, as a control.
Materials and methods
Animals and model establishment
Seventy-two male Wistar rats, weighing 250 g to 330 g, were purchased from Beijing Vital River Company (License No: SCXK Jing 2001-0007) and were raised by the SPF Lab Animal Center of PLA General Hospital. All of the rats were housed in a constant-temperature room (maintained at 25±2 °C) with a consistent light cycle (from 7:00 am to 7:00 pm) and were fed a standard rat diet (0.5% Na, 22% protein). After one week of adaptability feeding, 12 rats were chosen randomly to receive a sham operation, and 5/6 nephrectomy was performed in the other 60 rats.
The rats that underwent 5/6 nephrectomy were randomly divided into a model control group, a sulodexide group, an irbesartan group, and a group combining sulodexide and irbesartan. Drug treatment commenced one week after the operations. Sulodexide injections, supplied by Alfa Issermann Pharmaceutical Inc, China (batch No 1884), were administered at a dosage of 5 mg/kg every other day by muscular injection. Irbesartan was supplied by Hangzhou Sanofi-Aventis Minsheng Pharmaceutical Co, Ltd (batch No 1564) and was administrated by gastric perfusion at a dose of 20 mg/kg each day. The combined group was treated with the same methods as the sulodexide group and the irbesartan group. Distilled water was given to the sham operation group and to the model control group. The rats were weighed each week, and the dosages of drugs and distilled water were adjusted accordingly for the entire course of 12 weeks. After 12 weeks, the numbers of rats remaining in the analysis were: 7 (control), 10 (sulodexide), 11 (irbesartan) and 11 (combined) (Figure 1).
Figure 1.
Animal experiments design.
General condition and urine/blood tests
The animals' energy level, activity, hair luster and food intake were observed. Upon completion of the 12-week experiment, the rats' blood pressure was measured with an LE5002 blood pressure instrument (Panlab SL Inc, Barcelona, Spain). 24-h urine was collected before the operations and at 4, 8, and 12 weeks after drug treatment. Urine protein was detected with a BS-400 Biochemical Analyzer purchased from Mindray Bio-Medical Electronics Co Ltd, Shenzhen, China.
Prior to the operation and 4 and 8 weeks after drug administration, 0.5 mL of blood from the endocanthion was drawn for serum creatinine testing with the 7600 Type Automatic Biochemistry Analyzer (HITACHI, Japan). Upon completion of the 12-week experiment, serum creatinine, triglycerides and cholesterin were measured.
Kidney pathologic examinations
A portion of the renal tissue was fixed in 10% formalin, and another portion was quickly frozen and stored in liquid nitrogen. After fixation with 10% formalin, the renal tissue was routinely treated and paraffin-embedded, and 2-μm sections were made. After staining with periodic acid-Schiff stain, the following semiquantitative scores were obtained using light microscopy3.
Glomerular sclerosis score
The score was graded from 0 to 4 points (0 points: normal glomerulus; 1 point: area of mesangial expansion or sclerosis <25%; 2 points: area of sclerosis from up to 25% to 50%; 3 points: area of sclerosis from up to 50% to 75%; 4 points: area of sclerosis greater than 75%). Fifty glomeruli were observed from each specimen under a microscope with 400-fold magnification, and the mean value was referred to as the glomerular sclerosis index.
Renal tubule-interstitium score
The score was based on tubular atrophy, interstitial inflammation, and fibrosis area. The score was graded from 0 to 3 points (0 points: free of tubulointerstitial lesions; 1 point: changes affecting <25% of the section; 2 points, changes affecting 25% to 50% of the section; and 3 points, changes affecting an area greater than 50%). Ten fields of vision were observed for each specimen under a microscope with 100-fold magnification. The mean value was referred to as the tubulointerstitial lesion index.
Immunohistochemistry (IHC)
After fixation with 4% paraformaldehyde, the renal tissue was submitted to routine treatment and was then embedded with paraffin, and 2-μm-thick sections were made before incubation with monoclonal JG-12 mouse anti-rat antibody (Santa Cruz Company, Santa Cruz, CA, USA), by means of the streptavidin-peroxidase method (Zhongshan Goldenbridge Biotechnology Co, Ltd, Beijing, China). The number of capillaries was counted per 0.01 mm2 of glomerular area under a microscope with 400-fold magnification.
Expression of eNOS and tPA mRNA in the kidneys
Upon the thawing of the renal tissues that had been stored in liquid nitrogen, Trizol was added, and homogenization was conducted for 2–3 min with a supersonic crusher. After extraction by chloroform, settling with isopropanol, and cleansing with 75% ethanol, the sample was dissolved in DEPC water, and RNA was quantified with a UV spectrophotometer.
Five micrograms of RNA template was reverse transcribed (reverse transcription reagent box provided by GIBCO Company, MI, USA) into cDNA and was expanded by PCR. The primer sequences are shown in Table 1. The PCR reaction bulk was 25 μL, the sample cDNA product was 1 μL, the 10×PCR buffer was 2.5 μL; Tag DNA polymerase was 0.25 μL; and 2.5 mmol/L dNTP mix was 2.4 μL. We used 25 mmol/L Mg2+ 3.5 μL and 25 μmol/L primers (Beijing SBS Genetech Co, Ltd), 1 μL each; we also used 13.35 μL of deionized, distilled water.
Table 1. Primers sequences for PCR, annealing temperature, and predicted size.
| Primers | Sequences | Annealing temperature (°C) | Predicted size (bp) |
|---|---|---|---|
| eNOS | 5′-TAACACAGACAGTGCAGGGG-3′ | 62 | 380 |
| 5′-CCTGGAACATCTTCCGTCTG-3′ | |||
| tPA | 5′-AGAGAGGTTTCCACCCCATC-3′ | 58 | 248 |
| 5′-CTGTCCAGTCAGGGAGCTGT-3 | |||
| GAPDH | 5′-TGCACCACCAACTGCTTAGC-3′ | 58 | 191 |
| 5′-GGCATGGACTGTGGTCATGAG-3′ |
The PCR products were subjected to electrophoresis on a 1.2% agarose gel before photographs were taken, and semiquantitative analysis was carried out using Quantity One Software for gel quantitative analysis.
Evaluation of the expression of eNOS and tPA protein in the kidneys with Western blotting
The frozen renal tissue in liquid nitrogen was added to a suitable amount of buffer solution and was then homogenized. The protein concentration was measured by the Coomassie brilliant blue method. The proteins were denatured, electrophoresed, transferred to PVDF membrane, and blocked with 5% degreased milk. eNOS antibody, tPA antibody (rabbit anti-rat eNOS and tPA polyclonal antibody, Santa Cruz Biotechnology, Inc) and anti β-actin antibody (1:200 dilution) were added. Incubation took place overnight at 4 °C. After membrane washing, the secondary antibodies (dilution 1:1000) marked with horseradish peroxidase (HRP) were added. Quantitative analysis of eNOS and tPA was performed with Quantity One Software for gel quantitative analysis.
Statistical analysis
Statistical analysis was conducted with SPSS software, version 15.0. The data were expressed as the mean±standard deviation (mean±SD). ANOVA was adopted for the comparison between different groups at the same time points. The LSD method was adopted for comparisons between two groups. P<0.05 was considered statistically significant.
Results
General condition and blood pressure
In the beginning of the experiment, the rats in the sham operation group acted quickly, and their coats were lustrous and in good condition. Meanwhile, no abnormal conditions in their diets were observed. After the operations, the rats in all of the groups appeared listless, and they moved less. Their furs pricked up and did not show order or luster. As time went by, their entire skins gradually became white, especially the skin at the ears, nose, feet, back and tail. No hematomas or other adverse reactions at the injection sites were observed.
The mean blood pressure of the model control group (112±5 mmHg) was higher than that of the sham group (108±4 mmHg) at the 12th week of treatment (P<0.05). The blood pressure levels in the irbesartan-treated group (108±3 mmHg) and the group receiving combined sulodexide and irbesartan (105±4 mmHg) were lower than those of the model control group (P<0.05).
Body weight
There was no significant difference in body weight for the rats in any of the groups before the operations or after the 5/6 nephrectomy but prior to drug treatment (0 weeks). Body weight was lower in the experimental rats after the operations compared with the sham operation group (P<0.05). Body weight increased in all of the rats over time (Table 2).
Table 2. Rats body weight condition at all groups. Mean±SD. bP<0.05, cP<0.01 vs Sham.
| Group |
Body weight (g) |
||||
|---|---|---|---|---|---|
| Before operation | 0 week | 4 weeks | 8 weeks | 12 weeks | |
| Sham (n=12) | 282.84±18.51 | 364.89±25.17 | 447.36±32.08 | 491.46±53.68 | 532.01±56.84 |
| Untreated (n=7) | 282.45±20.28 | 312.26±20.47b | 381.68±28.53c | 431.46±37.02b | 454.44±56.65b |
| IRB (n=11) | 281.38±15.46 | 310.48±29.57b | 383.10±32.66c | 420.15±32.82b | 449.89±40.02b |
| SLX (n=10) | 275.50±12.57 | 317.47±20.04b | 384.48±28.11c | 431.54±32.41b | 445.40±53.52b |
| SLX/IRB (n=11) | 299.70±27.39 | 323.69±23.19b | 379.64±40.72c | 428.09±61.16b | 451.86±66.85b |
*Sham: sham group; Untreated: model control group; SLX: sulodexide treated group;
IRB: irbesartan treated group; SLX/IRB: combining group of sulodexide and irbesartan.
Urinary protein and serum creatinine
Urinary protein and serum creatinine were not different among the 5 groups at baseline. Compared with the model group, 24-h urine protein was clearly lower in the irbesartan group, the sulodexide group, and the combined sulodexide and irbesartan group than that in the control group after 8 and 12 weeks of treatment (P<0.05). Serum creatinine in the model control group increased gradually over time. From the 4th week after treatment, the levels of serum creatinine in the sulodexide group were lower than those of the model control group (P<0.05). After 12 weeks of treatment, the levels of serum creatinine in all of the treated groups were lower than those of the model control group (Table 3).
Table 3. Quantitative analysis of urine protein and serum creatinine. Mean±SD. bP<0.05, cP<0.01 vs Sham. eP<0.05, fP<0.01 vs Model.
| Group |
Urine protein quantity (mg/24 h) |
|||
|---|---|---|---|---|
| Baseline | 4 weeks | 8 weeks | 12 weeks | |
| Sham (n=12) | 9.86±4.57 | 11.34±3.57 | 19.21±5.94 | 17.83±4.22 |
| Model (n=7) | 9.57±5.35 | 34.79±15.11c | 119.91±23.60c | 159.19±66.51c |
| IRB (n=11) | 8.31±3.48 | 20.24±10.54 | 61.00±24.73cf | 65.70±20.98cf |
| SLX (n=10) | 9.33±4.41 | 31.00±21.10c | 65.16±24.50cf | 90.02±57.82ce |
| SLX/IRB (n=11) | 9.00±4.14 | 33.51±17.99c | 66.95±28.17cf | 59.13±32.30bf |
| Group | Serum creatinine (μmol/L) | |||
| Baseline | 4 weeks | 8 weeks | 12 weeks | |
| Sham (n=12) | 28.15±4.79 | 22.70±3.42 | 29.84±4.23 | 46.04±7.70 |
| Model (n=7) | 26.71±2.65 | 83.04±20.80c | 94.61±14.56c | 167.06±26.62c |
| IRB (n=11) | 25.74±1.16 | 76.74±19.67c | 86.94±20.96c | 130.09±29.84ce |
| SLX (n=10) | 25.62±1.49 | 60.31±14.33cf | 75.35±23.88ce | 125.84±60.58ce |
| SLX/IRB (n=11) | 26.11±3.40 | 71.47±24.75c | 85.47±28.58c | 121.39±33.55ce |
Serum cholesterin (CH) and triglycerides (TG)
In the 12th week after the operations, the level of serum cholesterin in the model control group was higher than in the sham group (P<0.01). The serum cholesterin level of the sulodexide-treated group was lower than that of the model control group (P<0.05). No differences were found in the levels of triglycerides among all of the groups (Table 4).
Table 4. CH and TG changes after 12 weeks of treatment. Mean±SD. bP<0.05, cP<0.01 vs Sham. eP<0.05 vs Model.
| Group | Cholesterin (CH) (mmol/L) | Triglyceride (TG) (mmol/L) |
|---|---|---|
| Sham (n=12) | 1.86±0.25 | 0.96±0.34 |
| Model (n=7) | 3.00±1.25c | 1.69±1.32 |
| IRB (n=11) | 2.70±0.37c | 1.39±0.63 |
| SLX (n=10) | 2.25±0.60e | 1.28±0.62 |
| SLX/IRB (n=11) | 2.50±0.50 | 0.96±0.49 |
Pathology and immunohistochemistry
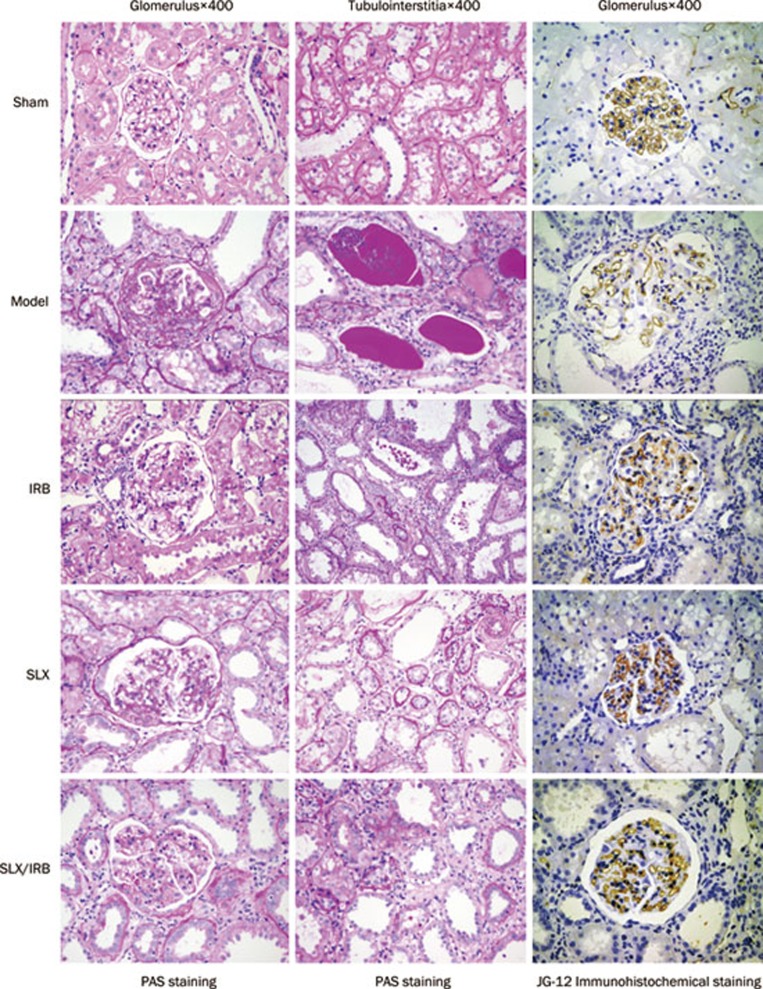
The model control group displayed glomerular hypertrophy, mesangial cell proliferation, mesangial matrix accumulation, telangiectasia or occlusions of the capillaries, thickening of the glomerular capsule wall, and focal or global sclerosis of some glomeruli. Furthermore, the renal tubules in this group showed dilation or atrophy, a large number of protein casts, interstitial widening, substantial infiltration of inflammatory cells, and focal distribution of renal interstitial microangiopathy, with narrowing and distortion of capillary cavities.
Compared with the model control group, the pathological changes in the sulodexide-treated group, the irbesartan-treated group, and the combination group were alleviated to different extents: lower glomerular sclerosis scores and tubulointerstitial scores were observed in these groups compared with the model control group (Figure 2, P<0.05).
Figure 2.
The glomerular and tubulointerstitial damage and loss of glomerular capillary loops were alleviated to different degrees in irbesartan (IRB), and sulodexide (SLX), and sulodexide combined with irbesartan (SLX/IRB) treated groups.
JG-12 staining showed far fewer glomerular capillary loops per 0.01 mm2 of cross-section area in the rats with 5/6 nephrectomy than in the sham group (P<0.01). There were more glomerular capillary loops per unit of area in the sulodexide-treated group and the combined sulodexide and irbesartan group than in the model control group (P<0.05) (Table 5).
Table 5. Pathological scores and JG-12 immunohistochemistry staining after 12 weeks of treatment. Mean±SD. bP<0.05, cP<0.01 vs Sham. eP<0.05, fP<0.01 vs Model.
| Groups | Glomerulus score | Tubulointerstitial score | Number of glomerular capillary loops (number/0.01 mm2) |
|---|---|---|---|
| Sham (n=12) | 0.05±0.02 | 0.05±0.05 | 12.99±4.02 |
| Model (n=7) | 1.83±0.32c | 2.10±0.12c | 4.72±2.91c |
| IRB (n=11) | 1.54±0.24cf | 1.71±0.30cf | 5.56±2.14c |
| SLX (n=10) | 1.58±0.21ce | 1.63±0.32cf | 7.28±3.01ce |
| SLX/IRB (n=11) | 1.50±0.27cf | 1.50±0.36cf | 7.12±4.45ce |
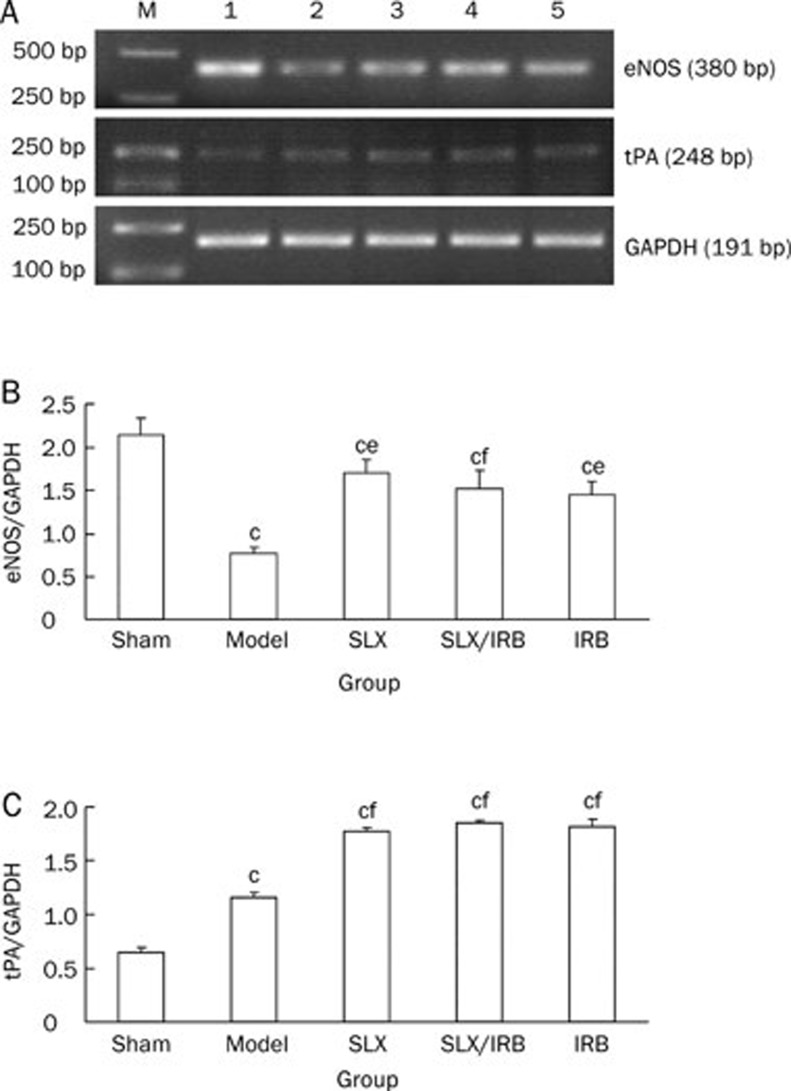
The mRNA expression of eNOS and tPA
Compared with the sham group, the expressions of eNOS in the model control group was reduced (P<0.01); compared with the model control group, the expressions of eNOS mRNA in the sulodexide-treated group, combined sulodexide and irbesartan therapy group, and irbesartan-treated group were increased (P<0.05, P<0.01, P<0.01, Figure 3A, 3B). The expression of tPA in the model control group was increased compared with Sham group, and the expression of tPA increased much more in the sulodexide-treated group, the combined sulodexide and irbesartan group, and irbesartan-treated group (all P<0.01, Figure 3A, 3C).
Figure 3.
Semiquantitative analysis of renal mRNA eNOS (A, B) and tPA (A, C) expression after 12 weeks of treatment. bP<0.05, cP<0.01 vs sham group. Mean±SD. Sham: n=12; Model: n=7; SLX: n=10; SLX/IRB: n=11; IRB: n=11. eP<0.05, fP<0.01 vs model control group. M: marker; 1: Sham group; 2: model control group; 3: sulodexide treated group; 4: combined group of sulodexide and irbesartan; 5: irbesartan treated group.
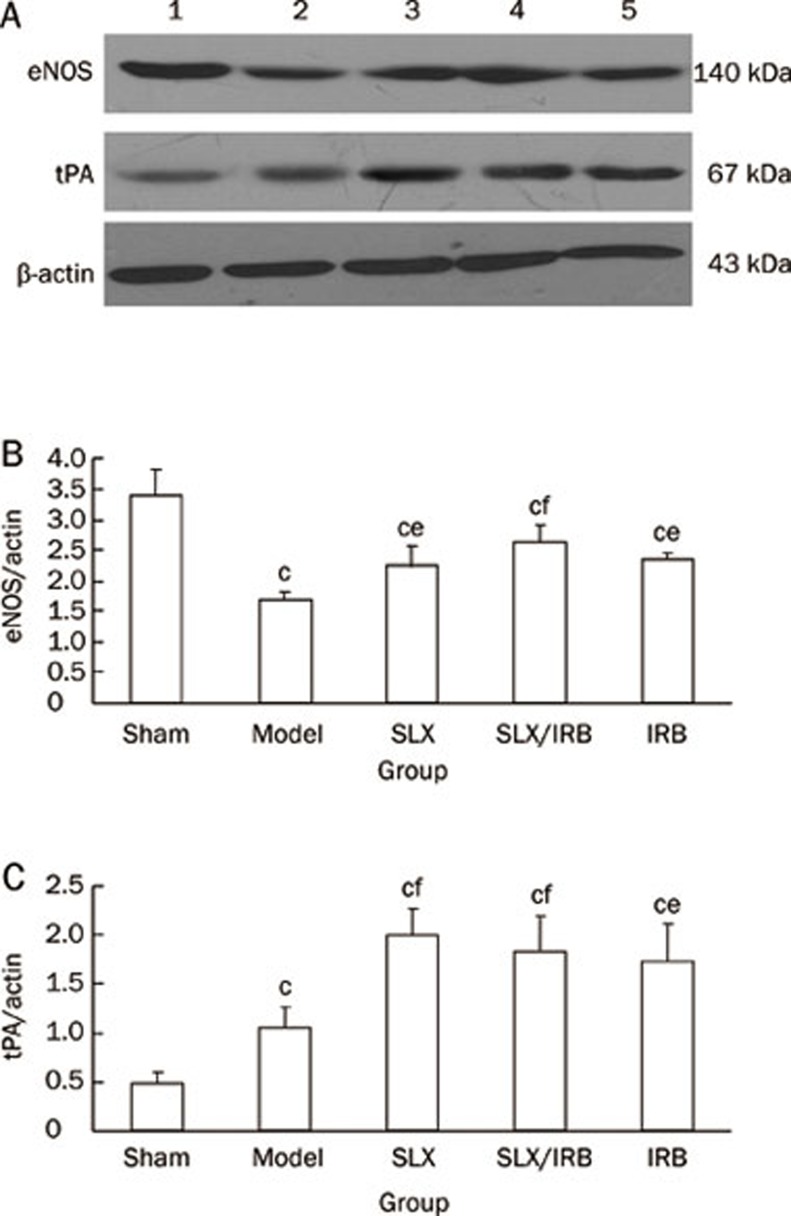
The protein expression of eNOS & tPA
Compared with the sham group, the protein expression of eNOS was reduced in the model control group (P<0.01). Compared with the model control group, the protein expression of eNOS was increased in the sulodexide-treated group, the combined sulodexide and irbesartan group, and the irbesartan-treated group (P<0.05) (Figure 4A, 4B).
Figure 4.
Analysis of renal eNOS (A, B) and tPA (A, C) protein expression after 12 weeks of treatment. 1: Sham group; 2: model control group; 3: sulodexide treated group; 4: combined therapy group of sulodexide and irbesartan; 5: irbesartan treated group. Mean±SD. Sham: n=12; Model: n=7; SLX: n=10; SLX/IRB: n=11; IRB: n=11. bP<0.05, cP<0.01 vs sham group; eP<0.05, fP<0.01 vs model control group.
The expression of tPA in the model control group was increased compared with Sham group (P<0.01), and it increased much more in the sulodexide-treated group, the combined sulodexide and irbesartan treated group, and irbesartan-treated group (P<0.01, P<0.01, P<0.05, Figure 4A, 4C).
Discussion
Sulodexide is a compound created from the fractional precipitation of glycosaminoglycans extracted from the mucosa of swine intestines, which mainly consists of fast-moving heparin (FMH) and dermatan sulfate (DS)14. Sulodexide has been used clinically in the treatment of patients with diabetic microvascular complications to reduce proteinuria in both type I and type II diabetes15. In a diabetic rat model induced by streptozotocin, sulodexide was reported to reduce the number of endothelial cells detached into the blood and to improve the diastolic function of the mesenteric artery16. However, whether sulodexide has a therapeutic function for the kidneys and for renal vascular endothelial cells in patients with CRF still needs to be demonstrated.
The 5/6 nephrectomy model is a common one for CRF17,18. Sclerosis of the residual nephrons gradually occurs in response to the high perfusion, high pressure, and high filtration caused by 5/6 nephrectomy. The renal function will deteriorate, along with the retention of substances such as serum creatinine, which should be discharged via the kidney19. These changes are similar to the major mechanisms of renal function regression in CRF. In this study, after the establishment of the 5/6 nephrectomy model, it was observed that the serum creatinine and urine protein levels increased continually, together with changes in the development of glomerular sclerosis and interstitial fibrosis, which suggested that the model was successfully created.
Many animal experiments and clinical trials have demonstrated a sound curative effect of sulodexide for proteinuria in diabetic nephropathy20,21,22,23,24. As sulodexide consists of GAGs abundant in anion electrical charges, its effect in reducing proteinuria may be related to the recovery by the glomerular barrier of electric charges22,25. Our results demonstrated that sulodexide was also able to reduce proteinuria in rats with 5/6 nephrectomy: 24-h urine protein was clearly lower in the sulodexide group and the combined sulodexide and irbesartan group than in the model control group after 8 or 12 weeks of treatment. In addition, the sulodexide group showed no difference in the degree of reduction of proteinuria compared with the irbesartan group, which resulted in the exciting finding that in rats with 5/6 nephrectomy, sulodexide was as effective as angiotensin receptor blocker (ARB)—the most approved agent in clinics for reducing proteinuria.
CRF is a disease that seriously endangers human health. Blocking renin-angiotensin-aldosterone system and controlling blood pressure are not sufficient to halt the progression of CRF1. It was in this study that sulodexide was first used in a 5/6 nephrectomy model. Over 4–12 weeks of treatment, sulodexide consistently decreased the levels of serum creatinine and steadily delayed the progression of renal failure. Furthermore, its effects were better than those of irbesartan in the early stages (fourth week). Unexpectedly, it was not observed that the combination of sulodexide and an ARB medicine had better effects. Currently, there is no proof that sulodexide and ARB medicines interact pharmacodynamically. The effect of sulodexide mainly focuses on the glomeruli, and sulodexide has an extremely high affinity for the vascular walls26, whereas ARB drugs decrease the high filtration, high perfusion and the high pressure of the glomeruli. It is not clear whether the abovementioned functions interfere with sulodexide locating the capillary loop and recovering the basement membrane, which requires further research and confirmation.
Our results showed that sulodexide is as effective as irbesartan in mitigating glomerular sclerosis and tubulointerstitial fibrosis in rats with 5/6 nephrectomy. There is damage of the vascular endothelium in CRF patients, which can lead to the gradual loss of the renal capillary bed area; then, ischemia/hypoxia is involved in the continuous progression of renal pathological lesions. Mitigation of injury to renal vascular endothelial cells may delay the progression of CRF pathological lesions20. Promoting the repair of the injured endothelium can achieve the effect of stabilizing renal function and delaying the progression of pathological changes that are not related to the control of blood pressure and proteinuria12. It has been found in animal models that sulodexide protects vascular endothelial cells from detachment26. JG-12 is an aminopeptidase that anchors onto the cell membrane with glycosyl-phosphatidylinositol (GPI) and that serves as a specific marker for the vascular endothelium27,28. Within the glomerular capsule, JG-12 is only expressed on the surface of the capillary endothelium26,29. In our experiments, JG-12 immunohistochemical (IHC) staining showed that sulodexide was able to abrogate injury due to the loss of renal capillaries. However, irbesartan did not show the same effect.
In this study, we explored sulodexide's impact on two factors that are related to the function of the vascular endothelium: eNOS and tPA. In the kidney, eNOS remains mainly in the vascular endothelium21,22. Previous studies have proved that nitric oxide, generated by eNOS, has a protective effect on the kidney23. NO is able to protect endothelial cells through its functions, including dilating blood vessels, inhibiting thrombocyte adhesion and aggregation, preventing white blood cells from attaching to the vascular wall, and inhibiting apoptosis of endothelial cells24. The expression of eNOS is reduced in damaged endothelial cells25. In this study, there was a decrease in both endothelial cells and eNOS expression in rats with 5/6 nephrectomy. After 12 weeks of treatment with sulodexide and irbesartan, renal eNOS expression clearly increased, suggesting a protective role for sulodexide in endothelial cells. Damaged endothelial cells also cause corresponding changes to their functions. One important physiological function of the endothelial cells is to regulate the proportion of blood-clotting substances and anti-clotting substances so the blood can flow smoothly in the vessels. Normal vascular endothelial cells manifest an “anti-clotting” phenotype. tPA and its specific inhibitor, type 1 plasminogen activator inhibitor (PAI-1), are mainly generated by vascular endothelial cells and are involved in the regulation of clotting and anti-clotting. The main function of tPA is to activate profibrinolysin in blood clots to produce fibrinolysin for the dissolution of thrombi. PAI-1 is a fast and specific physiological inhibitor of tPA. Both tPA and PAI-1 are markers for evaluating vascular endothelial function and injury. Under physiological conditions, tPA and PAI-1 are in states of dynamic equilibrium. Damaged endothelial cells transform from the anti-clotting type to the clotting type, with increased synthesis of PAI-1 and decreased synthesis of tPA4,30. In our experiments, the increase in the expression of tPA in the model control group indicated an abnormal state of the clotting/anti-clotting system in this 5/6 nephrectomy model; that the expression of tPA increased much more in the sulodexide-treated group and the combined sulodexide and irbesartan group indicated a potential protective role for sulodexide in the endothelium.
Blood lipid disorders are among the complications of CRF that are related to the reduction of lipoprotein lipase activity, as well as the reduced intake of lipids in circulation by the organ and tissues31. Increased blood cholesterol can bind to receptors on the mesangial cells, leading to cellular proliferation, matrix accumulation, and the production of cytokines, which can boost the progression of pathological changes in renal sclerosis32. In animals fed a diet containing cholesterin, sulodexide was able to reduce the level of plasma cholesterin12. In the present study, the serum cholesterin levels of the sulodexide-treated group were significantly lower than those of the model control group. However, the irbesartan group did not show the same effect, which indicated that the efficacy of sulodexide treatment was related to the inhibition of CH production.
The progression of CRF involves a variety of mechanisms, including glomerular hemodynamic changes, proteinuria, angiotensin II, inflammation reactions, cellular proliferation, matrix accumulation, abnormal blood lipid metabolism, and the formation of microthrombi inside the capillaries. It has been shown that a single agent is unable to stop the progression of renal pathological changes in CRF15. In this study, we found that sulodexide has the effect of halting the deterioration of renal function in 5/6 nephrectomy rats, and it has the same efficacy at proteinuria reduction as irbesartan. Sulodexide has a renal protective role in mitigating glomerular sclerosis and tubulointerstitial fibrosis in rats that have undergone 5/6 nephrectomy. These effects might be achieved via protection of the vascular endothelium.
Author contribution
Xiang-mei CHEN, Ru-juan XIE, Yuan-sheng XIE and Ri-bao WEI designed research; Lin-lin MA, Ping LI and Min YIN performed research; Ping LI and Lin-lin MA analyzed data; Ping LI, Lin-lin MA and Jian-zhong WANG wrote the paper.
Acknowledgments
This work was supported by the following grants: Key science and technology Program of the Beijing Academy of Sciences(D09050104310000); “Significant Creation of New Drugs” of National Science and Major Project (2010ZX09102-204); National Natural Sciences Foundation of China (81072914)
References
- Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52:249–55. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, et al. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–16. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- Gadola L, Noboa O, Márquez MN, Rodriguez MJ, Nin N, Boggia J, et al. Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int. 2004;65:1224–30. doi: 10.1111/j.1523-1755.2004.00496.x. [DOI] [PubMed] [Google Scholar]
- Vásquez J, Mathison Y, Romero-Vecchione E, Suárez C. Effect of sulodexide on aortic vasodilation capacity and associated morphological changes in rats with streptozotocin-induced diabetes. Invest Clin. 2010;51:467–77. [PubMed] [Google Scholar]
- Ceol M, Gambaro G, Sauer U, Baggio B, Anglani F, Forino M, et al. Glycosaminoglycan therapy prevents TGF-beta1 overexpression and pathologic changes in renal tissue of long-term diabetic rats. J Am Soc Nephrol. 2000;11:2324–36. doi: 10.1681/ASN.V11122324. [DOI] [PubMed] [Google Scholar]
- Shah SV. Progress toward novel treatments for chronic kidney disease. J Ren Nutr. 2010;20:S122–6. doi: 10.1053/j.jrn.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Cirujeda JL, Granado PC. A study on the safety, efficacy, and efficiency of sulodexide compared with acenocoumarol in secondary prophylaxis in patients with deep venous thrombosis. Angiology. 2006;57:53–64. doi: 10.1177/000331970605700108. [DOI] [PubMed] [Google Scholar]
- Coccheri S, Scondotto G, Agnelli G, Aloisi D, Palazzini E, Zamboni V, et al. Randomised, double blind, multicentre, placebo controlled study of sulodexide in the treatment of venous leg ulcers. Thromb Haemost. 2002;87:947–52. [PubMed] [Google Scholar]
- Karoń J, Połubinska A, Antoniewicz AA, Sumińska-Jasińska K, Breborowicz A. Anti-inflammatory effect of sulodexide during acute peritonitis in rats. Blood Purif. 2007;25:510–4. doi: 10.1159/000113011. [DOI] [PubMed] [Google Scholar]
- Fracasso A, Baggio B, Masiero M, Bonfante L, Bazzato G, Feriani M, et al. Effect of oral treatment with the glycosaminoglycan sulodexide on peritoneal transport in CAPD patients. Perit Dial Int. 2003;23:595–9. [PubMed] [Google Scholar]
- Crepaldi G, Fellin R, Calabrò A, Baiocchi MR, Rossi A, Lenzi S, et al. Preliminary results of sulodexide treatment in patients with peripheral arteriosclerosis and hyperlipidemia. A multicentre trial. Monogr Atheroscler. 1986;14:215–21. [PubMed] [Google Scholar]
- Radhakrishnamurthy B, Sharma C, Bhandaru RR, Berenson GS, Stanzani L, Mastacchi R. Studies of chemical and biologic properties of a fraction of sulodexide, a heparin-like glycosaminoglycan. Atherosclerosis. 1986;60:141–9. doi: 10.1016/0021-9150(86)90006-7. [DOI] [PubMed] [Google Scholar]
- Skrha J, Perusicová J, Kvasnicka J, Hilgertová J. The effect of glycosaminoglycan sulodexide on oxidative stress and fibrinolysis in diabetes mellitus. Sb Lek. 1998;99:103–9. [PubMed] [Google Scholar]
- Harenberg J. Review of pharmacodynamics, pharmacokinetics, and therapeutic properties of sulodexide. Med Res Rev. 1998;18:1–20. doi: 10.1002/(sici)1098-1128(199801)18:1<1::aid-med1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Perico N, Codreanu I, Schieppati A, Remuzzi G. Prevention of progression and remission/regression strategies for chronic renal diseases: can we do better now than five years ago. Kidney Int Suppl. 2005. pp. S21–4. [DOI] [PubMed]
- Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85–93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Ueda S, Yamagishi S, Matsuguma K, Shibata R, Fukami K, et al. Dimethylarginine dimethylaminohydrolase prevents progression of renal dysfunction by inhibiting loss of peritubular capillaries and tubulointerstitial fibrosis in a rat model of chronic kidney disease. J Am Soc Nephrol. 2007;18:1525–33. doi: 10.1681/ASN.2006070696. [DOI] [PubMed] [Google Scholar]
- Tsunenari I, et al. Renoprotective effects of telmisartan in the 5/6 nephrectomised rats. J Renin Angiotensin Aldosterone Syst. 2007;8:93–100. doi: 10.3317/jraas.2007.017. [DOI] [PubMed] [Google Scholar]
- Hostetter TH, Olson JL, Rennke HG., Venkatachalam MA, Brenner BM.Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation J Am Soc Nephrol 2001121315–25.11373357 [Google Scholar]
- Weiss R, Niecestro R, Raz I. The role of sulodexide in the treatment of diabetic nephropathy. Drugs. 2007;67:2681–96. doi: 10.2165/00003495-200767180-00004. [DOI] [PubMed] [Google Scholar]
- Heerspink HL, Greene T, Lewis JB, Raz I, Rohde RD, Hunsicker LG, et al. Effects of sulodexide in patients with type 2 diabetes and persistent albuminuria. Nephrol Dial Transplant. 2008;23:1946–54. doi: 10.1093/ndt/gfm893. [DOI] [PubMed] [Google Scholar]
- Gambaro G, Kinalska I, Oksa A, Pont'uch P, Hertlová M, Olsovsky J, et al. Oral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the Di.N.A.S. randomized trial. J Am Soc Nephrol. 2002;13:1615–25. doi: 10.1097/01.asn.0000014254.87188.e5. [DOI] [PubMed] [Google Scholar]
- Achour A, Kacem M, Dibej K, Skhiri H, Bouraoui S, El May M, et al. One year course of oral sulodexide in the management of diabetic nephropathy. J Nephrol. 2005;18:568–74. [PubMed] [Google Scholar]
- Lambers Heerspink HJ, Fowler MJ, Volgi J, Reutens AT, Klein I, Herskovits TA, et al. Rationale for and study design of the sulodexide trials in type 2 diabetic, hypertensive patients with microalbuminuria or overt nephropathy. Diabet Med. 2007;24:1290–5. doi: 10.1111/j.1464-5491.2007.02249.x. [DOI] [PubMed] [Google Scholar]
- Dedov I, Shestakova M, Vorontzov A, Palazzini E. A randomized, controlled study of sulodexide therapy for the treatment of diabetic nephropathy. Nephrol Dial Transplant. 1997;12:2295–300. doi: 10.1093/ndt/12.11.2295. [DOI] [PubMed] [Google Scholar]
- Kristova V, Liskova S, Sotnikova R, Vojtko R, Kurtansky A. Sulodexide improves endothelial dysfunction in streptozotocin-induced diabetes in rats. Physiol Res. 2008;57:491–4. doi: 10.33549/physiolres.931506. [DOI] [PubMed] [Google Scholar]
- Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–55. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciszewicz M, Polubinska A, Antoniewicz A, Suminska-Jasinska K, Breborowicz A. Sulodexide suppresses inflammation in human endothelial cells and prevents glucose cytotoxicity. Transl Res. 2009;153:118–23. doi: 10.1016/j.trsl.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Sulikowska B, Olejniczak H, Muszyńska M, Odrowaz-Sypniewska G, Gaddi A, Savini C, et al. Effect of sulodexide on albuminuria, NAG excretion and glomerular filtration response to dopamine in diabetic patients. Am J Nephrol. 2006;26:621–8. doi: 10.1159/000098195. [DOI] [PubMed] [Google Scholar]
- Messa G, La Placa G, Puccetti L, Di Perri T. Effectiveness and tolerability of heparan sulfate in the treatment of superficial thrombophlebitis. Controlled clinical study vs sulodexide. Minerva Cardioangiol. 1997;45:147–53. [PubMed] [Google Scholar]
- Kristová V, Kriska M, Babál P, Djibril MN, Slámová J, Kurtansky A. Evaluation of endothelium-protective effects of drugs in experimental models of endothelial damage. Physiol Res. 2000;49:123–8. [PubMed] [Google Scholar]
- Kristova V, Kriska M. Endothelial diseases and endothelium-protective agents. Bratisl Lek Listy. 1998;99:511–7. [PubMed] [Google Scholar]